Abstract
Leptin, one of the most important adipokines, is not only an energy regulator but also a regulator of innate immunity. Inflammation plays a key role in the tissue damage after intracerebral hemorrhage (ICH), and we sought to investigate whether leptin has a detrimental effect on ICH. After the injection of a high replacement dose (0.04 mg/kg) and two pharmacologic doses (4 and 8 mg/kg) of leptin, brain water contents increased significantly compared with that of control mice (P<0.05), which was confirmed when comparing the results with leptin-deficient ob/ob and wild-type (WT) mice (78.8%±0.6% versus 79.7%±0.6%, P<0.05). The number of Ox6-positive microglia/macrophages was increased in the leptin-injected group and decreased in ob/ob compared with WT mice. Among the candidate signal transducers, an increase in signal transduction and activator of transcription 3 (STAT3) levels was found after leptin injection. When we administered NSC74859, a specific inhibitor of phosphorylated STAT3 (pSTAT3), the water content became normalized. Activity of pSTAT3 was found mainly in Ox6-positive microglia/macrophages, but not in either neurons or astrocytes. We demonstrate that leptin plays a critical role in the secondary brain injury around a hematoma and is a novel mediator of the inflammation. This detrimental effect of leptin on ICH is mediated by the STAT3 signaling pathway in inflammatory cells.
Keywords: acute stroke, adipokine, brain edema, inflammation, intracerebral hemorrhage, leptin
Introduction
Intracerebral hemorrhage (ICH), which accounts for ∼15% of all strokes, has a higher incidence in the Hispanic, black, and Asian populations.1 It is the most devastating and least treatable subtype of stroke, causing severe disability and a high rate of acute mortality. Intracerebral hemorrhage volume is itself a key factor in brain tissue damage.2 However, secondary brain injury related to hemorrhage is also important,3, 4 which is reflected by the edema surrounding the hematoma, and has been a critical therapeutic target for ICH.
Leptin, one of the most important adipokines, has been established as a key regulator of energy balance and body weight.5 Clinical observations have indicated that hyperleptinemia is associated with risk of cardiovascular diseases6, 7 and is more closely related to hemorrhagic stroke than ischemic stroke.7 Recent clinical observations showed that endogenous leptin levels increased after ICH in humans, and that elevated leptin levels were associated with poor outcomes after ICH.8, 9 However, experimental evidence for this relationship is lacking, and the effects of leptin after ICH have never been elucidated. Leptin is not only an energy regulator but also a regulator of innate immunity.10 Leptin promotes phagocytosis by monocytes/macrophages and their secretion of proinflammatory cytokines.11, 12 Inflammation after ICH can cause disruption of the blood–brain barrier,13 elicit brain edema,14 and contribute to cell death after hemorrhage via cytotoxic mediators.15, 16 In this context, we have focused on leptin and inflammation after ICH, and brain edema is used as an important marker of brain injury related to inflammation. We sought to investigate whether leptin has a detrimental effect on ICH in terms of post-ICH inflammation. First, to evaluate the direct effects of leptin, exogenous leptin was injected into a mouse ICH model, and brain edema surrounding the hematoma and neurologic deficits were observed. Second, to investigate the effects of leptin deficiency after ICH, we induced ICH in leptin-deficient knockout mice. Finally, we investigated the intracellular messengers of leptin using a specific inhibitor for a candidate messenger.
Materials and methods
Animals and Experimental Groups
The study was performed in two stages. First, to examine if exogenous leptin influences the progression of ICH, male imprinting control regions mice (Koatech, Seoul, Republic of Korea) were divided into a leptin-injected group and a control group. Second, to determine the effects of leptin deficiency on ICH, male C57BL/6J strain Lepob/Lepob (ob/ob) mice and their wild-type (WT) littermates were used (Japan SLC, Shizuoka, Japan). Lepob/Lepob (ob/ob) mice have a mutated gene in ob to produce leptin, and they cannot secrete leptin in adipose tissue. These mice eat excessively, become obese, and develop high blood sugar. This study was carried out according to the National Institutes of Health Guide of the Care and Use of Laboratory Animals, and all experimental procedures were approved by the Institutional Animal Care and Use Committee of the Biomedical Research Institute at Seoul National University Hospital. Every effort was made to minimize animal suffering and to limit the number of animals used.
Induction of Intracerebral Hemorrhage and the Injection of Leptin
Experimental ICH was induced by the stereotaxic, intrastriatal administration of bacterial collagenase type IV (Sigma, St Louis, MO, USA). After inhalation anesthesia using 3% isoflurane in 30% oxygen and 70% air, the mice were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). A burr hole was made, and a 30-gauge Hamilton syringe needle was inserted into the striatum (location: 0.19 mm left lateral to the midline, 0.06 mm posterior to the bregma, 0.4 mm in depth below the skull). Collagenase type IV (0.23 U in 1 μL saline, Sigma) was administered over a 5-minute period. After infusion, the burr hole was sealed with bone wax, the wound was sutured, and the mice were allowed to recover. Physiological parameters, including mean arterial blood pressure, blood gases, and glucose concentration, were measured during the experiment. During the recovery period, the mice were assessed for forelimb flexion and contralateral circling to confirm the procedures. Seizure events were not observed during the experiments. Rectal temperature was maintained at 37°C±0.5°C with the use of a thermistor-controlled heating blanket. Free access to food and water was allowed after recovery from anesthesia.
We administered mouse recombinant leptin with replacement doses (0.01 and 0.04 mg/kg, Sigma) and pharmacologic doses (4 and 8 mg/kg) or phosphate-buffered saline (PBS) intraperitoneally just before the induction of ICH. The doses of leptin were determined on the basis of a previous experiment in a rodent stroke model17 and a leptin replacement study in leptin-deficient adults.18
Analysis of Brain Water Content
We analyzed brain water content 72 hours after ICH, when brain injury is maximal.19 The brain was divided into two hemispheres along the midline, and the cerebellum and brainstem were removed. The brain samples were immediately weighed on an electronic analytical balance to obtain the wet weight and were dried in a gravity oven at 100°C for 24 hours to obtain the dry weight. Water content was expressed as a percentage of wet weight using the following formula: (wet weight−dry weight)/(wet weight) × 100 (Song et al20).
