Abstract
Background and Aims
Bariatric surgery is increasingly performed worldwide to treat morbid obesity and is also known as metabolic surgery to reflect its beneficial metabolic effects especially with respect to improvement in type 2 diabetes. Understanding surgical weight loss mechanisms and metabolic modulation is required to enhance patient benefits and operative outcomes.
Methods
We apply a parallel and statistically integrated metagenomic and metabonomic approach to characterize Roux-en-Y gastric bypass (RYGB) effects in a rat model.
Results
We show substantial shifts of the main gut phyla towards higher levels of Proteobacteria (52-fold) specifically Enterobacter hormaechei. We also find low levels of Firmicutes (4.5-fold) and Bacteroidetes (2-fold) in comparison to sham-operated rats. Faecal extraction studies reveal a decrease in faecal bile acids and a shift from protein degradation to putrefaction through decreased faecal tyrosine with concomitant increases in faecal putrescine and diamnoethane. We find decreased urinary amines and cresols and demonstrate indices of modulated energy metabolism post-RYGB including decreased urinary succinate, 2-oxoglutarate, citrate and fumarate. These changes could also indicate renal tubular acidosis, which associates with increased flux of mitochondrial tricarboxylic acid cycle intermediates. A surgically-induced effect on the gut-brain-liver metabolic axis is inferred by increased neurotropic compounds; faecal γ-aminobutyric acid (GABA) and glutamate.
Conclusion
This profound co-dependence of mammalian and microbial metabolism, which is systematically altered following RYGB surgery, suggests that RYGB exerts local and global metabolic activities. The effect of RYGB surgery on the host metabolic-microbial crosstalk augments our understanding of the metabolic phenotype of bariatric procedures and can facilitate enhanced treatments for obesity-related diseases.
Keywords: bariatric surgery, NMR spectroscopy, UPLC-MS, obesity, bile acid
INTRODUCTION
Obesity is a global healthcare epidemic that affects all ages and socioeconomic groups. The World Health Organisation[1] projects a rising prevalence of obesity by approximately 7 million per year, which is associated with a concomitant rise in obesity-related co-morbidities such as diabetes[2], metabolic syndrome, heart disease,[3] and cancer.[4] These conditions lead to significant morbidity and mortality, which result in increased healthcare costs and medical resource consumption.[5] Although a number of treatment strategies have been applied to treat obesity including lifestyle, behavioral therapy and pharmacotherapies, the results of these strategies have only been marginally beneficial for morbidly obese individuals.[6]
Bariatric surgical approaches to reduce body fat have provided a definitive treatment for morbid obesity. These operations are successful in achieving and maintaining long-term weight loss,[7] but additionally achieve pronounced metabolic effects, including the resolution of type 2 diabetes in up to 75% of patients through both weight-dependent and weight-independent activities.[3, 8] As a result, these procedures are now considered as ‘metabolic operations’ that offer a significant beneficial impact on metabolism and physiology.[9, 10, 11]
The mechanisms of weight loss and metabolic augmentation through metabolic surgery remain poorly understood. RYGB is a multimodal surgical procedure that consists of Bile flow alteration, Reduction of gastric size, Anatomical gut rearrangement and altered flow of nutrients, Vagal manipulation and subsequent Enteric gut hormone modulation – the B.R.A.V.E effects.[4, 8, 9, 10] Although the modulation of gut hormones has been shown to contribute to the beneficial effects of surgery (primarily those of the hindgut such as the L-cell secreted Glucagon-like peptide-1 and Peptide YY to modify diabetes and appetite)[8, 11] they do not account for all the metabolic changes associated with these operations. Elucidating the beneficial mechanisms of metabolic surgery should a) help identify patients most likely to have a successful bariatric surgical outcomes through the application of pharmaco-metabonomic approaches[12] and b) identify biochemical mechanisms that might give new druggable antidiabetic and obesity targets.
A practical method to study the global metabolic effects of surgery is through a top-down systems biology and metabolic phenotyping approaches, which applied at individual and population levels could lead to advances both in personalized health care such as personalized chemotherapeutic decision making and molecular epidemiology including risk biomarkers and risk hypothesis evaluation.[13] This approach has been used extensively to study metabolic syndrome, cancer, infectious diseases and nutritional interventions.[14, 15] Many of the metabolic profiling studies of animal models of obesity to date have implicated a strong influence of gut microbial metabolism through the altered urinary co-metabolism including urinary phenols, bile acids and methylamines.[16, 17] Parallel studies using metagenomic data have also shown that obese and lean individuals have profound differences in the gut microbial landscape with a major shift towards an increased Firmicutes to Bacteroidetes ratio[18] in obese individuals (which is low calorie diet responsive) [19]. However, Duncan et al [20] have demonstrated that this ratio is not associated with obesity in all human studies. An increase in gut Actinobacteria in obese individuals has also been observed in a core gut microbiome study of obese and lean twins.[17] The study by Zhang et al further investigated the effect of bariatric bypass on three obese individuals and demonstrated an increase of γ-proteobacteria and a decrease of Firmicutes post surgery compared with 3 normal-weight controls and 3 obese patients.[21] Another recent study on 30 obese individuals and 13 lean control subjects have revealed the higher levels of Escherichia coli species 3 months following the RYGB operation, which is inversely associated with body fat mass, and reported that Faecalibacterium .prausnitzii species are shown to be correlated with reduced low-grade inflammation in obesity and diabetes.[22] A separate study in 14 female patients exploring the metabolic effects of bariatric surgery showed an increase in serum concentrations of p-cresyl sulfate, nervonic acid and lysophosphatidylcholine six months after post-operatively.[23] These metabolic changes may therefore represent the effects of metabolic surgery on renal function, insulin sensitivity and lipid metabolism respectively.
Since both mammalian and gut microbial metabolism exhibit an interdependence with respect to controlling energy balance, we use a parallel metagenomic and metabolic profiling strategy based on 454 Pyrosequencing technology and 1H NMR spectroscopy respectively to explore the impact of Roux-en-Y gastric bypass surgery on the interactions between the faecal and urinary metabolic phenotype, faecal bile acids and gut microbial modulation in non-obese Wistar rats.
MATERIALS AND METHODS
Animal model and sampling
Male Wistar rats (non-obese) were individually housed under a 12 hour /12 hour light-dark cycle at a room temperature of 21±2 °C. Water and standard chow were available ad libitum, unless otherwise stated. All experiments were performed under a license issued by the Home Office UK (PL 70-6669). Subjects were acclimatized for 1 week and were randomized to Roux-en-Y gastric bypass (RYGB) or sham operation according to our previously described technique.[9]
Preoperatively rats were food deprived overnight for 12 hours with water available ad libitum. Subjects were weighed, and then anesthetized with isofluorane (4% for induction, 3% for maintenance). Preoperative antibacterial prophylaxis was administered intra-peritoneally to both RYGB and sham groups (1 ml of an Amoxicillin/Flucoxacillin solution, both at 12.5 mg/ml). Surgery was performed on a heating pad to avoid decrease of body temperature during the procedure. The abdomen was shaved and disinfected with surgical scrub and a midline laparotomy was performed.
