SUMMARY
Sensory axon development requires concerted actions of growth factors for the precise control of axonal outgrowth and target innervation. How developing sensory neurons integrate different cues is poorly understood. We demonstrate here that Smad1 activation is required for neurotrophin-mediated sensory axon growth in vitro and in vivo. Through differential phosphorylation, Smad1 exerts transcriptional selectivity to regulate the expression and activity of Erk1 and Erk2—two key neurotrophin effectors. Specifically, BMPs signal through carboxy-terminal phosphorylation of Smad1 (pSmad1C) to induce Erk1/2 transcription for enhanced neurotrophin responsiveness. Meanwhile, neurotrophin signaling results in linker phosphorylation of Smad1 (pSmad1L), which in turn upregulates an Erk-specific dual-specificity phosphatase, Dusp6, leading to reduced pErk1/2, and constituting a negative feedback loop to prevent axon overgrowth. Together, BMP and neurotrophin pathways are integrated in a tightly regulated signaling network with balanced ratio of Erk1/2 and pErk1/2 to direct the precise connections between sensory neurons and peripheral targets.
Keywords: BMP, Neurotrophin/NGF, Differential phosphorylation of Smad1, Sensory neuron development, Axon growth of DRG neurons, Dusp, Erk/pErk
INTRODUCTION
During sensory circuit wiring, embryonic neurons need to integrate various extracellular cues for the precise control of initiation, elongation, and branching of their axons. It remains to be clarified how multiple signaling pathways converge to collaboratively mediate axon development.
The neurotrophin family plays a central role in stimulating axonal outgrowth. Nerve growth factor (NGF), a prototypic neurotrophin, is target-derived and modulates terminal sensory axon branching. In NGF knockout mice on a Bax−/− background that prevents neuronal cell death, terminal arborization and epidermal innervation are impaired (Patel et al., 2000). Neurotrophins bind to receptor tyrosine kinases of the Trk family and signal through well-conserved effectors—the mitogen-activated protein kinase/extracellular signal regulated kinase (MAPK/Erk) and the phosphatidylinositol-3-kinase/Akt (PI3K/Akt) (Reichardt, 2006). In cultured neurons, both pathways are required for peripheral axon outgrowth, while PI3K/Akt is more involved in cell survival (Atwal et al., 2000; Kaplan and Miller, 2000). Double deletion of either Braf/Raf1 or Erk1/Erk2 results in aberrant terminal branching of sensory axons in the NGF-expressing target field in vivo (Newbern et al., 2011; Zhong et al., 2007). Although the critical roles of NGF/TrkA pathways in sensory axon outgrowth have been amply demonstrated, it is less clear, however, how expression and activity of individual signaling components are regulated to ensure proper neurotrophin responsiveness in the developing neurons. Furthermore, safeguard mechanisms to prevent axon overgrowth remain to be uncovered.
Other growth factors involved in axonal outgrowth include the bone morphogenetic proteins (BMPs). BMPs, members of the TGFβ superfamily, play a plethora of roles in neural development (Liu and Niswander, 2005). BMPs activate receptor serine/threonine kinases (BMPR) and signal through C-terminal phosphorylation of Smads (pSmadC) (Feng and Derynck, 2005; Shi and Massague, 2003). Previously, we have shown that Smad1, one of the Smads mediating BMP signaling, is developmentally regulated (Zou et al., 2009). pSmad1C accumulates in the nuclei of embryonic sensory neurons in the dorsal root ganglia (DRGs) from E10.5 to E15.5 during active axonal outgrowth. Blocking BMP signaling by pharmacological inhibition of its receptors or by RNAi-mediated Smad1 knockdown results in an arrest of axonal outgrowth in cultures (Parikh et al., 2011). In vivo evidence has yet to be established to confirm the role of Smad1 in axonal growth. Importantly, when adult DRG neurons are triggered into a regenerative state, Smad1 is induced and activated, and it enhances regeneration of sensory axons after spinal cord injury (Parikh et al., 2011).
An intriguing aspect from the previous studies is that when BMP signaling is blocked, axonal outgrowth is arrested even though neurotrophin is present in the culture media, suggesting that BMP signaling is required for neurotrophin-mediated axonal outgrowth. However, one outstanding question remains as to whether the two pathways converge on a common node or function in parallel with distinct downstream targets. Precedent exists for a collaboration between BMP and neurotrophin—e.g. BMP7 requires NGF as a cofactor to optimally induce dendritic growth in sympathetic neurons (Lein et al., 1995)—but mechanistic understanding is lacking. One possible convergence point is Smads. Besides the C-terminal phosphorylation sites, Smads also have a linker area between the MH1 and MH2 domains, containing four conserved MAPK phosphorylation sites that can be targeted by members of the MAPK family—Erk, p38, or JNK (Hough et al., 2012; Pera et al., 2003), and several GSK phosphorylation sites. Integration of dorsoventral (BMP) and anteroposterior (Wnt/GSK3) patterning gradients reportedly occurs through differential phosphorylation of Smad1 in Xenopus embryos (Fuentealba et al., 2007). The in vivo function of linker phosphorylation of Smad1 (pSmad1L) in the mammalian nervous system is not known.
Here, we tested the hypothesis that Smad1 functions as a convergence node of the BMP and neurotrophin pathways during sensory axon development. We show that Smad1 is specifically expressed in the developing peripheral sensory neurons and that it is activated at both its C-terminus and linker region. By studying cultured embryonic DRG neurons and in vivo axon growth patterns in three Smad1 mutant alleles—two conditional deletions and one linker mutation, we demonstrate that Smad1 activation is required for NGF-mediated terminal axon branching. We identify Erk1 and Erk2 as novel pSmad1C transcriptional targets and reveal a pSmad1L-Dusp-based negative feedback loop regulating the intensity of Erk1/2 signaling, thus establishing distinct in vivo roles for pSmad1C and pSmad1L during sensory neuron development. Together, the previously unrecognized collaborative molecular events of the BMP and neurotrophin pathways contribute to a tightly regulated signaling network that directs the precise connections between sensory neurons and their targets.
RESULTS
Neurotrophin-induced Axonal Outgrowth Specifically Requires BMP Signaling
To investigate how the neurotrophin and BMP pathways collaboratively mediate sensory axon development, we isolated embryonic day 12.5 (E12.5) DRGs and monitored axonal outgrowth in explant cultures in media containing NGF and an increasing concentration of dorsomorphin (DM), an inhibitor of the type I BMP receptor that blocks activation of Smad1/5/8 (Yu et al., 2008). Axonal outgrowth was inhibited by DM treatment in a dose dependent manner (Figure 1A–B). At higher dose of DM, axonal outgrowth was completely arrested, confirming the requirement of BMP signaling for NGF-mediated axonal outgrowth. The inhibitory effect of DM was reversible since DRG neurons resumed growth after DM washout (Figure 1B), indicating no permanent adverse effect from DM treatment. Similar DM-mediated inhibition of axonal outgrowth was observed in dissociated E12.5 DRG neurons plated at low density to minimize the effect of glial cells (Figure 1C).