Measurement of Hemorrhage Volume
Seventy-two hours after ICH, the brains were cut coronally through the needle entry site (identifiable on the brain surface), and serial slices (1-mm thickness) both anterior and posterior to the needle entry site were obtained. Digital photographs of the serial slices were taken, and hemorrhage volume was measured using an image analysis program, Image J (National Institutes of Health, Bethesda, MD, USA). The total hemorrhage volume (mm3) was calculated by summing the clot area in each section and multiplying by the distance between sections.20, 21
Behavior Testing
Behavior testing (n=6, respectively) was performed using the modified Neurological Severity Score (mNSS) method.22 The evaluation was performed by an investigator (DR) masked to the experimental scheme. The mNSS is a composite of motor (six points), sensory (two points), reflexes (three points), and abnormal movements (one point), and beam balance tests were not included owing to the small size of mice compared with rats. The assessment was performed on day 1 before ICH, at 30 minutes, and on days 1 and 3 after ICH. Neurologic function was graded on a scale of 0–12 (normal score: 0; maximal deficit score: 12).
Enzyme-Linked Immunosorbent Assay for Measuring Leptin Levels in Serum and Brain
Using a commercially available enzyme-linked immunosorbent assay kit for measuring leptin levels (ALPCO Diagnostics, Salem, NH, USA), we checked leptin levels of serum and brain after ICH without meal. Before induction of ICH, baseline leptin levels in fasting states were measured, and after induction of ICH, leptin levels were checked at 10 minutes and 1 day in fasting states without meal (Figure 1A).
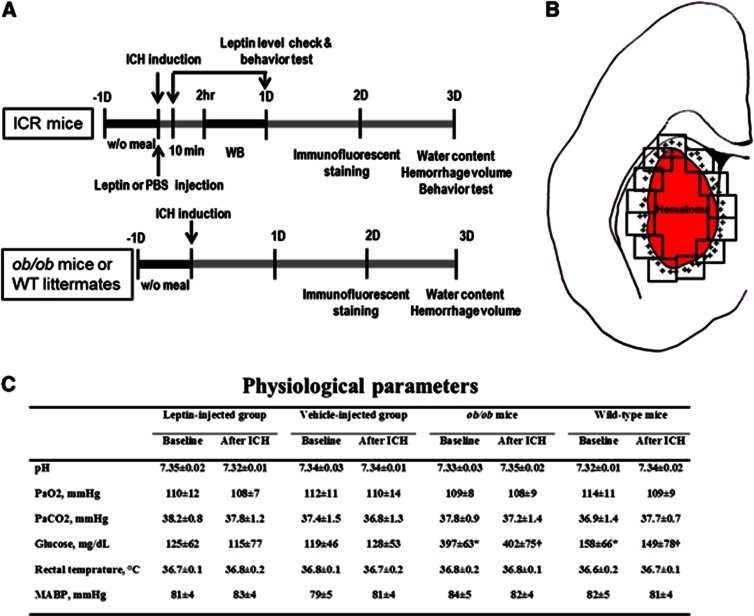
Figure 1.
Schematic diagram of the study protocols and regions of cell quantification. (A) For imprinting control regions (ICR) mice, leptin or phosphate-buffered saline (PBS) was administered just before the induction of intracerebral hemorrhage (ICH). At 2 and 24 hours, Western blot (WB) analyses were performed, and at 2 days, immunofluorescent staining was performed. Brain water content, hemorrhage volume, and functional deficits were evaluated 3 days after ICH induction. For ob/ob mice or their wild-type (WT) littermates, immunofluorescent staining was performed at 2 days, and water content and hemorrhage volume were evaluated at 3 days. (B) High-power field images that fully covered perihematomal areas were taken from sections stained through the center of the hemorrhagic lesion for counting activated microglia/macrophages and neutrophils. The plus signs indicate perihematomal inflammatory cells. (C) In baseline parameters, the glucose levels were higher in ob/ob mice than in WT mice, which are typical findings for ob/ob mice, but the other parameters did not differ between ob/ob and WT mice (*P<0.05 by unpaired Student's t-test for baseline values between ob/ob and WT mice and †P<0.05 for values after ICH). ‘After ICH' indicates the time point at 30 minutes after induction of ICH; MABP, mean arterial blood pressure. Values are expressed as mean±s.d.
Immunofluorescent Staining and Cell Quantification
Immunofluorescent staining of brain tissue was performed using cryopreserved 40-μm coronal sections. Each section was incubated with 0.5% bovine serum albumin/0.3% Triton-X followed by 10% normal serum in PBS for 1 hour for blocking. Sections with a primary antibody were placed at 4°C for 16 hours. After washing, each section was subsequently incubated for 2 hours at room temperature with the fluorophore-conjugated secondary antibody. The following primary antibodies were used: monoclonal antibodies against MHC class II Ia (Ox6; Santa Cruz Biotech., Santa Cruz, CA, USA) to label activated microglia/macrophages; MPO (myeloperoxidase; DAKO, Carpinteria, CA, USA) to stain neutrophils; NeuN (neuronal nuclei antigen; Chemicon, Temecula, CA, USA) to label mature neurons; GFAP (glial fibrillary acidic protein; Chemicon) to label astrocytes; and phosphorylated signal transduction and activator of transcription 3 (pSTAT3; Cell Signaling Tech., Danvers, MA, USA). Cell nuclei were visualized with 4,6-diaminodino-2-phenylindole staining. Stained cells were then examined under a confocal laser scanning biological microscope (LSM 410 META; Carl Zeiss, Jena, Germany). To analyze the expression of leptin in the brain, we performed immunohistochemistry assay using anti-leptin antibody (Abcam, Cambridge, UK) in hemorrhagic brain with or without leptin injection, or in nonhemorrhagic brain.
Quantitative analysis of the positively stained cells was performed in the perihematomal regions by two independent investigators (W-SR, BJK) who were masked to the group allocation. To count activated microglia/macrophages and neutrophils, 16 high-power fields were taken from the section staining through the center of the ICH lesion (Figure 1B) 48 hours after ICH, when the intensity of inflammation is maximal.23 Total counts in the measured sections were converted into cell densities for comparison between the ICH groups according to our established protocol.24 In addition, to locate the pSTAT3 site of action, we performed double-immunofluorescence staining with antibodies for pSTAT3 and cell type-specific markers (Ox6, NeuN, and GFAP).
