Abstract
Positron emission tomography (PET) imaging of monoamine oxidases (MAO-A: [11C]harmine, [11C]clorgyline, and [11C]befloxatone; MAO-B: [11C]deprenyl-D2) has been actively pursued given clinical importance of MAOs in human neuropsychiatric disorders. However, it is unknown how well PET outcome measures for the different radiotracers are quantitatively related to actual MAO protein levels. We measured regional distribution (n=38) and developmental/aging changes (21 hours to 99 years) of both MAOs by quantitative immunoblotting in autopsied normal human brain. MAO-A was more abundant than MAO-B in infants, which was reversed as MAO-B levels increased faster before 1 year and, unlike MAO-A, kept increasing steadily to senescence. In adults, regional protein levels of both MAOs were positively and proportionally correlated with literature postmortem data of MAO activities and binding densities. With the exception of [11C]befloxatone (binding potential (BP), r=0.61, P=0.15), correlations between regional PET outcome measures of binding in the literature and MAO protein levels were good (P<0.01) for [11C]harmine (distribution volume, r=0.86), [11C]clorgyline (λk3, r=0.82), and [11C]deprenyl-D2 (λk3 or modified Patlak slope, r=0.78 to 0.87), supporting validity of the latter imaging measures. However, compared with in vitro data, the latter PET measures underestimated regional contrast by ∼2-fold. Further studies are needed to address cause of the in vivo vs. in vitro nonproportionality.
Keywords: ageing, immunoblotting, monoamine oxidase, positron emission tomography, postmortem human brain
Introduction
Monoamine oxidase (MAO, EC 1.4.3.4) catalyzes the de-amination of serotonin (5-HT), noradrenaline (NA) and dopamine, monoamine neurotransmitters implicated in a variety of normal and abnormal brain functions (see ref. 1 for a review). Two subtypes of the enzyme (MAO-A and MAO-B) have been identified in the human, which show different substrate selectivity, inhibitor sensitivity, and tissue and brain regional distribution and also different clinical significances. For example, inhibitors of MAO-A, which preferentially metabolizes 5-HT, are used in treatment of mood disorders and the loss of MAO-A in the human is associated with aggression, whereas inhibitors of MAO-B, which is the major enzyme metabolizing dopamine in the human and is abundantly expressed in glial cells, are sometimes used in treatment of Parkinson's disease, a dopamine-deficiency disorder.
Given the clinical importance of MAOs, radioligands for in vivo investigations of MAOs by positron emission tomography (PET) have been actively developed, with [11C]harmine,2, 3, 4 [11C]clorgyline,5 and [11C]befloxatone6 for MAO-A and [11C]deprenyl-D2 for MAO-B7, 8 having been applied in human brain imaging studies. In particular, recent studies of mood disorders using [11C]harmine have highlighted the role of MAO-A in the development of human depression (see ref. 9 for a review) and depressed mood after cigarette withdrawal.10 [11C]deprenyl-D2 PET imaging has also been used for (nonspecific) assessment of brain astrogliosis under several pathological conditions.11, 12 Importantly, these quantitative in vivo PET techniques are being used in the clinical development of a new generation of MAO-targeting compounds.13, 14
An important question with PET imaging has been whether the outcome measures of binding obtained at tracer dose of a radioligand by kinetic modeling are quantitatively related to actual levels of its target in brain.15, 16 In this regard, regional values of the distribution volume (VT or VS, with the free and nonspecific portion removed) of the reversible radiotracer [11C]harmine, as a measure of MAO-A density in human brain, were previously correlated with MAO-A distribution in rat brain determined by in vitro radioligand binding.2 For the acetylenic suicide radiotracer [11C]clorgyline and [11C]deprenyl-D2, the composite parameter λk3 is the preferred outcome measure of activities of MAOs in vivo (although when arterial blood sampling was not possible, a modified reference-Patlak model has been used instead, with the obtained Patlak slope as the binding measure)11, 12 and the regional ranking order of this model term for both tracers in human brain is consistent with activities of MAO-A5 and MAO-B,7 respectively, determined in autopsied human brains. However, literature data on the regional distribution of both MAOs, either in terms of activity or that determined by a radioligand binding assay, in postmortem human brain have been fragmentary, with no single study providing MAO levels (activity or binding) for many of the brain regions reported in PET studies,5, 7 making a meaningful correlation analysis difficult. Further, the specificity of MAO activities determined with different substrates (e.g., 5-HT for MAO-A and benzylamine or phenylethanolamine for MAO-B) are relative but not absolute; binding assays with radioligands for either subtypes had the same issue of relative specificity. To our knowledge, there has been no quantitative study of the regional distribution of the MAO proteins in the human.
The present study was designed to address this literature deficiency by using quantitative immunoblotting of MAO-A and MAO-B in autopsied human brains. We calibrated the assays using commercial recombinant MAO-A and MAO-B so that the levels of the two isozymes in human brains can be directly compared with each other. In addition, developmental and ageing changes of the monoamine metabolizing enzymes were also examined in a total of 70 autopsied brains with age ranging from 21 hours to 99 years old.
Materials and methods
Subjects
This study was approved by Research Ethics Board of the Centre for Addiction and Mental Health. A total of six (four male/two female, five died of cardiovascular illnesses and one from multiple injuries by accident) autopsied brains from neurologically normal subjects (age: 48±0.3 (47 to 49) years; postmortem interval: 16±3 (5.25 to 23) hours; mean±s.e.m. (range)) were used in the MAO regional distribution study. To evaluate the age and development-related changes of MAOs, frontal cortex (Brodmann area 10) from a total of 70 subjects (43 males/27 females) were used (see Supplementary Table 1 for details and causes of death of the subjects), with age ranging from 21 hours to 99 years old and a mean postmortem interval of 13±1 (3 to 27) hours. One-half brain was used for neuropathological examination, whereas the other half was frozen for neurochemical analyses.
Tissue sample preparation, sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and western blotting
Cerebral cortical subdivisions were excised according to the Brodmann classification. Dissection of the subcortical areas from ∼3-mm-thick coronal sections followed published procedures.17 Brain tissue homogenates were used throughout this study. Sample preparation, sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and western blot followed published procedures.17 Protein concentration was determined using the Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA, USA) with bovine serum albumin as the standard. Five concentrations of tissue standard (1 to 15 μg of protein), consisting of a pooled human striatal samples, were run on each blot together with the samples (10 to 40 μg of protein, depending on regional levels of MAOs). After probing for MAO-A, the polyvinylidene difluoride membranes were stripped and reprobed for MAO-B. The antibodies used for quantitative determination of levels of MAO-A and MAO-B were rabbit polyclonal antibodies from Santa Cruz Biotechnology (Dallas, TX, USA) (sc-20156, H-70, raised against C-terminal amino acids 458 to 527 of human MAO-A) and Abcam (Cambridge, MA, USA) (ab67297, raised against amino acids 448 to 466 of human MAO-B), respectively. The secondary antibody used was the goat anti-rabbit IgG (H+L) horseradish peroxidase from SouthernBiotech (cat. no. 4050-05, Birmingham, AL, USA). Another monoclonal anti-human MAO-A antibody from Santa Cruz Biotechnology (sc-271123, clone G-10, IgG1, raised against the same peptide as that for the polyclonal H-70) was also used to help characterize the MAO-A immunoreactive protein bands. For antibody characterization and quantitative measurement, recombinant human MAO-A (M7316, lot#069K-1040) and MAO-B (M7441, lot#039K159) were obtained from Sigma-Aldrich (St Louis, MO, USA), with levels of the overexpressed enzymes in the microsome preparations calibrated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis against a serial concentration of bovine serum albumin (0.25 to 10 μg) according to ref. 18. Western blot of the ‘control' protein neuron-specific enolase followed published procedures.17
Data analyses
A linear five-point standard curve of the tissue standards was constructed for each blot. Levels of MAO-A and MAO-B in the tissue standard were first calibrated by using a serial dilution of the recombinant MAO proteins (Figure 1). The concentration of both MAOs in a sample was determined by interpolation from the respective standard curve and expressed as ng/μg protein. The within and between blots coefficient of variation were 6.7% and 11.5% for MAO-A and 7.0% and 6.4% for MAO-B, respectively. No significant correlation (Pearson) was observed between levels of either MAO-A or MAO-B protein and postmortem interval of the subjects examined.
