Abstract
Stroke patients are prone to life-threatening bacterial pneumonia. Previous experimental stroke studies have demonstrated that preventive antibiotic treatment (PAT) improves outcome compared with placebo treatment, which however does not model the clinical setting properly. Here we investigate whether PAT is superior to the current clinical ‘gold standard' for treating poststroke infections. Therefore, we modeled stroke care according to the current stroke guidelines recommending early antibiotic treatment after diagnosing infections. To reliably diagnose pneumonia in living mice, we established a general health score and a magnetic resonance imaging protocol for radiologic confirmation. Compared with standard treatment after diagnosis by these methods, PAT not only abolished pneumonia successfully but also improved general medical outcome. Both, preventive and standard antibiotic treatment using enrofloxacin improved survival in a similar way compared with placebo treatment. However, in contrast to standard treatment, only PAT improved functional outcome assessed by gait analysis. In conclusion, standard and preventive treatment approach reduced poststroke mortality, however at the cost of a worse neurologic outcome compared with preventive approach. These data support the concept of PAT for treating patients at risk for poststroke infections and warrant phase III trials to prove this concept in clinical setting.
Keywords: antibiotic prophylaxis, brain ischemia, gait analysis, lung MRI, mouse model of stroke, pneumonia, stroke-induced immunodepression
Introduction
Acute cerebral ischemia not only impairs neurologic function but also leads to medical complications, affecting up to 95% of patients in the first 3 months after stroke.1, 2 Infection and, in particular, pneumonia is one of the most important complications and a main cause of death after stroke, with an incidence of pneumonia ranging between 5 and 22%3, 4, 5, 6, 7 and the highest attributable mortality accounting for ∼30% of all deaths.3 By comparison, the incidence of pneumonia in non-stroke patients treated in a geriatric hospital averaged only 3.5%.8 Moreover, infectious complications after stroke also worsen neurologic outcome.9, 10, 11 Recent experimental and clinical data suggest that the deleterious combination of stroke-facilitated aspiration and stroke-induced immunodeficiency dramatically increases susceptibility to infection in stroke patients.12
Despite general measures to prevent aspiration, stroke-associated pneumonia remains a common and severe clinical challenge even for patients treated in specialized stroke units.11, 13 Thus, alternative treatment strategies are of urgent need. Using an experimental mouse model, we have previously demonstrated that poststroke infections and fever can be reduced effectively by preventive antibacterial therapy (PAT) with moxifloxacin. More importantly, PAT also reduces mortality, and improves neurologic outcome significantly.14 Three randomized controlled phase IIb trials on PAT after stroke have been performed. Whereas the PANTHERIS and MISS trials suggest superiority of a preventive antiinfective therapy over current standard therapy in terms of infection control, ESPIAS is questioning this approach.15, 16, 17 A metanalysis on these trials suggests that PAT reduces the occurrence of poststroke infections, but remains ineffective in terms of outcome.18 Consequently, the current ‘gold standard' by stroke guidelines is treatment immediately after diagnosis of infections. Two main reasons might account for the discrepancy between experimental and clinical findings. First, clinical trials were not powered to address treatment effects on neurologic outcome.18, 19 Second, our experimental model might not be predictive for the clinical setting. In fact, we tested PAT against ‘placebo' in our experimental model14 but not against the above-mentioned standard treatment, which is obviously not reflecting clinical practice properly. Thus, mice of the control group were not treated with antibiotics at all, independently of whether they developed an infection or not, in contrast to the stroke treatment guidelines in patients. To model the clinical situation appropriately, we here define diagnostic criteria for murine pneumonia, similar to the Centers for Disease Control criteria20 for human. Reasoned by the lack of an established method to reliably detect chest infections in living mice, we developed a general medical examination protocol and applied magnetic resonance imaging (MRI) for radiologic confirmation.21 Based on this protocol, we were able to investigate whether preventive antibiotic treatment (PAT) is superior in improving poststroke outcome compared with the standard treatment defined by the stroke guidelines in experimental stroke.
Materials and methods
Experimental Setup
In a mouse model of stroke, we imitated a randomized clinical trial investigating superiority of preventive antiinfective treatment of poststroke infections compared with the ‘gold standard' according to the stroke guidelines. Our primary aim was improvement of long-term outcome. Mice (n=80) underwent transient middle cerebral artery occlusion (MCAo) with reperfusion after 60 minutes as described below. After MCAo, animals were randomly assigned to receive either a daily preventive medication (n=26, enrofloxacin) starting at the day of MCAo or a therapeutic medication (n=25; enrofloxacin) after diagnosis of lung infection. Standard treatment started immediately after the appearance of clinical signs (general health score>6) usually between day 4 and 6 after stroke. In a pre-defined subgroup of the study population (n=39), pneumonia was confirmed by MRI on day 3 after experimental stroke. To reproduce our findings,14 we compared outcome of both antibiotic treatment approaches against placebo-treated animals (n=29, sodium chloride). General medical outcome was assessed every day. Neurologic outcome was measured by gait analysis at day 10. After 14 days, mice were euthanized and brains were removed for immunologic examination. (Figure 1) For all outcome measures, examiners were masked against treatment allocation.
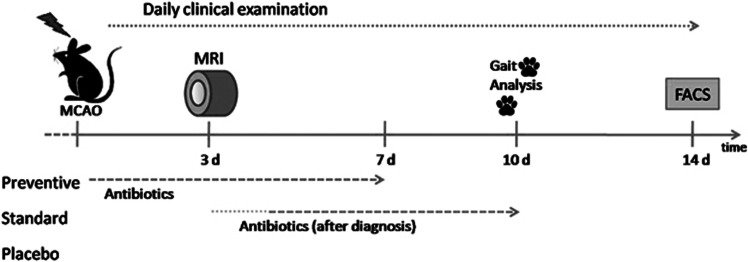
Figure 1.
Experimental design. After experimental stroke (middle cerebral artery occlusion (MCAo)), mice were closely monitored for signs of infection. A Mouse General Health Score (MGHS)>5 or an infiltration rate⩾5% in lung magnetic resonance imaging (MRI) was considered as sign for infection to start antibiotic treatment in the standard group (between day 4 and 6). Other groups received either antibiotics directly after MCAo (preventive) or no antibiotics (placebo).
Animals and Housing
We used 11- to 14-week-old C57Bl6/J male mice (Charles River Laboratories, Sulzfeld, Germany), housed in groups with chip bedding and environmental enrichment (mouse tunnel and igloo; Plexx BV, Elst, the Netherlands) on a 12-h light/dark cycle (change 7 o'clock) with ad libitum access to water and food (standard chow). All experiments were conducted in accordance with the European directive on the protection of animals used for scientific purposes and approved by the relevant local authority (Landesamt für Gesundheit und Soziales, Berlin, Germany).