The sham procedure consisted of a 7 mm gastrotomy on the anterior wall of the stomach with subsequent closure (interrupted prolene 5-0 sutures) and a 7 mm jejunotomy with subsequent closure (running prolene 6-0 suture). In the gastric bypass procedure, the proximal jejunum was divided 15 cm distal to the pylorus to create a biliopancreatic limb. After identification of the cecum, the ileum was then followed proximally to create a common channel of 25 cm. Here, a 7 mm side-to-side Jejuno-Jejunostomy (running prolene 7-0 suture) between the biliopancreatic limb and the common channel was performed. The gastric pouch and alimentary limb were anastomosed end-to side using a running prolene 7-0 suture. The gastric remnant was closed with interrupted prolene 5-0 sutures. The complete bypass procedure lasted approximately 60 minutes and the abdominal wall was closed in layers using 4-0 and 5-0 prolene sutures. Approximately 20 minutes before the anticipated end of general anesthesia, all rats were injected with 0.1 ml of 0.3% buprenorphine subcutaneously to minimize postoperative discomfort. Immediately after abdominal closure, all rats were injected subcutaneously with 5 ml of normal saline to compensate for intra-operative fluid loss. After 24 hours of wet diet (normal chow soaked in tap water), regular chow was offered on postoperative day 2.
Urine and Stool was collected for 24 hours at 2 weeks, 4 weeks, 6 weeks and 8 weeks post-operatively and stored at −80° C.
Nuclear magnetic resonance spectroscopy
Urine samples were thoroughly defrosted and vortexed for 15 s prior to mixing an aliquot of 400 μl with 250 μl of 0.2 M phosphate buffer (pH=7.4) containing 20% deuterium oxide (D2O) for the magnetic field lock, 0.01% 3-(trimethylsilyl)-[2,2,3,3-2H4]-propionic acid sodium salt (TSP) for the spectral calibration and 3 mM sodium azide (Na3N) for avoiding bacterial contamination. The resulting mixture was centrifuged at 10392 g for 10 min and 600 μl of supernatants was transferred into a NMR tube with a diameter of 5 mm pending for 1H NMR spectral acquisition.
A total of 1 faecal pellet was placed into a 2 ml Eppendorf containing 1.4 ml of phosphate buffer previously mentioned in urinary sample preparation. The sample was homogenised, vortexed for 15 s, sonicated for 30 min at the temperature of 298 K and centrifuged at 10392 g for 20 min. A total of 700 μl supernatant was taken into a 1.5 ml Eppendorf and centrifuged again under the same condition. The supernatant (600 μl) was taken into a NMR tube with a diameter of 5 mm pending for 1H NMR spectral acquisition. 1H NMR spectra of urine and fecal extract samples were obtained using a Bruker 600 MHz spectrometer (Bruker; Rheinstetten, Germany) at the operating 1H frequency of 600.13 MHz with a temperature of 300 K. A standard NMR pulse sequence (recycle delay [RD]−90°-t1-90°-tm-90°-acquisition) was applied to acquire 1-dimensional (1-D) 1H NMR spectral data, where t1 was set to 3 μs and tm (mixing time) was set to 100 ms. Tthe water peak suppression was achieved using selective irradiation during RD of 2 s and tm. A 90 degree pulse was adjusted to approximately 10 μs. A total of 128 scans were collected into 64 k data points with a spectral width of 20 ppm. A series of 2-D NMR spectra including 1H-1H correlation spectroscopy (COSY), 1H-1H total correlation spectroscopy (TOCSY), J-resolved spectroscopy, 1H-13C heteronuclear single quantum coherence (HSQC), 1H-13C heteronuclear multiple bond coherence (HMBC) were acquired on the selected urine and faecal extract samples for the purpose of metabolite annotations. The standard parameters for these spectral acquisitions were previously reported.[24, 25]
Multivariate data analysis
Multivariate data analyses were performed based on the pre-possessed NMR dataset. 1H NMR spectra obtained from urine and faecal extracts were automatically phased, referenced and baseline-corrected using a MATLAB script developed by Dr. T. Ebbels at Imperial College. The resulting NMR spectra (δ0-10) were imported to MATLAB software and digitized into 20 k data points with the resolution of 0.0005 using script developed in house (Dr. O. Cloarec). The water peak region δ 4.62-5.05 and in urine spectra and δ 4.7-4.9 in faecal water spectra were removed in order to minimise the effect of the disordered baseline. Additionally, regions δ 0-0.10, δ 5.47-6.24 and δ 9.90-10.00 in urine spectra, and regions δ 0-0.30 and δ 9.4-10.00 in faecal water spectra containing only noise were, therefore, removed, followed by normalization to the remaining spectral areas of NMR data in order to perform further analyses. Principal component analysis (PCA), O-PLS and O-PLS-DA were carried out based on the resulting NMR spectral datasets in SIMCA (P+11.5) and MATLAB (2009a) software.
Analysis of the gut microbiota by pyrosequencing
The composition on the gut microbiota was determined by undertaking a survey of the 16S rRNA genes in each animal. DNA was extracted from fecal pellets (250mg) using a modified protocol based on the Qiagen Stool Kit (Qiagen, Crawly, UK) with an additional bead beating step to homogenise and lyse bacteria in the samples (0.1 g 0.1mm sterile glass beads, 3 × 4500 rpm for 30 secs with 5 mins on ice in between cycles). DNA obtained from this extraction was quantified using the Invitrogen Qubit platform and diluted to a working concentration of 10 ng μl−1. The PCR was used to amplify the V1-V3 regions of the 16S rRNA gene from each DNA sample using the primers shown in Table S1. The PCR was performed in triplicate on all DNA extracts using a MJ Research PTC-200P Thermal Cycler (MJ Research, USA). PCR mixtures (25 μl) contained 1 X Buffer (20 mM Tris pH 8.4, 50 mM KCl), 1.5 mM MgCl2, 200 μM of each dNTP, 1.25 U of Taq polymerase (NEB, UK), 5 pmol of each primer and 10 ng of DNA. The PCR conditions were: 95°C for 5 min initial denaturation, followed by 25 cycles of amplification at 95°C denaturation for 30 s, annealing at 55°C for 40 s and extension of 72°C for 1 min, with a final extension of 72°C for 5 min. PCR products were pooled for each sample, purified using a Qiagen PCR purification kit, quantified and equimolar amounts pooled prior to running on a ¼ of a PTP (Pico titre plate) using titanium chemistry (AGOWA, Berlin, Germany). The sequences were binned according to their sample source and processed via the RDP’s pyropipeline[26, 27] to remove any reads that were less than 250 bp and which contained any ambiguities. The filtered sequences were classified using the RDP classifier and the relative proportions of phyla and families determined. Community analysis of the data was undertaken using MOTHUR.