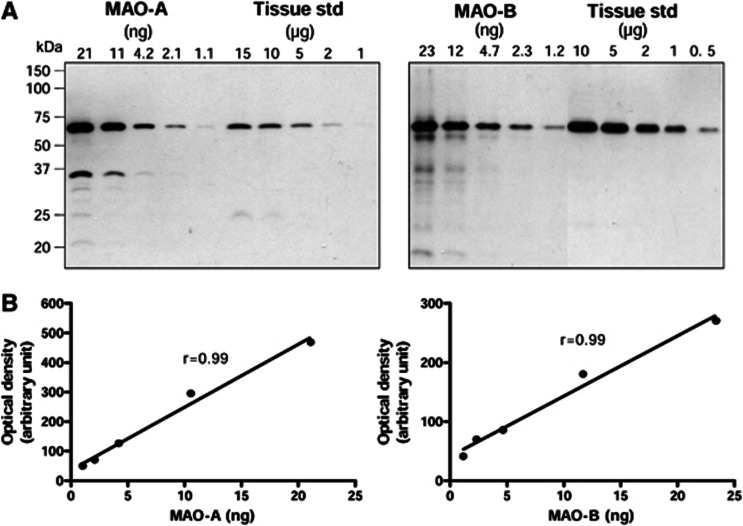
Figure 1.
Quantification of monoamine oxidases (MAOs) in autopsied human brain. (A) immunoblots of MAO-A and MAO-B in the pooled striatum tissue standards (0.5 to 15 μg) and in the commercial overexpressed recombinant enzymes (1 to 25 ng). (B) Standard curves for the recombinant MAOs.
Statistical analyses were performed by using StatSoft STATISTICA 7.1 (Tulsa, OK, USA). Monoamine oxidase regional distribution was examined by one-way repeated measures analysis of variance with MAO subtype as the repeated factor followed by post hoc Bonferroni adjustments. Possible correlations between MAO levels vs. age of the subjects and between regional MAO levels vs. literature data of MAO activities and binding densities (see below) were examined by Pearson product-moment correlation or by Spearman rank-order correlation as indicated in the text. F-tests of the null-hypothesis intercept=0 for the linear correlations, i.e., proportional, were performed by using GraphPad Prism 4.0.
Results
Characterization of monoamine oxidase-immunoreactive protein bands in western blots of normal autopsied human striatum
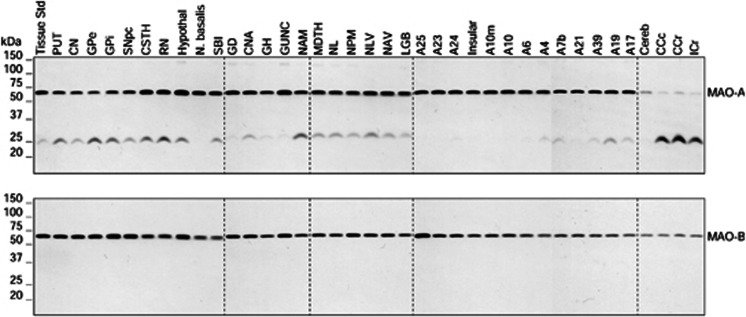
The polyclonal anti-MAO-A and MAO-B antibodies detected in human striatal samples one major protein band with an apparent molecular weight of approximately 65 kDa and 64 kDa, respectively (Figure 1). Strip-and-reprobe of the same blot with the anti-MAO-B antibody after probe with anti-MAO-A confirmed that the MAO-B immunoreactive protein band ran slightly faster than that for MAO-A. This is consistent with the relative theoretical size of the two MAO isozymes (MAO-A: 59.7 kDa; MAO-B: 58.0 kDa)1 and the slightly larger than expected apparent molecular weight of the holoenzymes in sodium dodecyl sulfate–polyacrylamide gel electrophoresis. With recombinant MAOs as the positive controls, the two closely located protein bands in brain tissues migrated at the same positions as the respective recombinant proteins (Figure 1), confirming correct identification of MAO-A and MAO-B in brain by the two antibodies, respectively. As expected, there was no cross-reaction of the two antibodies with the opposite isozyme when switching primary antibodies for detection of the recombinant proteins even after longer exposure and higher amount of protein loaded (data not shown), again confirming their specificity. However, a minor protein band of molecular weight ∼25 kDa was also detected by the anti-MAO-A antibody in brain samples, in particular in white matters (Figure 3), likely a N-terminal truncated fragment of MAO-A that was not included in the quantitative analyses below. The recombinant proteins also showed several minor MAO-immunoreactive protein bands on higher loading and longer exposure, likely partially degraded products.
A monoclonal anti-MAO-A antibody was also tested and detected the same protein band as the polyclonal one, although with much lower sensitivity (data not shown). Therefore, the polyclonal antibody was selected for the quantitative study.
Quantification of monoamine oxidases in human brain tissues
Levels of MAO-A and MAO-B protein in the commercial overexpressed recombinant preparations were determined to be 0.211±0.010 and 0.234±0.010 ng/μg protein (six determinations). Thereafter, the recombinant enzymes were used to calibrate the concentration of both MAOs in the pooled striatum tissue standard used in the quantitative immunoblotting assays, which were determined to be 0.399±0.042 (MAO-A) and 3.096±0.192 (MAO-B) ng/μg protein, respectively (see Figure 1 for blots and calibration curves of a representative experiment of three).
