Abstract
Dementia is a major cause of morbidity in the western society. Pharmacological therapies to delay the progression of cognitive impairments are modestly successful. Consequently, new therapies are urgently required to improve cognitive deficits associated with dementia. We evaluated the effects of physical and cognitive activity on learning and memory in a rat model of vascular dementia (VasD). Male Sprague-Dawley rats (6 months old) were exposed to either regular chow or a diet rich in saturated fats and sucrose and chronic bilateral common carotid artery occlusion or sham surgery. First, this model of VasD was validated using a 2 × 2 experimental design (surgery × diet) and standard cognitive outcomes. Next, using identical surgical procedures, we exposed animals to a paradigm of cognitive rehabilitation or a sedentary condition. At 16 weeks post surgery, VasD animals demonstrated significant learning and memory deficits in the Morris water maze, independent of diet. Rehabilitation significantly attenuated these cognitive deficits at this time point as well as at 24 weeks. Further, rehabilitation normalized hippocampal CA1 soma size (area and volume) to that of control animals, independent of cell number. Importantly, these findings demonstrate beneficial neuroplasticity in early middle-aged rats that promoted cognitive recovery, an area rarely explored in preclinical studies.
Keywords: Cognition, cognitive rehabilitation, dementia, hippocampus, hypoperfusion, learning and memory
Introduction
Cognitive dysfunction as a result of Alzheimer's disease or vascular dementia (VasD) is a major contributor to morbidity in Western society.1, 2 Although the underlying etiology of dementia is likely multifactorial, dysfunction of the cerebrovasculature is undoubtedly a contributing factor, especially with respect to VasD. To date, pharmacological and immunologic interventions have met with limited success in reducing mild cognitive impairments and dementia in patient populations.3 More promising, however, is the theory that physical exercise,4 cognitive activity,5 or a combination of both, improves cognition and may potentially delay the progression of mild cognitive impairments.6
In a prospective study of cognitively normal participants, baseline physical activity was associated with lower rates of dementia at 5 years, and there was a negative correlation between the intensity of exercise and cognitive decline.7 Furthermore, participants who completed the lowest levels of exercise were more likely to have co-morbid conditions and experienced higher rates of attrition. This is further supported by complementary studies and meta-analyses demonstrating cognitive benefits associated with physical exercise.4, 8
With respect to cognitive activity, however, improvements in cognition have been demonstrated with increases in the number of cognitive activities (e.g., cards, board games, reading, and so on) rather than intensity (the total time spent engaged in cognitive integration and activities).5 Although the above findings were demonstrated in cognitively intact post-menopausal women, it appears that they also extend to individuals at risk for developing cognitive impairment. The Bronx Aging Study of at-risk individuals demonstrated that cognitive, but not physical, activity decreased the likelihood of developing vascular cognitive impairment.9
With respect to VasD, age is thought to be one of the most important risk factors associated with endothelial and, subsequently, vascular disruption.3 However, other modifiable lifestyle choices may significantly influence vascular health as well. For example, high-fat diets accelerate vascular senescence10 and exacerbate ischemic stroke damage when exposure extends from the early post-weaning period.11 Preclinical studies often overlook the importance of modeling co-morbid conditions (e.g., old age, unhealthy diet) when assessing intervention efficacy12 and this failure to reflect key aspects of the clinical setting undoubtedly contributes to the so-called translational roadblock.13
The model of VasD employed in the current study is a commonly used model that induces chronic cerebral hypoperfusion.14 Initially cerebral blood flow is dramatically reduced, but returns to near normal levels over several months.15 Changes in blood–brain barrier permeability,16 neuroinflammatory sequelae,17 neuronal physiology,18 white matter,19 and learning and memory abilities20 also accompany hypoperfusion. These changes, taken together, are thought to model several of the underlying neuropathological perturbations observed in clinical VasD.21
Although there is a growing understanding and acceptance of the positive effects of physical and cognitive activity on brain health, traditional preclinical studies using relevant brain injury animal models have focused primarily on pharmacological interventions.22 Fortunately, experimental studies have recently emphasized the importance of more global treatments on recovery of function after brain injury.23 This has increased our understanding of the mechanisms underlying neuronal plasticity and recovery of function after neuronal damage.24 The present study expands on this literature in several ways: first, we developed and validated an animal model of VasD that incorporates novel co-morbid conditions (diet and age) that are not commonly used in preclinical stroke research. Second, we tested a cognitive rehabilitation paradigm previously shown to be effective in young, intact animals.25 Notably, the findings reported herein demonstrate that a combination of physical and cognitive activity in early middle-aged animals with cognitive impairment improves learning and memory abilities and restores hippocampal cell morphology to control levels.
Materials and methods
Subjects
Forty-five male Sprague-Dawley rats (Vivarium, St. John's, Newfoundland and Labrador, Canada) were used in this study. Animals ∼6 months of age (∼710 g) on arrival, were placed on 12:12 hour light to dark cycles with lights on at 0600 hours and food and water provided ad libitum. Animals were housed in pairs in standardized cages, unless otherwise indicated. All procedures were approved by the Memorial University of Newfoundland Animal Care Committee and conformed to the Canadian Council on Animal Care guidelines.
Experimental Conditions
Animal pairs were pseudorandomized into one of five conditions: Sham-control diet (n=6); hypoperfusion (2-vessel occlusion; 2-VO)-control diet (n=12); Sham-high-fat, high-sugar diet (HFS; n=6); 2-VO-HFS diet (n=10); and 2-VO+physical and cognitive (PA/CA) activity-control diet (n=11). The 2-VO+PA/CA animals were individually given 24-hour access to voluntary wheel running (physical activity; 36 cm diameter wheels) on alternate days (3 day/week) combined with 24 hour exposure to modified Hebb–Williams mazes (100 × 100 × 20 cm3)26 on alternate days with a cagemate present (3 days/week). The Hebb–Williams maze configuration was changed weekly so as to increase novelty. Additionally, novel objects and food rewards were scattered throughout the maze and the water bottle and food hopper were separated to encourage exploration. PA/CA animals were housed in these conditions throughout the study except during behavioral testing and exposure was initiated after surgery.