Western Blot Analysis
The mice were killed via decapitation, and the brains were immediately extracted 2 or 24 hours after the induction of ICH. After the centrifugation of hemisphere homogenates, 50 μg of protein was separated on a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and transferred to nitrocellulose membranes. These membranes were incubated in blocking buffer (5% skim milk in 50 mmol/L Tris pH 7.5, 0.15 mmol/L NaCl, 0.05% Tween-20), and the blots were probed with antibodies recognizing STAT3, pSTAT3 (Cell Signaling Tech.), extracellular signal-regulated kinase (ERK; Santa Cruz Biotech.), pERK (Cell Signaling Tech.), Akt (Santa Cruz Biotech.), pAkt (Cell Signaling Tech.), cyclooxygenase-2 (COX-2; BD Biosciences, Franklin Lakes, NJ, USA), and inducible nitric oxide synthase (iNOS; NOVUS Biologicals, Littleton, CO, USA). Immunoreactivity was visualized by enhanced chemiluminescence, and the relative optical densities were determined by comparison of the measured values with the mean values of the control group.
Inhibition of Phosphorylated Signal Transduction and Activator of Transcription 3
The pSTAT3 inhibitor NSC74859 (Merck, Darmstadt, Germany), dissolved in 50% dimethyl sulfoxide and 50% PBS in a 0.2-mL volume, was administered intraperitoneally after the injection of leptin in the leptin-injected group. To investigate the effect of NSC74859 in the absence of exogenous leptin, NSC74859 was also administered to the control group at the same time. The pSTAT3-inhibiting effect of NSC74859 was analyzed using Western blot analysis.
Statistical Analysis
The values are presented as the mean±s.d. Data were analyzed with the unpaired Student's t-test and ANOVA (analysis of variance) test when appropriate. Otherwise, the nonparametric Mann–Whitney U test or Wilcoxon signed-rank test was used for unpaired or paired samples, respectively. A two-tailed value of P<0.05 was considered significant. All statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL, USA).
Results
Physiological Parameters and Leptin Levels After Intracerebral Hemorrhage
The physiological parameters, including mean arterial blood pressure, blood gases, serum glucose, and body temperature, were similar between the leptin-injected and control groups (Figure 1C). The glucose level and body weight were higher in ob/ob mice than in WT mice (glucose: 397 versus 158 mg/dL, P<0.05; body weight: 43.2 versus 21.2 g, P<0.05), which are typical findings for leptin-deficient ob/ob mice, but the other parameters did not differ between ob/ob and WT mice.
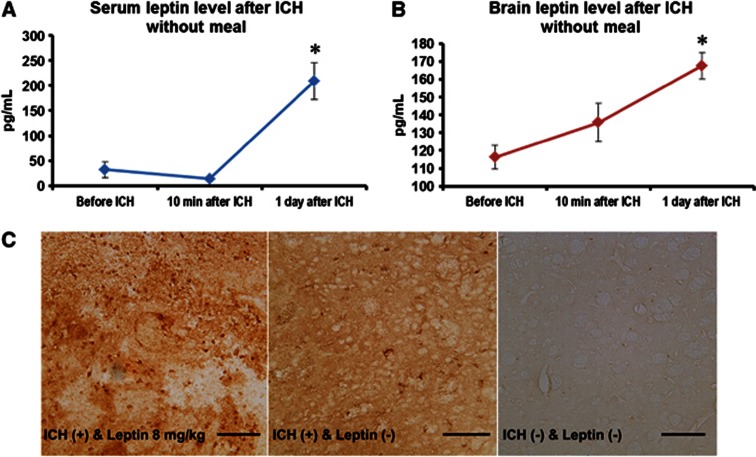
Compared with leptin levels in serum and brain in fasting state, leptin increased significantly 1 day after ICH in serum and brain without meal (208±38 versus 32±16 pg/mL in serum, and 167±7 versus 116±6 pg/mL in brain, P<0.05; Figures 2A and 2B). In immunohistochemistry assay, 1 day after ICH, leptin was more frequently detected in the hemorrhagic hemisphere than in nonhemorrhagic brain (Figure 2C).
Figure 2.
Leptin levels after intracerebral hemorrhage (ICH). (A) Compared with serum leptin level before ICH, leptin increased 1 day after ICH in serum without meal (*P<0.05, n=4). (B) Compared with brain leptin level before ICH, leptin increased 1 day after ICH in brain without meal (*P<0.05, n=4). (C) In immunohistochemistry assay, leptin was more frequently detected in hemorrhagic hemisphere 1 day after ICH than nonhemorrhagic brain. Scale bar=100 μm.