RESULTS AND DISCUSSION
Effect of RYGB surgery on weight loss
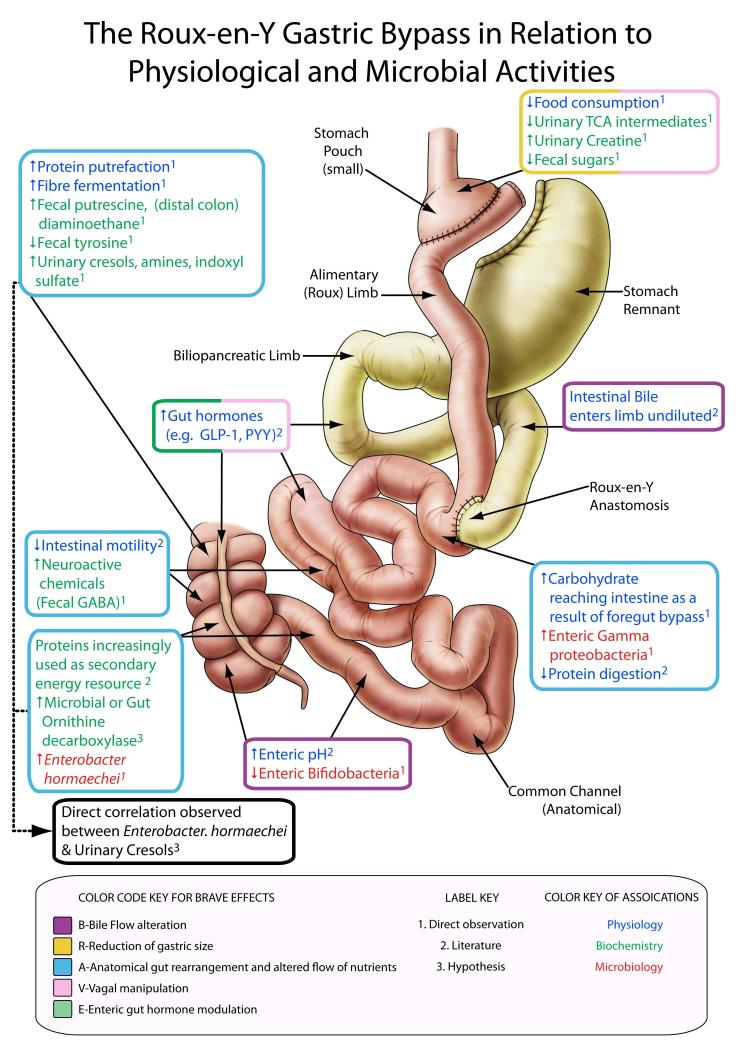
The multimodal effects of RYGB on the systemic metabolic and microbiotic phenotype can be described in terms of the B.R.A.V.E effects (figure 1) [4, 8, 9, 10], which led to a decrease in post-operative body weight and food consumption (table 1) in a non-obese surgical rat model (to objectively assess the metabolic effects of surgery indpendent to any pre-existing metabolic dysfucntion). These metabolic changes result in altered gastric and intestinal conditions including a reduction of acid production with a resultant increase in pH[28] and a suggested alteration in gut oxygen bioavailability,[21] and exert a substantial impact not only on the host metabolism but also on gastrointestinal ecology, mainly manifesting in a reduction in Firmicutes (includes the Clostridial family) and Bacteroidetes with a corresponding increase in Proteobacteria (γ-proteobacteria family, figure 2). These changes are consistent with findings in a previous study of 3 human surgical subjects and 6 controls.[21] The surgically altered anatomy, flow of nutrients and weight loss was associated with a global change in the urinary and faecal profiles reflecting: (a) an increased activity of oligosaccharide fermentation in the gut; (b) biogenesis of p-cresol and related compounds; and (c) the generation of amines which may mechanistically contribute to body weight loss and metabolic enhancements. We summarize the effects of RYGB surgery in male Wistar rats (n=14) over an 8-week period post surgery in comparison with a sham operated control group (n=15) according to the B.R.A.V.E framework[4, 8, 9, 10] and define microbial-metabolic interactions which contribute to the current understanding of the mechanism by which RYGB surgery operates to reduce obesity and type 2 diabetes.
Figure 1.
Metabolic modulation following Metabolic Roux-en-Y Gastric Bypass Surgery (RYGB). The surgical diagram of our animal model of RYGB is categorized according to the B.R.A.V.E. effects (coloured box outlines) at relevant anatomical sites. Within each box we describe the physiological, biochemical and microbiological effects of surgery at each site (coloured text), and further describe whether these effects were noted in this study, derived from the literature or our hypothesis (numbered label key).
Table 1.
Surgical outcomes, body weight and food consumption in the study population.
| Time Points |
Number of rats | Number for NMR analysis |
Number for UPLC-MS analysis |
Number for metagenomic analysis |
Body weight (g) | T-test P Value |
Food intake RYBG (g) |
T-test P Value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | Bypass | Sham | Bypass | Sham | Bypass | Sham | Bypass | Sham | Bypass | Sham | Bypass | |||
| Week -1 (Pre- op) |
18 | 18 | 18 | 18 | 6 | 6 | 0 | 0 | 341.9±30.7 | 346.6±35.8 | 0.71 | 24.7±2.8 | 25.7±2.7 | 0.31 |
| 2 (Post- op) |
16 | 15 | 16 | 15 | 0 | 0 | 6 | 6 | 358.6±22.6 | 288.6±29.1 | <0.001 | 29.5±2.8 | 14.3±3.2 | <0.001 |
| 4 (Post- op) |
16 | 15 | 16 | 15 | 0 | 0 | 383.8±21.7 | 275.6±25.4 | <0.001 | 30.9±2.7 | 22.4±3.1 | <0.001 | ||
| 6 (Post- op) |
16 | 14 | 16 | 14 | 6 | 6 | 0 | 0 | 400.3±22.8 | 270.9±32.7 | <0.001 | 25.7±3.2 | 18.9±5.1 | <0.001 |
| 8 (Post- op) |
16 | 14 | 16 | 14 | 6 | 6 | 6 | 6 | 419.8±25.3 | 259.1±36.1 | <0.001 | 20.9±4.9 | 14.6±6.5 | 0.007 |
NMR = Nuclear magnetic resonance, UPLC-MS = Ultra-performance liquid chromatography-tandem mass spectrometry, RYGB = Roux-en-Y Gastric Bypass.
Figure 2.
A heatmap (A) shows pairwise similarities between gut communities generated using MOTHUR and a cutoff of 0.10. Microbial composition of individual rat from sham control and RYGB-operated groups at week 2 and 8 (B). The pie chart (C) shows the mean of each bacterial class level within the control (N=6) and RYGB (N=6) groups at week 2 and 8. * The Student’s t test was used to calculate the difference of each bacterial class between 2 groups for each time point. Of note was the anomalous behaviour of one of the sham rats (S06) which exhibited a high level of Proteobacteria at week 2, more consistent with the response of the RYGB-operated animals. This animal was reported to be unwell immediately following the sham surgical intervention, but subsequently recovered by week 8 post surgery, at which stage the microbial profile of this animal was similar to that of the other sham operated animals.