Measuring Lung Infection with Magnetic Resonance Imaging
In an independent experiment (n=15), we established MRI for radiologic confirmation of murine pneumonia based on a recently published protocol.22 Magnetic resonance imaging was performed 3 days after MCAo using a 7 Tesla rodent scanner (Pharmascan 70/16, Bruker BioSpin, Ettlingen, Germany) and a 1H-RF volume resonator with an inner diameter of 38 mm for imaging. We examined 20 axial slices of the lung with a slice thickness of 0.5 mm using a T1-weighted FLASH sequence with TR 500 ms, TE 2.604 ms and 4 averages. With a field of view (FOV) of 1.28 × 1.28 cm and a matrix size of 128 × 128, we attained an in-plane image resolution of 100 μm. Bruker software Paravision 4.0 served for data acquisition and image processing. Anesthesia was induced and maintained with 2.5% and 1.5% to 2.0% isoflurane (Forene, Abbot, Wiesbaden, Germany), respectively, delivered in 0.5 L/min of 1:2 mixture oxygen/nitrous oxide via a face mask under constant ventilation. To ensure a steady body temperature of 37°C during examinations, mice were placed on a heated circulating water blanket. Motion artefacts were minimized using cardiac and respiratory gating (Small Animal Monitoring and Gating System, SA Instruments, Stony Brook, NY, USA). Images were analyzed with ImageJ (ImageJ 1.42q Wayne Rasband, National Institutes of Health, and USA) with a semi-automated threshold procedure. According to previous studies,22 we manually outlined lung borders on all slices to calculate the area on the images. For each slice, intensity was measured in a certain lung area with physiologic appearance to set background noise. Subsequently, we considered a threshold with a signal-to-noise ratio larger than 3.5 as signals of lung inflammation and expressed this as a fraction of whole lung area.
Bacterial Examination
To verify the MRI infection measurements, the bacterial burden of lung was measured as described elsewhere.12 Anesthetized (ketamine 100 mg/kg, Deltaselect, Dreieich, Germany, and xylazin 10 mg/kg, Bayer Vital, Leverkusen, Germany) mice were killed by cervical dislocation and subsequently washed with 70% ethanol under sterile conditions. After thoracotomy, lungs were removed and homogenized in 400 μL sterile phosphate-buffered saline using a sterile pestle. A volume of 50 μL of this tissue homogenate was plated on LB agar plates in serial dilutions. For determination of colony-forming units, plates were incubated (37°C, 5% CO2, 20% O2 in N2) overnight and bacterial colonies were counted the next morning.
Stroke Model
The surgical procedure of MCAo was performed as described in detail elsewhere,23 according to standard operating procedures of our lab.24 Briefly, after anesthesia induction with isoflurane (Abott, Wiesbaden, Germany) in a 1:2 mixture oxygen/nitrous oxide, a ventral cervical midline incision was made and a 12-mm silicon-coated nylon filament was introduced over the common carotid artery further to the internal carotid artery, to bolt the origin of the middle cerebral artery. After an ischemia time of 60 minutes, the filament was removed for reperfusion. Body temperature was controlled throughout the procedure. We verified surgical success by using the Bederson score.24
Drug Administration
Enrofloxacin (Baytril 2.5% oral solution, Bayer Vital) was dispensed in saline (2 mg/mL), antibiotic-treated animals received a daily orally dispensed dose of 10 mg per kg body weight via feeding needle every 12 hours over a period of 7 days, while placebo animals received the same amount of saline via feeding needle. Animals of preventive antibiotic group obtained enrofloxacin after waking from reperfusion anesthesia (ca. 1 hour after operation). Therapeutic antibiotic treatment was given immediately after appearance of clinical signs (general health score>5) and confirmation of lung infection by MRI (signal rate⩾5%). The group allocation was randomized.
Mice General Health Score
For assessment of rodent health status, we monitored the mice for 14 days by using a score that considers indicators of general well-being, such as behavior, posture, grooming, or body weight changes (Table 1). Examinations were performed at the same time every day and the investigator was masked for group assignment.
Table 1. Mice General Health Score.
| Score | 0 | 1 | 2 |
|---|---|---|---|
| Behavior | Alert, high advertence, responds quickly to environment | Reduced advertence, mouse reacts slowly to moderate stimulation | Lethargic, no movement after stimulation, no interest in environment |
| Posture | Physiologic | Betimes relieving posture or unstable movements | Bended back, many unstable movements, possibly trepidation |
| Skin and fur | Smooth, shiny and tight fur, regular grooming | Slightly scrubby, dull fur, reduced grooming | No grooming, fur scrubby |
| Eyes | Physiologic | Eyes open, clear but serous ocular discharge, beginning conjunctivitis | Eyes almost closed, serous-mucous ocular discharge, conjunctivitis |
| Breathing | Physiologic | Serous nose defluxion | Possibly forced breathing or hyperventilation, mucous nose defluxion |
| Gastrointestinal tract | Normal dejection | Tender dejection consistency | Diarrhea |
| Body temperature | 35°C–39°C | 33°C–35°C or 39°C–40°C | <33°C or >40°C |
| Weight | Stable | 5–10% decreased weight | >10% decreased weight |
| Min. 0 points | Max. 16 points |
Neurologic Outcome Measurement with Gait Analysis
Neurologic outcome was determined by assessing characteristic stroke-induced changes in gait parameters as described elsewhere25 using an automated computer-assisted method (CatWalk, Noldus Information Technology, Wageningen, the Netherlands) according to manufacturer instructions and published procedures. In brief, the system consists of an elevated 1.3-m-long glass plate illuminated with fluorescent light. Contact of animal paws with the glass plate lead to a changed refractive index of the internally reflected fluorescent light, which then leaves the glass plate and is reflected downwards. A high-speed camera underneath the glass plate captures the images, which are subsequently analyzed by the connected computer software. Home cage served as bait at the end of the walkway, and animals were trained in three sessions before first measurement. We acquired a minimum of three compliant runs, which had to fulfill minimum run duration of 0.5 seconds, maximum run duration of 5 seconds, and a maximum speed variation of 60%. Runs, where animals turned or walked backwards, were not used for analysis. When software was unable to calculate phase dispersion, for instance anchor paw being undetected, runs were excluded from statistical analysis.