Figure 1. BMP/Smad Signaling is Required for NGF-Dependent Axon Growth.
(A–B) E12.5 DRG explants were cultured in NGF-containing media for 48hrs with DM (1 or 5µM) or SB431542 (5µM) and axonal length was determined by Tuj1 immunostaining and averaged over 6 independent experiments (n=6). w.o.: DM wash off.
(C–D) Low-density dissociated cultures of E12.5 DRG neurons were treated with DM (1µM) for 48hrs. Quantification of the average axonal length is shown in (C) and pSmad1C immunostaining in (D). DM treatment reduced pSmad1C nuclear levels (n=3).
(E) Western blotting of DRG extracts from E12.5 embryos with antibodies indicated (Histone 4, H4, as loading control).
(F) Immunohistochemistry of P1 DRGs and quantification showed that pSmad1C was present specifically in wild-type DRGs but absent in Smad1 cKONes DRGs.
(G) E12.5 DRG explants from Smad1 cKONes or control embryos were cultured in NGF-containing media for 48 hrs and axonal length was determined by Tuj1 immunostaining and averaged over 3 independent experiments.
Scale bar: 200µm (A, G), 50µm (D), and 100µm (F)
See also Figure S1.
To examine if the requirement for NGF-mediated axonal outgrowth is specific to the BMP branch of the TGFβ superfamily, E12.5 DRG explants were treated with SB431542, a specific TGFβ signaling inhibitor, and no inhibitory effect was observed (Figure 1A–B). Application of a PKA inhibitor also did not affect axonal length (Figure S1A), as previously reported (Liu and Snider, 2001).
pSmad1C is Present Specifically in the Embryonic DRG Neurons and is Required for Neurotrophin-mediated Axonal Outgrowth
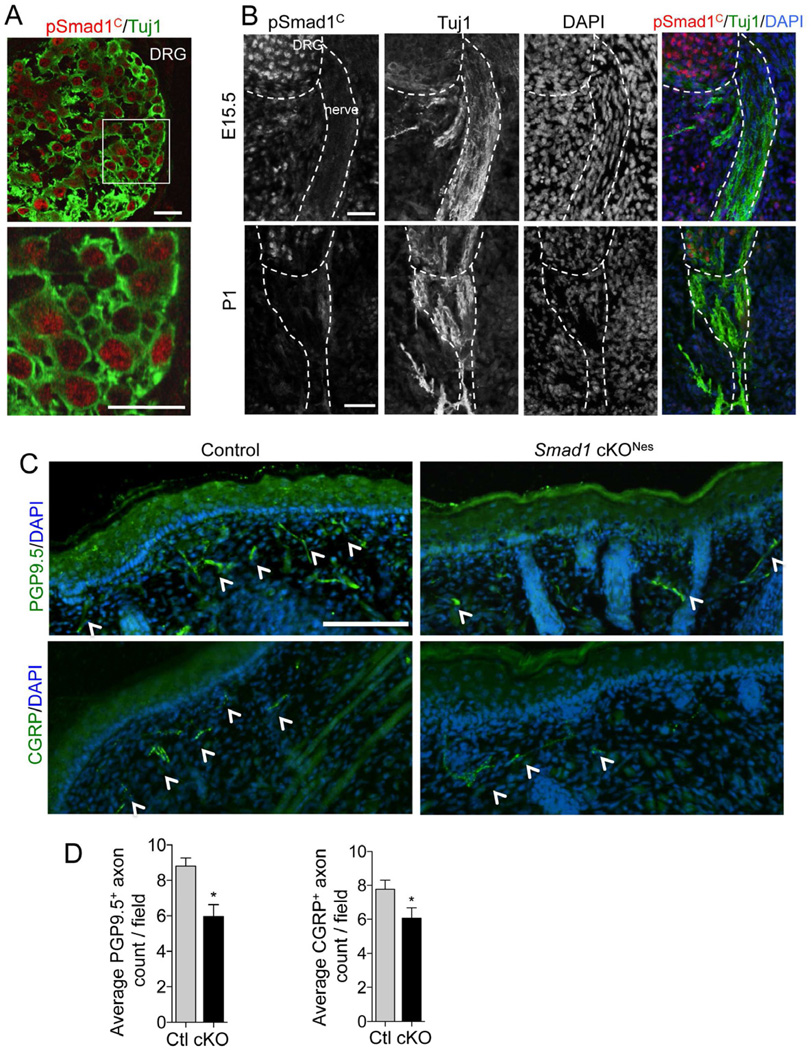
We next investigated whether it is the canonical BMP pathway signaling through pSmad1C that is involved in NGF-dependent axonal outgrowth. The axonal growth arrest with DM treatment occurs in parallel with a marked reduction of pSmad1C in embryonic DRG neurons (Figure 1D), suggesting the involvement of pSmad1C in axonal outgrowth. To rule out non-specific effects of DM and to further confirm the requirement of pSmad1C for NGF-mediated axon growth, we decided to repeat the study using E12.5 DRGs from mutant mice with a genetic deletion of Smad1. Because of early lethality of Smad1−/− embryos (Lechleider et al., 2001), conditional mice (Smad1fl/−; Nestin-Cre) were generated (hereafter referred to as Smad1 cKONes). Nestin-Cre induces recombination in neural progenitor cells, and is expected to ablate Smad1 specifically in DRG sensory neurons as early as E11.5, but sparing Schwann cells (Dubois et al., 2006; Kao et al., 2009; Tronche et al., 1999), which was confirmed using Rosa26-EYFPSTOP reporter line (Figure S1C–E). First, Western blotting confirmed that Smad1 was markedly reduced in E12.5 mutant DRGs as compared to Smad1fl/+;Nestin-Cre control littermates (Figure 1E). Immunostaining showed that pSmad1C was abundantly present in the nuclei of 95.6 ± 5.5 % of the sensory neurons in the wild-type DRGs at postnatal day 1 (P1), but not in Smad1 cKONes DRGs (Figure 1F). Notably, co-labelling with pSmad1C and β-tubulin III (Tuj1), a pan-neuronal marker, demonstrated that the pSmad1C immuno-signals were specifically detected in sensory neurons, but not in satellite glial cells in DRGs, nor in developing Schwann cells populating the peripheral nerves (Woodhoo et al., 2009) (Figure 2A–B), thus supporting a role for Smad1 in the development of sensory neurons but not Schwann cells. We then compared axonal outgrowth of E12.5 DRGs explants from Smad1 cKONes and control littermates in NGF-containing media. Mutant DRGs with Smad1 deletion grew shorter axons than wild-type DRGs, with an average axonal length of 346.6µm ± 29.0 s.e.m and 431.7µm ± 16.2 s.e.m, respectively, after 24 hrs in culture (Figure 1G). We therefore conclude that pSmad1C-mediated BMP signaling is required for NGF-dependent axonal outgrowth.