Developmental and ageing changes of monoamine oxidase in human frontal cortex
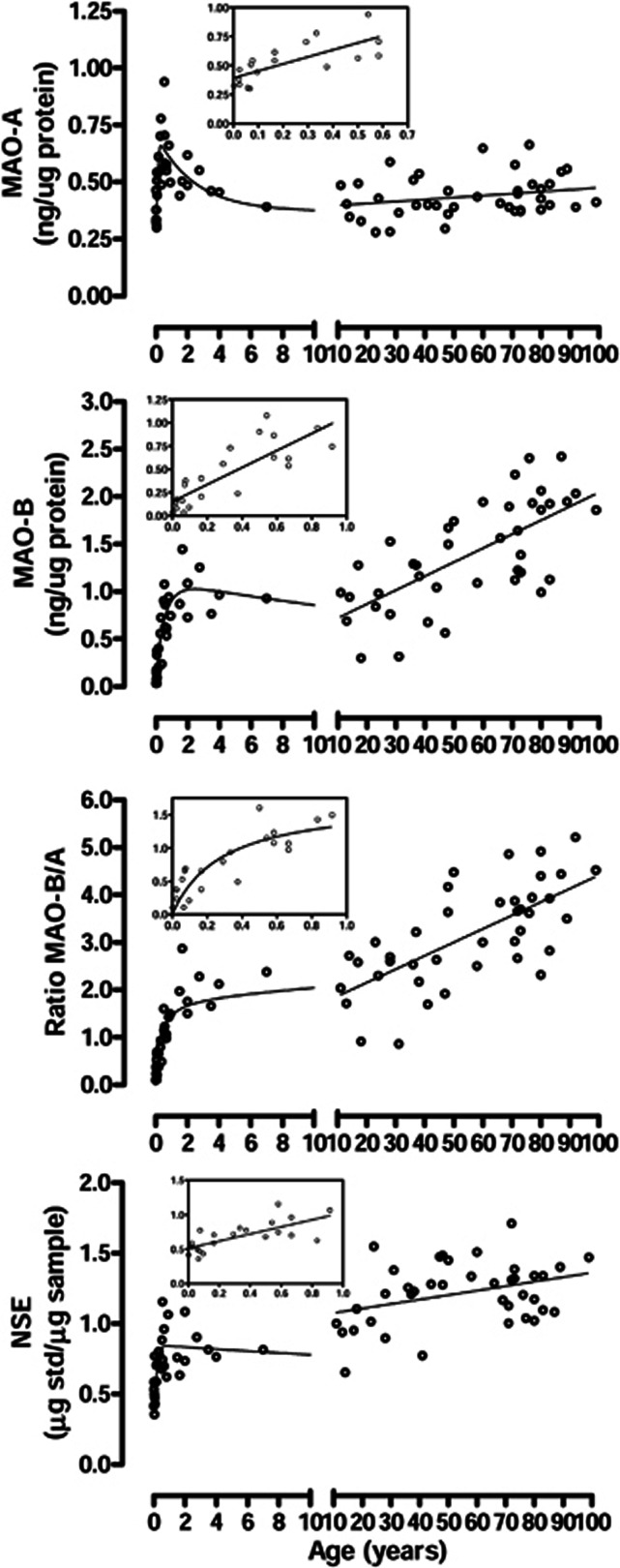
Overall, there were significant positive correlations with age (from 21 hours to 99 years old, n=70) for levels of MAO-B (Pearson) and the ratio of MAO-B vs. MAO-A (Spearman) (r=0.78 and 0.90, respectively, P<0.0001) but not for MAO-A (Pearson r=−0.21, P>0.05).
A close examination showed that the age-related changes could be separated into three phases—infant (within 1 year of age), toddler (1 to 4 years), and thereafter (Figure 2). During infancy, both MAOs increased rapidly with age (MAO-A: r=0.73, P=0.0005, n=18 (21 hours to 7 months); MAO-B: r=0.82, P<0.0001, n=22 (21 hours to 11 months); Figure 2, insets). However, the two subtypes showed quite different patterns of neonatal growth. MAO-B was barely detectable in brain at birth but increased quickly (0.076 ng/μg/month) during the first year, reaching a high at around 2 years of age, which then decreased slightly during the next few years. In contrast, levels of MAO-A at birth were already 78% of adult levels and increased with a rate of 0.050 ng/μg/month to reach a high of ∼50% above those of adults by 7 months, which then declined during the next couple of years and remained stable thereafter. Thus, levels of MAO-B were lower than those of MAO-A before ∼6 months of age but with the ratio increased hyperbolically during infancy and then linearly after 1 year. During adulthood (above 18 years), levels of MAO-A remained stable (r=0.29, P>0.05), whereas those of MAO-B increased at a rate of 0.014 ng/μg/year or 18% per decade (18 to 99 years old, r=0.68, P<0.0001). Consequently, the ratio of MAO-B vs. MAO-A increased from 1.8 at 18 years of age to 4.6 at 99 years in the frontal cortex.
Figure 2.
Ageing and neonatal developmental changes of levels of monoamine oxidases (MAOs), the ratio of MAO-B vs. MAO-A, and the control protein neuronal-specific enolase (NSE) in autopsied human brain. The insets show enlarged portion below 1 year of age.
As a control protein, levels of neuron-specific enolase were significantly and positively correlated (Pearson) with age (r=0.73, n=69, P<0.0001), although similar to those of MAOs, there were an early quick-increase phase during infancy and an adjustment phase in the next couple of years (Figure 2).
We also examined the partial correlation between levels of the two MAO subtypes after controlling for the effect of age, with significant positive correlations (P<0.0005) observed either for the full age range (21 hours to 99 years, r=0.54), adult only (⩾18 years, r=0.64), childhood only (<18 years, r=0.61), or infancy only (<1 year, r=0.82). In contrast, no significant correlation existed between levels of either MAO and those of neuron-specific enolase after controlling for the ageing effects during the full age range (r=0.09 and 0.16 for MAO-A and MAO-B, respectively) or adulthood (r=−0.004 and 0.01 for MAO-A and MAO-B, respectively), although there were positive correlations (<18 years, r=0.55 and 0.53 for MAO-A and MAO-B, respectively) or trends (<1 year, r=0.42, P=0.06 and r=0.33, P=0.14 for MAO-A and MAO-B, respectively) during childhood, likely reflecting brain/neuronal development.
Regional distribution of monoamine oxidases in normal human brain
A total of 38 brain regions sampling the human forebrain were examined in six subjects of 47 to 49 years old with the exception of samples of lateral geniculate body, which were available for only two subjects (see Figure 3 for representative immunoblots).
Figure 3.
Representative immunoblots of the regional distribution of monoamine oxidases (MAO-A and MAO-B) in autopsied human brain. A23, cingulate gyrus posterior; A24, cingulate gyrus anterior; A25, paraolfactory/subgenual gyrus; CCc, corpus callosum caudal; CCr, corpus callosum rostral; cereb, cerebellar cortex; CN, caudate; CNA, hippocampal Ammon's horn; CSTH, subthalamic nucleus; GD, dentate gyrus; GH, hippocampal gyrus; GPe, globus pallidus external; GPi, globus pallidus internal; GUNC, gyrus of uncus; hypothal, hypothalamus; ICr, internal capsule rostral; LGB, lateral geniculate body; MDTH, mediodorsal thalamus; NAM, amygdala; NAV, anterior ventral nucleus of thalamus; N. basalis, nucleus basalis; NL, nucleus lateralis of thalamus; NLV, lateral ventral nucleus of thalamus; NPM, medial pulvinar of thalamus; PUT, putamen; RN, red nucleus; SBI, substantia innominata; SNpc, substantia nigra pars compacta.