Isolation of Mononuclear Cells from the Brain
Mice were anesthetized with a ketamine (Ketavet, Pfizer, Berlin, Germany; dose 150 mg/kg) and xylazine (Rompun Bayer Vital; dose 15 mg/kg) mixture 14 days after MCAo surgery and brains were removed after transcardial perfusion with saline and decapitation. Brains were kept in ice cold complete RPMI1640 medium (Biochrom, Berlin, Germany) enriched with 10% fetal calf serum (FCS), 50 U/mL penicillin and 50 μg/mL streptomycin (Biochrom), 2 mmol/L ℒ-alanyl-ℒ-glutamine (Biochrom). Ipsi- and contralateral hemispheres were separated with sterile scalpel and only ipsilateral hemisphere was used for further processing. Single-cell suspension was achieved by using a 70 -μm pore size cell strainer. Isolated cells were washed once in complete RPMI1640 medium. Cell pellet was resuspended in 6 mL of 35% Easycoll gradient (Biochrom) in complete RPMI1640 medium and pipetted carefully on a top of 4 mL of 70% Easycoll (Biochrom) gradient in complete RPMI1640 medium. Brain cell gradients were centrifuged at room temperature (21°C) for 30 minutes at 900 g, without acceleration and brake. The fraction of mononuclear cells was removed and washed once in complete RPMI1640.
Flow Cytometry
Mononuclear cells, harvested from the brain, were subjected to flow cytometry (FACS). Cells were washed once in FACS washing buffer (2% FCS and 0.1% sodium azide in phosphate-buffered saline). Thereafter, cell surfaces were stained with following monoclonal antibodies: FITC-conjugated anti-CD8a, PE-conjugated anti-CD44, Alexa Fluor 700-conjugated anti-CD4, PE-Cy7 conjugated anti-CD45 (all from BD Pharmingen, San Diego, CA, USA), Pacific Blue-conjugated anti-Gr1, Pacific Orange-conjugated anti-CD45RO (both from Caltag Laboratories, Life Technologies, Carlsbad, CA, USA), Alexa Fluor 647-conjugated anti-CD3 (eBioscience, San Diego, CA, USA), APC-Cy7 conjugated anti-CD11b, PE-Texas Red conjugated anti-CD11c. Samples were measured on LSR II flow cytometer (BD Pharmingen) and using with FACSDiva software (BD Pharmingen). Data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
Statistical Analysis
Data are expressed as mean ± s.d. or, for ordinal data, as median ± range (general health score). The required number of animals per group was calculated with respect to functional outcome in gait analysis (primary endpoint), and 50% improvement in outcome parameters was considered as a relevant effect.25 Four gait parameters were included as independent measures in the power calculation, and calculations were performed using statistical tables and self-written macros in Microsoft Excel. For power calculation, we have chosen a power of 80%. Secondary endpoints were survival, immunologic examination of the brain and clinical outcome. Subgroups with and without lung MRI were compared for differences. Not normally distributed data were analyzed by Kruskall–Wallis analysis and pairwise Dunn's method or Mann–Whitney U-test respectively, using SPSS Statistics 18.0 software (IBM Somers, NY, USA). Where applicable, we used Student's t-test and analysis of variance with Bonferroni correction. Survival was calculated according to Kaplan Meier and compared univariately with Log Rank test and layerwise correction for two experiments. For analyzing gait parameters, the comparison for effect sizes described by Matthews and Altman26 was applied. Examining relationship, we applied Spearman's rank correlation coefficient ρ, being less dependent from assumptions on normality. Comparisons between methods were performed according to Bland and Altman with the adoption for measurements in different units.27, 28 In brief, we looked here for a 95% probability to predict the value of the old method with the new method.
Results
Lung MRI as Diagnostic Tool for Diagnosis of Poststroke Pneumonia
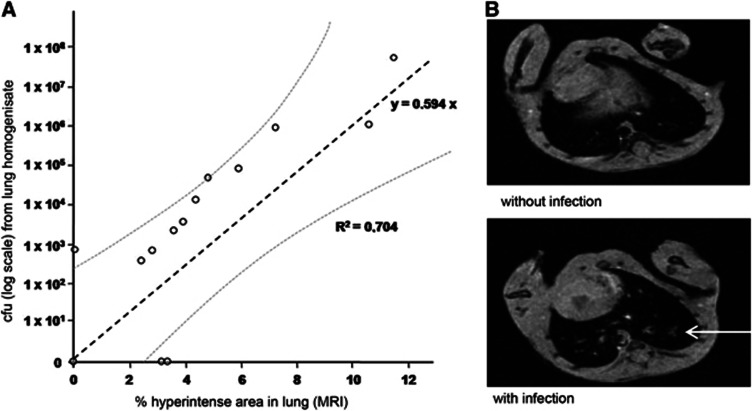
To assess the effects of different treatment approaches of poststroke infections on long-term outcome, we established a lung MRI protocol to diagnose pneumonia in living mice reliably. This validation study of the MRI protocol was completely independent from the other study. The method is based on measuring the fraction of hyperintense areas in the whole lung reflecting and summarizing areas in the lung tissue with edema as a marker of inflammation and bacterial infections. At 3 days after experimental stroke, lungs of infected mice typically exhibited hyperintense areas between 6% and 7% of total lung volume, whereas lungs from uninfected mice had areas smaller than 3%. Using a microbiological analysis for bacterial quantification (colony-forming units) of lung tissue as ‘gold standard' and comparing measurements in different units, we found a significant linear regression between bacterial burden of the lung and MRI (Pearson's R=0.893, R2=0.704; y=0.594x with s.e.=0.107 and P<0.001; n=15). The prediction of colony-forming unit in lung homogenisate from lung MRI values achieved 95% confidence (Figure 2; Bland–Altman comparisons for measurements in different units individually calculated). A signal rate of 5% or greater was considered as a marker of infection. Thus, lung MRI is a reliable method to diagnose bacterial pneumonia in living mice.
Figure 2.
Investigating poststroke pneumonia in mice with lung magnetic resonance imaging (MRI). (A) Lung MRI is a reliable method for investigating poststroke pneumonia compared with the current standard method based on the quantitative bacterial analysis from lung homogenisate (n=15, linear regression R2=0.704, y=0.594x; Bland–Altman analysis of comparability: 95% limits of agreement; cfu, colony-forming units). (B) Representative lung MRI of a healthy mouse (above) and a mouse with pneumonia (below, 3 days after middle cerebral artery occlusion). Mice rested in supine position, section of heart visible. The arrow indicates infiltration of the lung parenchyma.