Figure 2. pSmad1C is Present Specifically in DRG Sensory Neurons and is Required for Terminal Axon Branching.
(A–B) Immunostaining of E16.5 DRGs (A, confocal images) or E15.5 and P1 DRGs and peripheral nerves (B) from wild-type mice showed that immuno-signals of pSmad1C were specifically detected in the majority of neurons but not in satellite glial cells (A) or in developing Schwann cells populating the nerves (Tuj1, green) (B).
(C–D) Representative images and quantification of cutaneous innervation of mystacial pads of P1 pups with immunostaining for PGP9.5 (green, arrows) (top panel) or CGRP (green, arrows) (bottom panel) and DAPI counterstaining. Average number of axon terminal branches in the upper dermis per field examined was calculated from five independent 20x images from each pup and then averaged over 5 pairs of littermates (D).
Scale bars: 50µm (A), 100µm (B), and 50µm (C).
See also Figure S2.
Smad1 Deletion Hinders Terminal Branching of Peripheral Axons
Next, we investigated the in vivo role of pSmad1C in sensory neuron development by first examining sensory axon projections in Smad1 cKONes mice. We focused on cutaneous innervation—a well-characterized NGF-dependent process—in mystacial pads of newborn pups by immunostaining for a pan-axonal marker, protein gene product 9.5 (PGP9.5), and a small-caliber axon marker, calcitonin gene-related peptide (CGRP). Smad1 cKONes newborn pups displayed a mild but significant truncated distal network of axons and diminished terminal arborization in the upper dermis as compared to littermate controls (Figure 2C and S2). The average number of axonal branches at the junction of upper dermis and epidermis over a similar field examined was 8.8 in control pups and 6.0 in Smad1 cKONes pups, a 32% decrease (Figure 2D). Similarly, the number of CGRP+-terminal axonal branches per similar field examined was reduced from 7.8 in control pups to 6.1 in Smad1 cKONes pups, a significant 22% decrease (Figure 2C–D). The decrease in terminal axon branches is unlikely from a reduced expression of PGP9.5 or CGRP in mutant pups as their immunoreactivity in sensory neuron soma appeared comparable and the total number of PGP9.5+ or CGRP+ neurons in P1 DRGs from the same thoracic spinal segment was also similar regardless of the genotypes (Figure 3D–E). Because the terminal axon defects were detected in P1 mice, they are more likely originated from stunted distal axonal branching than axonal retraction. The phenotypes are reminiscent of those in NGF−/−; Bax−/− or TrkA−/−; Bax−/− mice, although milder, which may be attributed to compensatory mechanisms from different Smads or other molecules involved in sensory axon growth (Patel et al., 2000).
Figure 3. BMP/Smad1 is not Required for NGF-mediated Cell Survival or Differentiation of Peripheral Sensory Neurons.
(A) Images of spinal cords of P1 Smad1 cKONes or control pups immunostained for PV or CGRP (red) and counterstained with DAPI (blue).
(B) Apoptotic sensory neurons in E15.5 DRGs were revealed by antibody against activated caspase-3 (a-casp3) and quantified.
(C) E12.5 DRG explants were cultured for 48hrs and immunostained for a-casp3. Quantification was averaged over duplicate DRGs cultures from 3 littermate pairs (n=3, left). Quantification of E12.5 DRG explants cultured for 48hrs with or without DM was shown in graph on the right (n=6).
(D–E) Sensory neuron differentiation in P1 DRGs was determined by immunostaining for the indicated markers and quantified in randomly selected fields of similar sizes from thoracic DRGs of the same spinal segments.
Scale bars: 200µm (A), 50µm (B-D-E), and 100µm (C).
See also Figure S3.
We also examined whether Smad1 is required for directing central projections of sensory axons. In Smad1 cKONes P1 pups, the small-caliber CGRP+ nociceptive axons entered the dorsal spinal laminae in a normal pattern; and the big-caliber parvalbumin (PV)+ proprioceptive afferent axons projected toward the ventral motor pools also in a normal fashion (Figure 3A). Therefore, Smad1 appears to be dispensable for the central axon projection in vivo. Taken together, Smad1 signaling is specifically required for proper NGF-mediated target innervation of sensory axons.
Smad1 is not Required for Neurotrophin-mediated Survival and Differentiation of Sensory Neurons
We next asked whether BMP/Smad1 is required for the two other key processes that are neurotrophin-dependent: survival and differentiation of sensory neurons. The survival of TrkA+ DRG neurons is dependent upon successful competition for target-derived NGF (White et al., 1996), whereas the survival of TrkC+ neurons depends on NT3 (Wyatt et al., 1999). We first assessed the extent of cell death in embryonic DRGs by immunostaining for activated-caspase 3, a marker of apoptosis. Loss of Smad1 did not have an impact on neuronal survival at E15.5, one of the peak times of apoptosis in the developing DRGs (White et al., 1996) (Figure 3B). The number of total DRG neurons (Tuj1+ or PGP9.5+) from the same thoracic spinal segment of P1 mice was also comparable regardless of the genotypes (Figure 3D). Additionally, TrkA+ small-diameter neurons and PV+ big-diameter neurons were similar in number in P1 DRGs (Figure 3E). To rule out a compensatory mechanism by other Smads, E12.5 wild-type DRG explants were cultured with NGF for 2 days with or without DM treatment, and no change in the percentage of apoptotic cells in DRG explants was found (Figure 3C). Similar results were demonstrated in E12.5 DRG explants from Smad1 cKONes or control embryos after 2 days of culture (Figure 3C).
We then investigated whether loss of Smad1 might affect the neurotrophin-dependent neuronal differentiation. We did not detect a difference in the number of TrkA+, CGRP+, or PV+ sensory neurons in P1 thoracic DRGs from mutant or control pups (Figure 3E). Together, Smad1 appears to be specifically required for neurotrophin-mediated axonal outgrowth, but not survival nor differentiation of sensory neurons in vivo.
In further support of our conclusion, we confirmed all our findings in another conditional mutant mouse, Smad1fl/+;Wnt1-Cre (Smad1 cKOWnt1). Wnt1-Cre induces recombination in premigratory pluripotent neural crest cells, at about E8.5, thus is expected to affect both Schwann cells and DRG sensory neurons (Danielian et al., 1998) (Figure S1C–E). We observed no changes in the central branch innervation, cell death, or differentiation of DRG neurons in Smad1 cKOWnt1 mice (Figure S3); whereas the average number of terminal axon branches per field inspected in mystacial pads was significantly decreased by about 43% in Smad1 cKOWnt1 pups as compared to control littermates (Figure S3B–C).
