Clonal deletion of the chromosome 5 long arm (del(5q)) has important prognostic implications for patients with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML).1 Even standard intensive therapy including induction chemotherapy and allogeneic hematopoietic stem cell transplantation is often associated with treatment failure.2 Combination azacitidine plus lenalidomide represents a potential innovative approach in these patients, and is also supported by existing data from patients with higher-risk non-del(5q) MDS or AML.3, 4
The primary objective of the Azacitidine-Lenalidomide multicenter phase I study was to identify the dose-limiting toxicity and maximum tolerated dose of lenalidomide in combination with a fixed regimen of azacitidine during treatment cycle 1 in patients with higher-risk MDS or AML and del(5q). Secondary endpoints were clinical and cytogenetic response rates, safety and mutational analyses. A standard 3+3 design was used to determine the maximum tolerated dose. Eligible patients were: aged ⩾18 years with del(5q) karyotypic abnormalities; had an Eastern Cooperative Oncology Group performance status score of 0–3; had previously treated or untreated International Prognostic Scoring System Intermediate-2- or high-risk MDS or AML (>30% bone marrow (BM) blasts according to French-American-British classification); and were ineligible for immediate allogeneic hematopoietic stem cell transplantation due to donor unavailability. The study was conducted in accordance with the Declaration of Helsinki, and received approval from appropriate institutional review boards or ethics committees. All patients provided written informed consent. ClinicalTrials.gov identifier NCT00923234.
The effects of azacitidine are cell-cycle S-phase-dependent, whereas lenalidomide inhibits cell-cycle progression; therefore, we considered sequential administration of azacitidine followed by lenalidomide to maximize their additive effects.5 In accordance with a previous study,4 and to avoid potentially higher hematotoxicity associated with azacitidine in direct combination with lenalidomide, patients received azacitidine 75 mg/m2/day subcutaneously (days 1–5) then oral lenalidomide (days 6–19) starting at 10 mg/day, escalating in 5 mg increments to 25 mg/day. Patients who experienced dose-limiting toxicity could continue at the next lower dose level. In patients with partial response or stable disease, induction therapy was continued up to eight cycles, or until progressive disease, unacceptable toxicity, or complete response (CR). To prevent continued hematotoxicity, patients who achieved complete BM blast clearance (<5% BM blasts or CR) after ⩾2 cycles of induction therapy received maintenance therapy, which comprised azacitidine 30 mg/m2/day subcutaneously (days 1–5) followed by lenalidomide at maximum tolerated dose (days 6–19) every 8 weeks, up to six cycles or until progressive disease. Response was evaluated using International Working Group 2003 criteria for AML and 2006 criteria for MDS.6, 7
Conventional chromosome banding analyses from BM cultures and fluorescence in situ hybridization analyses of peripheral blood CD34+ cells were performed. Evaluation of molecular mutations in candidate genes including TP53 was performed using next-generation deep-sequencing of whole-BM cells before the study.8 Screening for ASXL1 mutations was performed using Sanger capillary sequencing. Changes in TP53 mutation levels were evaluated in patients who achieved both complete hematologic and cytogenetic responses.
Twenty patients (median age 69 years; 55% male) were enrolled, including 35% of patients with World Health Organization-defined AML. Del(5q) was present as an isolated abnormality in 15% of patients, and as part of a complex karyotype in 80% 11 of 17 patients (65%) evaluable for molecular analysis had a TP53 mutation at baseline. All patients were wild-type for ASXL1, EZH2 and ETV6. Azacitidine (30%), allogeneic hematopoietic stem cell transplantation (15%), lenalidomide (10%) and low-dose cytarabine (10%) were the most common regimens among previously treated patients; 9 (45%) were previously untreated. Median time from diagnosis to screening was 11.3 months (range, 0.7–112.5). Median follow-up duration was 15.3 months (range, 0.4–33.7).