Preventive but not Standard Antibiotic Treatment Reduced Lung Inflammation
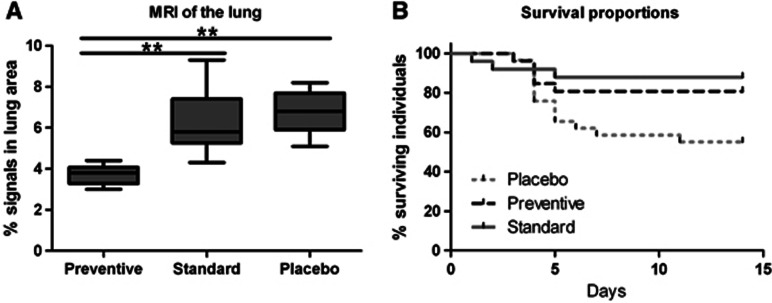
In the placebo group, MRI detected hyperintense areas affecting 6.8%±1.1% (mean±s.d.) of total lung volume and in the standard group 6.3%±1.5%. In contrast, the hyperintense area fraction was significantly reduced to 3.7%±0.4% in the prevention group (n=32, analysis of variance with Bonferroni post-hoc; preventive vs. standard P<0.001; preventive vs. placebo P<0.001, standard vs. placebo, not significant; Figure 3A). Thus, preventive treatment abolished lung inflammation compared with standard and placebo treatment.
Figure 3.
(A) Preventive antibiotic treatment reduced inflammation markers in lung magnetic resonance imaging (MRI) compared with standard treatment. Hyperintense area fractions of the total lung parenchyma were measured as markers of inflammation 3 days after middle cerebral artery occlusion in placebo (n=9), standard (n=13), and preventive (n=10) treatment group (analysis of variance with Bonferroni post-hoc; preventive vs. standard P<0.001; preventive vs. placebo P<0.001, standard vs. placebo not significant). (B) Effect of antibiotic treatment on poststroke survival in mice. Mortality in placebo-treated mice is highest between day 3 and day 7 after stroke onset (gray line). Standard antibiotic treatment (after diagnosis of pneumonia; gray dotted line) improves survival similarly as PAT (black interrupted line). (Kaplan–Maier analysis of survival; n=80; preventive vs. placebo P=0.03; standard vs. placebo P=0.007).
Prevention and Treatment of Poststroke Pneumonia Improved Survival
Survival in placebo-treated animals was 55%. In contrast, prevention and standard treatment of poststroke pneumonia with enrofloxacin improved survival significantly to 81% and 88%, respectively (Figure 3B; preventive vs. placebo P=0.03; standard vs. placebo P=0.007 Kaplan–Maier analysis of survival). Both antibiotic treatment regimes were similar in terms of survival (P=0.44).
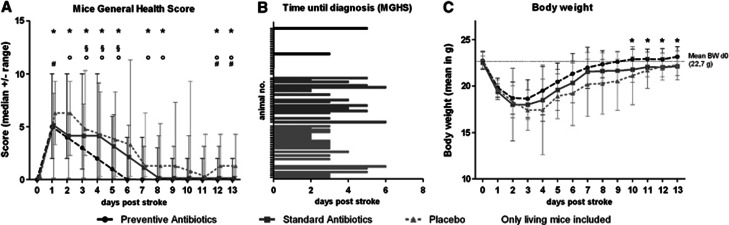
Preventive Antibiotic Treatment Improved General Health of Mice
Mice were daily monitored using the Mice General Health Score (MGHS). Preventively treated mice recovered without complications within 6 days in median (Figure 4A). In comparison, mice treated with placebo or antibiotic standard treatment had a significantly worse health status between day 4 and day 6, and needed a longer recovery period.
Figure 4.
(A) Effect of antibiotic treatment on medical condition of mice after stroke The Mice General Health Score (MGHS) as a marker for the general medical condition of mice was measured daily during the observation period. Mice treated in a preventive manner recovered faster compared with the standard and placebo group. Both, preventive and standard treatment had a better long-term outcome compared with placebo-treated mice. (Kruskall–Wallis test *P>0.05; Dunn's post-hoc P<0.05 between § preventive vs. standard; ° preventive vs. placebo; # standard vs. placebo). (B) Time until mice reached the diagnostic criterion in MGHS. Animals that died before day 2 (direct effect of stroke, e.g., brain edema) and mice that did not reach the diagnostic criterion are censored. Most mice showed onset of symptoms around day 3. (C) Effect of antibiotic treatment on poststroke body weight in mice. Preventive but not standard antibiotic treatment accelerates regain of body weight after stroke, compared with placebo treatment, with a statistically significant difference at the last 4 days of the observation period (day 10 to 13, Kruskall–Wallis test *P<0.05).
Pneumonia was diagnosed by the MGHS in median 4 days after MCAo (only placebo and standard group; Figure 4B) and confirmed by MRI. Accordingly, standard treatment started in mean 4.7 days after MCAo (range 4 to 6 days). Although the MGHS was significantly correlated to signs of infections in lung MRI on day 3 (Spearman Rho; day 3: ρ=0.400, P=0.023; day 4: ρ=0.523, P=0.004; day 5: ρ=0.431, P=0.031), some animals revealed a high lung infiltration measured by MRI despite a rather medium MGHS (under 5 points). Almost all mice in the standard and placebo group showed an increased signal in MRI (cutoff⩾5%) as a marker for lung infection. Only two animals in the standard group failed with 4.3 and 4.8%. However, these mice showed clinical symptoms of infection (MGHS>5) 2 days after the scan. In the standard and placebo group 31% of the animals did not reach the clinical cutoff (MGHS>5), although most of these animals had signs of lung infection measured by MRI. Thus, a significant proportion of animals in the placebo and standard group had subclinical courses of pneumonia. Therefore, we applied antibiotics on day 6 for those animals in the standard group, which did not reached the clinical cutoff (MGHS>5) in the days before.
Preventive but not Standard Antibiotic Treatment Expedites Recovery of Body Weight After Stroke
There was no significant difference between the three groups concerning weight loss (20.8%±8%) within the first 3 days after stroke onset. However, preventively treated mice regained their body weight faster compared with standard or placebo-treated animals, leading to a significant difference in the last 4 days of the observation period (Figure 4B; day 10 to 13, Kruskall–Wallis test P<0.05).