Next, we investigated whether a delay or deficit in the initial phase of axon outgrowth would be the reason for the aberrant terminal branching in mutants. Notably, early outgrowth of sensory axons in vivo is neurotrophin-independent (Davies et al., 1987; O'Connor and Tessier-Lavigne, 1999). We postulated that BMP might regulate this early process. To test this hypothesis, whole-mount immunostainings for neurofilament marker 2H3 were performed on E11.5-E12.5 embryos. Since Nestin-Cre induces recombination starting from E11.5, we therefore focused on Smad1 cKOWnt1 for evaluation of early peripheral axonal projections. In Smad1 cKOWnt1 embryos, peripheral trigeminal sensory axons initiated in correct directions and with similar axonal length as in control embryos (Figure S4A). The axonal projections to face, trunk and limb buds also exhibited similar length, fasciculation and branching patterns (Figure S4B–D). Thus, the initial axon growth did not appear to be affected in the Smad1 cKO mice. Note that even though in vivo exposure to NGF occurs much later in DRG neurons, E12.5 DRG neurons are already capable to respond to NGF in vitro. Therefore, while blocking BMP signaling in E12.5 DRG explants attenuated axon growth stimulated by exogenous NGF; early phase of axon growth in vivo appeared normal in E12.5 Smad1 cKO mutants.
pSmad1C Directly Regulates Erk1/2 Transcription in Embryonic Sensory Neurons
We next investigated the mechanism by which Smad1 is involved in the neurotrophin-dependent sensory axonal outgrowth. Since Smad1 is a transcription factor, we searched for transcriptional targets of Smad1 that may play a role in neurotrophin signaling. We took a candidate approach by analyzing mRNA levels of neurotrophin effectors. After normalized to Gapdh, a housekeeping gene, Erk1, Erk2, and TrkA were significantly down-regulated in DM-treated E12.5 DRGs, while transcription of another Trk effector, Akt, was not changed (Figure 4A). Transcripts of Rpl13a, another housekeeping gene remained unchanged, indicating that BMP inhibition does not lead to global transcriptional repression. A time course study showed that mRNAs of Erk1 and Erk2 began to decline 6 hrs after DM treatment and remained down-regulated after 48 hrs (Figure 4B). Consistent with the DM treatment results, in freshly collected DRGs from wild-type or Smad1 cKONes embryos, both Erk1 and Erk2 were reduced in mutant DRGs (Figure 4C). Conversely, BMP stimulation led to induction of Erk1/2 in cultured E12.5 DRG neurons (Figure 4D).
Figure 4. BMP pathway is involved in Regulating Erk1/2 Transcription.
(A) qRT-PCR of mRNA extracts from dissociated E12.5 DRG neurons cultured for 48hrs with or without DM (1µM). Data were normalized to Gapdh (n=6).
(B) Time-course analysis of Erk1 and Erk2 transcripts by qRT-PCR from E12.5 dissociated DRG neurons cultured with DM (1µM) for the indicated period as compared with no DM treatment (at 0 hr) (n=6).
(C) qRT-PCR of mRNA extracts from DRGs of E12.5 Smad1 cKONes or control littermates.
(D) mRNA levels of Erk1/2 from dissociated DRG treated with or without BMP (10ng/ml) for 24hrs.
(E–G) Western blots of protein extracts from dissociated E12.5 DRG neurons cultured for 48hrs in the specific neurotrophin-containing media with or without DM (1µM) (E–F) or with SB431542 (SB, 5µM) (G), with antibodies against indicated proteins (H3 as loading control). (H–I) Western blots of protein extracts of DRGs from E12.5 Smad1 cKONes (H) or Smad1 cKOWnt1 (I) littermates with antibodies against the indicated proteins showed a decrease in Erk1/2 and pErk1/2 in both mutant DRGs (H3 and H4 as loading control). Asterisks in (H) denote the Erk2 band.
See also Figure S4.
We subsequently focused on Erk1/2 regulation. Western blotting confirmed a marked decrease of Erk1/2 in DM-treated E12.5 DRG neurons and a concordant depletion of phosphorylated Erk1/2 (pErk1/2) (Figure 4E). Erk1/2 was similarly down-regulated after DM treatment in BDNF- and NT3-dependent DRG neurons (Figure 4F). In contrast, inhibiting TGFβ or PKA did not alter Erk1/2 nor pErk1/2 levels (Figure 4G and Figure S1B), in agreement with earlier findings that axonal outgrowth was unaffected by these two inhibitors. In addition, we confirmed a decrease of Erk1/2 and pErk1/2 in E12.5 DRGs from both Smad1 cKONes and Smad1 cKOWnt1 embryos as compared to controls by Western blotting (Figure 4H–I), although the decrease is to a smaller extent than in cultured DM-treated DRG neurons. This may be explained by in vivo compensatory mechanisms that maintain a tight transcriptional regulation of Erk1/2. Also notable is that the magnitude of reduction of Erk1/2 or pErk1/2 was smaller in E12.5 DRGs from Smad1 cKONes than Smad1 cKOWnt1 embryos, a finding perhaps related to an earlier Smad1 deletion in the latter.
To establish a direct link between pSmad1C and Erk1 and Erk2 transcription, analysis of the Erk1 and Erk2 promoter regions identified multiple conserved GC-rich Smad-binding elements (SBEs) within 1-kilobase upstream of the transcription start site (TSS) (Figure 5A and S5A) (Morikawa et al., 2011). Chromatin immunoprecipitation (ChIP) on freshly dissected E12.5 DRG neurons showed preferential binding of pSmad1C to the SBE-containing region of the Erk2 promoter as compared to the internal coding or promoter regions not containing SBEs (Figure 5B). pSmad1C occupancy on the SBE-containing Erk2 promoter is a direct result of BMP stimulation as we observed an 8-fold increase of pSmad1C binding in a ChIP assay performed in BMP-treated Neuro-2A cells and a concomitant 3-fold increase in Erk2 expression (Figure 5C–D). Changes of pSmad1C binding were not detected in the internal coding region of Erk2 or on the promoter of a housekeeping gene, Gapdh (Figure 5C). Similar findings were observed for Erk1 (Figure S5C–E). Collectively, our data support a direct role of BMP/pSmad1C in regulating Erk1 and Erk2 transcription, thereby sensitizing developing axons to neurotrophins (Figure 5E).
Figure 5. pSmad1C RegulatesErk1/2Transcription.
(A) Erk2 contains conserved Smad-binding elements (SBEs) in its promoter region. Human, mouse and rat Erk2 promoter sequences were aligned and one GC-rich SBE was highlighted in grey. The schematic representation of Erk2 gene shows the location of SBEs in Erk2 promoter. Only one SBE is shown here for clarity. Locations of the qPCR primer sets covering specific promoter or coding regions are shown at the bottom.