Experimental Diet
Animals were acclimated to the animal care facility for 1 week before condition assignment. After this acclimation period, animals were pseudorandomized into one of two diet conditions: control or HFS. The HFS diet (high-fat/high-sucrose purified diet meal, TestDiet, 57NZ, Richmond, IN, USA) consisted primarily of protein (21% of energy (kcal)), fat (39% lard) and carbohydrates (40% 45% refined sugar (sucrose), and 3% fiber). Control diet consisted of standardized rat chow provided by Memorial University. High-fat and -sugar diet exposure was initiated, ad libitum, after surgical procedures, before which all animals were administered standard laboratory chow ad libitum.
Surgery
Animals underwent either bilateral common carotid artery occlusion (cerebral hypoperfusion; 2-VO) or sham surgery under isoflurane (4% induction; 2.5% maintenance; CDMV Canada, St. Hyacinthe, Quebec, Canada) anesthesia in a 70/30% nitrous oxide/oxygen mixture as previously described.20 After anesthesia and under asepetic surgical techniques, a midline neck incision was made and both the left and right common carotid arteries were carefully isolated from surrounding muscle and adjacent nerve bundles. Each artery was doubly ligated (2-VO) with 4-0 silk sutures, checked for lack of patency and returned to the neck cavity. Sham surgery consisted of similar procedures including neck incision and isolation of arteries without ligation. Animals received topical 2% Xylocaine (AstraZeneca, Mississauga, Ontario, Canada) on the midline neck incision and were placed in a standard cage on a heating blanket until recovered from surgery.
Behavioral Assessments
A timeline for all behavioral assessments is shown in Figure 1.
Figure 1.
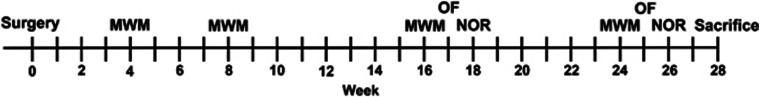
Experimental timeline of behavioral assessments. MWM, Morris water maze; OF, Open field; NOR, novel object recognition.
Morris Water Maze
Morris water maze (MWM) testing occurred at 4, 8, 16, and 24 weeks post surgery.27 The maze consisted of a black pool (180 cm diameter) filled with water (∼50 cm depth; ∼22 °C). A circular platform (20 cm diameter) was submerged ∼2.5 cm below the water surface and placed 25 to 30 cm from the edge of wall within a quadrant, chosen at random. In an effort to avoid reliance on visual spatial abilities due to possible optic tract dysfunction sometimes observed with this model,19 we attempted to use an auditory spatial paradigm of the MWM. The first two MWM sessions consisted of this paradigm (4 and 8 weeks post surgery; see below) and the remaining two sessions consisted of a more conventional multimodal maze (16 and 24 weeks post surgery).27 During the auditory maze testing, a curtain surrounded the maze to remove distal room cues and an automated ‘in-house' computerized program emitted sounds at a frequency of 7,000, 8,000, 9,000 and 10,000 Hz from speakers, arranged on the maze frame, centered in each quadrant. Each tone was played for 3 seconds with a 1-second interval between sounds. The tones were presented in a randomized block order so that each speaker was activated in a block of four but the order within each block was pseudorandomized. The multimodal maze configuration used at 16 and 24 weeks post surgery consisted of spatial, olfactory, and auditory domains. The curtain was removed and the room cues displayed (e.g., computer, bookshelves, shapes), with a radio emitting white noise (auditory) in one corner of the room and a scented marker (olfactory) placed within one quadrant. All maze cues remained constant throughout each test session and subsequent probe trials (i.e., week 16; see below), but were rearranged between sessions. Similarly, platform locations remained constant within each test session, but were changed between sessions.
A similar protocol was used for each test session where animals were tested for four trials/day over 4 days (acquisition), with the exception of Trial 1, Day 1 (week 4). Before initiating this trial, each animal was placed on the platform for 20 seconds to orient the animal to the escape platform location. Each subsequent trial lasted a maximum of 90 seconds (unable to locate platform) or until animals located the hidden platform. Following the location of the platform, animals remained there for an additional 10 seconds and were then placed in a holding cage for a further 60 seconds, when each animal was returned to the maze for the next trial. Animals were placed into the maze facing the wall of the pool and quadrant starting positions were pseudorandomized. Latency to locate the platform was the dependent measure for the MWM.
On the day after acquisition trials, animals were assessed in a probe trial where the platform was removed from the maze. Animals were placed into the pool, directly opposite the platform location facing the wall, and were allowed 60 seconds to freely swim. The dependent measure for probe tests included the total amount of time spent in the target quadrant.
To rule out visual deficits potentially caused by chronic carotid occlusion,19 we assessed animals' visual abilities using a visible platform paradigm after the probe trial (day 5) at post-surgery week 24 (PSW 24). Briefly, the platform was elevated (∼2 cm) above the surface of the water and brightly colored tape surrounded the edge of the platform to increase visibility.
Open Field
At 1 week after the MWM probe session at weeks 17 and 25 post surgery, animals were placed into an open-field chamber (72 × 76 × 57 cm) in a soundproof room for 5 minutes and videotaped from above.28 The dependent measure was the number of grid crosses using a 3 × 3 grid (24 × 25 cm).