Preventive but not Standard Treatment Improves Neurologic Outcome
Treatment Effect on Spatial and Kinetic Characteristics of Gait
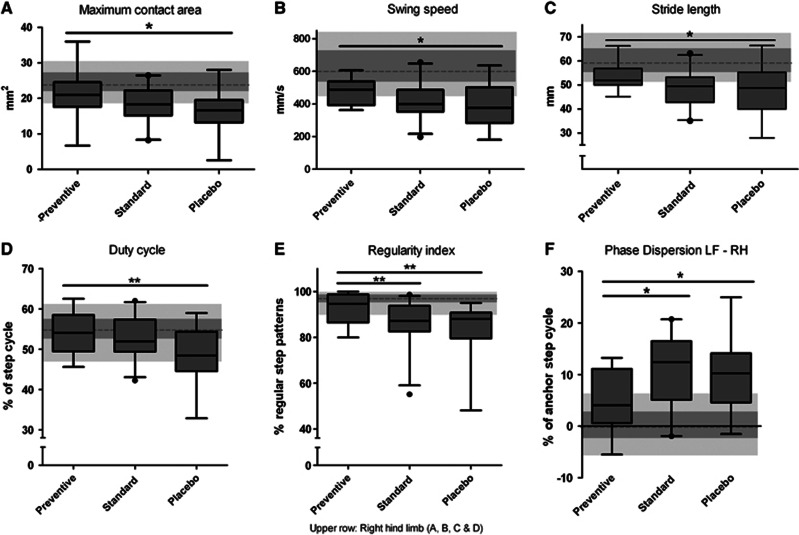
Compared with the prestroke performance, the maximum contact area of hind limbs decreased 10 days after left-sided MCAo, with largest effects on right hind limb. Placebo-treated mice showed a significant worse gait performance with respect to this parameter compared with preventively treated animals, but not to standard treated ones (Comparison of effect sizes, P<0.05; Figure 5A). Furthermore, the right hind limb possesses significant decelerated swing speed in placebo-treated animals, which was significantly improved in the preventive treatment group but not in the standard group (speed of a paw while it moves from one to the next contact with the glass plate, checked against walking speed, P<0.05; Figure 5B). The distance between consecutive steps (stride length) with the right hind paw was significantly shortened in placebo but not preventively treated animals (P<0.05; Figure 5C). In contrast to the preventive treatment, standard treatment improved none of these gait parameters significantly compared with placebo.
Figure 5.
Functional recovery in gait after middle cerebral artery occlusion (MCAo) depends on antibiotic treatment regime. (A) Comparing maximal contact area of the right hind limb before and 10 days after MCAo placebo-treated mice had significantly lower contact areas. In contrast to the standard group, mice treated in the preventive group recovered significantly better compared with placebo. (B) Swing speed, (C) stride length, and (D) duty cycle of the right hind limb were significantly decreased in placebo group compared with preventively treated animals, whereas standard treatment group did not reach any significance compared with placebo group. (E) According to regularity index, placebo and standard group animals show a less regular, improper coordinated walk 10 days after stroke, whereas preventively treated animals have a significant more regular gait pattern. (F) Comparing phase dispersion from left front to right hind paw (LF–RH) before and 10 days after MCAo placebo-treated mice were impaired by ischemia. The functional recovery was significantly better in mice treated preventively compared with both placebo and standard treated mice. (Data are presented as box plots with 5 to 95 percentile whiskers. Values before stroke are represented in the background as gray boxes, in parallel to the box plots in darker gray the 1st to 3rd quartile and in light gray the 5 to 95 percentile. The dotted line indicates the median. Comparison of effect sizes; n=60; *P<0.05; **P<0.01).
Treatment Effects on Gait Coordination
Duty cycle, the relative stand phase as part of the step cycle, was significantly more impaired after stroke in the right hind limb of placebo-treated compared with preventively treated mice (P<0.01), but not in comparison with standard treated mice (Figure 5D). The interpaw coordination during gait is based on a normal step sequence pattern in each animal. The regularity index, presenting the overall paw coordination, was significantly reduced by stroke. Preventive treatment improved regularity index significantly compared with placebo and standard treatment (P<0.01; Figure 5E). Another valuable parameter for assessing interpaw coordination is the phase dispersion, characterizing the timing for the placement of two paws (defined as target and anchor). The phase dispersion from left front to right hind paw (LF–RH) and right front to left hind paw (RF–LH; data not shown) was significantly improved in preventive treatment group compared with placebo and standard treatment (P<0.05; Figure 5F). Taken together, preventive but not standard treatment improved neurologic outcome 10 days after stroke onset compared with placebo treatment.
Worse Functional Outcome is Associated with Increased Infiltration of Leukocytes into the Brain
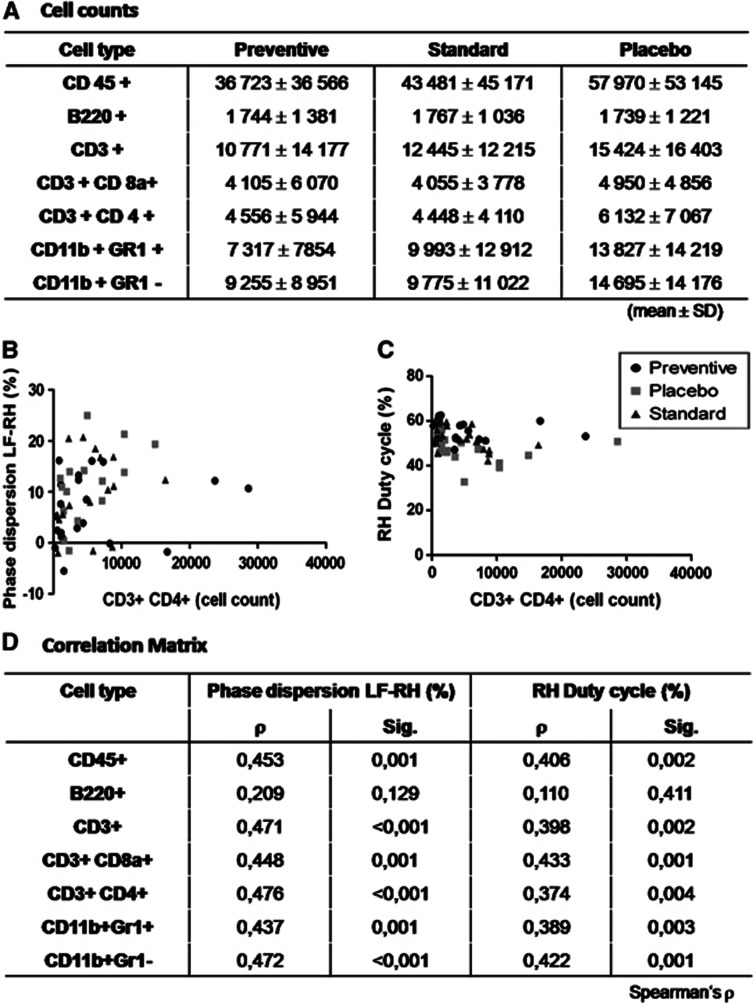
Hypothesizing that infection alters the inflammatory response in the ischemic brain, we investigated the number of infiltrating leukocytes into the affected hemisphere. The dominant cell populations among all leukocytes (CD45+) infiltrating the brain 10 days after stroke onset were T lymphocytes (CD3+), macrophages (CD11b+Gr1−), granulocytes (CD11b+ Gr1+) and to lower extent B lymphocytes (B220+). While there was a trend towards lower leukocyte numbers in antibiotic-treated animals, we observed no significant differences for any cell subset between the treatment groups (Kruskall–Wallis P>0.05; n=58; Figure 6A).