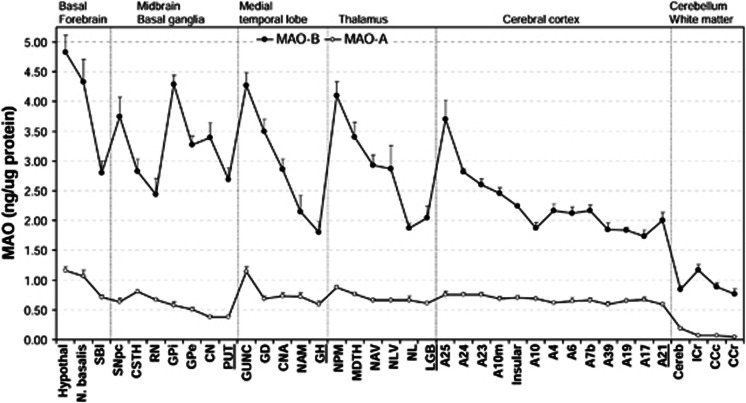
Overall, repeated measures analysis of variance (excluding lateral geniculate body) showed that the distribution of both MAOs was highly heterogeneous (F37, 186=31, P<0.0001), the difference between levels of MAO-A and MAO-B was highly significant (F1, 186=4533, P<0.0001), and there was a significant subtype × brain region interaction (F37, 186=23, P<0.0001). As shown in Figure 4, brain regions examined were grouped according to major divisions, including basal forebrain areas, basal ganglia, medial temporal lobe, thalamus, cerebral cortices, cerebellar cortex, and white matters. Highest levels of both MAOs (in ng/μg protein, mean±s.e.m.) were observed in a few small brain areas including hypothalamus (A: 1.16±0.07; B: 4.83±0.28), nucleus basalis (A: 1.06±0.10; B: 4.33±0.37), and the hippocampal uncus (A: 1.14±0.08; B: 4.27±0.21), whereas the lowest levels among the gray matter regions were found in the cerebellar cortex (A: 0.19±0.02; B: 0.84±0.06). The striatum and globus pallidus (GP) contained low levels of MAO-A (caudate: 0.38±0.03; putamen: 0.37±0.03; GP external: 0.51±0.04; GP internal: 0.57±0.06), but rather high levels of MAO-B (caudate: 3.39±0.25; putamen: 2.69±0.20; GP external: 3.27±0.15; GP internal: 4.29±0.15). In contrast, cerebral cortices had high and evenly distributed levels of MAO-A (0.59 to 0.75) but relatively low levels of MAO-B in most of the cortical areas (1.73 to 2.24) with the exception of medial frontal (2.46±0.09) and posterior (A23: 2.60±0.09), anterior (A24: 2.82±0.05), and subgenual (A25: 3.70±0.32) cingulate cortices, which also had the highest levels of MAO-A (0.75) among the cortical areas. The thalamus and medial temporal lobe (hippocampus and amygdala) had quite variable levels of MAOs in their subdivisions, with hippocampal uncus (above) and medial pulvinar thalamus (A: 0.88±0.04; B: 4.10±0.23) among areas containing the highest levels of both MAOs, whereas the hippocampal gyrus (A: 0.59±0.06; B: 1.80±0.19) and lateral thalamus (A: 0.66±0.07; B: 1.87±0.08) having levels similar to the bulk of the cerebral cortical areas. Three white matter samples were examined, which all contained a negligible amount of MAO-A (0.04 to 0.07) but, surprisingly, a significant level of MAO-B (0.77 to 1.17), in particular in the internal capsule (A: 0.07±0.01; B: 1.17±0.10).
Figure 4.
Regional distribution (mean±s.e.m.) of monoamine oxidases (MAO-A and MAO-B) in autopsied human brain (n=6 with the exception of n=2 for LGB). A23, cingulate gyrus posterior; A24, cingulate gyrus anterior; A25, paraolfactory/subgenual gyrus; CCc, corpus callosum caudal; CCr, corpus callosum rostral; cereb, cerebellar cortex; CN, caudate; CNA, hippocampal Ammon's horn; CSTH, subthalamic nucleus; GD, dentate gyrus; GH, hippocampal gyrus; GPe, globus pallidus external; GPi, globus pallidus internal; GUNC, gyrus of uncus; hypothal, hypothalamus; ICr, internal capsule rostral; LGB, lateral geniculate body; MDTH, mediodorsal thalamus; NAM, amygdala; NAV, anterior ventral nucleus of thalamus; N. basalis, nucleus basalis; NL, nucleus lateralis of thalamus; NLV, lateral ventral nucleus of thalamus; NPM, medial pulvinar of thalamus; PUT, putamen; RN, red nucleus; SBI, substantia innominata; SNpc, substantia nigra pars compacta.
Ratio of monoamine oxidase-B to monoamine oxidase-A and correlations between the two isoforms
Post hoc analyses with Bonferroni adjustments for multiple comparison (37 brain regions) showed that levels of MAO-B were significantly higher than those of MAO-A in every brain region examined (P<0.0005), with the ratio of MAO-B to -A ranging from 2.6 (A17, occipital cortex) to 17.8 (corpus callosum caudal). The highest ratios were found in white matters (13.6 to 17.8), containing a negligible concentration of MAO-A (above). Among gray matters, highest ratios were in the basal ganglia, including caudate (9.0), globus pallidus internal (7.5), putamen (7.2), globus pallidus external (6.5), and substantia nigra pars compacta (5.9). All other brain regions had a quite homogeneous MAO-B/MAO-A ratio (on average 3.7 (2.6–5.1)), despite variance in absolute levels of the enzymes. In general, there were significant positive correlations (Pearson P<0.0001) between regional levels of the two MAO subtypes in the whole regional data set (r=0.68, n=224), after excluding the basal ganglia areas that had higher MAO-B/MAO-A ratios (r=0.81, n=194), after excluding additional extreme areas with lowest (white matters and cerebellar cortex) and highest (hypothalamus, hippocampal uncus, and nucleus basalis) levels of both MAOs to avoid outlier effects (r=0.53, n=152), and in the basal ganglia only (r=0.69, n=30).
Correlation between regional levels of monoamine oxidases and monoamines
By using postmortem brain regional (n=27) monoamine neurotransmitter data, previously reported from our laboratory,19 significant or trends for positive correlations (Pearson) were observed between regional levels of MAO-A vs. NA (r=0.56, P=0.003), 5-HT (r=0.31, P=0.11), and NA+5-HT (r=0.56, P=0.003) and between MAO-B vs. 5-HT (r=0.53, P=0.004), NA (r=0.51, P=0.006), and NA+5-HT (r=0.61, P=0.0007). There was no significant correlation between levels of either MAOs and those of dopamine or total monoamine neurotransmitters, primarily because of unmatched high levels of dopamine in the caudate and putamen (at least sixfold higher than those of any other brain regions).