Leptin Increases Brain Water Content and Neurological Deficits after Intracerebral Hemorrhage
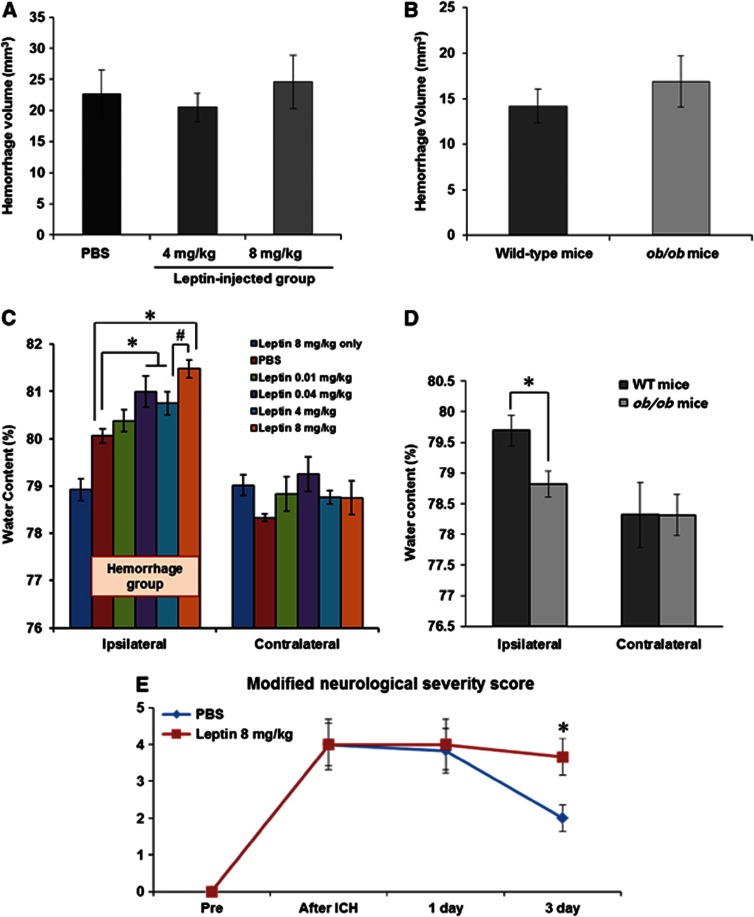
To investigate the dose-dependent effects of leptin, we compared the results of water content at replacement doses (0.01 and 0.04 mg/kg) and pharmacologic doses of leptin (4 and 8 mg/kg), and then determined an optimal dose for this study protocol. We replicated the same procedures in leptin-deficient ob/ob mice and their WT littermates to confirm the effects of leptin in ICH. First, hemorrhage volumes were not different between control and both pharmacologic dose groups of leptin injection (22.7±11.4 mm3 for control; 20.5±7.4 mm3 for 4 mg/kg, P=0.62; 24.6±12.1 mm3 for 8 mg/kg, P=0.75; Figure 3A), which was confirmed by comparing WT with ob/ob mice (14.2±5.3 mm3 for WT versus 16.9±7.9 mm3 for ob/ob mice, P=0.44; Figure 3B). After injection of high pharmacologic dose (8 mg/kg) of leptin without ICH, the brain water content was not different from that of nonhemorrhagic hemisphere, and in low replacement dose (0.01 mg/kg) of leptin after induction of ICH, the brain water content was not increased compared with the PBS-injected control group. However, high replacement dose (0.04 mg/kg) and both pharmacologic doses (4 and 8 mg/kg) induced significant increases of brain water content compared with values in the control group (80.1%±0.8% for control, 80.9%±0.5% for 0.04 mg/kg, 80.7%±0.9% for 4 mg/kg and 81.5%±0.5% for 8 mg/kg; P<0.01 by ANOVA test and P<0.05 by Mann–Whitney U test, respectively; Figure 3C), and the injection of 8 mg/kg leptin was more detrimental than the 4-mg/kg injection (P<0.05). Based on these results, all of the following experiments (leptin-injected group) were performed with 8 mg/kg of leptin. Furthermore, the brain water content of ob/ob mice decreased more than that of their WT littermates (Figure 3D; 79.7%±0.6% in WT versus 78.8%±0.6% in ob/ob mice, P<0.05). Leptin injection was not associated with brain water content in the contralateral hemisphere.
Figure 3.
Measurements of brain water content, hemorrhage volume, and neurologic deficits. (A, B) Mean hemorrhage volumes were not different between the control and leptin-injected groups (n=10 each) or between the wild-type (WT) and ob/ob mice (n=8 each). (C) High replacement dose (0.04 mg/kg) and both pharmacologic doses (4 and 8 mg/kg) of leptin increased the brain water content in the hemorrhagic hemisphere compared with the control group (n=10 for pharmacologic doses and n=6 for replacement doses; P<0.01 by analysis of variance (ANOVA) test and *P<0.05 by Mann–Whitney U test, respectively). The water content in the 8-mg/kg group was higher than in the 4-mg/kg group (#P<0.05). (D) The water content of leptin-deficient ob/ob mice was lower than that of WT mice (n=7 each, *P<0.05). (E) Leptin injection aggravated neurologic deficits in intracerebral hemorrhage (ICH) injury. In all mice (n=12), the modified Neurological Severity Score (mNSS) was 0 before the ICH, indicating normal neurologic function. In the control group (n=6), the mNSS was peaked at 30 minutes after ICH and decreased on day 3. In the leptin-injected group (n=6), the mNSS was not reduced on days 1 and 3 compared with 30 minutes after ICH, and was significantly higher than that of control group on day 3 after ICH (*P<0.05).
Leptin injection aggravated neurologic deficits in ICH injury (Figure 3E). In all mice, the mNSS was 0 before the ICH, indicating normal neurologic function. In the control group, the mNSS was peaked at 4±1.4 at 30 minutes after ICH and decreased to 2±0.9 on day 3. In the leptin-injected group, the mNSS was not reduced on days 1 and 3 compared with 30 minutes after ICH (4±1.7 at 30 minutes, 4±1.7 on 1 day, and 3.7±1.2 on day 3), and was significantly higher than that of control group on day 3 after ICH (P<0.05).
Leptin Increases the Number of Inflammatory Cells in the Perihematomal Area
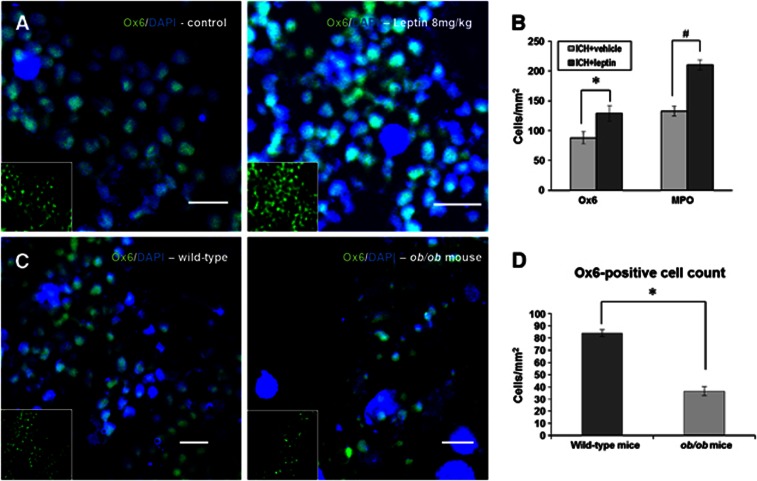
Ox6-stained microglia/macrophages were frequently found in the periphery around the lesion, and the density in the leptin-injected group was higher than that in the control group (Figure 4A). In quantitative analysis, the leptin-injected group exhibited a higher number of Ox6-positive cells (128.9±13.1 versus 88.0±20.7 cells/mm2, P<0.05) and MPO-positive neutrophils (210.4±16.8 versus 132.8±8.0 cells/mm2, P<0.05) than the control group (Figure 4B). To compare the density of microglia/macrophages around the hemorrhagic lesion in a leptin-deficiency state, we stained Ox6-positive cells in ob/ob and WT mice. The number of Ox6-positive cells was decreased in ob/ob mice compared with WT mice (Figures 4C and 4D; 36.6±6.3 versus 84.1±5.8 cells/mm2, P<0.05).