Figure 6.
Lymphocytes in ipsilateral hemisphere. (A) At 14 days after experimental stroke, we did not observe a statistical difference neither in total lymphocyte number nor in subpopulations (mean±s.d.). (B) CD3+ CD4+ T cells show a relationship to impaired gait parameters as phase dispersion from left front to right hind paw (LF–RH) or (C) duty cycle in right hind paw. (D) Correlation matrix reveals relationship between infiltrating lymphocytes and gait parameters, here exemplarily shown for two parameters, except for B cells. (Spearman's rank correlation coefficient).
However, noticing the quite large interindividual variance in cell infiltration, we examined whether the magnitude of immune cell infiltration into the brain is probabilistically independent from functional outcome. Applying Spearman's rank correlation coefficient, the number of brain-infiltrating CD4+ and CD8+ T cells, macrophages and granulocytes, but not B220+ B cells were correlated to gait parameters. This holds true both for the analysis over all groups as well as for the subgroups placebo and standard treatment. We did not observe a significant correlation between lung MRI and brain immune cell counts (data not shown). Thus, worse functional outcome after stroke appears to be positively correlated with an increased number of infiltrating immune cells in the affected brain hemisphere.
Discussion
Recently, we have demonstrated in the same MCAo mouse model that poststroke infections can be prevented effectively by antibacterial therapy with moxifloxacin, either immediately or 12 hours after experimental stroke. Consequently, PAT was shown to improve outcome after experimental stroke with a reduction in mortality by 40%. Moreover, the proportion of animals in treated groups with only mild or no deficit was almost twice the number as untreated controls.14 However, untreated controls or placebo treatment are not reflecting the clinical practice. Mice of the control (‘placebo') group were not treated with antibiotics at all, independently of whether they developed an infection or not, being in contrast to the stroke treatment guidelines in patients. In the present study, we took a step further modeling this clinically relevant issue in our mouse model of stroke. We established a reliable diagnostic procedure to detect lung inflammation in living mice by MRI enabling investigations on the impact of poststroke infections on long-term outcome in mice. Thereby, we demonstrated that compared with ‘placebo' treatment standard and PAT reduced poststroke mortality. However, only PAT but not standard treatment improved neurologic long-term outcome measured by gait analysis. This is an example for the importance of modeling current clinical ‘stroke care', which needs to be implemented in preclinical stroke studies.29
Pneumonia was reliably detected in lung MRI, and with a lower sensitivity in general health score (MGHS). As mice tend to hide sickness behavior because of their ecological niche,30 it seems coherent that a less dramatic course of infection might be overlooked by just applying clinical parameters such as a general health score.
In the clinical situation, 30% of all stroke patients experience infection, in most cases pneumonia or urinary tract infection. The incidence is even higher with 45%. in severely affected stroke patients treated on intensive care units.31 In our stroke model, resembling severe MCA stroke,23 mice without PAT were prone to develop infection and showed a worse general clinical picture.
A metanalysis of clinical studies demonstrated that antibacterial prophylaxis reduced the occurrence of poststroke infections, but a clear conclusion about mortality and functional outcome is lacking.18 Similarly, preventively treated mice were also protected from infections in our study. However, treatment with antibiotics improved survival significantly, independent of whether it was given preventively or immediately after diagnosis of pneumonia. The effect of the preventive approach is in accordance with a previous report demonstrating a reduction of mortality from nearly around 60% (placebo) to around 15%.14 In our study, especially animals with signs of infection had a lower chance for survival, which is corresponding to the clinical situation, where mortality is approximately threefold higher in infected patients than in noninfected.4 Especially, pneumonia is one of the most prominent reasons for death after stroke.3, 31
Clinical evidence and animal experiments indicate weight loss as a common phenomenon after stroke, and several pathways of metabolic imbalance were proposed to contribute to this phenomenon.32 We also observed a substantial weight loss in mice after MCAo, independent of the treatment group. However, preventively treated mice regained their body weight faster than mice from other treatment groups. In patients, weight loss is correlated with poor functional outcome,33 hence faster weight recovery might also be partially related to the improved functional outcome in preventively treated mice.
Poststroke infection is associated with poor neurologic outcome in patients.31, 34 In our experimental study, we observed a similar effect concerning functional outcome. Spatial and kinetic gait parameters were significantly improved in the preventive group compared with placebo treatment. A precedent study reported an improved basic functional outcome measured by Bederson score for a short observation period.14 Taken together, our observations match those from clinical trials, where patients without infection had a superior outcome compared with patients with infection.10, 16 Importantly, in our study the occurrence of infection accounts for poor functional outcome, regardless of whether mice received antiinfective treatment after diagnosis of chest infection.
One may speculate that the improved neurologic outcome is because of direct neuroprotective effects of the antibiotic drug. For example, neuroprotective effects have been shown for the antibiotics ceftriaxone35 and minocycline, notwithstanding that the latter failed to prove efficacy in a recent elaborate preclinical study.36 However, in a previous study, treatment with a gyrase inhibitor had no further neuroprotective effect when infections were prevented by restoring immune function via adoptive cell transfer.14 Thus, neuroprotection by antibiotics may be mediated rather indirectly by preventing or mitigating the infection-induced systemic inflammatory response.
As stroke induces local inflammation at the site of the insult,6, 37 and infection alters the systemic inflammatory profile in the brain,38 we hypothesized that the differences in functional outcome might be associated with a change in the inflammatory profile in the brain. Accordingly, we analyzed the number of leukocytes in the affected hemisphere. We observed a significant relation between the number of infiltrating T lymphocytes and myeloid cells, and neurologic outcome. We did not observe a significant correlation between lung MRI and brain immune cell counts. However, in this analysis sample size was smaller and most observations derived from antibiotic-treated animals, so that the distribution lacks animals with most severe infections because of mortality. These data suggest that worse functional outcome after stroke is associated with, although not necessarily caused by an increased brain infiltration of peripheral immune cells. However, there are several limitations of this observation. Most importantly, we only performed quantitative analysis of brain-infiltrating immune cells restricted to merely one (late) time point after stroke. While there was a trend towards less immune cells in ischemic brain tissue in antibiotic-treated animals, a mere reduction in cell infiltration cannot solely explain the better functional outcome in preventively treated animals. Even though an earlier T-cell infiltration into the brain would be diminished by antibiotic prevention of poststroke infections, T-cell infiltration into the brain is not necessarily causative for worse outcome and might be merely a surrogate parameter for inflammation. A detailed characterization of systemic and local immunologic response, in particular the assessment of the inflammatory profile in the ischemic brain and of the functional phenotype of brain-infiltrating immune cells, in the wake of poststroke infectious complications, and its relation to functional outcome may provide further clues as to why an incipient infection distant from the brain may impair neurologic outcome. However, such analysis demands an elaborate study design and was outside the focus of this study.