A median of two induction cycles (range, 1–6) were administered; two patients received maintenance cycles. Three of six patients treated with lenalidomide 25 mg experienced a dose-limiting toxicity, including: one patient with absence of hematologic recovery despite achieving a BM CR (suggesting response, but no recovery of counts due to drug-associated toxicity); one patient with pneumonia considered probably related to the study drugs; and one patient with grade 3 deep-vein thrombosis considered possibly related to lenalidomide, which required temporary treatment interruption then dose reduction. Of an additional three patients to receive lenalidomide 20 mg, one patient had a treatment delay of >4 weeks, resulting from a grade 4 sepsis considered probably related to study drugs. Although the lenalidomide maximum tolerated dose of 20 mg in combination with azacitidine was lower than in previous reports,3, 9 the heavily pretreated patient population together with the sequential approach may have potentiated the hematologic toxicity profile of both compounds. In addition, del(5q) progenitors are more sensitive to the antiproliferative effects of lenalidomide compared with other cytogenetic aberrations.5
The adverse event profile of sequential azacitidine and lenalidomide was consistent with that of the individual drugs.10, 11 The most common non-hematologic grade ⩾3 AEs were pneumonia (40%) and sepsis (15%). The most common treatment-related grade 3–4 hematologic AEs were thrombocytopenia (45%) and neutropenia (35%). Overall, 13 of 29 serious AEs were considered to be possibly, probably, or definitely related to the study drugs, including: three events of grade 3 febrile neutropenia; one of grade 4 pancytopenia; two of pneumonia; and one event each of grade 4 sepsis and grade 3 herpes zoster (in one patient), grade 3 deep-vein thrombosis, grade 3 nausea and vomiting, grade 4 thrombocytopenia, grade 3 anemia and grade 4 cerebral ischemia. Seven patients with serious AEs died (median one cycle), including two patients with Aspergillus pneumonia, one patient with atypical pneumonia, one patient with infectious sepsis and three patients with pneumonia. However, only one serious AE resulting in death (pneumonia in a patient who received one cycle of lenalidomide 25 mg) was considered related to study drugs. Overall, 10 patients survived ⩾6 months after treatment initiation.
Despite most patients having a complex aberrant karyotype and heavily pretreated, response rates were encouraging (Table 1). Of 19 patients evaluable for response, 26 and 42% achieved hematologic (CR, CR with incomplete recovery of peripheral blood counts or partial response) and cytogenetic responses, respectively. Among previously untreated patients, hematologic and cytogenetic response rates were 44 and 56%, respectively. All three patients who achieved a cytogenetic response without hematologic response discontinued treatment early due to AEs. Median duration of hematologic and cytogenetic responses was 2.3 months (range, 0.9–8.1) and 3.2 months (range, 1.9–6.4), respectively. All responders achieved an initial response after cycle one, in contrast to data with azacitidine alone.12 Although other studies of sequential azacitidine 75 mg/m2 and lenalidomide (up to 75 mg) have reported higher response rates (up to 60%), among untreated patients with higher-risk MDS or AML, they enrolled a comparatively low percentage of patients with adverse baseline cytogenetics.3, 9 Furthermore, the majority of our patients displayed a TP53 mutation. This is consistent with the high-rate of TP53 mutations observed in advanced del(5q) MDS, which appears to underlie MDS pathophysiology in some patients with del(5q).13