Figure 4.
Immunofluorescent staining of inflammatory cells. (A) Ox6-stained microglia/macrophages (red) were frequently found in the periphery around the lesion, and the density in the leptin-injected group was higher than in the control group (n=4 each). Nuclear staining using 4,6-diaminodino-2-phenylindole (DAPI) (blue) was performed. Scale bar=10 μm. (B) In quantitative analysis, the leptin-injected group exhibited a higher number of Ox6+ cells (*P<0.05) and MPO+ neutrophils (#P<0.05) than the control group. (C) The density of Ox6-stained cells (green) in ob/ob mice was lower than in wild-type (WT) mice (n=4 each). Scale bar=10 μm. (D) The number of Ox6+ cells was decreased in ob/ob mice compared with WT mice (*P<0.05). MPO, myeloperoxidase.
The Signal Transduction and Activator of Transcription 3 Signaling Pathway Plays a Critical Role in Inflammatory Cells
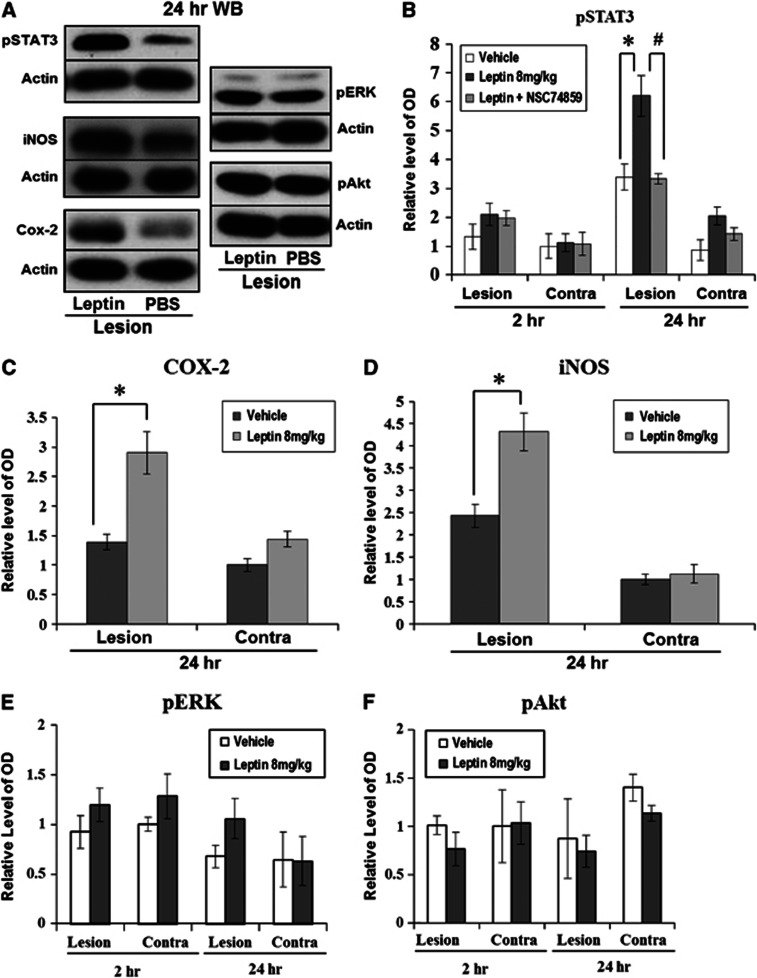
Phosphorylated STAT3 was minimally detected 2 hours after ICH, but at 24 hours, a 1.8-fold increase in pSTAT3 density was detected in the leptin-injected group compared with the control group, and the density in the hemorrhagic hemisphere was also higher than that in the nonhemorrhagic hemisphere (Figure 5B; P<0.05). After the injection of the pSTAT3 inhibitor, NSC74859, the pSTAT3 density of the hemorrhagic hemisphere in the leptin-injected group was significantly decreased to the level of the control group (Figure 5B; P<0.05). The densities of COX-2 and iNOS, target inflammatory proteins of STAT3, in the hemorrhagic hemisphere were 2.1- and 1.8-fold higher in the leptin-injected group compared with the control group, respectively (Figures 5C and 5D; P<0.05). However, the densities of pERK and pAkt, which have been identified as candidate intracellular messengers related to tyrosine kinase receptor of leptin, did not differ between the leptin-injected and control groups (Figures 5E and 5F).
Figure 5.
Western blot analyses for signal transduction and activator of transcription 3 (STAT3), extracellular signal-regulated kinase (ERK), Akt, and cyclooxygenase-2 (COX-2). (A) At 24 hours after intracerebral hemorrhage (ICH), the density of pSTAT3 and COX-2 in the leptin-injected group was higher than in the control group (n=4 each). (B) In quantitative analyses, pSTAT3 was minimally detected at 2 hours after ICH, but 24 hours later, a 1.8-fold increase of pSTAT3 density was detected in the leptin-injected group (*P<0.05). After the injection of NSC74859, a specific inhibitor of pSTAT3, the pSTAT3 density in the leptin-injected group was significantly decreased, nearly to the level of the control group (#P<0.05). (C) The density of COX-2 in the hemorrhagic hemisphere showed a 2.1-fold increase in the leptin-injected group compared with the phosphate-buffered saline (PBS)-injected group. (D) The density of inducible nitric oxide synthase (iNOS) in the hemorrhagic hemispheres showed a 1.8-fold increase in the leptin-injected group compared with the PBS-injected group. (E, F) The densities of pERK and pAkt were not different between the leptin-injected and control groups.