Correlation between regional protein levels of monoamine oxidases and enzyme activities or binding densities in the literature
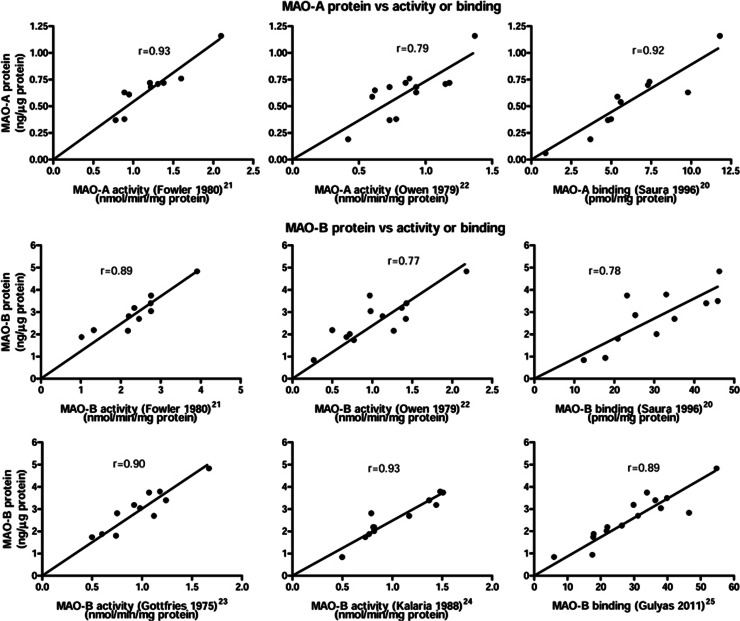
By using literature reports of regional postmortem brain MAO activities and binding densities (Bmax) of autoradiography, we assessed their correlation (Pearson) with our regional data. The studies included in this analysis (Supplementary Table 2 for details of subject number, age, postmortem interval, and regional data in these studies as compared with the current report) examined at least 10 different and widespread forebrain areas and used relatively specific substrates (MAO-A: 5-HT; MAO-B: benzylamine or phenylethylamine) or radioligands for the assays. In cases where subdivisional data were reported (e.g., CA1-3 of hippocampal Ammon's horn, subregions of hypothalamus, and layers of cerebellar cortex in autoradiography of Saura et al;20 subregions of thalamus (including medial pulvinar, mediodorsal, anterior ventral, lateral ventral, and nucleus lateralis), hippocampus (including dentate gyrus, CNA, and hippocampal gyrus), GP (globus pallidus internal and external), and white matters (internal capsule rostral, corpus callosum rostral, and corpus callosum caudal) in the present study), averages of the subdivisional data were used. As shown in Figure 5, significant (P<0.0001) and proportional (P>0.05 for the null-hypothesis intercept=0) correlations existed between regional levels of MAO-A protein determined by immunoblotting in our study vs. MAO-A/5-HT (substrate used) activity of Fowler et al21 (r=0.93, n=10), MAO-A/5-HT activity of Owen et al22 (r=0.79, n=13), and MAO-A/[3H]Ro41-1049 Bmax of Saura et al20 (r=0.92, n=11) and between MAO-B protein vs. MAO-B/benzylamine activity of Fowler et al21 (r=0.89, n=10), MAO-B/benzylamine activity of Owen et al22 (r=0.77, n=13), MAO-B/phenylethylamine activity of Gottifries et al23 (r=0.90, n=11), MAO-B/benzylamine activity of Kalaria et al24 (r=0.93, n=12), MAO-B/[3H]lazabemide Bmax of Saura et al20 (r=0.78, n=11), and MAO-B/[11C]deprenyl binding of Gulyas et al25 (r=0.89, n=15).
Figure 5.
Correlations (Pearson) between regional protein levels of monoamine oxidases (MAO-A and MAO-B) determined by immunoblotting in this study vs. activities and binding densities of the respective isozymes reported in the literature.
Correlation between regional protein levels of monoamine oxidases and positron emission tomography outcome measures
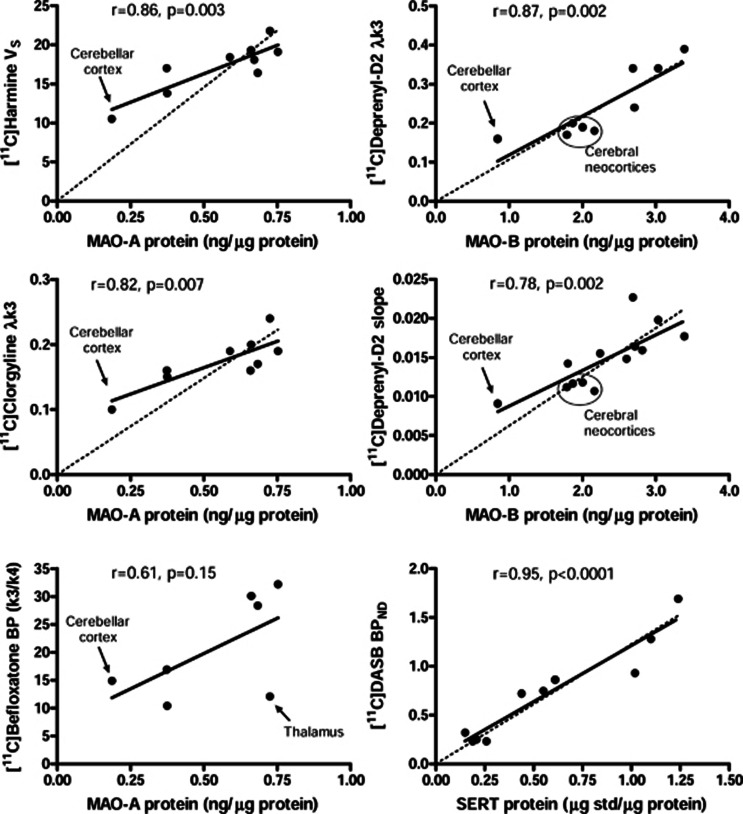
The literature disclosed brain imaging studies of MAO-A ([11C]harmine,2, 3 [11C]clorgyline,5, 26 and [11C]befloxatone6) and MAO-B ([11C]deprenyl-D2).7, 11, 12, 26 As shown in Figure 6, significant positive correlations (Pearson) were observed between regional levels of MAO-A protein vs. [11C]harmine VS2, 3 (r=0.86, n=9, P=0.003) and [11C]clorgyline λk3 (ref. 26) (r=0.82, n=9, P=0.007) and between those of MAO-B protein vs. [11C]deprenyl-D2 λk3 (ref. 26) (r=0.87, n=9, P=0.002) and modified Patlak slope11, 12 (r=0.78, n=13, P=0.002). For [11C]harmine, similar correlations were obtained when using VS, VT, or k3/k4 with constraints on K1/k2 (methods C and D in ref. 2) but not when using k3/k4 without constraints on K1/k2 (method B of ref. 2). However, the correlation between regional BP (k3/k4) of [11C]befloxatone6 and MAO-A protein levels was relatively poor (r=0.61, n=7, P=0.15) due in large part to the low BP values reported in the thalamus (Figure 6). Further examination of those showing good correlations disclosed a lack of proportionality. Thus, for MAO-A, the null-hypothesis intercept=0 was rejected for both [11C]harmine (P=0.002) and [11C]clorgyline (P=0.01). The ratios between thalamus and cerebellar cortex were 3.9 for protein levels vs. only 2.1 for [11C]harmine VS and 2.4 for [11C]clorgyline λk3. For MAO-B, although the intercepts were not significantly different from zero, the cerebral neocortical areas had unusually low in vivo binding, expressed either in λk3 or in modified Patlak slope, that was not significantly different from the cerebellar cortex, and the ratios between caudate and cerebellar cortex were 4.0 for protein levels vs. 2.4 for [11C]deprenyl-D2 λk3 and 1.95 for modified Patlak slope. For comparison, we show the correlation between regional brain levels of the serotonin transporter17 and [11C]DASB PET measurement of serotonin transporter densities (BPND),27 both obtained in our laboratory, in which the correlation between protein and PET measures is high (r=0.95, n=10, P<0.0001) and proportional (intercept=0).
Figure 6.
Correlations (Pearson) between regional protein levels of monoamine oxidases (MAO-A and MAO-B) determined in this study vs. outcome measures of density ([11C]harmine distribution volume VS2, 3 and [11C]befloxatone binding potential k3/k4)6 or activity (λk326 or modified Patlak slope11, 12 for [11C]clorgyline and/or [11C]deprenyl-D2) of the respective isozymes reported by positron emission tomography (PET) in the literature. For comparison, the correlation between regional protein levels of serotonin transporter (SERT)17 and [11C]DASB PET measurement of SERT density (BPND)27 is also shown. The dotted lines show expected proportional correlations. Note the poor correlation for [11C]befloxatone, the lack of proportionality for MAO-A PET, and similar binding of MAO-B PET despite twofold differences in MAO-B protein concentrations between cerebellar and cerebral neocortices.