Bile flow changes
The gastric bypass procedure modifies the anatomical location at which bile enters the upper gastrointestinal tract via the biliopancreatic limb of the Roux-en-Y construction and increases primary and secondary serum bile acid levels,[29] including taurochenodeoxycholic, taurodeoxycholic, glycocholic, glychochenodeoxycholic and glycodeoxycholic acid.[30] A targeted ultra-performance liquid-chromatography (UPLC)-MS method[31] was used to profile the faecal bile acids in 6 selected animals in each group at 3 time points (1 week pre-op, 6 and 8 weeks post operation). We detected decreased faecal bile acids, mainly unconjugated, post RYGB (>6 weeks), but not pre-operatively (figure S1). The relative concentration of taurine-conjugated and several unconjugated faecal bile acids in sham operated rats was significantly greater than in the RYGB operated animals at weeks 6 and 8 (taurine-conjugated: 15.6× p=0.049, unconjugated: 3.6×, p=0.026). This modulation of bile acids may trigger the gut-brain-liver axis to achieve earlier satiety after meal consumption and improved glucose regulation.[32]
Restriction of gastric size
The main effect of RYGB surgery is to bypass the main body of the stomach and duodenum lower down the gastrointestinal tract so that nutrients are exposed to the small intestine (jejunum) immediately after transit through the esophagus and a subtracted small gastric remnant (figure 1). In rats that underwent RYGB surgery (n=14, mean pre-operative weight of 347g), we measured an average weight loss of 87.5 g +/− 50.8 g over an 8 week post surgical period in contrast to a matched control group of sham operated rats (n=16, mean pre-operative weight of 342g) that gained 77.9 g +/− 39.7 g with a corresponding food consumption of 14.6 g/day for the RYGB group compared with 20.9 g/day consumed by the sham group. These effects provide a degree of caloric restriction which is associated with a specific metabolic profile. We have previously demonstrated[33] that caloric restriction in rats result in a decrease of 2-oxoglutarate and an increase in creatine. The urinary metabolites (including succinate, 2-oxoglutarate, citrate and fumarate) of the RYGB operated rats, as measured by 1H nuclear magnetic resonance (NMR) spectroscopy at 2, 4, 6 and 8 weeks were decreased over all observation time points (table 2). This observation persistent up to 8 weeks post the operation indicates a long-term modulation of cellular energy metabolism through increased utilization of tricarboxylic acid (TCA) cycle intermediates. These findings agree with the findings of caloric restriction where substrates are upregulated as a result of an acidotic effect, although we did not find a clear link between TCA cycle intermediates and animal body weight. These changes could therefore indicate renal tubular acidosis which associates with an increased flux of tricarboxylic acid cycle intermediates in the mitochondrion[34] and may contribute as a post-operative mechanism of weight loss and metabolic enhancement. Further studies of cellular energy metabolism after gastric bypass surgery demonstrate the down regulation of mitochondrial complex I-IV post-RYGB in diet-induced obese Sprague-Dawley rats and post-operative human subjects.[35, 36] Rhabdomyolysis (muscle lysis and metabolism; RML) is a recognized complication of gastric bypass surgery[37] and is also observed in extreme caloric restriction.[38] The criteria for a RML diagnosis is >5 fold increase of normal creatine kinase levels in plasma. The higher urinary level of creatine in this study may therefore reflect the increased muscle protein catabolism following surgery.
Table 2.
Summary of metabolic changes in urinary (u) and fecal (f) NMR profiles
| Class | Metabolites | O-PLS-DA models of RYGB-operated and sham control rats |
|||
|---|---|---|---|---|---|
| 2-week post-op | 4-week post-op | 6-week post-op | 8-week post-op | ||
| TCA cycle | succinate | −0.6977(u) | −0.6591(u); +0.5879(f) | −0.8562(u) | −0.7674(u); +0.6286(f) |
| 2-oxoglutarate | −0.6509(u) | −0.6333(u); *+0.6170(u) | −0.8084(u) | −0.8153(u) | |
| citrate | −0.6090(u) | −0.4856(u) | −0.6150(u) | −0.5045(u) | |
| fumarate | −0.6518(u) | −0.6374(u); | −0.8703(u) | −0.7200(u) | |
| Amines | trimethylamine N-oxide | +0.5183(u); *+0.7718(u) | +0.5755(u); *+0.6994(u) | +0.6311(u); *+0.6241(u) | +0.6937(u); *+0.7320(u) |
| trimethylamine | +0.6016(f) | +0.8156(f) | +0.6865(f) | ||
| methylamine | +0.5196(f) | +0.7819(f) | +0.6933(f) | +0.7167(f) | |
| putrescine | +0.6133(f) | +0.6601(f) | +0.7048(f) | +0.7328(f) | |
| diaminoethane | +0.5170(f) | +0.6573(f) | +0.7230(f) | ||
| Microbial activity | oligosaccharides | −0.6144(f) | −0.6461(f) | ||
| acetate | +0.5415(f) | +0.5413(f) | |||
| propionate | +0.5916(f) | +0.5186(f) | |||
| p-cresol glucuronide | +0.7785(u) | +0.6304(u) | +0.7116(u) | +0.7225(u); *+0.6367(u) | |
| p-cresol sulfate | +0.7980(u) | +0.6911(u) | +0.6501(u) | +0.7303(u) | |
| 5-aminovalerate | +0.6977(u) | +0.5518(u) | +0.6297(u) | +0.6921(u) | |
| phenylacetylglycine | +0.9192(u); *+0.5416(u) | +0.7038(u) | +0.8432(u); *+0.5497(u) | +0.7533(u) | |
| p-hydroxyphenylacetate | +0.7129(u) | +0.5207(u) | +0.6546(u) | +0.6176(u) | |
| hippurate | −0.8768(u) | −0.6692(u) | |||
| methanol | +0.5561(f) | +0.5167(f) | |||
| formate | +0.6820(f) | +0.5907(f) | |||
| tyrosine | −0.6139(f) | −0.6726(f) | −0.6543(f); *+0.5820(f) | −0.6065(f) | |
| Peripheral neuroactive intermediate |
γ-amino-N-butyrate | +0.7036(f) | +0.7373(f) | +0.7038(f) | +0.6806(f) |
| glutamate | +0.5514(f) | +0.5914(f) | |||
| Urea cycle | aspartate | −0.6653(f) | −0.7314(f) | −0.6881(f) | −0.6999(f) |
| Muscle metabolism | creatine | +0.6619 (u) | +0.4473(u) | +0.7781(u) | +0.7294(u) |
| creatinine | *+0.6337(u) | −0.7473(u); *+0.6424(u) | |||
| lactate | +0.5871(f) | ||||
| Renal metabolism | indoxylsulfate | +0.8598(u) | +0.7271(u) | +0.6550(u); *+0.7770(u) | +0.6542(u) |
| Amino acids | valine | +0.5822(f) | |||
| phenylalanine | +0.5849(f); *+0.5719(f) | ||||
| alanine | *+0.5636(f) | ||||
| glycine | +0.6577(f) | +0.6317(f) | +0.5698(f); *+0.6521(f) | +0.5377(f) | |
| others | N-acetyl glycoproteins 2.06(s)** | −0.7523(f) | −0.6160(f); *-0.5648(f) | ||
| ethanol | +0.6365(f) | +0.6904(f) | |||
| phenylalanine | +0.5849(f); *+0.5719(f) | ||||
| 2-oxoadipate | −0.8260(u) | −0.6238(u) | −0.9030(u) | −0.9045(u) | |
indicates a higher level of metabolites in RYGB-operated rats;
indicates a lower level of metabolites in RYGB-operated rats.