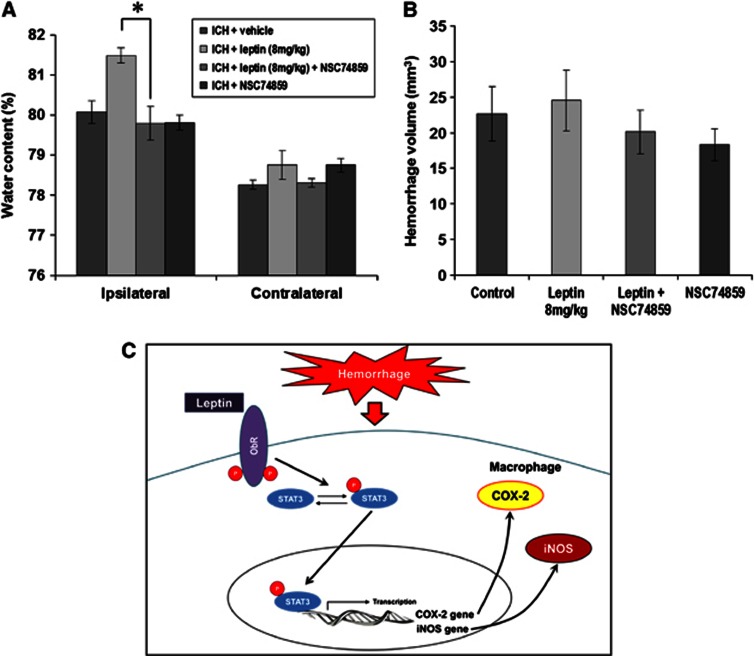
After the inhibition of pSTAT3 using NSC74859 in the leptin-injected group, the brain water content decreased to the level of the control group (Figure 6A; leptin+NSC74859: 79.8%±0.8% versus leptin-only group: 81.5%±0.5%, P<0.05). However, the injection of NSC74859 in the control group did not decrease the brain water content compared with the vehicle-only group, and the hemorrhage volume was not different between the groups (Figure 6B). From the results of Western blot analyses and experiments with STAT3 inhibitor, tyrosine kinase receptor of leptin pSTAT3 in cytosol, and translocated pSTAT3 into nucleus initiated transcription of COX-2 and iNOS genes. After that, COX-2 and iNOS were excessively expressed after ICH in leptin-injected group (Figure 6C).
Figure 6.
Changes in brain water content and hemorrhage volume after the injection of a phosphorylated signal transduction and activator of transcription (pSTAT) inhibitor (NSC74859). (A) After the inhibition of pSTAT3 using NSC74859 in the leptin-injected group (n=5), the brain water content was decreased to the level of the control group (*P<0.05 by Mann–Whitney U test to directly compare between the leptin+intracerebral hemorrhage (ICH) group and the leptin+NSC74859+ICH group). However, the injection of NSC74859 in the control group (n=5) did not induce a change in brain water content compared with the vehicle-only group. (B) The hemorrhage volumes were not different between the groups (n=5 each). (C) Tyrosine kinase receptor of leptin (ObRb) pSTAT3 in cytosol, and translocated pSTAT3 into nucleus initiated transcription of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) genes. After that, COX-2 and iNOS were excessively expressed after ICH in leptin-injected group.
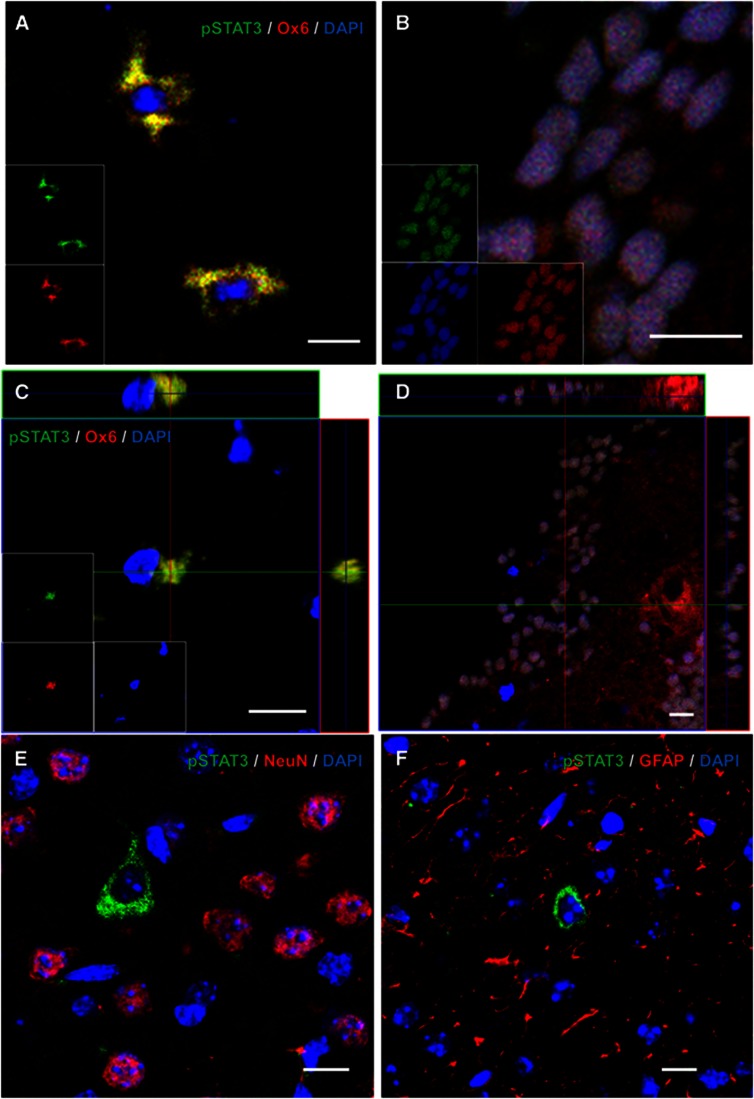
Ox6-positive microglia/macrophages were stained by pSTAT3 in nucleus or cytosol (Figures 7A and 7B), and we confirmed the identity of double-stained cells with three-dimensional analysis (Figures 7C and 7D). However, the cells labeled by pSTAT3 were not stained by NeuN or GFAP in the periphery around a hemorrhagic lesion (Figures 7E and 7F).
Figure 7.
Double staining of phosphorylated signal transduction and activator of transcription 3 (pSTAT3) and cell-specific markers (n=4). (A) pSTAT3-positive cells (green) were colocalized with Ox6-positive microglia/macrophages (red) in cytosol. (B) pSTAT3-positive nuclei were colocalized with Ox6-positive cells. (C, D) Ox6 and pSTAT3 double labeling was confirmed using a three-dimensional reconstruction of the confocal images. (E) The cells labeled by pSTAT3 (green) were not stained by neuronal nuclei antigen (NeuN), a marker for mature neurons (red). (F) The cells stained by pSTAT3 (green) did not colocalize with glial fibrillary acidic protein (GFAP) (red). Scale bar=10 μm.