Object Recognition
Object recognition testing occurred 1 week after each open-field test session (i.e., weeks 18 and 26 post surgery) using the same chamber located in the soundproof room.29 Rats received three 5-minute trials, each separated by 7 minutes and returned to the home cage between trials. The rat was placed in the chamber with two identical objects, one in the ‘upper-left' and the other in the ‘upper-right' quadrant. Next, the rat was returned to the chamber with the two identical objects, but the ‘upper-right' object was relocated to the ‘lower-right' quadrant. Finally, during the last test trial, the rat was returned to the chamber with a novel object replacing the original ‘upper-left' object, whereas the ‘lower-right' object remained in the same location. Each session was videotaped from above and the dependent measure included time spent investigating an object. The rat was considered to be investigating an object when it was actively sniffing the object or if the nose was within ∼2 cm of the object (touching the object with its vibrissae).
Hippocampal CA1 Cell Quantification
After completion of the behavioral assessments, animals were deeply anesthetized with 4.0% isoflurane in a 30/70% O2/N2O mixture and transcardially perfused with ice-cold 0.9% heparinized saline and 4.0% paraformaldehyde. Animals were decapitated, brains were removed and stored overnight at 4 °C in 4.0% paraformaldehyde. Brains were then transferred to 20% sucrose in 1 × phosphate-buffered saline solution and stored at 4 °C until saturated, then frozen and stored at −20 °C until further processing.
Coronal slices were cut at 40 μm using a cryostat and slide-mounted for cresyl violet staining. Unbiased stereological procedures were used to estimate dorsal hippocampal CA1 pyramidal cell number and size using a Leica DMRXE microscope (Leica Microsystems Inc, Richmond Hill, ON, Canada) and the Optical Fractionator method of Stereo Investigator (MBF Bioscience, Williston, VT, USA). A three-dimensional optical dissector counting frame (x, y, and z dimensions of 35 × 35 × 17 μm, respectively) was applied to systematic random sample sites resulting in an average of 15 to 20 sampling sites superimposed on the circumscribed area of dorsal CA1.30 CA1 cell counts were conducted between coronal planes −2.30 mm and −4.52 mm relative to bregma31 corresponding to the rostral and caudal dorsal hippocampus, respectively. The dorsal CA1 region was outlined using a × 5 objective and this allowed for the generation of sampling sites using the optical fractionator method (300 μm in the x direction and 100 μm in the y direction). Seven equally spaced sections were analyzed in each brain and only the left hippocampus was analyzed because there were no anticipated asymmetric differences. A border guard of 2 μm was set at the top and bottom of each section and CA1 cell number and size (calculated using the nucleator method of Stereo Investigator) estimates were calculated using a × 40 objective and the serial section manager method of Stereo Investigator. On average, ∼480 cells were counted/brain with a coefficient of error of 0.09.
Statistical Analyses
All data were analyzed using the Statistical Package for Social Sciences (IBM SPSS Statistics; v 19.0.0, Armonk, NY, USA). Assessments of functional deficits after 2-VO were analyzed with a 2 × 2 (diet × surgery) analysis of variance (ANOVA), with or without repeated measures where appropriate. Similarly, histologic data were analyzed with a 2 × 2 ANOVA or independent t-tests with Bonferroni correction. After these analyses, the effects of rehabilitation on functional recovery and CA1 cell number and size were analyzed using either a one-way ANOVA with or without repeated measures where appropriate or an independent t-test. Performance in the novel object test was analyzed with a 2 × 2 ANOVA as well as a paired t-test to assess exploratory behavior. In cases where the homogeneity of variance or sphericity assumptions were violated, the Brown-Forsythe or Huynh-Feldt correction was used, respectively. All data were analyzed by an experimenter masked to experimental conditions. Statistical significance was considered at P≤0.05.
Results
Experiment 1: Effects of 2-VO Surgery and Dietary Manipulation
Two animals were removed from the analysis because their calculated means for histologic assessments were >5 s.d. from the group's mean (2-VO). Additionally, one animal died before completion of functional assessments (2-VO). Animals' weights are displayed in Table 1.
Table 1. Animals' body weight (g) (mean±s.e.m.).
| Condition | Pre | Week 4 | Week 8 | Week 16 | Week 24 | Kill |
|---|---|---|---|---|---|---|
| C-Sham | 825 (23) | 847 (33) | 870 (34) | 894 (31) | 829 (49) | 790 (70) |
| C-2-VO | 742 (23) | 720 (23) | 732 (24) | 777 (28) | 790 (31) | 786 (33) |
| HFS-Sham | 734 (31) | 766 (25) | 795 (26) | 841 (36) | 904 (53)* | 912 (47)* |
| HFS-2-VO | 748 (28) | 755 (31) | 789 (38) | 858 (47) | 900 (53)* | 890 (49)* |
| PA/CA | 717 (31) | 673 (14) | 678 (25) | 705 (28) | 735 (34) | 737 (35) |
Pre, Pre-surgery; C, Control diet; HFS, high-fat, high-sugar diet; 2-VO, 2-vessel occlusion; PA/CA, physical activity combined with cognitive activity.
*P<0.03 vs control diet.
Visible platform
To exclude the possibility of visual deficits after 2-VO,19 1 day after the probe test at week 24, animals were assessed in a visible platform paradigm. A 2 × 2 ANOVA showed that there was no difference among conditions and no interactions (P>0.05) indicating similar visual abilities among animals.
Morris water maze: post surgery week 4 & 8 (PSW 4 & 8)
A 2 × 2 ANOVA with repeated measures of animals' acquisition latency in the MWM revealed a significant effect of time during both assessments (F2.978,83.394=29.780, P<0.01; F2.758,77.229=4.305, P<0.01, PSW 4 and 8, respectively) indicating that animals learned the platform location during each test, but no effect of surgery or diet and no significant interactions (P>0.05; Table 2). Further, there were no differences among groups during the 24-hour probe trials with respect to the amount of time spent in the platform quadrant (P>0.05; Table 2).