(B) Relative enrichment of ChIP values. The pSmad1/5/8C-immunoprecipitated DNA from freshly dissected E12.5 DRGs was amplified by qPCR using primer sets shown in (A) (n=3).
(C) ChIP assays with antibody against pSmad1/5/8C using Neuro-2A cells treated with BMP (50ng/ml) or DM (2µM) for 1hr before cross-linking. (n=3). Gapdh is a housekeeping gene.
(D) qRT-PCR for Erk2 expression in Neuro-2A cells treated with BMP4 (50ng/ml) or DM (2µM).
(E) Schematic model of pSmad1C, activated by BMP, drives Erk1/2 transcription, leading to enhanced NGF responsiveness of developing sensory axons.
See also Figure S5.
Neurotrophin Signaling Results in Linker Phosphorylation of Smad1 in Developing Sensory Neurons
We have so far established that pSmad1C-mediated transcriptional regulation of Erk1/2 serves to integrate BMP and neurotrophin signaling. To identify additional convergence points, particularly in light of the multiple MAPK sites in Smad1 linker region, we investigated the functional roles of pSmad1L during sensory axon development. To this end, we studied a mouse mutant that harbors phosphorylation-resistant mutations in all four MAPK phosphorylation sites (but not GSK phosphorylation sites) in the Smad1 linker region. Smad1L/L mutant mice are viable and exhibit subtle defects in gastric epithelial homeostasis (Aubin et al., 2004), but their neural development has never been studied.
We first examined whether Smad1 linker region is phosphorylated in the developing sensory neurons. Immunostaining demonstrated persistent presence of pSmad1L in the nuclei of developing DRG neurons from E12.5 to P1 but absent in the developing Schwann cells (Figure 6A and S6F–H). The pSmad1L antibody was specific because its immunoreactivity was absent in either Smad1L/L or Smad1 cKONes mutant DRGs (Figure 6A–B).
Figure 6. pSmad1L is Activated by Neurotrophins and Dampens its Signaling Intensity.
(A–B) Immunohistochemistry of pSmad1L in DRGs from P1 pups of the indicated genotypes and quantification.
(C) Immunocytochemistry of pSmad1L in dissociated E12.5 wild-type DRG neurons cultured for 24hrs with or without indicated neurotrophin. Caspase inhibitor was included in all conditions to prevent cell death. The percentage of nuclei that were pSmad1L+ was quantified from wild-type DRGs only, as no pSmad1L immuno-signals were detected in Smad1L/LDRGs.
(D) Immunocytochemistry of pSmad1/5/8C in dissociated E12.5 DRG neurons cultured with or without BMP (10ng/ml) for 24hrs. The percentage of DRG neurons with strong nuclear pSmad1C immuno-signals remained similar in Smad1L/L vs. Smad1L/+ DRGs (n=3). n.s.:non statistically significant.
(E) pSmad1C levels were not affected in P1 DRGs from Smad1L/L as shown by immunohistochemistry.
(F) qRT-PCR for expression of components of the neurotrophin pathway from E12.5 DRGs from Smad1+/+ and Smad1L/L embryos.
(G) Western blot of protein extracts from E12.5 DRGs from linker mutant or control littermate showed that levels of pErk1/2 were increased in Smad1L/L DRGs.
(H) Summary depicting that pSmad1L is involved in regulating pErk levels.
Scale bars: 50µm (A-C, E), and 100µm (D). (* p<0.05, ** p<0.01, *** p<0.001)
See also Figure S6.
To establish a direct link between neurotrophin stimulation and linker phosphorylation of Smad1, dissociated E12.5 DRG neurons were cultured in media with or without NGF for 24 hrs, caspase inhibitor being included to prevent cell death. Neurotrophin stimulation by NGF, BDNF, or NT3 indeed led to a marked increase in pSmad1L in wild-type but not Smad1L/L neurons (Figure 6C).
Linker Phosphorylation of Smad1 Functions to Attenuate Neurotrophin/Erk Signaling
We next investigated the functional role of pSmad1L in sensory neuron development. Previous studies in non-neuronal cell cultures reported conflicting results in regards to whether Smad1 linker phosphorylation antagonizes BMP activity by tethering pSmad1C in the cytoplasm (Aubin et al., 2004; Kretzschmar et al., 1997). We found that linker mutation did not impact the nuclear accumulation of pSmad1C at baseline or upon BMP stimulation in E12.5 DRG neurons (Figure 6D). Additionally, pSmad1C stability was not altered, as the steady-state level of pSmad1C was comparable in Smad1L/L and control E12.5 DRGs by WB or immuno-staining (Figure 6E and 6G).
We then considered the possibility that Smad1 requires phosphorylation at both the linker region and C-terminus for the optimal transcriptional activity of pSmad1C, as shown previously in non-neuronal cell lines (Alarcon et al., 2009; Gao et al., 2009). Since Erk1/2 are identified as pSmad1C target genes, Erk1/2 mRNA levels would serve as a read-out of the pSmad1C transcriptional activity. We therefore compared mRNA levels of Erk1 or Erk2 in E12.5 Smad1L/L and control DRGs and found no significant difference (Figure 6F). In addition, when pSmad1C activation was blocked with DM, both Erk1 and Erk2 were down-regulated by a similar magnitude in Smad1L/L and control DRG neurons (Figure S6A). Thus, linker phosphorylation of Smad1 does not affect pSmad1C transcriptional activity, at least for Erk1/2. Consistently, Erk1/2 protein levels remained unaltered in Smad1L/L DRGs (Figure 6G).
Unexpectedly, we found that pErk1/2 was elevated in E12.5 Smad1L/L DRGs compared to controls (Figure 6G), suggesting that linker phosphorylation of Smad1 may affect the balance between Erk1/2 and pErk1/2, favoring a decrease in pErk1/2. Since Erk can activate linker phosphorylation of Smad1, we hypothesize that Erk/pSmad1L may be part of a negative feedback loop that dampens the signaling intensity of the neurotrophin/Erk signaling (Figure 6H). To evaluate this model in vivo, terminal axon branches in Smad1L/L newborn pups were examined. If pSmad1L function as a brake for neurotrophin stimulation, lacking such a brake, as in Smad1L/L DRGs, would lead to exaggerated growth of terminal branches. Indeed, in mystacial pads of newborn Smad1L/L pups, even though the number of terminal axon branches at the upper dermis remained the same, a significantly more axonal branches extended beyond the upper dermis and into the epidermal layer, resulting in longer average axonal length in the cutaneous axon network (Figure 7C and S7A). This change was not due to alterations in the expression level of PGP9.5, nor an increased number of PGP9.5+ neurons, as cell differentiation and survival were unaffected (Figure S6B–E).
Figure 7. pSmad1L MediatesDusp6Expression.