–labelled metabolites are found to be changed in sham control rats in comparison to pre-operation, where “+”/“−“ indicates higher/lower levels of metabolites post-sham.
Putative assignment.
Altered gastrointestinal anatomy
The RYGB procedure results in a large stomach remnant (up to 1000 ml in humans and 5 ml in rats) with an associated segment of small bowel being bypassed lower down the upper gastrointestinal tract (figure 1). As a result, ingested nutrients only have exposure to a small stomach pouch (approximately 15-30 ml in man and 0.25 ml in rats) before entering the small intestine earlier than the standard gastrointestinal configuration (figure 1, animal model paper). Consequently the distal small intestine receives a higher load of nutrients that have not been exposed to the standard volume of stomach mucosa. RYGB profoundly disrupts the gut microbial ecology as evidenced in the 454 sequencing data of 6 randomly selected animals from each group at 2 and 8 weeks post-surgery. We show a dramatic increase (52-fold) in the relative proportion of phylum Proteobacteria (mainly the order Enterobacteriaceae) with a corresponding but less dramatic fall in the level of phylum Firmicutes (4.5-fold reduction - mainly the family Peptostreptococcaceae) in RYGB-operated individuals (figure 2B). The most striking alteration in microbial ecology following RYGB surgery was the growth of the class γ-proteobacteria, particularly the species Enterobacter hormaechei (figures 2C and S2) which has not been reported previously. This is a Gram-negative bacterium with extended-spectrum beta-lactamase activity that has previously been described as a nosocomial pathogen, although its role in contributing to gut and systemic metabolism require furthers investigation.[39] The selection for this bacterium is likely to derive from the effects of surgically altered gastrointestinal anatomy and inherent bacterial beta-lactamase activity. The RYGB operation provides an anatomical path where carbohydrates directly enter the ileum without prior exposure to the main body of stomach. This favours the growth of enterobacterial species as they display a high level of flexibility in fermenting carbohydrates.[40] The faecal profile also showed evidence of altered energy metabolism with the spectral region containing oligosaccharides and other sugars deceasing in relative percentage in RYGB group that is consistent with an altered microbial ecology favouring oligosaccharide use in the post-operative gut.[41] Furthermore, the increased concentrations of various amines (methylamine, trimethylamine) in faecal extracts (table 2) reflects the fundamental role for the intestinal microflora after RYGB surgery in the provision of methylamine and trimethylamine from the catabolism of precursors such as choline.[42]
A further consequence of the RYGB surgery is to modulate gastric emptying and intestinal mobility, which have been demonstrated to decrease post-operatively such that approximately 40% of human patients demonstrate very slow or no emptying on upper gastrointestinal contrast studies at one year post-operatively[43, 44] and rodents demonstrate an approximate 40% decrease in 30-minute-intestinal transit time after RYGB compared to controls. This is associated with the effects of proximal surgical vagotomy[45] and the action of gut peptides such as ghrelin, Glucagon-like peptide-1 (GLP-1) and Peptide YY (PYY) on gastric and intestinal motility.[46] Reduced upper-gastrointestinal motility can lengthen intestinal exposure times to promote the conditions for protein putrefaction. Consequently, incompletely digested proteins reaching the colon due to the surgical bypass of the foregut are likely to result in a high bioavailability of proteins in the hindgut. As a result, several polyamines such as putrescine, diaminoethane (aliphatic biogenic amine) are derived from microbial catabolic processes[47] as demonstarted from our fecal extract results. In our study, the majority of fecal metabonomic changes occured by week 2 and continued with this profile thereafter. The neuroactive peptide γ-aminobutyric acid (GABA) is also increased in post-RYGB faecal samples and has also been demonstrated to derive from the microbial processing of putrescine.[48] Increased expression of faecal GABA is consistent with the well-defined increase of GLP-1 following RYGB.[11] GABA can stimulate GLP-1 release from intestinal cells[49] and raised GLP-1 levels in turn promote GABA formation by pancreatic β-cells to provide autocrine and paracrine effects including α-cell regulation to inhibit glucagon release, acinar cells activity to amplify cholecystokinin release and also immunomodulatory actions on infiltrating T cells to suppress autoimmune damage.[45] Increased brain GABA levels can also reflect the hypothalamic GABA-mediated control of food intake.[50, 51]
Vagal nerve effects and enterohumoural (gut hormone) activity
Vagal nerve innervation and neurohormonal processes play a key role in the short-term regulation of food intake and the release of key gastrointestinal hormones including gastrin and secretin.[11] Due to the variation in vagal anatomy, operative surgical dissection near the stomach can lead to vagotomies of the gastric pouch and gastric remnant with varying results.[52] Surgical vagal nerve stimulation can attenuate weight gain[53] and we have previously demonstrated that distal vagal sparing bariatric procedures can enhance weight loss.[54] Some of the weight loss effects of gastric bypass may reflect the surgical regulation of vagal activity in the gut-brain-liver axis in addition to the role of the vagus nerve in transmitting some of the appetite-suppression signals of modulated gut hormones after surgery.[11]
Cross talk between mammalian metabolism and the intestinal microbiome and its impact on weight loss following RYGB operation
The most striking metabolic feature of RYGB operated rats is an increase in the diversity and complexity of signals in the aromatic region of the 1H NMR urinary spectra (figure S3), corresponding to increased concentrations of p-cresyl glucuronide, p-cresyl sulfate, 5-aminovalerate, phenylacetylglycine, p-hydroxyphenylacetate and indoxyl sulfate (figures 3A and B, table 2). The aromatic region of the 1H NMR spectrum provides a convenient spectral window on gut microbial activity[55] and many of the metabolites with signals in the range δ6.5-8.0 derive from mammalian-microbial co-metabolism.[55]
Figure 3.