In the clinical situation, stroke patients receive standard antibiotic regimes on diagnosis of infection, which normally includes β-lactam antibiotics, such as acylaminopenicillins and cephalosporins as well as fluoroquinolons. The current treatment guidelines for stroke patients do not recommend preventive use of antibiotics.39 In our experimental study, we have demonstrated that this standard approach is as effective as the preventive treatment concept in consideration of mortality reduction, compared with the omission of antibiotic treatment at all (placebo). Concerning gait analysis, our data suggest that PAT is superior compared with the standard approach. Hence, standard treatment might be behind time to controvert negative effects of infection on neurologic outcome.
Superiority of prevention compared with standard treatment might be because of difficulties in detecting pneumonia in our experimental stroke model. However, even in the clinical setting, the early diagnosis of poststroke pneumonia is a major challenge. Centers for Disease Control criteria for the diagnosis of pneumonia require pathologic findings in clinical and laboratory examinations as well as in chest X-ray. In particular, pathologic signs in chest X-ray will be often detected only in the late course of pneumonia.40 Consequently, a delayed treatment of pneumonia may contribute to worse outcome in stroke patients.
Our experimental data argue for further elaborate investigation of the impact of poststroke infections on functional outcome in the clinical situation. In fact, at least three large clinical trials are ongoing to prove whether preventive antiinfective treatment improves stroke outcome.41, 42 Although not detected in a phase II trial,16 the preventive use of antibiotics might have negative effects because of potential promotion of antibiotic resistance in common bacteria. Hence, early identification of stroke patients at risk for poststroke infections is of paramount importance to tailor preventive antiinfective treatment. Blood-based biomarkers could help to identify these patients and guide physicians in antibiotic treatment. The STRAWINSKI trial investigates whether a biomarker-guided antibiotic treatment improves functional outcome after severe ischemic stroke by immediate early antibiotic treatment. The method is based on the rapid identification of infections by ultrasensitive procalcitonin measurement.43 Summarising our current knowledge, patients might benefit from prevention of infectious complications after stroke, and the next step is to prove whether and under which circumstances PAT is effective in patients. As poststroke infections are common even in stroke models and are known to have a negative effect on outcome after stroke, prophylactic antibiotic treatment has to be taken into account for preclinical studies where ‘side effects' from infectious complications may interfere with mechanisms or treatments under research.44
Acknowledgments
The authors would like to thank Sabine Kolodziej, Mareike Thielke and Claudia Conert for the excellent technical support.
A patent application on antiinfective agents and immunomodulators used for preventative therapy after an acute cerebrovascular accident has been filed to the European Patent Office (PCT/EP03/02246): patent owner Charite Universitaetsmedizin Berlin, patent inventors Andreas Meisel and Christian Meisel. Andreas Meisel has received speaker's honoraria from Bayer Vital GmbH and Wyeth Pharma GmbH. The other authors declare no conflict of interest.
Footnotes
This work was supported by the European Union's Seventh Framework Programme (FP7/2008-2013) under Grant Agreements 201024 and 202213 (European Stroke Network); the Deutsche Forschungsgemeinschaft (NeuroCure Cluster of Excellence, Exc 257, Collaborative Research Centres SFB-TRR 43 and SFB-TRR 84); the Bundesministerium für Bildung und Forschung (Center for Stroke Research Berlin, 01 EO 08 01); the Helmholtz Gemeinschaft (SO-022NG).
References
- Davenport RJ, Dennis MS, Wellwood I, Warlow CP. Complications after acute stroke. Stroke. 1996;27:415–420. doi: 10.1161/01.str.27.3.415. [DOI] [PubMed] [Google Scholar]
- Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–118. doi: 10.1016/S1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- Heuschmann PU, Kolominsky-Rabas PL, Misselwitz B, Hermanek P, Leffmann C, Janzen RW, et al. Predictors of in-hospital mortality and attributable risks of death after ischemic stroke: the German Stroke Registers Study Group. Arch Intern Med. 2004;164:1761–1768. doi: 10.1001/archinte.164.16.1761. [DOI] [PubMed] [Google Scholar]
- Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60:620–625. doi: 10.1212/01.wnl.0000046586.38284.60. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38 (2 Suppl:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- Ionita CC, Siddiqui AH, Levy EI, Hopkins LN, Snyder KV, Gibbons KJ. Acute ischemic stroke and infections. J Stroke Cerebrovasc Dis. 2011;20:1–9. doi: 10.1016/j.jstrokecerebrovasdis.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Rothan-Tondeur M, Meaume S, Girard L, Weill-Engerer S, Lancien E, Abdelmalak S, et al. Risk factors for nosocomial pneumonia in a geriatric hospital: a control-case one-center study. J Am Geriatr Soc. 2003;51:997–1001. doi: 10.1046/j.1365-2389.2003.51314.x. [DOI] [PubMed] [Google Scholar]
- Vermeij FH, Scholte op Reimer WJ, de Man P, van Oostenbrugge RJ, Franke CL, de Jong G, et al. Stroke-associated infection is an independent risk factor for poor outcome after acute ischemic stroke: data from the Netherlands Stroke Survey. Cerebrovasc Dis. 2009;27:465–471. doi: 10.1159/000210093. [DOI] [PubMed] [Google Scholar]
- Rocco A, Fam G, Sykora M, Diedler J, Nagel S, Ringleb P.Poststroke infections are an independent risk factor for poor functional outcome after three-months in thrombolysed stroke patients Int J Strokee-pub ahead of print 28 May 2012doi: 10.1111/j.1747-4949.2012.00822.x [DOI] [PubMed]
- Koennecke HC, Belz W, Berfelde D, Endres M, Fitzek S, Hamilton F, et al. Factors influencing in-hospital mortality and morbidity in patients treated on a stroke unit. Neurology. 2011;77:965–972. doi: 10.1212/WNL.0b013e31822dc795. [DOI] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Kuklina EV, Gillespie C, George MG. Medical complications among hospitalizations for ischemic stroke in the United States from 1998 to 2007. Stroke. 2010;41:980–986. doi: 10.1161/STROKEAHA.110.578674. [DOI] [PubMed] [Google Scholar]