(A) Neurite outgrowth assay on E12.5 DRG explants. Axonal length of Smad1L/L neurons was compared to control DRGs at 24hrs in the presence of 12.5 ng/ml of NGF with or without DM treatment (n=4).
(B) Immunocytochemistry and quantification of dissociated E12.5 DRG neurons cultured with caspase inhibitor alone or with NGF for 48hrs showed that the increased Dusp6 after NGF treatment was observed in wild-type but not Smad1L/L DRGs (n=3).
(C) Immunostaining of mystacial pads from P1 pups for PGP9.5 (green) demonstrated exaggerated growth of terminal axons beyond the upper dermis and into the epidermis (white arrows point to several examples) in Smad1L/L compared to Smad1+/+ pups. Dotted white lines depict the dermis/epidermis boundary. The average number of axon branches per field of inspection, the percentage of cutaneous axons extending beyond the upper dermis, and the mean axonal length in the terminal network of axons were quantified from five independent 20x images for each pup and average over 4 littermate pairs.
(D–E) qRT-PCR of Dusp transcript levels in E12.5 DRGs from embryos of the indicated genotypes (n=3).
(F) qRT-PCR of Dusp6 and Dusp3 transcript levels in E12.5 DRGs from Smad1+/+ or Smad1L/L embryos cultured for 48hrs in caspase inhibitor-containing media with or without NGF. Dusp6 was induced by NGF only in control DRGs.
(G) Working model. During DRG sensory neuron development, BMP activates pSmad1C to induce Erk1/2 transcription that enhances neurotrophin responsiveness; while neurotrophin activates pSmad1L to regulate Erk-specific Dusp, which dampens the NGF/Erk signaling intensity.
Scale bars: 50 mm (C); 100 mm (A,B,D,E).
See also Figure S7.
The Smad1 linker mutation is present in both neurons and glial cells, but the absence of pSmad1L in the developing Schwann cells suggests that the overgrowth of terminal axon branches is more likely associated with enhanced growth state of Smad1L/L mutant DRG neurons, perhaps due to the elevated ratio of pErk1/2 over Erk1/2. We thus conducted a neurite outgrowth assay in DRG explant cultures. With stimulation of exogenous NGF (12.5ng/ml), axonal outgrowth appeared similar regardless of the genotypes (Figure 7A). However, when the total Erk1/2 was reduced with a low dose of DM (1mM) so that pErk1/2 would not reach saturating levels, Smad1L/L DRG neurons grew longer axons as compared to controls (Figure 7A). These results provided a direct link between linker mutation and enhanced growth state of developing sensory neurons. Together, our data suggest that pSmad1L is involved in reducing pErk1/2 levels, leading to attenuated axonal outgrowth.
pSmad1L Induces Erk1/2-specific Dusps to Modulate the Intensity of Erk1/2 Signaling
We next sought to address the cause of elevation of pErk1/2 in Smad1L/L mutant DRGs. The level of Erk1/2 phosphorylation is a result of balanced activities between Erk-specific kinases and phosphatases. Because components of the NGF signaling cascade, such as TrkA, Akt, and Erk, remained unchanged in Smad1L/L DRGs (Figure 6F), we thus examined Erk-specific phosphatases. Dual-specificity (threonine/tyrosine) phosphatases (Dusps) are a subclass of phosphatases that specifically dephosphorylate MAPKs (Jeffrey et al., 2007). Among the 16 mammalian Dusps that show catalytic activity for MAPK, we examined the Dusps that have substrate preference for Erk1/2 over JNK or p38. The mRNA levels of Dusp2, 4, and 6 were significantly decreased in E12.5 Smad1L/L DRGs, whereas mRNA levels of Dusp5 and 9 remained the same (Figure 7D). Dusp3 and 8 do not act upon Erk1/2 and their mRNA levels were not affected (Figure 7D). Consistently, Dusp4 and 6, but not Dusp3, were also down-regulated in Smad1 cKONestin DRGs compared with controls (Figure 7E). Taken together, our data suggest that pSmad1L is involved in the regulation of Erk1/2-specific Dusps levels.
We subsequently focused on Dusp6 as it functions as a negative feedback regulator of FGF/Erk signaling during mouse development (Li et al., 2007). We first investigated whether neurotrophin/Erk signaling induces Dusp6. Neurotrophin stimulation of E12.5 DRGs led to a 4-fold induction of Dusp6. Importantly, the induction was abolished in Smad1L/L DRGs (Figure 7F). Dusp6 induction was evident by increased immunoreactivity in wild-type, but not Smad1L/L, DRG neurons treated with NGF (Figure 7B). Taken together, our data suggest that Dusp6 is specifically regulated in embryonic DRGs by neurotrophin-activated pSmad1L and acts as a brake to attenuate neurotrophin/Erk signaling (Figure 7G).
DISCUSSION
By studying two Smad1 cKO and Smad1L/L mutants, we have revealed novel in vivo roles of two phosphorylated forms of Smad1 during sensory axon development: pSmad1C mediating Erk1/2 transcription to sensitize neurotrophin response, and pSmad1L regulating Dusp6 induction to dampen neurotrophin signaling through a negative feedback loop mechanism. BMP and neurotrophin pathways are thus tightly integrated into a signaling network to ensure proper responses to the growth stimulation from growth factors (Graphical abstract).
Balanced Ratio of Erk1/2 and pErk1/2 in the Developing Sensory Neurons Ensures Proper Response to Neurotrophin Stimulation
Sensory neurons in DRGs are exposed to neurotrophins throughout development, which mediate their survival, differentiation and axonal outgrowth (White et al., 1996). Early axonal outgrowth is NGF-independent, while terminal arborization is regulated by target-derived NGF. En route to target fields, developing axons also encounter other members of the neurotrophin family such as BDNF and NT3. Along the axon trajectory, developing axons may encounter fluctuating concentration of neurotrophins. How to ensure proper level of neurotrophin responsiveness is an important yet unanswered question. Here, we demonstrate that in the developing DRG neurons, transcription of Erk1 and Erk2, two key neurotrophin effectors, is regulated by Smad1. Furthermore, activity of Erk1/2 is also modulated by Smad1 through Dusp-mediated dephosphorylation of Erk. Notably, the former process is controlled by pSmad1C and the latter by pSmad1L. The pSmad1L/Dusp6-based negative feedback loop mechanism may prevent large fluctuation of the signaling intensity of neurotrophin by maintaining a balanced ratio of Erk1/2 and pErk1/2 (Figure 7G). Of note, both BMP4 and BMP receptors are expressed in E12.5 DRGs Figure S7B). Our working model thus reveals the molecular basis of how the growth stimulation from neurotrophins can be constantly modulated and swiftly turned off once target innervation approaches completion.