Discussion
To our knowledge, this is the first detailed quantitative examination of MAO-immunoreactive species in human brain. The major finding, from a practical neuroimaging perspective, is that the previously published regional brain PET outcome measures of levels of both MAO subtypes correlated generally well with actual postmortem brain levels of MAO proteins with the exception of that using the MAO-A radiotracer [11C]befloxatone. Nevertheless, in vivo PET underestimates somewhat the regional contrast as observed in vitro. We also examined developmental and ageing changes of both MAOs and found that although MAO-B is much more expressed than MAO-A in brain from adolescence to senescence, this relationship is reversed in neonatal developing brain.
Levels of monoamine oxidases and ratio of monoamine oxidase-B/monoamine oxidase-A in the human brain
In postmortem human brain, levels of MAOs have previously been determined by activity titrations with subtype-specific substrates and the irreversible inhibitors (clorgyline, deprenyl, J-508 or pargyline)21, 28 or radioligand binding assays with [3H]pargyline (combined with clorgyline or deprenyl),28 [3H]Ro41-1049, and [3H]lazabemide.20, 29, 30 The reported MAO concentrations have been quite variable, for example, for cerebral cortex, ranging from 1.6 to 33 pmol/mg protein for MAO-A (equivalent to 0.1 to 2 ng/μg protein) and from 1.8 to 31 pmol/mg protein for MAO-B (equivalent to 0.11 to 1.9 ng/μg protein), likely because of uncertain nonspecific binding profile and cross-reaction of these ligands/substrates, in particular for activity titration.28 In the present study, specificity of antibodies was confirmed by recombinant human MAOs and no cross-reactivity of the antibodies to the other subtypes was observed even after overloading and long exposure. Further, calibration with the recombinant proteins allowed for direct comparison of relative levels of the two subtypes, confirming the prevalent view that adult human brain contains higher levels of MAO-B than those of MAO-A. The ratios of MAO-B/MAO-A among the brain regions examined were indeed in the same range as those reported by Saura et al20 by autoradiography, e.g., 15.9 vs. 16 in white matters, 9.0 vs. 8.5 in caudate, 7.2 vs. 7.0 in putamen, and 3.4 vs. 5.7 in temporal cortex.
It is noteworthy though that, in general, individuals or brain areas having high levels of one MAO subtype also tended to have high levels of another after controlling for ageing effects, except when comparing regions with high dopamine levels (basal ganglia) to other brain areas. This was somewhat corroborated by generally positive correlations between regional levels of MAOs and those of NA, 5-HT, or both but not dopamine. Although it is well known that the 5-HT neuronal cell body region raphe area contains mainly MAO-B, whereas the NA neuronal cell body region locus ceruleus contains mainly MAO-A,1 studies on the cellular and subcellular localization of MAOs in the human forebrain monoamine terminal fields have been scanty. Animal data indicate that in telencephalon and diencephalon MAO-B appears to be widely expressed by different types of neurons (e.g., histamine, acetylcholine) and nonneuronal cells (e.g., astroglials), whereas MAO-A is localized to all three monoaminergic terminals and also to other neuronal types (see ref. 31 and references therein). The dopamine-rich areas, in particular caudate and putamen, have unmatched ‘low' levels of MAOs, which might be related to a recent observation that 5-HT neurotransmission is largely reuptake and metabolism controlled, whereas dopamine levels are regulated mainly by synthesis, reuptake, and repackaging.32
Aging and developmental changes of monoamine oxidases
Confirming numerous previous studies of MAO activities21, 30 and radioligand binding,30 adult aging is associated with a marked increase in protein levels of MAO-B but not MAO-A (see ref. 1 for a review). Correspondingly, the ratio of MAO-B/MAO-A also increases linearly with aging. In the examined frontal cortex, the rate of increase for MAO-B during adulthood and senescence is 18% per decade from 18 years old, which is higher than that obtained with [11C]deprenyl-D2 PET (6%/decade)33, but in the range of those reported in postmortem studies of MAO-B activity (11%, ref. 21; 29%, ref. 30) and binding (33%, ref. 30).
However, the relative abundance and age-related growth rate of the two MAO isoforms in neonatal brain development were quite different from those of adults. Thus, MAO-A was already high in brain at birth, with MAO-B barely detectable; however, expression of MAO-B increased much faster after birth and overtook MAO-A at ∼6 months of age. This early phase of MAO-B growth was 66-fold faster than during adulthood. For MAO-A, an early increase was followed by a quick adjustment back to adult levels at approximately 1 year old, which was consistent with a previous report of postnatal MAO-A activity decreases in human frontal cortex.34 Our observations of neonatal changes of proteins levels of MAO-A and MAO-B are also in line with those reported for MAO activities in rodent postnatal development (see ref. 1 for references), suggesting conserved but distinctive role of the two subtypes in brain development, e.g., recent data showing a role of MAO-A but not MAO-B in modulating developmental apoptosis,35 even though the relative abundance of MAO-B vs. MAO-A in rodent brains can be opposite to that in the human.29
Monoamine oxidase regional brain distribution and implication for positron emission tomography imaging
Comparing regional concentrations of MAO proteins with the postmortem literature data of MAO activity and binding density, the correlations were generally ‘proportional' (i.e., if MAO activity in region X was twice that in region Y, protein levels are also twice as high; Figure 5) even though the absolute values of MAO activity and Bmax can be variable from lab to lab, and none of the previous studies examined all of the different gray and white matter regions reported here. This suggests that for both isoforms of MAO, enzyme activity, ligand binding and the protein amount are directly related to each other at least in vitro.
With the exception of [11C]befloxatone (BP: k3/k4), the regional PET outcome measures of binding for MAO-targeting radiotracers [11C]harmine and [11C]clorgyline for MAO-A and [11C]deprenyl-D2 for MAO-B showed good correlations with the concentration of respective MAO proteins (Figure 6), providing support for the validity of the latter brain imaging measurements. For [11C]befloxatone, the poor correlation was primarily driven by similarly low BP (k3/k4) values in thalamus, caudate, putamen, and cerebellar cortex despite marked difference in MAO-A protein levels among the brain regions. Although [11C]befloxatone is a highly specific reversible MAO-A inhibitor in vitro, its in vivo kinetics at a PET tracer dose in humans might not be optimal, or it may be that the parameter selected (k3/k4 with constraints on K1/k2) was not optimally identifiable.6 Some (minor) discrepancies might be attributable to limitations of autopsied brain studies. For example, whereas thalamus had the highest PET binding of MAO-A with either [11C]harmine or [11C]clorgyline, the average concentration of MAO-A protein in postmortem brain, including all thalamic subregions examined, was no different from that of hippocampus or anterior cingulate cortex. In principal, this could be related to subregionally heterogeneous distribution of MAOs, in particular in thalamus and hippocampus (Figure 4), and limited tissue sampling in our immunoblotting study. The thalamic subregions are not equal in volume, with the pulvinar nucleus dominant and having the highest levels of MAOs; thus, simple mathematical average of MAO protein levels in the subregions would have underestimated the overall concentration.