Table 2. Animals' latency (mean±s.e.m.) to locate hidden platform in MWM.
| Condition | Day 1 | Day 2 | Day 3 | Day 4 | Probe |
|---|---|---|---|---|---|
| Week 4 | |||||
| C-Sham | 69 (6) | 36 (7) | 31 (6) | 26 (6) | 14 (1) |
| C-2-VO | 49 (5) | 32 (7) | 29 (4) | 21 (3) | 15 (1) |
| HFS-Sham | 60 (8) | 37 (12) | 30 (10) | 18 (5) | 12 (1) |
| HFS-2-VO | 61 (5) | 34 (7) | 32 (7) | 37 (8) | 16 (1) |
| PA/CA | 44 (5) | 27 (4) | 31 (5) | 27 (5) | 14 (1) |
| Week 8 | |||||
|---|---|---|---|---|---|
| C-Sham | 24 (2) | 19 (4) | 14 (4) | 21 (5) | 16 (1) |
| C-2-VO | 24 (5) | 17 (4) | 20 (4) | 20 (4) | 15 (2) |
| HFS-Sham | 19 (3) | 12 (3) | 13 (2) | 13 (2) | 16 (1) |
| HFS-2-VO | 34 (6) | 17 (2) | 18 (3) | 16 (3) | 16 (1) |
| PA/CA | 24 (4) | 17 (2) | 17 (2) | 13 (2) | 16 (1) |
| Week 24 | |||||
|---|---|---|---|---|---|
| C-Sham | 22 (6) | 21 (10) | 9 (4) | 10 (3) | 21 (4) |
| C-2-VO | 25 (9) | 24 (6) | 21 (7) | 21 (8) | 20 (2) |
| HFS-Sham | 17 (4) | 10 (2) | 10 (4) | 6 (1) | 25 (2) |
| HFS-2-VO | 30 (7) | 20 (6) | 22 (7) | 19 (5) | 20 (1) |
| PA/CA | 10 (2) | 10 (2) | 9 (2) | 7 (1) | 21 (2) |
Probe, Average time in target quadrant; C, Control diet; HFS, high-fat, high-sugar diet; 2-VO, 2-vessel occlusion; PA/CA, Physical activity combined with cognitive activity.
Post-surgery week 16
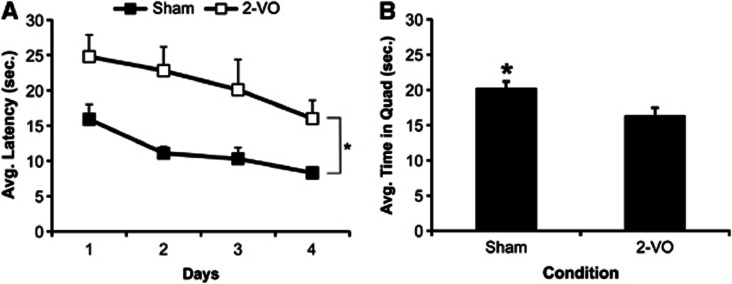
During the PSW 16 MWM assessment, the protocol was changed from an auditory maze to a multimodal maze with distal visual spatial, auditory, and olfactory cues. Repeated measures ANOVA revealed a significant effect of time (F3,84=4.540, P<0.01) and a significant effect of surgery (F1,28=6.316, P<0.02; Figure 2A), but no effect of diet and no interactions (P>0.05). Post-hoc analyses showed that animals exposed to the 2-VO surgery required significantly more time to locate the hidden platform than sham animals, regardless of dietary consumption. Visual inspection of Figure 2 reveals a similar learning curve between conditions. Further stratification of the data to assess potential differences in learning within each day, especially Day 1, showed that there was no effect of trial, surgery or diet and no significant interactions among the variables.
Figure 2.
Performance in the Morris water maze at 16 weeks post surgery (mean±s.e.m.), validating the 2-vessel occlusion (2-VO) model of vascular dementia. (A) Animals exposed to the 2-VO exhibited significant learning impairments (*P<0.05) manifested by longer latencies to locate the hidden platform than sham animals during acquisition. (B) Additionally, animals exposed to 2-VO spent significantly less time in the target quadrant during the probe test, thus indicating a significant memory impairment (*P<0.05).
Assessment of the amount of time spent in the target quadrant during the 24-hour probe test using a 2 × 2 univariate ANOVA demonstrated a significant effect of surgery (F1,28=4.324, P<0.05), but no effect of diet and no interaction (P>0.05). Animals exposed to the sham surgery spent significantly more time in the target quadrant during the probe test than animals exposed to the 2-VO surgery (Figure 2B).
Post-surgery week 24
At PSW 24, animals were assessed in a similar MWM paradigm as PSW 16. A 2 × 2 ANOVA with repeated measures at this time point showed that there was a significant effect of time (F2.253,58.576=5.950, P<0.01) but no effect of surgery or diet and no significant interactions (P>0.05; Table 2). Similarly, there were no differences among conditions with respect to the amount of time spent in the target quadrant during the probe assessment (P>0.05; Table 2).
Open Field
Assessment of the number of squares crossed during a 5-minute trial at weeks 17 and 25 revealed no effect of time, surgery or diet (P>0.05) and no significant interactions (P>0.05).
Novel object recognition
A paired t-test of the amount of time spent exploring objects during Trial 1 at both 18 and 26 weeks showed that animals spent similar amounts of time with each object (P>0.05). During Trial 2 (change in object location) of each test period (i.e., week 18 and 26), paired t-tests showed that animals spent either similar (week 18) or significantly less (week 26) time exploring the object in the new location (i.e., ‘lower right' P>0.05 and t28=3.052, P<0.01, respectively). Similar analyses of Trial 3 data (novel object) however, demonstrated that animals spent significantly more time exploring the novel object compared with the object located in the lower-right quadrant at each time point of 18 and 26 weeks (t29=3.520, P<0.01 and t28=5.247, P<0.01, respectively).