Erk1/2 also play critical roles in developing Schwann cells (Newbern et al., 2011), however, since immunoreactivity of pSmad1C or pSmad1L is not detectable in the developing Schwann cells, regulation of Erk1 and Erk2 in these cells may rely on entirely different transcription factors. For a similar reason, a neuronal cell-autonomous effect is more likely for the phenotypes observed in Smad1 mutant mice. Two additional observations further favor a neural rather than glial defect in Smad1 mutant mice: i) Both Smad1 cKONes and Smad1 cKOWnt-1 mutants have similar phenotypes. Because Nestin-Cre leads to recombination more specifically in neurons in the DRGs, the sensory neurons therefore are the more likely cellular components responsible for the phenotypes. ii) In cultured explants of DRGs from Smad1 cKONes mice, axon outgrowth is affected. An indirect effect from glial cells remains a possibility.
Besides the well-studied NGF in the peripheral nerve system (PNS) development, other members of the neurotrophin family, BDNF in particular, play a plethora of roles in the central nervous system (CNS), ranging from axonal and dendritic growth, synaptogenesis, to learning and memory (Aguado et al., 2003; Cheng et al., 2011; Yamada et al., 2002). Whether there is a similar Smad1-based regulatory mechanism in the CNS to maintain a balanced ratio of Erk1/2 and pErk1/2 awaits future studies.
pSmad1C-mediated Erk1/2 Transcription Sensitizes Sensory Neurons to Neurotrophins
The present study extends beyond previous understanding of a modulatory role of BMP on the functions of neurotrophin in revealing pSmad1C-mediated Erk1/2 transcription as the basis for this collaboration in developing sensory neurons. Along with Erk1/2, blocking Smad1C activation results in a decrease in the transcription of other neurotrophin signaling components, such as TrkA, suggesting a broader role for Smad1 in regulating neurotrophin signaling. The functional link between Smad1 and Erk1/2 is highly significant given the prominent role of Erk1/2 in the growth and differentiation of a wide variety of cells, and that their aberrant regulation contributes to neoplastic transformation (Roberts and Der, 2007).
Here, we establish that BMP/Smad1 signaling is specifically required for NGF-mediated axon growth but not cell survival or differentiation of developing sensory neurons. CGRP expression in sensory neurons is reportedly dependent on TrkA (Snider and McMahon, 1998) and can be induced by BMP in cultured DRGs in vitro (Ai et al., 1999). However, no significant change was detected in the percentage or the total number of CGRP+ DRG neurons in Smad1 cKO pups, arguing against an indispensable role for Smad1 in mediating CGRP expression in DRG neurons in vivo, although compensatory effects from Smad5 or Smad8 remain a possibility. Indeed, Smad1, 5 and 8 are all expressed in the trigeminal sensory neurons (Ji and Jaffrey, 2012).
The phenotype of the stunted terminal axonal branches in Smad1 cKO pups is milder than in Erk1/2 knockout mice (Newbern et al., 2011), which may be attributed to a modest reduction of Erk1/2 in Smad1 cKO DRGs as compared to the dramatic reduction in cultured DM-treated DRG neurons. The difference between in vivo and in vitro results may be related to: i) a compensatory increase in transcriptional activity of other Smads or additional transcriptional factors to maintain a tight transcriptional regulation of Erk1/2 in vivo, and ii) Smad1 deletion results in not only Erk1/2 reduction, but also compromised Dusp induction, leading to a smaller reduction in pErk1/2 levels. It is also worth noting that trigeminal sensory neurons are derived from both neural crest progenitors and epithelial placodal progenitors (Streit, 2011), but Wnt1-Cre affects only the former, which may explain the partial decrease in terminal axon branches in the mutants.
A Negative Feedback Loop of Neurotrophin/Erk Signaling Relies on pSmad1L-mediated Expression of Erk-specific Dusp
Once sensory axons complete target innervation, growth signals need to be effectively switched off to avoid overgrowth. Previous studies have focused mostly on activation of the neurotrophin-Ras-Raf-MEK-MAPK cascade, with little attention paid to inactivation mechanisms. Our study uncovers a pSmad1L/Dusp-based negative feedback mechanism that enables developing sensory neurons to attenuate neurotrophin/Erk signaling intensity. Phosphatases are powerful negative regulators. In fact, the enzymatic power of a phosphatase is estimated as 100–1000 times greater than that of a kinase (Reth, 2002), and computational analyses confirmed a dominant role of Dusps over kinases on the extent of MAPK phosphorylation (Bhalla et al., 2002). Our results are in agreement with Dusp induction upon MAPK activation to dampen mitogenic signaling (Patterson et al., 2009). Moreover, a similar functional link between Smad1 and Dusp has been identified in murine embryonic stem cells for setting appropriate Erk activity level to maintain pluripotency (Li et al., 2012). Different Dusps have distinct substrate specificities, subcellular localizations, and functions (Patterson et al., 2009), and the present study suggests that Dusp6 is involved in attenuating axonal outgrowth in developing sensory neurons. Future studies will uncover the roles of each individual Dusp during neural development.
Distinct Roles of pSmad1C and pSmad1L During Sensory Axon Development
Our study has assigned distinct in vivo roles for the two phosphorylated forms of Smad1. Thus, differential post-translational modifications of the same transcription factor can lead to distinct transcription programs. This has been demonstrated for other transcription factors such as Nkx2-1 (Silberschmidt et al., 2011), Sap-1a (Janknecht and Hunter, 1997), and NF-κB (Anrather et al., 2005). In the case of NF-κB, differential phosphorylation of p65, one of the subunit of NF-κB, results in distinct gene expression profiles.
We demonstrate that pSmad1C binds to SBE on Erk1 and Erk2 promoters upon BMP stimulation. It remains to be determined whether Dusp6 induction is directly regulated by pSmad1L, and if so, whether pSmad1L and pSmad1C bind to the same or different SBEs, and collaborate with the same or distinct co-factors to mediate transcription. Indeed, precedent exists for specific recruitment of the transcriptional coactivator—the yes-associated protein (YAP)—to the phosphorylated linker region of Smad1 (Alarcon et al., 2009). YES acts downstream of the Hippo pathway to control organ size (Zhao et al., 2008). Our findings suggest versatilities of Smad1 in regulating different sets of genes through differential phosphorylation, thereby enabling neurons to respond to combinations of instructive cues in a context-specific manner.
Conclusions
We have identified novel collaborative signaling events of BMP and NGF pathways during sensory axon development. Through differential phosphorylation, Smad1 potentiates neurotrophin signaling by pSmad1C-dependent transcription of Erk1/2, and then dampens its signaling intensity by pSmad1L-mediated Dusps expression. The concerted integration of the BMP and neurotrophin pathways forms a tightly regulated signaling network key to sensory axon development.