Table 1. Individual patient characteristics and responses in 19 patients evaluable for response.
| Universal patient no. | Dose cohort | Disease (FAB/ WHO) | IPSS risk | Age, years | Cytogenetics additional to del(5q) | TP53 mutation (exon) | No. of induction (maintenance) cycles received | Best hematologic response | Best cytogenetic response |
|---|---|---|---|---|---|---|---|---|---|
| 1 | I | RAEB/ RAEB-2 | High | 69 | del(1)(p36p32),+8 | No | 2 (3) | CRi | PR |
| 2 | I | AML/ AML | — | 45 | t(4;17)(q12;q11.2), t(6;8)(p12;q24), −7, +der(7)t(7;13)(p10;q10), −12, +der(12)t(X;12)(q2?;q13), −13, −22 | No | 2 | SD | SD |
| 3 | I | RAEB/ RAEB-2 | High | 69 | −9, +der(9)t(1;9)(q21;q22), del(11)(q14q24) | NA | 2 | SD | PR |
| 4 | I | RAEB/ RAEB-2 | Int-2 | 73 | None | No | 5 | SD | SD |
| 5 | II | RAEB/ RAEB-2 | High | 57 | t(2;9)(p14;q22), −3, +der(3)t(17;3;18;3;18;4;2)(17?-> 17?::3?->3cen->::18?->18?::3?-> 3?::18?->18?::4p13?->4q31?::2?->2?), −4, +der(4)t(4;17)(4p11-> 4q21::17q12->17q23::?), −7, +der(7)t(7;19)(q34;?), iso(11)(q10), −17, +der(17)t(17;19)(p11.2;?), −18, +der(18)t(18;3)(18pter-> 18q11.2::3?), −19 | Yes (6) | 1 | SD | PR |
| 6 | II | AML/ AML | — | 66 | None | Yes (7, 8) | 3 | SD | SD |
| 7 | II | RAEB/ RAEB-2 | Int-2 | 49 | +der(3)t(3;6)(p10;q10), −6, −7, del(12)(p13p11.2), −17, −20, +der(20)t(17;20)(q10;p10) | Yes (6) | 5 | SD | PR |
| 8 | II | AML/ AML | — | 67 | +11, −17 | No | 2 | PD | PD |
| 9 | III | RAEB/ RAEB-2 | High | 75 | der(3)t(3;5)(p11;p13), der(11)t(11;12)(p15;q13), −12, der(17)t(17;20)(p11.2;p11.2), der(18;20)(p10;q13?), −20, +der(20)t(10;18;20)(18pter?-> 18p11.2?::20p11.2::10q24-> 10qter?) | Yes (5) | 3 | SD | SD |
| 10 | III | AML/ sAML | — | 68 | r(11)hrs(11)(q23) | Yes (6) | 4 | SD | SD |
| 11 | III | RAEB/ RAEB-1 | High | 72 | del(7), (q11.2?), −16, +der(16) t(16;21)(p10?;q10?)x2,−21, i(21)(q10), der(22) t(1;22)(p11;p11.1) | NA | 6 | SD | SD |
| 12 | III | AML/ AML | — | 55 | 40–42, X, −X, −3, der(4) t(X;4)(p?;q21), t(4;11)(q11;q21?), der(6)t(6;7;11)(qter->p?::?->?), del(6)(q?), t(7;11)(pter->q22?::?), −11, der(11)t(4;11)(qter->?::q21?-> pter), −14, −16, del(17)(q?), +20, der(20)t(11;20)(?::q13->pter), +22 [cp21] | Yes (5) | 2 | PR | PR |
| 13 | III | RAEB/ RAEB-1 | Int-2 | 60 | −7, −13, +der(13)(q14?q22?), der(17)t(17;22)(p13;p11.2?), −22, +2mar | Yes (5, 6, 7) | 3 (4) | CR | CR |
| 14 | III | RA/ RCMD | Int-2 | 80 | −7, der(17)t(5;17)(q13;p13) | Yes (8) | 1 | CRi | PR |
| 15 | IV | RAEB/ RAEB-2 | Int-2 | 60 | dic(6;18)(p23;p11.1)del(18)(q21), +8 | Yes (5) | 1 | CRi | CR |
| 16 | IV | RAEB/ RAEB-2 | High | 78 | −7, −16, +der(16) dic(16;17)(q13?;p12?), −17 | No | 1 | SD | SD |
| 17 | IV | RAEB/ RAEB-2 | High | 76 | t(1;6)(q42;p22), t(3;4)(p14;p16), −12, der(16), der(18)t(5;18)(q13;q21.3) | Yes (6) | 3 | SD | PD |
| 18 | IV | RAEB/ RAEB-1 | Int-2 | 71 | del(7q), +8, del20(q), +17p, del(18q), del(21q) | No | 3 | SD | SD |
| 19 | IV | AML/ AML | — | 63 | None | Yes (5) | 2 | SD | SD |
Abbreviations: AML, acute myeloid leukemia; CR, complete response; CRi, CR with incomplete recovery of peripheral blood counts; FAB, French-American-British; Int, Intermediate; IPSS, International Prognostic Scoring System; NA, not applicable; PD, progressive disease; PR, partial response; RA, refractory anemia; RAEB, RA with excess blasts; RCMD, refractory cytopenia with multilineage dysplasia; sAML, secondary AML; SD, stable disease; WHO, World Health Organization.