However, as can be seen from Figure 6, perhaps a more subtle ‘discrepancy' between results of our in vitro measurement of MAO proteins and the in vivo PET outcome measures of the ‘good' radiotracers is in the lack of quantitative proportionality, in particular for MAO-A. Thus, considering only the major gray matter regions (i.e., excluding the small areas with very high levels of MAOs, e.g., hypothalamus, uncus, and subgenual gyrus A25, which are delineated with difficulty by PET), the difference between, for example, cerebellar cortex, the area with the lowest amount of both MAO proteins, and areas with high levels of MAOs (thalamus for MAO-A and caudate nucleus for MAO-B) were ∼4-fold for both MAOs in vitro but 2- to 2.5-fold in vivo. Further, [11C]deprenyl-D2 binding for the cerebellar cortex was not significantly different from those of many cerebral neocortical areas7, 8, 11, 12, 26 despite more than 2-fold differences in levels of MAO-B protein (Figure 6). Part of this discrepancy could be related to larger partial volume effects in cerebral cortices than in cerebellar cortex. In this respect, it is interesting to note that whereas MAO-A is evenly distributed across layers of cerebral cortex, MAO-B is particularly enriched in the superficial layer,29 which might make PET imaging of cerebral cortical MAO-B more susceptible to partial volume effects. In addition, noise sensitivity of the radiotracer and modeling outcome measures, in particular in brain regions of low signal-to-noise ratio, e.g., cerebellar and cerebral cortices for MAO-B, may also contribute to the difference between PET and postmortem measures. It could be argued that this lack of proportionality between in vitro and in vivo measures of enzyme concentrations is related to differences in the assumptions made between these techniques. However, there is evidence from the studies of other PET radiotracers that in vivo and in vitro parameter of binding density can be proportionally (well) correlated, e.g., for [11C]DASB/serotonin transporter (Figure 6), [11C]MP4A/acetylcholinesterase,36 [18F]fallypride/D2-like receptor,16 and this proportionality was suggested to be ‘required' for validity of a radiotracer in previous studies.15 That the flattened contrast effect was similar for all three ‘good' MAO radiotracers of different mechanisms of binding (reversible vs. irreversible) does suggest that the explanation might not be trivial.
A possible explanation is that there are differences in nonspecific binding profiles of the MAO tracer among different brain regions, especially as the outcome measure of binding for the irreversible tracers [11C]clorgyline and [11C]deprenyl-D2 includes free and nonspecific binding. However, Fowler et al37 have estimated the percentage of nonspecific binding for [11C]clorgyline λk3 to be from 16% in the cerebellum to 2.5% in the thalamus. The free and nonspecific binding for [11C]harmine total distribution volume (VT) was estimated to be approximately only 15% to 20% throughout the brain regions and has been subtracted for the specific term VS.2 This suggests that nonspecific binding might not account for the whole in vitro vs. in vivo differences. MAO protein levels determined in autopsied brain in vitro might not correspond to that available for binding of the radioligand in vivo. A number of uncertain factors could contribute, including differential cellular/subcellular localization (e.g., neurons vs. glia, axon terminal vs. cell body), posttranslational modification status of the enzyme, e.g., phosphorylation and dimerization,38 and influence by endogenous MAO inhibitors.39 Positron emission tomography outcome measures obtained at a single tracer dose are determined not only by density (Bmax) but also by affinity (Kd), which can be influenced by the above-mentioned in vivo variables. In this respect, [11C]harmine binding to MAO-A does not appear to be influenced by endogenous substrates as increase or decrease in the availability of substrates caused opposite changes in VT to that expected from a competition model.40
In conclusion, we report for the first time detailed regional distribution of protein levels of both MAO subtypes in the human brain. The presence of good correlations between in vivo PET ([11C]harmine, [11C]clorgyline, and [11C]deprenyl-D2) outcome measures of MAO density/activity with in vitro measurement of MAO protein levels support the validity of the major current MAO-targeting radiotracers for PET; however, the poor correlation between [11C]befloxatone PET binding and MAO-A protein regional distribution suggests caution in interpretation of data obtained with this radiotracer. The lack of proportionality between our in vitro regional data and in vivo PET outcome measures of MAO levels also suggests further studies to explain this finding and understand its implication for clinical MAO PET imaging.
Drs Meyer, Wilson and Houle have received operating grant funding for other studies from Eli-Lilly, Lundbeck, GlaxoSmithKline, BristolMyersSquibb, Takeda, Mylan, and SK Life Sciences, and Dr Meyer has consulted to several of these companies. Dr Meyer is developing natural health products to treat high MAO-A states. Dr Meyer is applying for a patent to apply measures of MAO to diagnose or treat mood disorders.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported in part by the USA NIH NIDA DA07182.
Supplementary Material
References
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginovart N, Meyer JH, Boovariwala A, Hussey D, Rabiner EA, Houle S, et al. Positron emission tomography quantification of [11C]-harmine binding to monoamine oxidase-A in the human brain. J Cereb Blood Flow Metab. 2006;26:330–344. doi: 10.1038/sj.jcbfm.9600197. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, et al. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry. 2006;63:1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Miler L, Rusjan P, Bloomfield PM, et al. Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch Gen Psychiatry. 2009;66:1304–1312. doi: 10.1001/archgenpsychiatry.2009.156. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, et al. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci USA. 1996;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy C, Bragulat V, Berlin I, Gregoire MC, Bottlaender M, Roumenov D, et al. Cerebral monoamine oxidase A inhibition in tobacco smokers confirmed with PET and [11C]befloxatone. J Clin Psychopharmacol. 2009;29:86–88. doi: 10.1097/JCP.0b013e31819e98f. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Wang GJ, Logan J, Xie S, Volkow ND, MacGregor RR, et al. Selective reduction of radiotracer trapping by deuterium substitution: comparison of carbon-11-L-deprenyl and carbon-11-deprenyl-D2 for MAO B mapping. J Nucl Med. 1995;36:1255–1262. [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- Meyer JH. Neuroimaging markers of cellular function in major depressive disorder: implications for therapeutics, personalized medicine, and prevention. Clin Pharmacol Ther. 2012;91:201–214. doi: 10.1038/clpt.2011.285. [DOI] [PubMed] [Google Scholar]
- Bacher I, Houle S, Xu X, Zawertailo L, Soliman A, Wilson AA, et al. Monoamine oxidase A binding in the prefrontal and anterior cingulate cortices during acute withdrawal from heavy cigarette smoking. Arch Gen Psychiatry. 2011;68:817–826. doi: 10.1001/archgenpsychiatry.2011.82. [DOI] [PubMed] [Google Scholar]
- Carter SF, Scholl M, Almkvist O, Wall A, Engler H, Langstrom B, et al. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J Nucl Med. 2012;53:37–46. doi: 10.2967/jnumed.110.087031. [DOI] [PubMed] [Google Scholar]