Discussion
In this study, leptin increased the degree of brain edema, neurologic deficits, recruitment of inflammatory cells, and production of inflammatory end products after ICH, and these results were confirmed in leptin-deficient ob/ob mice. Phosphorylated STAT3, the signaling protein of leptin, was increased in the leptin-injected group compared with the control group, and inhibition of pSTAT3 activated by leptin reduced edema after ICH. In addition, pSTAT3 activation was mainly found in the activated microglia/macrophages.
The early phase of secondary brain injury after ICH is mainly mediated by clot-derived thrombin, which is associated with local injury such as direct cellular toxicity, blood–brain barrier breakdown, and inflammatory cell infiltration.13 After the early phase, subsequent inflammatory reaction plays an important role in tissue damage that occurs after ICH. As one of the critical mediators of the immune system, leptin promotes the secretion of proinflammatory cytokines such as tumor necrosis factor-α, interleukin-6, and interferon-γ.11 Functional receptors for leptin are expressed not only in the hypothalamus, where it regulates energy homeostasis, but also in various cell types related to immunity. Leptin binding to the functional receptor recruits Janus tyrosine kinase, which then serves as a docking site for cytoplasmic adaptors such as STAT3, ERK, and Akt, candidate signal transducers in this study.25 In this study, leptin increases inflammation after ICH via the STAT3 pathway, and the target gene related to inflammation of STAT3 might be COX-2 and iNOS, which were highly expressed after injection of leptin in hemorrhagic hemisphere.26 According to previous studies about the role of STAT3 in inflammatory processes, STAT3 is involved in the expression of iNOS and is partly dependent on Ser727 phosphorylation, which is also necessary for nuclear translocation and DNA binding,27 and STAT3 upregulated COX-2 for late preconditioning in cardioprotection.28
In the current study, leptin-deficient ob/ob mice showed a protective effect against secondary tissue damage after ICH via decreased inflammation. Compared with WT littermates, ob/ob mice were obese by chronic overnutrition, and had hyperglycemia as well as leptin deficiency. Because leptin is an important modulator of immunity, ob/ob mice were in immunocompromised state compared with WT mice.29 It is difficult to delineate the acute effects of leptin deficiency in ob/ob mice from chronic hyperglycemia, obesity, and immunocompromised state. However, our previous study showed that hyperglycemia increased brain water content after ICH,20 and the incidence of hemorrhagic transformation after cerebral ischemia, not tissue damage after spontaneous hemorrhage, was increased in ob/ob mice, because of increased matrix metalloproteinase-9 level related to obesity.30 It is noteworthy that beneficial effects in ob/ob mice were achieved as the potential harmful influence of hyperglycemia and obesity was overcome. According to immunocompetence, leptin deficiency confers resistance to the induction for several experimentally induced autoimmune diseases such as autoimmune encephalomyelitis,31 and experimentally induced colitis,32 hepatitis,33 and nephritis.34 Combined with the result that leptin injection aggravated ICH-induced inflammation and secondary tissue damage, acute leptin deficiency may contribute some aspect of protection against secondary ICH injury related to inflammation in ob/ob mice.
Janus tyrosine kinase 2-STAT3 signaling is an important pathway mediating the effects of leptin on immune cells.35 It is well established that leptin stimulation leads to the tyrosine phosphorylation of STAT3 and its translocation to the nucleus in human or murine macrophages.36 This pathway has not been observed in stroke models, but we found that the detrimental effect of leptin on ICH disappeared after the injection of a pSTAT inhibitor (NSC74859) and that, after ICH, pSTAT3 was located in microglia/macrophages and not in neurons or astrocytes. The STAT3 activation was higher at 24 hours than at 2 hours after ICH in our study; this delayed activation is highly correlated with the time course of inflammation after ICH because the infiltration of inflammatory cells begins at 12 hours and is maximal 48 hours after ICH.23 In this context, we postulate that STAT3 is a key molecule of the leptin-induced detrimental effect on ICH. However, the inhibitor of STAT3, NSC74859, only works in the presence of the pharmacologic dose of leptin (8 mg/kg), and it did not reduce brain edema in mice administered ICH+inhibitor without injection of leptin. This result shows that the protective effects of reducing activation of STAT3 without hyperleptinemia against perihematomal edema and inflammation after ICH are still elusive.
Leptin is an adipokine with physiologic function, and increased leptin in stressful conditions associated with various diseases is not always harmful. After focal ischemia in the brain or heart, increased leptin showed protective effects via the antiapoptotic pathway, but a possible proinflammatory effect of hyperleptinemia was not evaluated in these studies.17, 37 As demonstrated in the current study, increased leptin in inflammatory diseases was harmful, and in an experimental ICH model, leptin showed a detrimental effect through the enhancement of inflammation. The protective or harmful effect of leptin after various diseases is not seen uniformly, and it may depend on the specific disease conditions, action timing, or doses of leptin.
According to glucose homeostasis, leptin acts on peripheral leptin receptors expressing various tissues. The direct action of leptin on the pancreas inhibits insulin and glucagon secretion, and leptin suppresses insulin signaling and action in adipocytes and liver. The centrally mediated action of leptin is that autonomic efferents increase direct leptin action on the pancreas and insulin-sensitive tissues via activation of the hypothalamus.38 From these reasons, in the leptin-deficient state such as in ob/ob mice, hyperglycemia is induced with combined overnutrition, obesity, and hyperinsulinemia.
Taken together, we have demonstrated that leptin is a novel mediator of inflammation after ICH and plays a critical role in secondary brain injury around the hematoma via the STAT3 signaling pathway in microglia/macrophages. Specific medical or surgical treatment for ICH patients does not exist: the benefit of surgical treatment has not been proven,39 and furthermore, hemostatic medical treatment using the early introduction of recombinant factor VIIa to inhibit the expansion of hematoma failed in a large clinical trial.40 As the incidence of ICH is very high worldwide and increases up to 30–50% in Asian countries, specific treatment to improve the outcomes of ICH patients must be discovered and immediately applied to clinical practice. However, whether there is a key molecule that modulates the ICH-induced inflammation is still controversial. We believe that the perihematomal inflammation mediated by leptin can be a new therapeutic target for ICH treatment.