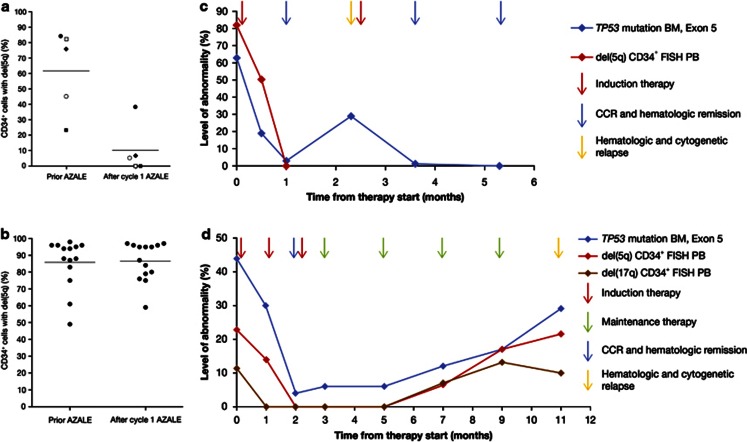
An interesting preliminary finding suggested that monitoring of peripheral blood del(5q)-positive CD34+ cells may be a surrogate marker of response. Compared with baseline, the percentage of peripheral blood CD34+ cells with the del(5q) clone was reduced in the five hematologic responders (including three with TP53 and one with DNMT3A mutations) after one cycle of therapy (P=0.001) (Figure 1a), but remained unchanged in nonresponders (Figure 1b). Furthermore, for the first time we performed minimal residual disease monitoring in consecutive BM samples of patients with complete cytogenetic and hematologic responses using next-generation deep-sequencing. Sequential treatment resulted in a rapid decline and disappearance of the TP53-mutant clone in one patient (Figure 1c), and decline followed by steady re-emergence in the other (Figure 1d). Re-emergence of the TP53 clone might have occurred owing to treatment continuation by consolidation (lower doses, cycles every 8 instead of 4 weeks), and preceded hematologic and cytogenetic relapse by several months. In addition, del(5q) and del(17p) levels in peripheral blood CD34+ cells increased concomitantly with the re-emergence of the TP53-mutated clones in the BM (Figure 1d). Intensification of combination treatment especially for the first six cycles under molecular minimal residual disease monitoring might require further investigation. Effects on TP53 mutated clonal cells have not been reported with lenalidomide alone, reinforcing the potential benefit of its combination with azacitidine.14, 15 Both lower- and higher-risk del(5q) MDS patients harboring TP53 mutations have lower response rates to lenalidomide and a high-risk of AML evolution compared with unmutated patients.14, 15 Whether the addition of azacitidine improves treatment outcomes needs to be evaluated in future studies.
Figure 1.
Percentage of CD34+ cells in PB with del(5q) by fluorescence in situ hybridization analysis in (a) hematologic responders (n=5; P=0.001; every patient is marked by a unique symbol to allow for individual pre and posttreatment comparisons) and (b) nonresponders (n=14). (c and d) Levels of TP53 mutation load and percentage of FISH+ cells at the respective sampling time points are reported on the y axis. (c) Universal patient number 15 who achieved CCR and hematologic remission: changes in TP53 mutation levels in the BM and changes in del(5q) CD34+ FISH+ cells in the PB. The patient achieved a CR with incomplete recovery of peripheral blood counts and CCR after the first cycle of induction therapy. The second cycle of treatment had to be delayed due to prolonged cytopenia. Upon recovery from cytopenia the patient displayed a hematologic and cytogenetic relapse. A CCR was obtained again after the second course of treatment. Six months after the first cycle of induction therapy the patient underwent an allogeneic hematopoietic stem cell transplantation. (d) Universal patient number 13 who achieved CCR and hematologic remission: changes in TP53 mutation levels in the BM, and changes in del(5q) and del(17p) CD34+ FISH+ cells in the PB. The patient underwent three cycles of induction therapy, which were followed by four cycles of maintenance therapy every 8 weeks. CR and CCR lasted for 9 months. During this time we observed the re-emergence of the TP53 mutated clone in the BM, as well as the del(5q) and del(17p) CD34+ blood cells, months before hematologic and cytogenetic relapse.
In summary, in a population of del(5q) higher-risk MDS and AML patients, including a majority with complex karyotypes, the sequential combination of azacitidine and lenalidomide was shown to be a feasible and potentially effective treatment strategy, even in patients with TP53-mutated clones. We observed a correlation between the percentage of peripheral CD34+ cells with del(5q) and response, suggesting that monitoring of this cell population may be a surrogate marker of response. Our results encourage application of sequential azacitidine and lenalidomide as first-line therapy for MDS patients with del(5q) in future trials, using a maximum lenalidomide dose of 20 mg, and with close surveillance of hematologic side-effects during the first two induction cycles.