A 2 × 2 ANOVA of the time spent exploring the ‘lower-right' object (Trial 2) revealed no effect of surgery or diet (P>0.05) and no significant interactions (P>0.05). Similarly, there were no differences among conditions with respect to the time exploring the novel object during Trial 3 (P>0.05).
Hippocampal CA1 quantification
A 2 × 2 ANOVA of hippocampal CA1 cell numbers revealed that there was no effect of surgery or diet and no significant interaction (P>0.05). There was however, a significant effect of surgery on soma area (F1,7=6.767, P<0.04) but no effect of diet and no interaction. Similarly, there was a significant effect of surgery on soma volume (F1,7=7.034, P<0.04) but no effect of diet and no interaction. In both instances, 2-VO animals had significantly larger CA1 somas than Sham animals with respect to area and cell volume (P<0.04; Table 3).
Table 3. Analysis of hippocampal CA1 pyramidal cell number, area and volume (mean±s.e.m.).
| Condition | Cell number | Area (μm2) | Volume (μm3) |
|---|---|---|---|
| Sham | 1.66 × 105 (±1.02 × 104) | 58.4 (±2.6) | 365.2 (±23.1) |
| 2-VO | 1.75 × 105 (±2.1 × 104) | 66.2 (±0.9)*,# | 442.9 (±10.9)*,# |
| PA/CA | 1.73 × 105 (±1.27 × 104) | 58.7 (±1.4) | 368.4 (±12.6) |
2-VO, 2-vessel occlusion; PA/CA, physical activity combined with cognitive activity.
*P<0.04 vs Sham animals; #P<0.01 vs PA/CA animals.
Experiment 2: Effects of Rehabilitation on 2-VO Outcomes
The effects of rehabilitation (physical and cognitive activity) on functional deficits and hippocampal CA1 changes were assessed by comparing animals (2-VO vs 2-VO+PA/CA) in the MWM, open field and novel object tests. Because there was no effect of diet in Experiment 1, data were collapsed across diet to assess rehabilitation efficacy after 2-VO.
Visible platform
Independent samples t-test of animals' visual abilities in the MWM tested at PSW 24 showed that there was no difference between conditions in latency to locate the visible platform (P>0.05).
Post-surgery week 4 and 8
Repeated measures ANOVA of animals' acquisition latency showed that there were no effects of time at PSW 4, but an effect of time (F2.356,73.023=3.359, P<0.03) at PSW 8, and no effect of condition and no interactions (P>0.05; Table 2) at either time point. There was also no difference between conditions with respect to the amount of time spent in the target quadrant during the probe trials (P>0.05; Table 2).
Post-surgery week 16
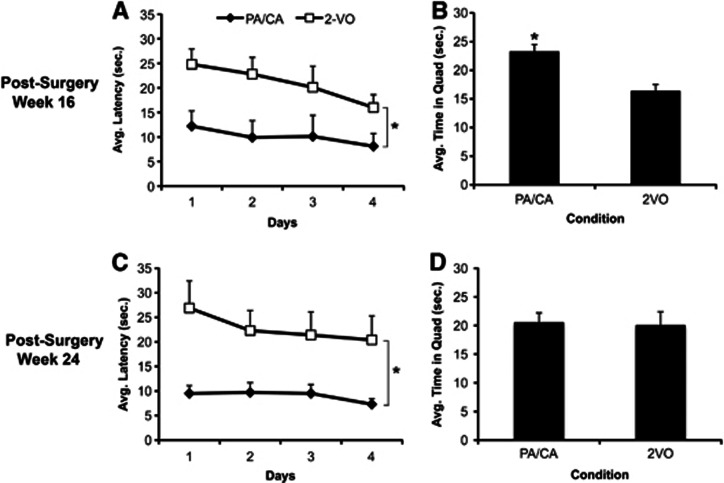
A repeated measures ANOVA revealed a significant effect of time (F3,84=2.94, P<0.04), condition (F1,23.476=11.744, P<0.01), and no interaction. Follow-up analysis showed that animals exposed to rehabilitation reached the platform in significantly less time than 2-VO-alone animals (Figure 3A). Further stratification of the data to assess acquisition curves within Day 1 failed to demonstrate a significant effect of Trial or Rehabilitation and no significant interaction between the independent variables. In the probe trial, the effect observed during acquisition also held true where 2-VO+PA/CA animals spent significantly more time in the target quadrant than their 2-VO-only counterparts (t29=3.497, P<0.01) (Figure 3B).
Figure 3.
Performance in the Morris water maze at 16 and 24 weeks post surgery (mean±s.e.m.), assessing rehabilitation efficacy. (A) Animals exposed to 2-vessel occlusion (2-VO) plus the physical and cognitive activity (PA/CA) rehabilitation paradigm displayed significantly shorter latencies to locate the hidden platform during the acquisition trials indicating superior learning abilities compared with 2-VO animals that did not receive rehabilitation at PSW 16 (*P<0.01). (B) Rehabilitation also significantly improved memory performance at this time point in the probe trial where 2-VO+PA/CA animals spent more time in the target quadrant than animals not exposed to rehabilitation (*P<0.01). (C) Similar effects were observed at PSW 24 (*P<0.01) where 2-VO+PA/CA animals exhibited significantly lower latencies than 2-VO-alone animals to locate the hidden platform. (D) There were no differences in memory performance at PSW 24 as assessed in the probe trial.
Post-surgery week 24
Repeated measures ANOVA showed that there was no effect of time but a significant effect of condition (F1,21.956=9.788, P<0.01) and no interaction during the acquisition trials (P>0.05). PA/CA animals had significantly lower latencies, indicating improved performance during the acquisition trials (P<0.01; Figure 3C). Independent t-tests revealed that there were no differences between conditions with respect to the amount of time spent in the target quadrant tested 24 hours after the last acquisition trial (Figure 3D).