The authors declare no conflict of interest.
Footnotes
This work was supported by grants of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A111014).
References
- Kase CS, Mohr JP, Caplan LR.Intracerebral hemorrhageIn: Mohr JP, Choi DW, Grotta JC, Weir B, Wolf PA, (eds). Stroke Philadelphia, PA, USA: Churchill Livingstone; 2004327–376. [Google Scholar]
- Caplan LR. Intracerebral haemorrhage. Lancet. 1992;339:656–658. doi: 10.1016/0140-6736(92)90804-c. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- Lok J, Leung W, Murphy S, Butler W, Noviski N, Lo EH. Intracranial hemorrhage: mechanisms of secondary brain injury. Acta Neurochir Suppl (Wien) 2011;111:63–69. doi: 10.1007/978-3-7091-0693-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- Soderberg S, Ahren B, Stegmayr B, Johnson O, Wiklund PG, Weinehall L, et al. Leptin is a risk marker for first-ever hemorrhagic stroke in a population-based cohort. Stroke. 1999;30:328–337. doi: 10.1161/01.str.30.2.328. [DOI] [PubMed] [Google Scholar]
- Dong XQ, Huang M, Hu YY, Yu WH, Zhang ZY. Time course of plasma leptin concentrations after acute spontaneous basal ganglia hemorrhage. World Neurosurg. 2010;74:286–293. doi: 10.1016/j.wneu.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Zhao QJ, Sun M, Zhang XG, Wang LX. Relationship between serum leptin levels and clinical outcomes of hypertensive intracerebral hemorrhage. Clin Exp Hypertens. 2012;34:161–164. doi: 10.3109/10641963.2011.561902. [DOI] [PubMed] [Google Scholar]
- La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- Zarkesh-Esfahani H, Pockley G, Metcalfe RA, Bidlingmaier M, Wu Z, Ajami A, et al. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001;167:4593–4599. doi: 10.4049/jimmunol.167.8.4593. [DOI] [PubMed] [Google Scholar]
- Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol. 2002;168:4018–4024. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Res. 2000;871:57–65. doi: 10.1016/s0006-8993(00)02427-6. [DOI] [PubMed] [Google Scholar]
- Mayne M, Fotheringham J, Yan HJ, Power C, Del Bigio MR, Peeling J, et al. Adenosine A2A receptor activation reduces proinflammatory events and decreases cell death following intracerebral hemorrhage. Ann Neurol. 2001;49:727–735. doi: 10.1002/ana.1010. [DOI] [PubMed] [Google Scholar]
- Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53:731–742. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38:2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O'Kirwan F, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA. 2004;101:4531–4536. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Ito U, Ohno K, Hirakawa K. Chronological changes in brain edema induced by experimental intracerebral hematoma in cats. Acta Neurochir Suppl (Wien) 1994;60:558–560. doi: 10.1007/978-3-7091-9334-1_154. [DOI] [PubMed] [Google Scholar]
- Song EC, Chu K, Jeong SW, Jung KH, Kim SH, Kim M, et al. Hyperglycemia exacerbates brain edema and perihematomal cell death after intracerebral hemorrhage. Stroke. 2003;34:2215–2220. doi: 10.1161/01.STR.0000088060.83709.2C. [DOI] [PubMed] [Google Scholar]
- Chu K, Jeong SW, Jung KH, Han SY, Lee ST, Kim M, et al. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J Cereb Blood Flow Metab. 2004;24:926–933. doi: 10.1097/01.WCB.0000130866.25040.7D. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neurosci Lett. 2000;283:230–232. doi: 10.1016/s0304-3940(00)00971-x. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Jeong SW, Han SY, Lee ST, Kim JY, et al. HMG-CoA reductase inhibitor, atorvastatin, promotes sensorimotor recovery, suppressing acute inflammatory reaction after experimental intracerebral hemorrhage. Stroke. 2004;35:1744–1749. doi: 10.1161/01.STR.0000131270.45822.85. [DOI] [PubMed] [Google Scholar]
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Strong R, Zhang J, Grotta JC, Aronowski J. Distinct patterns of intracerebral hemorrhage-induced alterations in NF-kappaB subunit, iNOS, and COX-2 expression. J Neurochem. 2007;101:652–663. doi: 10.1111/j.1471-4159.2006.04414.x. [DOI] [PubMed] [Google Scholar]
- Schuringa JJ, Schepers H, Vellenga E, Kruijer W. Ser727-dependent transcriptional activation by association of p300 with STAT3 upon IL-6 stimulation. FEBS Lett. 2001;495:71–76. doi: 10.1016/s0014-5793(01)02354-7. [DOI] [PubMed] [Google Scholar]
- Xuan YT, Guo Y, Zhu Y, Han H, Langenbach R, Dawn B, et al. Mechanism of cyclooxygenase-2 upregulation in late preconditioning. J Mol Cell Cardiol. 2003;35:525–537. doi: 10.1016/s0022-2828(03)00076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom P. The physiology of obese-hyperglycemic mice [ob/ob mice] Scientific World J. 2007;7:666–685. doi: 10.1100/tsw.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BW, Rose N, Robson FH, Rothwell NJ, Lawrence CB. Increased brain microvascular MMP-9 and incidence of haemorrhagic transformation in obese mice after experimental stroke. J Cereb Blood Flow Metab. 2010;30:267–272. doi: 10.1038/jcbfm.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna V, Di Giacomo A, La Cava A, Lechler RI, Fontana S, Zappacosta S, et al. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111:241–250. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–2025. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci USA. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarzi RM, Cook HT, Jackson I, Pusey CD, Lord GM. Leptin-deficient mice are protected from accelerated nephrotoxic nephritis. Am J Pathol. 2004;164:385–390. doi: 10.1016/S0002-9440(10)63128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- Sanchez-Margalet V, Martin-Romero C. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell Immunol. 2001;211:30–36. doi: 10.1006/cimm.2001.1815. [DOI] [PubMed] [Google Scholar]
- Smith CC, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol. 2006;149:5–13. doi: 10.1038/sj.bjp.0706834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denroche HC, Huynh FK, Kieffer TJ. The role of leptin in glucose homeostasis. J Diabetes Invest. 2012;3:115–129. doi: 10.1111/j.2040-1124.2012.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]