Acknowledgments
We thank Professor Ch. Thiede (Dresden) for providing samples for the molecular analyses and G Hoppe/Dr C Steudel (Celgene) for supporting the concept and logistics of the study. Next-generation deep-sequencing data analyses were performed by Dr A Kohlmann and colleagues at the MLL Munich Leukemia Laboratory, Munich (Germany). We received editorial/writing support provided by Alessia Piazza, PhD, from Excerpta Medica, funded by Celgene.
Disclaimer
We had full access to the data and are fully responsible for content and editorial decisions for this manuscript.
Author Contributions
UP designed and performed research, collected data, analyzed and interpreted data, performed statistical analysis, and/or wrote the manuscript. FB, AK, KG, GB, CR, RN, KS, JN, AG, W-KH, UG, DH, GE, CS, MB and MW performed research, collected data, analyzed and interpreted data. All authors edited the paper, were involved in analyzing and interpreting data, and approved the final version of the manuscript.
U Platzbecker, U Germing and D Haase have received honoraria and research funding from Celgene Corporation. A Kündgen, K Götze, M Bornhäuser, A Giagounidis and W-K. Hofmann have received honoraria from Celgene Corporation. The remaining authors declare no conflict of interest.
References
- Giagounidis AA, Germing U, Aul C. Biological and prognostic significance of chromosome 5q deletions in myeloid malignancies. Clin Cancer Res. 2006;12:5–10. doi: 10.1158/1078-0432.CCR-05-1437. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G, Daver NG, Borthakur G, Konopleva M, Ravandi F, Wierda WG, et al. Phase I study of the combination of 5-azacitidine sequentially with high-dose lenalidomide in higher-risk myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML) Blood. 2011;118:abstract 2613. [Google Scholar]
- Sekeres MA, Tiu RV, Komrokji R, Lancet J, Advani AS, Afable M, et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higher-risk myelodysplastic syndromes. Blood. 2012;120:4945–4951. doi: 10.1182/blood-2012-06-434639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise C, Carter T, Schafer P, Chopra R. Pleiotropic mechanisms of action of lenalidomide efficacy in del(5q) myelodysplastic syndromes. Expert Rev Anticancer Ther. 2010;10:1663–1672. doi: 10.1586/era.10.135. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, Clinical application and proposal for modification of the International Working Group et al. (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- Kohlmann A, Klein HU, Weissmann S, Bresolin S, Chaplin T, Cuppens H, et al. The Interlaboratory RObustness of Next-generation sequencing (IRON) study: a deep sequencing investigation of TET2, CBL and KRAS mutations by an international consortium involving 10 laboratories. Leukemia. 2011;25:1840–1848. doi: 10.1038/leu.2011.155. [DOI] [PubMed] [Google Scholar]
- Pollyea DA, Kohrt HE, Gallegos L, Figueroa ME, Abdel-Wahab O, Zhang B, et al. Safety, efficacy and biological predictors of response to sequential azacitidine and lenalidomide for elderly patients with acute myeloid leukemia. Leukemia. 2011;26:893–901. doi: 10.1038/leu.2011.294. [DOI] [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adès L, Boehrer S, Prebet T, Beyne-Rauzy O, Legros L, Ravoet C, et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood. 2009;113:3947–3952. doi: 10.1182/blood-2008-08-175778. [DOI] [PubMed] [Google Scholar]
- Seymour JF, Fenaux P, Silverman LR, Mufti GJ, Hellstrom-Lindberg E, Santini V, et al. Effects of azacitidine compared with conventional care regimens in elderly (⩾75 years) patients with higher-risk myelodysplastic syndromes. Crit Rev Oncol Hematol. 2010;76:218–227. doi: 10.1016/j.critrevonc.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejar R, Stevenson K, Abdel-Wahab O, Galili M, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möllgård L, Saft L, Treppendahl MB, Dybeda Il, Nørgaard JM, Astermark J, et al. Clinical effect of increasing doses of lenalidomide in high-risk myelodysplastic syndrome and acute myeloid leukemia with chromosome 5 abnormalities. Haematologica. 2011;96:963–971. doi: 10.3324/haematol.2010.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jädersten M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Göhring G, et al. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol. 2011;29:1971–1979. doi: 10.1200/JCO.2010.31.8576. [DOI] [PubMed] [Google Scholar]