Cross validation plots and O-PLS-DA coefficient plots of urinary (A, B (R2X =32%; Q2Y =0.86)) and faecal (C, D, (R2X =33.5%; Q2Y =0.84)) NMR spectral data obtained from sham control (blue) and Roux-en-Y Gastric Bypass-operated rats (red) at week 8, reflecting the discrimination between these two groups. Keys: AP: 2-oxoadipate; Asp: aspartate; AV: 5-aminovalerate; Cre: creatine; Crn: creatinine; DE: diaminoethane; ET: ethanol; FA: formate; FM: fumarate; GABA: γ-animo N-butyrate; Gly: glycine; GT: 2-oxoglutarate; HA: p-hydroxyphenylacetate; HP: hippurate; IS: indoxyl sulfate; Lac: lactate; MA: methylamine; OS: oligosaccharides; PAG: phenylacetylglycine; PG: p-cresyl glucuronide; PS: p-cresyl sulfate; PT: putrescine; Suc: succinate; TMA: trimethylamine; TMAO: trimethylamine N-oxide; Ura: uracil.
Fermentation is one of the many functions provided by gut microbes, where those of the proximal colon are mainly responsible for enhanced calorific recovery from otherwise indigestible polysaccharides.[56] The lower concentrations of oligosaccharides and higher concentrations of short chain fatty acids (table 2), which are major products of fermentation, suggest increased microbial fermentation activity. There was a positive trend between fermentation-generated compounds and lower body weight although this was not significant, but may suggest a role in metabolic modulation and metabolic syndrome resolution after surgery.[3, 8] The efficiency of microbially-mediated energy recovery is determined by the composition of the microbiome.
In order to probe the deeper association between the changes in the metagenome and metabolic phenotype, key discriminatory bacterial classes and selected species were correlated directly with the metabolic profiles. A significant proportional increase in the γ-proteobacteria in RYGB-operated rats was positively correlated with urinary p-hydroxyphenylacetate, p-cresyl glucuronide, p-cresyl sulfate, creatine, phenylacetylglycine (PAG) and indoxyl sulfate and with faecal succinate, putrescine, diaminoethane, uracil, glycine, methylamine and formate (figure 4). Clostridia demonstrated an association with decreased urinary p-cresyl glucuronide, PAG and creatine, and with decreased fecal succinate, glycine, uracil and formate and increased faecal oligosaccharides (figure 4). The main contributor to the increase in γ-proteobacteria was Enterobacter hormaechei which manifested a 200 and 42.8 fold increase at weeks 2 and 8 post RYGB surgery respectively. In the case of E. hormaechei strong direct covariation with p-cresyl derivatives, p-cresyl glucuronide and p-cresyl sulfate, PAG and creatine were detected (figure S5). Thus, although some studies indicate that some species of Clostridia produce phenol and p-cresol together with ammonia and hydrogen via the anaerobic degradation of aromatic amino acid such as tyrosine,[57] our findings suggest that γ-proteobacteria (predominantly in this case E. hormaechei), may also contribute to the pool of cresol metabolites in the urine. This finding is consistent with previous studies reporting that changes in gut Enterobacter are associated with corresponding alterations in p-cresol metabolite levels.[58]
Figure 4.

O-PLS regression loadings plot shows the correlation between the combination of urinary and faecal NMR spectral data and γ-proteobacteria (Q2Y=0.58; R2X=18.0%) and Clostridia Q2Y=0.45; R2X=18.7%) levels.
Urinary concentrations of PAG, p-cresyl glucuronide, 5-aminovalerate, p-cresyl sulfate, creatine and p-hydroxyphenylacetate, and faecal concentrations of uracil, putrescine and methylamine increased as body weight reduced (figure S6A). High intensities of Enterobacteriaceae and Pasteurellaceae are associated with weight loss, whereas Lachinospiraceae, Incertae Sedis XIII and Prevotellaceae increased with weight gain (figure S6B). The Enterobacteriaceae levels exhibit a strong correlation with surgical weight loss and urinary PAG, putrescine, uracil, p-cresyl glucuronide, creatine and methylamine levels whereas the urinary excretion of p-cresyl sulfate correlates with Bifidobacteriaceae and Micrococcaceae (figure S7).
Does bariatric surgery have a short-term gain and a long-term loss?
Bariatric surgery is currently the most effective long-term treatment for morbid obesity. It is associated with decreased cardiovascular risk and cancer incidence compared to obese controls.[3, 7, 59] Bariatric surgery-induced microbial and metabolic alterations can contribute to surgical weight loss, beneficial metabolic outcomes and decreased mortality. However a shift towards the γ-proteobacteria as a major component of the microbiota and elevation in p-cresol derivatives and another uremic toxin, indoxyl sulfate could have long-term impacts on host health with unpredictable outcomes. Recent evidence also identifies the bacterium E. Hormaechei as an apoptosis-inducing agent in human epithelial cells.[60] A deeper understanding of the disease-modifying mechanisms of bariatric surgery is therefore essential. Studying such metabolic interactions through a top-down systems biology approach can thus improve our current understanding of surgery to provide improved outcomes and novel treatments for obesity and metabolic disorders.
At 8 weeks post-RYGB operation, urinary creatinine levels were lower and may reflect improved creatinine clearance post-operatively or surgically induced kidney or muscle injury as the uremic toxin, indoxyl sulfate, was also found to increase in the RYGB group. The role of these metabolic changes on kidney function require further investigation as clinical studies demonstrate the beneficial effects of RYGB[61] and GLP-1[62] on renal function.
The current work represents a systematic and dynamic investigation of the metabolic and microbial effects of the RYGB procedure in Wistar rats using metagenomic and NMR-based metabonomic approaches. Both longitudinal (time-effect) and horizontal (surgery-effect) results are prominent in metabolic and microbial signatures where we also provide triangular (body weight loss, metabolite profiles and bacterial compositions) correlations. We uncover mechanistic insights into the role of the Roux-en-Y gastric bypass to achieve weight loss and metabolic enhancement. This includes the surgically effect of modulating the biochemical crosstalk between the gut microbiome and systemic host metabolism. Here we identify E. hormaechei as a long-term key contributor to gut ecology post-operatively. This organism is an excellent beta-lactamase producer[39] and therefore may be resistant to the pre-operative antibiotics (Amoxycillin and Flucloxacillin, both beta-lactams) thus enhancing its opportunity to colonise soon after the operation – however interestingly in the RYGB population bacterial levels were maintained high, whereas this was not the case in sham animals. These changes are associated with our findings of increased protein putrefaction after surgery. Furthermore, we support the increased awareness that these operations enhance weight loss through metabolic regulation of the gut-liver-brain axis and the enhancement of mitochondrial energetic efficiency. These multi-system effects of surgical intervention suggest a profound contribution of integrated host and microbial metabolism underlying the sophisticated mechanisms behind the metabolic benefits of bariatric surgery.
Supplementary Material
SUMMARY BOX.
What is already known about this subject?
Bariatric surgery, also-called metabolic surgery, is an effective approach to treat morbid obesity and can achieve pronounced metabolic effects including the resolution of type 2 diabetes resolution.