Open field
Assessment of the number of square crosses in the open field revealed no effect of time, condition, and no significant interaction (P>0.05).
Object recognition
A paired t-test of the amount of time spent exploring objects during Trial 1 at both 18 and 26 weeks showed that animals spent similar amounts of time with each object (P>0.05). During Trial 2 (change in object location) of each test period (i.e., week 18 and 26), a paired t-test revealed that there was no difference in the amount of time exploring the object in the new location at either testing period (P>0.05; two-tailed) but there was a significant difference in the amount of time exploring the new object during Trial 3 at both 18 (t27=5.215, P<0.01) and 26 weeks post surgery (t26=4.973, P<0.01) where all animals were more likely to explore the new object.
Independent t-tests with Bonferroni correction were then used to assess possible effects of rehabilitation on novel object location and recognition. There was no difference between conditions with respect to the amount of time spent exploring the novel location at either 18 or 26 weeks post surgery (P>0.05). Similarly, there were no differences between conditions with respect to the amount of time spent exploring the novel object at either 18 or 26 weeks post surgery.
Hippocampal CA1 cell quantification
Independent t-tests (two-tailed) with Bonferroni corrections showed that there were similar numbers of CA1 cells between animals in the 2-VO and PA/CA+2-VO conditions. Interestingly, however, as with the analyses between 2-VO and Sham animals in Experiment 1, there was a significant difference between the 2-VO and PA/CA+2-VO animals in Experiment 2 with respect to cell body size. Animals in the rehabilitation condition had significantly smaller hippocampal CA1 cells with respect to soma area (t12=4.522, P<0.01) and volume (t12=4.479, P<0.01; Table 3), comparable to the sham animals in Experiment 1 (ad hoc comparison, P>0.05).
Discussion
The purposes of this study were to validate a model of VasD that incorporated co-morbid conditions and to assess the efficacy of a rehabilitation paradigm that utilizes both physical and cognitive activity25 in ameliorating memory deficits in this model. Importantly, we demonstrated significant learning and memory deficits in the MWM (Experiment 1) using a bilateral carotid artery occlusion model of VasD with mid-early old-age animals,19, 20 thus validating our co-morbid VasD model with other laboratories using younger, healthy animals.32, 33 Next, we implemented a rehabilitative therapy (Experiment 2) that included alternating days of physical exercise (voluntary wheel running) and cognitive activity (modified Hebb–Williams maze exposure). At both 16 and 24 weeks post surgery, we observed marked improvements in both learning (acquisition) and memory abilities in animals exposed to the rehabilitative paradigm compared with sedentary controls. Further, the hypertrophy of the hippocampal CA1 pyramidal soma observed in 2-VO rats was normalized in 2-VO animals exposed to rehabilitation.
Physical exercise is known to improve both cardiovascular and brain health.2 The possible mechanisms underlying improvements in brain health include the upregulation of growth factors such as brain-derived neurotrophic factor and its receptor TrkB,34 increases in angiogenesis35 and neurogenesis36 as well as a reduction in oxidative damage.23 Certainly, activation of some or all these processes may have contributed to the ameliorative effects of the PA/CA intervention observed in the current study.
Further, cognitive activity and learning have some of the same effects as exercise on the molecular mechanisms associated with brain health.37 Indeed, it has been hypothesized that physical and cognitive activity may have synergistic roles in improving brain health and potentially ameliorating cognitive impairments and dementia.6, 38 This hypothesis was confirmed in a previous study conducted in our laboratory. Young, naïve rats exposed to a combination of physical and cognitive activity displayed superior memory performance relative to rats exposed to either activity alone, independent of enrichment duration, explaining our rationale to combine both activities in the current study.25 Thus, this theory has been extended and confirmed in the present study using a model of VasD.
Although this model of VasD has been well characterized, it is unclear whether the learning and memory deficits occur soon after carotid artery occlusion,19 or are more progressive in nature and more closely mimic a time-dependent deterioration of function20 as seen in humans with mild cognitive impairments. Unfortunately, it is difficult to dissect the onset of the functional deficits observed in the current study. An auditory version of the MWM was initially implemented to avoid potential confounding differences in visual abilities due to optic tract white matter injury sometimes reported with this model.17, 19 Because animals did not acquire platform location in the auditory MWM paradigm in a spatially dependent manner, we implemented a more traditional paradigm using distal spatial cues during the subsequent sessions (i.e., post-surgery weeks 16 and 24). As a result, one cannot conclude that memory impairments observed at 16 weeks post surgery evolved over months and are not instead due to the immediate reduction in cerebral blood flow after 2-VO because animals failed to learn the platform location using the auditory cues during this version of the water maze. Although generalized white matter injury (specifically, optic tract) was not assessed, we believe that the observed memory deficits were not because of visual problems, because visible platform assessments conducted at the end of behavioral testing (24 weeks after 2-VO surgery) revealed similar latencies among conditions. As a result, differences in MWM latency and platform target quadrant time more likely reflect learning (acquisition) and memory (probe) differences. Although path length was not directly measured in this study, similar visible platform latencies also help exclude the possibility of confounding variables such as motivational differences among conditions and locomotor deficits or enhancements from surgical or rehabilitative interventions, respectively. As the 2-VO surgical procedure mainly affects the hippocampus, one would not expect an effect on motor ability. This conclusion is further supported by findings from others where increased MWM path length and no effect on swim speeds19, 39 was found using this model of VasD, thus excluding locomotor deficits.32 Additionally, we have previously shown that a similar model of physical and cognitive activity improved learning and memory abilities in a task independent of locomotor abilitiy.25