- Johansson A, Engler H, Blomquist G, Scott B, Wall A, Aquilonius SM, et al. Evidence for astrocytosis in ALS demonstrated by [11C](L)-deprenyl-D2 PET. J Neurol Sci. 2007;255:17–22. doi: 10.1016/j.jns.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Azzaro AJ, Fielding RM, Zhu W, Poshusta AK, et al. Reversible inhibitors of monoamine oxidase-A (RIMAs): robust, reversible inhibition of human brain MAO-A by CX157. Neuropsychopharmacology. 2010;35:623–631. doi: 10.1038/npp.2009.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Kailajarvi M, Haltia T, Koskimies S, Nagren K, Virsu P, et al. Assessment of MAO-B occupancy in the brain with PET and [11C]-L-deprenyl-D2: a dose-finding study with a novel MAO-B inhibitor, EVT 301. Clin Pharmacol Ther. 2009;85:506–512. doi: 10.1038/clpt.2008.241. [DOI] [PubMed] [Google Scholar]
- Cunningham VJ, Hume SP, Price GR, Ahier RG, Cremer JE, Jones AK. Compartmental analysis of diprenorphine binding to opiate receptors in the rat in vivo and its comparison with equilibrium data in vitro. J Cereb Blood Flow Metab. 1991;11:1–9. doi: 10.1038/jcbfm.1991.1. [DOI] [PubMed] [Google Scholar]
- Rieck RW, Ansari MS, Whetsell WO, Deutch AY, Kessler RM. Distribution of dopamine D2-like receptors in the human thalamus: autoradiographic and PET studies. Neuropsychopharmacology. 2004;29:362–372. doi: 10.1038/sj.npp.1300336. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Furukawa Y, Chang LJ, Tong J, Ginovart N, Wilson A, et al. Regional distribution of serotonin transporter protein in postmortem human brain: is the cerebellum a SERT-free brain region. Nucl Med Biol. 2005;32:123–128. doi: 10.1016/j.nucmedbio.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Geha RM, Rebrin I, Chen K, Shih JC. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276:9877–9882. doi: 10.1074/jbc.M006972200. [DOI] [PubMed] [Google Scholar]
- Tong J, Hornykiewicz O, Furukawa Y, Kish SJ. Marked dissociation between high noradrenaline versus low noradrenaline transporter levels in human nucleus accumbens. J Neurochem. 2007;102:1691–1702. doi: 10.1111/j.1471-4159.2007.04636.x. [DOI] [PubMed] [Google Scholar]
- Saura J, Bleuel Z, Ulrich J, Mendelowitsch A, Chen K, Shih JC, et al. Molecular neuroanatomy of human monoamine oxidases A and B revealed by quantitative enzyme radioautography and in situ hybridization histochemistry. Neuroscience. 1996;70:755–774. doi: 10.1016/s0306-4522(96)83013-2. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Wiberg A, Oreland L, Marcusson J, Winblad B. The effect of age on the activity and molecular properties of human brain monoamine oxidase. J Neural Transm. 1980;49:1–20. doi: 10.1007/BF01249185. [DOI] [PubMed] [Google Scholar]
- Owen F, Cross AJ, Lofthouse R, Glover V. Distribution and inhibition characteristics of human brain monoamine oxidase. Biochem Pharmacol. 1979;28:1077–1080. doi: 10.1016/0006-2952(79)90307-1. [DOI] [PubMed] [Google Scholar]
- Gottfries CG, Oreland L, Wiberg A, Winblad B. Lowered monoamine oxidase activity in brains from alcoholic suicides. J Neurochem. 1975;25:667–673. doi: 10.1111/j.1471-4159.1975.tb04386.x. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Mitchell MJ, Harik SI. Monoamine oxidases of the human brain and liver. Brain. 1988;111 (Pt 6:1441–1451. doi: 10.1093/brain/111.6.1441. [DOI] [PubMed] [Google Scholar]
- Gulyas B, Pavlova E, Kasa P, Gulya K, Bakota L, Varszegi S, et al. Activated MAO-B in the brain of Alzheimer patients, demonstrated by [11C]-L-deprenyl using whole hemisphere autoradiography. Neurochem Int. 2011;58:60–68. doi: 10.1016/j.neuint.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Wang GJ, Volkow ND, Logan J, Franceschi D, Franceschi M, et al. Evidence that gingko biloba extract does not inhibit MAO A and B in living human brain. Life Sci. 2000;66:PL141–PL146. doi: 10.1016/s0024-3205(99)00660-8. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Lerch J, Furukawa Y, Tong J, McCluskey T, Wilkins D, et al. Decreased cerebral cortical serotonin transporter binding in ecstasy users: a positron emission tomography/[(11)C]DASB and structural brain imaging study. Brain. 2010;133:1779–1797. doi: 10.1093/brain/awq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll AM, Anderson MC, Tobbia I, Phillips JP, Tipton KF. Determination of the absolute concentrations of monoamine oxidase A and B in human tissues. Biochem Pharmacol. 1989;38:901–905. doi: 10.1016/0006-2952(89)90278-5. [DOI] [PubMed] [Google Scholar]
- Saura J, Kettler R, Da Prada M, Richards JG. Quantitative enzyme radioautography with 3H-Ro 41-1049 and 3H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci. 1992;12:1977–1999. doi: 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galva MD, Bondiolotti GP, Olasmaa M, Picotti GB. Effect of aging on lazabemide binding, monoamine oxidase activity and monoamine metabolites in human frontal cortex. J Neural Transm Gen Sect. 1995;101:83–94. doi: 10.1007/BF01271547. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Fouquet C, Alvarez C, Seif I, Price D, Gaspar P, et al. Developmental expression of monoamine oxidases A and B in the central and peripheral nervous systems of the mouse. J Comp Neurol. 2002;442:331–347. doi: 10.1002/cne.10093. [DOI] [PubMed] [Google Scholar]
- Hashemi P, Dankoski EC, Lama R, Wood KM, Takmakov P, Wightman RM. Brain dopamine and serotonin differ in regulation and its consequences. Proc Natl Acad Sci USA. 2012;109:11510–11515. doi: 10.1073/pnas.1201547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Logan J, Pappas N, Shea C, et al. Age-related increases in brain monoamine oxidase B in living healthy human subjects. Neurobiol Aging. 1997;18:431–435. doi: 10.1016/s0197-4580(97)00037-7. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Konradi C, Mack-Burkhardt F, Riederer P, Heinsen H, Beckmann H. Ontogenesis of monoamine oxidase-A and -B in the human brain frontal cortex. Brain Res. 1989;499:81–86. doi: 10.1016/0006-8993(89)91136-0. [DOI] [PubMed] [Google Scholar]
- Wang CC, Borchert A, Ugun-Klusek A, Tang LY, Lui WT, Chu CY, et al. Monoamine oxidase a expression is vital for embryonic brain development by modulating developmental apoptosis. J Biol Chem. 2011;286:28322–28330. doi: 10.1074/jbc.M111.241422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyo M, Namba H, Fukushi K, Shinotoh H, Nagatsuka S, Suhara T, et al. Measurement of acetylcholinesterase by positron emission tomography in the brains of healthy controls and patients with Alzheimer's disease. Lancet. 1997;349:1805–1809. doi: 10.1016/S0140-6736(96)09124-6. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Ding YS, Franceschi D, Wang GJ, Volkow ND, et al. Non-MAO A binding of clorgyline in white matter in human brain. J Neurochem. 2001;79:1039–1046. doi: 10.1046/j.1471-4159.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- Upadhyay AK, Wang J, Edmondson DE. Comparison of the structural properties of the active site cavities of human and rat monoamine oxidase A and B in their soluble and membrane-bound forms. Biochemistry. 2008;47:526–536. doi: 10.1021/bi7019707. [DOI] [PubMed] [Google Scholar]
- Medvedev AE, Glover V. Tribulin and endogenous MAO-inhibitory regulation in vivo. Neurotoxicology. 2004;25:185–192. doi: 10.1016/S0161-813X(03)00098-6. [DOI] [PubMed] [Google Scholar]
- Sacher J, Rabiner EA, Clark M, Rusjan P, Soliman A, Boskovic R, et al. Dynamic, adaptive changes in MAO-A binding after alterations in substrate availability: an in vivo [(11)C]-harmine positron emission tomography study. J Cereb Blood Flow Metab. 2012;32:443–446. doi: 10.1038/jcbfm.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.