The Roux-en-Y gastric bypass (RYGB) operation is a bariatric procedure that achieves its physiological benefits through the B.R.A.V.E effects: Bile flow alteration, Reduction of gastric size, Anatomical gut rearrangement and altered flow of nutrients, Vagal manipulation and subsequent Enteric gut hormone modulation.
Recent studies demonstrate the impact of bariatric bypass on the microbial composition of obese individuals including an increase of γ-proteobacteria and a decrease of Firmicutes after RYGB surgery
Some metabolic profiling studies have identified the altered concentrations of serum biochemical components such as p-cresyl sulfate, nervonic acid and lysophosphatidylcholine after RYGB surgery.
What are the new findings?
The RYGB operation induces substantial shifts of the main gut phyla towards higher levels of Proteobacteria (52-fold) specifically Enterobacter hormaechei and low levels of Firmicutes (4.5-fold) and Bacteroidetes (2-fold) in comparison to sham-operated rats.
The surgically altered anatomy and flow of nutrients is associated with a global change in the urinary and faecal profiles reflecting: (a) an increased activity of oligosaccharide fermentation in the gut; (b) biogenesis of p-cresol and related compounds; (c) the generation of amines; and (d) lower levels of urinary TCA intermediates, which may mechanistically contribute to body weight loss and metabolic enhancements.
Faecal profiles reveal a decrease in faecal bile acids and a shift from protein degradation to putrefaction through decreased faecal tyrosine with concomitant increases in faecal putrescine and diamnoethane.
How might it impact on clinical practice in the foreseeable future?
Understanding host-microbial crosstalk and the metabolic phenotype of bariatric procedures can facilitate enhanced management strategies for obesity-related disease. This can lead to improved operative procedures and novel treatments for obesity and metabolic disease.
Acknowledgements
We thank Dr. E. Want for help in performing UPLC experiments and data analysis. We would also like to thank Catherine Sulzmann for her artwork.
Funding This study received financial support from the Imperial College London Junior research fellowship to J.V.L and from the Wellcome Trust Research Training Fellowship to H.A. We are grateful for support from the NIHR Biomedical Research Centre Funding Scheme.
Footnotes
Competing interests The authors declare no conflict of interest.
Ethics approval All animal experiments were approved by Charing Cross Research Ethics Committee, London, UK.
All authors agree that “The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Gut editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence http:/gut.bmj.com/site/about/licence.pdf”
REFERENCES
- 1.World_Health_Organization Obesity and overweight - Fact Sheet N°311. 2006.
- 2.International_Diabetes_Federation Diabetes Atlas - Prevalence and Projections. 2008.
- 3.Ashrafian H, le Roux CW, Darzi A, et al. Effects of bariatric surgery on cardiovascular function. Circulation. 2008;118:2091–102. doi: 10.1161/CIRCULATIONAHA.107.721027. [DOI] [PubMed] [Google Scholar]
- 4.Ashrafian H, Ahmed K, Rowland SP, et al. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer. 2011 doi: 10.1002/cncr.25738. [DOI] [PubMed] [Google Scholar]
- 5.Ashrafian H, Darzi A, Athanasiou T. Bariatric surgery - can we afford to do it or deny doing it? (in press) Frontline Gastroenterol. 2011 doi: 10.1136/fg.2010.002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puterbaugh JS. The emperor’s tailors: the failure of the medical weight loss paradigm and its causal role in the obesity of America. Diabetes Obes Metab. 2009;11:557–70. doi: 10.1111/j.1463-1326.2009.01019.x. [DOI] [PubMed] [Google Scholar]
- 7.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 8.Ashrafian H, Athanasiou T, Li JV, et al. Diabetes resolution and hyperinsulinaemia after metabolic Roux-en-Y gastric bypass. Obes Rev. 2010 doi: 10.1111/j.1467-789X.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- 9.Ashrafian H, Bueter M, Ahmed K, et al. Metabolic surgery: an evolution through bariatric animal models. Obes Rev. 2009 doi: 10.1111/j.1467-789X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 10.Ashrafian H, Darzi A, Athanasiou T. Autobionics: a new paradigm in regenerative medicine and surgery. Regen Med. 2010;5:279–88. doi: 10.2217/rme.10.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashrafian H, le Roux CW. Metabolic surgery and gut hormones - a review of bariatric entero-humoral modulation. Physiol Behav. 2009;97:620–31. doi: 10.1016/j.physbeh.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Clayton TA, Lindon JC, Cloarec O, et al. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature. 2006;440:1073–7. doi: 10.1038/nature04648. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson JK, Lindon JC. Systems biology: Metabonomics. Nature. 2008;455:1054–6. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 14.Holmes E, Loo RL, Stamler J, et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453:396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134:714–7. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Waldram A, Holmes E, Wang Y, et al. Top-down systems biology modeling of host metabotype-microbiome associations in obese rodents. J Proteome Res. 2009;8:2361–75. doi: 10.1021/pr8009885. [DOI] [PubMed] [Google Scholar]
- 17.Williams R, Lenz EM, Wilson AJ, et al. A multi-analytical platform approach to the metabonomic analysis of plasma from normal and Zucker (fa/fa) obese rats. Mol Biosyst. 2006;2:174–83. doi: 10.1039/b516356k. [DOI] [PubMed] [Google Scholar]
- 18.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 19.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan SH, Lobley GE, Holtrop G, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 2008;32:1720–4. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106:2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutch DM, Fuhrmann JC, Rein D, et al. Metabolite profiling identifies candidate markers reflecting the clinical adaptations associated with Roux-en-Y gastric bypass surgery. PLoS One. 2009;4:e7905. doi: 10.1371/journal.pone.0007905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckonert O, Keun HC, Ebbels TM, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2:2692–703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson JK, Foxall PJ, Spraul M, et al. 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal Chem. 1995;67:793–811. doi: 10.1021/ac00101a004. [DOI] [PubMed] [Google Scholar]
- 26.Cole JR, Wang Q, Cardenas E, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–5. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–41. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melissas J, Kampitakis E, Schoretsanitis G, et al. Does reduction in gastric acid secretion in bariatric surgery increase diet-induced thermogenesis? Obes Surg. 2002;12:236–40. doi: 10.1381/096089202762552412. [DOI] [PubMed] [Google Scholar]