- Meisel C, Prass K, Braun J, Victorov I, Wolf T, Megow D, et al. Preventive antibacterial treatment improves the general medical and neurological outcome in a mouse model of stroke. Stroke. 2004;35:2–6. doi: 10.1161/01.STR.0000109041.89959.4C. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Horcajada JP, Obach V, Vargas M, Revilla M, Torres F, et al. The Early Systemic Prophylaxis of Infection After Stroke study: a randomized clinical trial. Stroke. 2005;36:1495–1500. doi: 10.1161/01.STR.0000170644.15504.49. [DOI] [PubMed] [Google Scholar]
- Harms H, Prass K, Meisel C, Klehmet J, Rogge W, Drenckhahn C, et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS ONE. 2008;3:e2158. doi: 10.1371/journal.pone.0002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Al-Shajlawi F, Sick C, Meairs S, Hennerici MG. Effects of prophylactic antibiotic therapy with mezlocillin plus sulbactam on the incidence and height of fever after severe acute ischemic stroke: the Mannheim infection in stroke study (MISS) Stroke. 2008;39:1220–1227. doi: 10.1161/STROKEAHA.107.499533. [DOI] [PubMed] [Google Scholar]
- van de Beek D, Wijdicks EF, Vermeij FH, de Haan RJ, Prins JM, Spanjaard L, et al. Preventive antibiotics for infections in acute stroke: a systematic review and meta-analysis. Arch Neurol. 2009;66:1076–1081. doi: 10.1001/archneurol.2009.176. [DOI] [PubMed] [Google Scholar]
- Meisel A, Meisel C. Stroke-induced immunodepression: consequences, mechanisms and therapeutic implications. Fut Neurol. 2008;3:551–563. [Google Scholar]
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Marzola P, Sbarbati A. Magnetic resonance imaging in animal models of pathologies. Methods Enzymol. 2004;386:177–200. doi: 10.1016/S0076-6879(04)86007-5. [DOI] [PubMed] [Google Scholar]
- Sheth VR, van Heeckeren RC, Wilson AG, van Heeckeren AM, Pagel MD. Monitoring infection and inflammation in murine models of cystic fibrosis with magnetic resonance imaging. J Magn Reson Imaging. 2008;28:527–532. doi: 10.1002/jmri.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel O, Kolodziej S, Dirnagl U, Prinz V. Modeling Stroke in Mice—Middle Cerebral Artery Occlusion with the Filament Model. J Vis Exp. 2011. p. e2423. [DOI] [PMC free article] [PubMed]
- Dirnagl U, Members of the MCAO-SOP Group Standard operating procedures (SOP) in experimental stroke research: SOP for middle cerebral artery occlusion in the mouse. Nature Precedings2010 . http://precedings.nature.com/documents/3492/version/2 .
- Hetze S, Romer C, Teufelhart C, Meisel A, Engel O. Gait analysis as a method for assessing neurological outcome in a mouse model of stroke. J Neurosci Methods. 2012;206:7–14. doi: 10.1016/j.jneumeth.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG. Interaction 3: How to examine heterogeneity. BMJ. 1996;313:862. doi: 10.1136/bmj.313.7061.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Bland M (2004). How do I compare methods of measurement which give results in different units? Frequently Asked Questions on the Design And Analysis of Measurement Studies. . http://www-users.york.ac.uk/~mb55/meas/diffunit.htm .
- Mergenthaler P, Meisel A. Do stroke models model stroke. Dis Model Mech. 2012;5:718–725. doi: 10.1242/dmm.010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizard I. Sickness behavior, its mechanisms and significance. Anim Health Res Rev. 2008;9:87–99. doi: 10.1017/S1466252308001448. [DOI] [PubMed] [Google Scholar]
- Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, de Beek D. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherbakov N, Dirnagl U, Doehner W. Body weight after stroke: lessons from the obesity paradox. Stroke. 2011;42:3646–3650. doi: 10.1161/STROKEAHA.111.619163. [DOI] [PubMed] [Google Scholar]
- Jonsson AC, Lindgren I, Norrving B, Lindgren A. Weight loss after stroke: a population-based study from the Lund Stroke Register. Stroke. 2008;39:918–923. doi: 10.1161/STROKEAHA.107.497602. [DOI] [PubMed] [Google Scholar]
- Johnston KC, Li JY, Lyden PD, Hanson SK, Feasby TE, Adams RJ, et al. Medical and neurological complications of ischemic stroke: experience from the RANTTAS trial. RANTTAS Investigators. Stroke. 1998;29:447–453. doi: 10.1161/01.str.29.2.447. [DOI] [PubMed] [Google Scholar]
- Thone-Reineke C, Neumann C, Namsolleck P, Schmerbach K, Krikov M, Schefe JH, et al. The beta-lactam antibiotic, ceftriaxone, dramatically improves survival, increases glutamate uptake and induces neurotrophins in stroke. J Hypertens. 2008;26:2426–2435. doi: 10.1097/HJH.0b013e328313e403. [DOI] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Cox SF, Van Raay L, Aleksoska E, Donnan GA, et al. Preclinical drug evaluation for combination therapy in acute stroke using systematic review, meta-analysis, and subsequent experimental testing. J Cereb Blood Flow Metab. 2011;31:962–975. doi: 10.1038/jcbfm.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nature reviews. Immunology. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Harms H, Halle E, Meisel A. Post-stroke infections – diagnosis, prediction, prevention and treatment to improve patient outcomes. Eur Neurol Rev. 2010;5:39–43. [Google Scholar]
- Harms H, Hoffmann S, Malzahn U, Ohlraun S, Heuschmann P, Meisel A. Decision-making in the diagnosis and treatment of stroke-associated pneumonia. J Neurol Neurosurg Psychiatry. 2012;83:1225–1230. doi: 10.1136/jnnp-2012-302194. [DOI] [PubMed] [Google Scholar]
- Nederkoorn PJ, Westendorp WF, Hooijenga IJ, de Haan RJ, Dippel DW, Vermeij FH, et al. Preventive antibiotics in stroke study: rationale and protocol for a randomised trial. Int J Stroke. 2011;6:159–163. doi: 10.1111/j.1747-4949.2010.00555.x. [DOI] [PubMed] [Google Scholar]
- Kalra L (2012). A cluster randomised trial of different strategies of antibiotic use to reduce the incidence and consequences of chest infection in acute stroke patients with swallowing problems (Identifier ISRCTN37118456). ClinicalTrials.gov. Bethesda, MD: National Library of Medicine, US. . www.controlled-trials.com/ISRCTN37118456 .
- Ulm L, Ohlraun S, Harms H, Hoffmann S, Klehmet J, Ebmeyer S, et al. STRoke Adverse outcome is associated WIth NoSocomial Infections (STRAWINSKI): procalcitonin ultrasensitive-guided antibacterial therapy in severe ischaemic stroke patients—rationale and protocol for a randomized controlled trial Int J Strokeadvance online publication, 28 August 2012;doi: 10.1111/j.1747-4949.2012.00858.x [DOI] [PubMed]
- Engel O, Dirnagl U, Meisel A. Infection—An amendment to the Stroke model guidelines. JESTM. 2010;3:29–32. [Google Scholar]