The learning improvements observed at PSW 24 in our rehabilitation condition was interesting given that there were no statistical differences between sham and 2-VO animals in Experiment 1. One explanation for this discrepancy has a statistical basis. Our original experiment used multiple conditions and this likely decreased the statistical power to detect a difference among conditions, especially because our assessment of rehabilitation demonstrated striking differences between the two conditions. Calculation of the effect size for both analyses revealed a similar ‘medium' effect size (ω2=0.07 in Experiment 1; ω2=0.12 in the rehabilitation study), thus indicating that indeed, given a larger sample size, statistical significance would likely be achieved at this time point.40
In the current study, we observed no reduction in CA1 cell number as noted by several other laboratories41, 42 (but see Farkas et al17). To our surprise, we detected a significant hypertrophy of CA1 pyramidal cells reflected by increases in cell area and volume in animals exposed to carotid artery occlusion. Interestingly, this hypertrophy was reversed (or possibly prevented) in animals exposed to our rehabilitation paradigm such that cell volumes were found to be comparable to sham animals. To the best of our knowledge, this represents a novel finding although an abnormal spatial arrangement of the CA1 cell layer has been reported using a slightly different model of chronic hypoperfusion.18 In that study, hypoperfused animals appeared to lack the typical compact grouping of CA1 cell bodies, with numerous neurons located above or below the CA1 pyramidal layer. The authors also noted that there was variation in cell body size (although not quantified) that was most pronounced in CA1 with less obvious alterations in CA3 and no change in the dentate gyrus. In another long-term 2-VO study,43 T-2-weighted imaging revealed evidence of edema; that is consistent with altered cell volume regulation.44 Typically large alterations in cell volume area, if not corrected, result in activation of apoptotic pathways.44 In contrast, subtle neuronal alterations found in the present study could result in significant neuronal dysfunction as a result of remodeling of the plasma membrane, dendritic modifications and altered ion channel function45, 46 and manifest as selective behavioral deficits. In support of this notion, chronic hypoperfusion impairs both tetanic and calcium-induced long-term potentiation, a model of long-term memory formation.47
It is noteworthy that a diet high in fats and refined sugars (HFS) did not exacerbate histopathological damage or behavioral impairments as previously reported in focal ischemia models.10, 11 Although it is possible that a HFS diet does not have an effect on pathologic or behavioral injury in the present 2-VO model, other explanations for this lack of effect exist. For example, it has been demonstrated that ischemic damage is substantially increased when animals are exposed to an unhealthy diet for a prolonged period before the ischemic insult, early in life.11 In the current study, we initiated exposure to the HFS diet after surgery when animals were 6 months old and thus potentially reducing the increased susceptibility of neuronal damage. Consequently, exposing animals to an unhealthy diet during a critical developmental period, early in life, when the central nervous system is still developing before insult may have more deleterious effects on VasD outcome.
In conclusion, dementia has profound effects at the individual, familial, and societal levels. There are relatively few treatments to delay or mitigate the memory impairments that accompany dementia and pharmacological strategies have not had a major impact in this area.3 In the current study, we describe a paradigm of physical and cognitive activity that reduces memory impairments and normalizes altered hippocampal CA1 pyramidal cell structure in a model of VasD using older animals. Whether these findings support a ‘use-it or lose-it'6 or ‘cognitive reserve'48 hypothesis is difficult to ascertain. These data strongly support the need for further research to explore the merits of lifestyle alterations in improving cognitive outcome associated with dementia. Further, it is essential that future studies elucidate the underlying mechanisms associated with these memory improvements to optimize therapies to mitigate the cognitive dysfunction that increasingly threatens our aging population.
Acknowledgments
The authors thank Dr John Evans, Garry Chernenko, Kathy McKay, Suzanne Evans and Andrew Orsborne for their technical assistance and Dr Crystal MacLellan for helpful comments on the manuscript.
The authors declare no conflict of interest.
Footnotes
This research was supported by a special Vascular Health and Dementia strategic initiative through a partnership involving the Heart and Stroke Foundation of Canada, Alzheimer Society of Canada, Canadian Institutes of Health Research and Pfizer Inc (DC) and NSERC operating grant awarded to DC. KDL was supported by a studentship from the Canadian Stroke Network.
References
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5:649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging. 2010;31:2047–2057. doi: 10.1016/j.neurobiolaging.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Eskes GA, Longman S, Brown AD, McMorris CA, Langdon KD, Hogan DB, et al. Contribution of physical fitness, cerebrovascular reserve and cognitive stimulation to cognitive function in post-menopausal women. Front Aging Neurosci. 2010;2:137. doi: 10.3389/fnagi.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberber U. Enrichment effects on adult cognitive development: can the functional capacity of older adults be preserved and enhanced. Psychol Sci Public Interest. 2009;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Verghese J, Cuiling W, Katz MJ, Sanders A, Lipton RB. Leisure activities and risk of vascular cognitive impairment in older adults. J Geriatr Psychiatry Neurol. 2009;22:110–118. doi: 10.1177/0891988709332938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Kim HH, Hiroi Y, Sawada N, Salomone S, Benjamin LE, et al. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal. 2009;2:ra11. doi: 10.1126/scisignal.2000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon KD, Clarke J, Corbett D. Long-term exposure to high fat diet is bad for your brain: exacerbation of focal ischemic brain injury. Neuroscience. 2011;182:82–87. doi: 10.1016/j.neuroscience.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Engelhardt B, Koistinaho J, Lindvall O, Meairs S, Mohr JP, et al. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc Dis. 2008;25:268–278. doi: 10.1159/000118039. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007;54:162–180. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Otori T, Katsumata T, Muramatsu H, Kashiwagi F, Katayama Y, Terashi A. Long-term measurement of cerebral blood flow and metabolism in a rat chronic hypoperfusion model. Clin Exp Pharmacol Physiol. 2003;30:266–272. doi: 10.1046/j.1440-1681.2003.03825.x. [DOI] [PubMed] [Google Scholar]
- De Jong GI, Farkas E, Stienstra CM, Plass JR, Keijser JN, de la Torre JC, et al. Cerebral hypoperfusion yields capillary damage in the hippocampal CA1 area that correlates with spatial memory impairment. Neuroscience. 1999;91:203–210. doi: 10.1016/s0306-4522(98)00659-9. [DOI] [PubMed] [Google Scholar]
- Farkas E, Donka G, de Vos RA, Mihaly A, Bari F, Luiten PG. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 2004;108:57–64. doi: 10.1007/s00401-004-0864-9. [DOI] [PubMed] [Google Scholar]
- Sekhon LH, Morgan MK, Spence I, Weber NC. Chronic cerebral hypoperfusion: pathological and behavioral consequences. Neurosurgery. 1997;40:548–556. doi: 10.1097/00006123-199703000-00025. [DOI] [PubMed] [Google Scholar]
- Pappas BA, de la Torre JC, Davidson CM, Keyes MT, Fortin T. Chronic reduction of cerebral blood flow in the adult rat: late-emerging CA1 cell loss and memory dysfunction. Brain Res. 1996;708:50–58. doi: 10.1016/0006-8993(95)01267-2. [DOI] [PubMed] [Google Scholar]
- Ni J, Ohta H, Matsumoto K, Watanabe H. Progressive cognitive impairment following chronic cerebral hypoperfusion induced by permanent occlusion of bilateral carotid arteries in rats. Brain Res. 1994;653:231–236. doi: 10.1016/0006-8993(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Corbett D, Nurse S. The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog Neurobiol. 1998;54:531–548. doi: 10.1016/s0301-0082(97)00078-6. [DOI] [PubMed] [Google Scholar]
- Cechetti F, Worm PV, Elsner VR, Bertoldi K, Sanches E, Ben J, et al. Forced treadmill exercise prevents oxidative stress and memory deficits following chronic cerebral hypoperfusion in the rat. Neurobiol Learn Mem. 2012;97:90–96. doi: 10.1016/j.nlm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Langdon KD, Corbett D. Improved working memory following novel combinations of physical and cognitive activity. Neurorehabil Neural Repair. 2012;26:523–532. doi: 10.1177/1545968311425919. [DOI] [PubMed] [Google Scholar]
- Hebb DO, Williams K. A method of rating animal intelligence. J Gen Psychol. 1946;34:59–65. doi: 10.1080/00221309.1946.10544520. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Corbett D, Evans S, Thomas C, Wang D, Jonas RA. MK-801 reduced cerebral ischemic injury by inducing hypothermia. Brain Res. 1990;514:300–304. doi: 10.1016/0006-8993(90)91424-f. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, Nguyen KP, et al. Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci USA. 2010;107:1624–1629. doi: 10.1073/pnas.0914207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press: San Diego, CA; 1997. [Google Scholar]
- Farkas E, Institoris A, Domoki F, Mihaly A, Luiten PG, Bari F. Diazoxide and dimethyl sulphoxide prevent cerebral hypoperfusion-related learning dysfunction and brain damage after carotid artery occlusion. Brain Res. 2004;1008:252–260. doi: 10.1016/j.brainres.2004.02.037. [DOI] [PubMed] [Google Scholar]
- He XL, Wang YH, Bi MG, Du GH. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur J Pharmacol. 2012;680:41–48. doi: 10.1016/j.ejphar.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, So V, Kesslak JP. Spatial learning induces neurotrophin receptor and synapsin I in the hippocampus. Brain Res. 2001;904:13–19. doi: 10.1016/s0006-8993(01)02394-0. [DOI] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for. Trends Neurosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Liu HX, Zhang JJ, Zheng P, Zhang Y. Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res. 2005;139:169–177. doi: 10.1016/j.molbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: a researcher's handbood. Prentice Hall: Englewood Cliffs, NJ; 1982. [Google Scholar]
- Schmidt-Kastner R, Aguirre-Chen C, Saul I, Yick L, Hamasaki D, Busto R, et al. Astrocytes react to oligemia in the forebrain induced by chronic bilateral common carotid artery occlusion in rats. Brain Res. 2005;1052:28–39. doi: 10.1016/j.brainres.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Farkas E, Institoris A, Domoki F, Mihaly A, Bari F. The effect of pre- and posttreatment with diazoxide on the early phase of chronic cerebral hypoperfusion in the rat. Brain Res. 2006;1087:168–174. doi: 10.1016/j.brainres.2006.02.134. [DOI] [PubMed] [Google Scholar]
- de la Torre JC, Fortin T, Park GA, Butler KS, Kozlowski P, Pappas BA, et al. Chronic cerebrovascular insufficiency induces dementia-like deficits in aged rats. Brain Res. 1992;582:186–195. doi: 10.1016/0006-8993(92)90132-s. [DOI] [PubMed] [Google Scholar]
- Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007;26:613S–623S. doi: 10.1080/07315724.2007.10719667. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Baraban SC, Hochman DW. Osmolarity, ionic flux, and changes in brain excitability. Epilepsy Res. 1998;32:275–285. doi: 10.1016/s0920-1211(98)00058-8. [DOI] [PubMed] [Google Scholar]
- Shulyakova N, Fong J, Diec D, Nahirny A, Mills L. Cell surface area regulation in neurons in hippocampal slice cultures is resistant to oxygen-glucose deprivation. Cell Health and Cytoskelet. 2010;2:69–81. [Google Scholar]
- Sekhon LH, Spence I, Morgan MK, Weber NC.Chronic cerebral hypoperfusion inhibits calcium-induced long-term potentiation in rats Stroke 1997281043–1047.discussion 1047–1048. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Mental exercise and mental aging: Evaluating the validity of the ‘‘use it or lose it'' hypothesis. Perspect Psychol Sci. 2006;1:68–87. doi: 10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]