- 29.Nakatani H, Kasama K, Oshiro T, et al. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–7. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–7. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Want EJ, Coen M, Masson P, et al. Ultra performance liquid chromatography-mass spectrometry profiling of bile acid metabolites in biofluids: application to experimental toxicology studies. Anal Chem. 2010;82:5282–9. doi: 10.1021/ac1007078. [DOI] [PubMed] [Google Scholar]
- 32.Wang PY, Caspi L, Lam CK, et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–6. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- 33.Veselkov KA, Lindon JC, Ebbels TM, et al. Recursive segment-wise peak alignment of biological (1)h NMR spectra for improved metabolic biomarker recovery. Anal Chem. 2009;81:56–66. doi: 10.1021/ac8011544. [DOI] [PubMed] [Google Scholar]
- 34.Nissim I, Yudkoff M. Adaptation of renal tricarboxylic acid cycle metabolism to various acid-base states: study with [3-13C,5-15N]glutamine. Miner Electrolyte Metab. 1991;17:21–31. [PubMed] [Google Scholar]
- 35.Guijarro A, Osei-Hyiaman D, Harvey-White J, et al. Sustained weight loss after Roux-en-Y gastric bypass is characterized by down regulation of endocannabinoids and mitochondrial function. Ann Surg. 2008;247:779–90. doi: 10.1097/SLA.0b013e318166fd5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Feuers RJ, Desai VG, et al. Surgical caloric restriction ameliorates mitochondrial electron transport dysfunction in obese females. Obes Surg. 2007;17:800–8. doi: 10.1007/s11695-007-9146-7. [DOI] [PubMed] [Google Scholar]
- 37.Koffman BM, Greenfield LJ, Ali II, et al. Neurologic complications after surgery for obesity. Muscle Nerve. 2006;33:166–76. doi: 10.1002/mus.20394. [DOI] [PubMed] [Google Scholar]
- 38.Worley G, Claerhout SJ, Combs SP. Hypophosphatemia in malnourished children during refeeding. Clin Pediatr (Phila) 1998;37:347–52. doi: 10.1177/000992289803700603. [DOI] [PubMed] [Google Scholar]
- 39.Townsend SM, Hurrell E, Caubilla-Barron J, et al. Characterization of an extended-spectrum beta-lactamase Enterobacter hormaechei nosocomial outbreak, and other Enterobacter hormaechei misidentified as Cronobacter (Enterobacter) sakazakii. Microbiology. 2008;154:3659–67. doi: 10.1099/mic.0.2008/021980-0. [DOI] [PubMed] [Google Scholar]
- 40.Le Bouguenec C, Schouler C. Sugar metabolism, an additional virulence factor in enterobacteria. Int J Med Microbiol. 2010 doi: 10.1016/j.ijmm.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Claus SP, Tsang TM, Wang Y, et al. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol Syst Biol. 2008;4:219. doi: 10.1038/msb.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.al-Waiz M, Mikov M, Mitchell SC, et al. The exogenous origin of trimethylamine in the mouse. Metabolism. 1992;41:135–6. doi: 10.1016/0026-0495(92)90140-6. [DOI] [PubMed] [Google Scholar]
- 43.Akkary E, Sidani S, Boonsiri J, et al. The paradox of the pouch: prompt emptying predicts improved weight loss after laparoscopic Roux-Y gastric bypass. Surg Endosc. 2009;23:790–4. doi: 10.1007/s00464-008-0069-8. [DOI] [PubMed] [Google Scholar]
- 44.Horowitz M, Collins PJ, Harding PE, et al. Gastric emptying after gastric bypass. Int J Obes. 1986;10:117–21. [PubMed] [Google Scholar]
- 45.Urbain JL, Penninckx F, Siegel JA, et al. Effect of proximal vagotomy and Roux-en-Y diversion on gastric emptying kinetics in asymptomatic patients. Clin Nucl Med. 1990;15:688–91. [PubMed] [Google Scholar]
- 46.Suzuki S, Ramos EJ, Goncalves CG, et al. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery. 2005;138:283–90. doi: 10.1016/j.surg.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Welters CF, Dejong CH, Deutz NE, et al. Effects of parenteral arginine supplementation on the intestinal adaptive response after massive small bowel resection in the rat. J Surg Res. 1999;85:259–66. doi: 10.1006/jsre.1999.5607. [DOI] [PubMed] [Google Scholar]
- 48.Kurihara S, Kato K, Asada K, et al. A putrescine-inducible pathway comprising PuuE-YneI in which gamma-aminobutyrate is degraded into succinate in Escherichia coli K-12. J Bacteriol. 2010;192:4582–91. doi: 10.1128/JB.00308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gameiro A, Reimann F, Habib AM, et al. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J Physiol. 2005;569:761–72. doi: 10.1113/jphysiol.2005.098962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nobrega JN, Snow J, Dixon LM, et al. Graded increases in brain GABA: differential effects on feeding and other behaviours in rats. Behav Brain Res. 1988;31:135–47. doi: 10.1016/0166-4328(88)90017-4. [DOI] [PubMed] [Google Scholar]
- 51.Turenius CI, Charles JR, Tsai DH, et al. The tuberal lateral hypothalamus is a major target for GABAA--but not GABAB-mediated control of food intake. Brain Res. 2009;1283:65–72. doi: 10.1016/j.brainres.2009.05.064. [DOI] [PubMed] [Google Scholar]
- 52.Sapala JA, Wood MH, Schuhknecht MP. Vagotomy at the time of gastric bypass: can it be harmful? Obes Surg. 2004;14:575–6. doi: 10.1381/096089204323093327. [DOI] [PubMed] [Google Scholar]
- 53.Sobocki J, Fourtanier G, Estany J, et al. Does vagal nerve stimulation affect body composition and metabolism? Experimental study of a new potential technique in bariatric surgery. Surgery. 2006;139:209–16. doi: 10.1016/j.surg.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 54.Bueter M, Lowenstein C, Ashrafian H, et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg. 2010;20:616–22. doi: 10.1007/s11695-010-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicholson JK, Connelly J, Lindon JC, et al. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov. 2002;1:153–61. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- 56.Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol. 2005;3:431–8. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 57.Elsden SR, Hilton MG, Waller JM. The end products of the metabolism of aromatic amino acids by Clostridia. Arch Microbiol. 1976;107:283–8. doi: 10.1007/BF00425340. [DOI] [PubMed] [Google Scholar]
- 58.Hida M, Aiba Y, Sawamura S, et al. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron. 1996;74:349–55. doi: 10.1159/000189334. [DOI] [PubMed] [Google Scholar]
- 59.Ashrafian H, Ahmed K, Rowland SP, et al. Metabolic Surgery and Cancer: The Protective Effects of Bariatric Procedures (in press) Cancer. 2010 doi: 10.1002/cncr.25738. [DOI] [PubMed] [Google Scholar]
- 60.Krzyminska S, Koczura R, Mokracka J, et al. Isolates of the Enterobacter cloacae complex induce apoptosis of human intestinal epithelial cells. Microb Pathog. 2010;49:83–9. doi: 10.1016/j.micpath.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Serpa Neto A, Bianco Rossi FM, Dal Moro Amarante R, et al. Effect of weight loss after Roux-en-Y gastric bypass, on renal function and blood pressure in morbidly obese patients. J Nephrol. 2009;22:637–46. [PubMed] [Google Scholar]
- 62.Bueter M, Ahmed A, Ashrafian H, et al. Bariatric surgery and hypertension. Surg Obes Relat Dis. 2009;5:615–20. doi: 10.1016/j.soard.2009.03.218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.