EXPERIMENTAL PROCEDURES
Generation of Mice
Smad1flox/flox mice (Huang et al., 2002) were used to generate a heterozygous germ line deletion of Smad1 (Smad1+/−). Smad1fl/− mice were then mated to Nestin-Cre mice (Jackson Laboratory) (Tronche et al., 1999) to generate Smad fl/−; Nestin-Cre conditional mutant mice, or to Wnt1-Cre mice (Danielian et al., 1998) to generate Smad fl/−; Wnt1-Cre mice. The Smad1L/L mutant (Aubin et al., 2004) and Rosa26-EGFPSTOP report line (Srinivas et al., 2001) were obtained from the Jackson Laboratory. At least 5 littermate pairs were used for each of the phenotype studied.
DRG Cultures and Neurite Outgrowth Assay
DRGs from E12.5 embryos were dissected for explant cultures or low-density (50,000 cells/cm2) dissociated cultures in Neurobasal media supplemented with B27 (Gibco) and NGF (12.5ng/ml). The length of at least 20 axons from each explant, or over 100 dissociated neurons, was measured and averaged for each experiment. A minimum of three replicates was performed for each condition.
Chromatin Immunoprecipitation (ChIP)
ChIP-IT enzymatic kit was used per manufacturer’s instructions (Active Motif). Briefly, cross-linked chromatin from Neuro-2A cells or freshly dissected E12.5 DRGs was enzymatically digested to yield 150–200 bp fragments and incubated overnight with magnetic beads plus 5 mg of pSmad1/5/8C (Cell Signaling, see Table S1) antibody or water at 4°C. This was followed by crosslinking reversal, proteinase K digestion, DNA extraction (PCR purification kit, Qiagen), and qPCR with primer sets listed in Table S2. The specific binding of pSmad1/5/8C to promoters was calculated by subtracting the noise determined by water and then normalized to diluted input (1:10). The relative enrichment was then calculated as arbitrary units relative to the ChIP value at the coding region.
Statistical Analysis
Prism Graphpad software was used for Student’s t-test, one-way ANOVA, or two-way ANOVA, followed by Bonferroni’s post hoc test for multiple comparisons. Data were presented as mean ± SEM. (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001).
Supplementary Material
HIGHLIGHTS.
-
*
BMP and Neurotrophin signaling converge on Smad1 in developing sensory neurons.
-
*
Smad1 through differential phosphorylation regulates balanced ratio of Erk/pErk.
-
*
pSmad1C regulates Erk1/2 transcription for enhanced neurotrophin responsiveness.
-
*
A pSmad1L-Dusp-based negative feedback loop regulates MAPK/Erk signaling intensity.
ACKNOWLEDGEMENTS
We thank Yuhan Hao and Dr. Trent Watkins for maintaining mouse colonies, and lab members for comments on the manuscript. This work was supported by the Whitehall, Craig H. Neilsen, and Irma T. Hirschl / Monique Weill-Caulier Foundations as well as NINDS NS073596 (H.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− co-transporter KCC2. Development. 2003;130:1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- Ai X, Cappuzzello J, Hall AK. Activin and bone morphogenetic proteins induce calcitonin gene-related peptide in embryonic sensory neurons in vitro. Mol Cell Neurosci. 1999;14:506–518. doi: 10.1006/mcne.1999.0798. [DOI] [PubMed] [Google Scholar]
- Alarcon C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139:757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anrather J, Racchumi G, Iadecola C. cis-Acting element-specific transcriptional activity of differentially phosphorylated nuclear factor-κB. Journal of Biological Chemistry. 2005;280:244–252. doi: 10.1074/jbc.M409344200. [DOI] [PubMed] [Google Scholar]
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–277. doi: 10.1016/s0896-6273(00)00035-0. [DOI] [PubMed] [Google Scholar]
- Aubin J, Davy A, Soriano P. In vivo convergence of BMP and MAPK signaling pathways: impact of differential Smad1 phosphorylation on development and homeostasis. Genes Dev. 2004;18:1482–1494. doi: 10.1101/gad.1202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla US, Ram PT, Iyengar R. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science. 2002;297:1018–1023. doi: 10.1126/science.1068873. [DOI] [PubMed] [Google Scholar]
- Cheng P-L, Song A-H, Wong Y-H, Wang S, Zhang X, Poo M-M. Self-amplifying autocrine actions of BDNF in axon development. Proceedings of the National Academy of Sciences. 2011;108:18430–18435. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Davies AM, Lumsden AGS, Rohrer H. Neural crest-derived proprioceptive neurons express nerve growth factor receptors but are not supported by nerve growth factor in culture. Neuroscience. 1987;20:37–46. doi: 10.1016/0306-4522(87)90004-2. [DOI] [PubMed] [Google Scholar]
- Dubois NC, Hofmann D, Kaloulis K, Bishop JM, Trumpp A. Nestin-Cre transgenic mouse line Nes-Cre1 mediates highly efficient Cre/loxP mediated recombination in the nervous system, kidney, and somite-derived tissues. genesis. 2006;44:355–360. doi: 10.1002/dvg.20226. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in TGF-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating Patterning Signals: Wnt/GSK3 Regulates the Duration of the BMP/Smad1 Signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Alarcon C, Sapkota G, Rahman S, Chen PY, Goerner N, Macias MJ, Erdjument-Bromage H, Tempst P, Massague J. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol Cell. 2009;36:457–468. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough C, Radu M, Doré JJE. TGF-Beta Induced Erk Phosphorylation of Smad Linker Region Regulates Smad Signaling. PLoS ONEs. 2012;7:e42513. doi: 10.1371/journal.pone.0042513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janknecht R, Hunter T. Activation of the Sap-1a transcription factor by the c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase. Journal of Biological Chemistry. 1997;272:4219–4224. doi: 10.1074/jbc.272.7.4219. [DOI] [PubMed] [Google Scholar]
- Jeffrey KL, Camps M, Rommel C, Mackay CR. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov. 2007;6:391–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- Ji S-J, Jaffrey SR. Intra-axonal translation of SMAD1/5/8 mediates retrograde regulation of trigeminal ganglia subtype specification. Neuron. 2012;74:95–107. doi: 10.1016/j.neuron.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S-C, Wu H, Xie J, Chang C-P, Ranish JA, Graef IA, Crabtree GR. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science. 2009;323:651–654. doi: 10.1126/science.1166562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massagu J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- Lechleider RJ, Ryan JL, Garrett L, Eng C, Deng C, Wynshaw-Boris A, Roberts AB. Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion. Dev Biol. 2001;240:157–167. doi: 10.1006/dbio.2001.0469. [DOI] [PubMed] [Google Scholar]
- Lein P, Johnson M, Guo X, Rueger D, Higgins D. Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron. 1995;15:597–605. doi: 10.1016/0896-6273(95)90148-5. [DOI] [PubMed] [Google Scholar]
- Li C, Scott DA, Hatch E, Tian X, Mansour SL. Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development. 2007;134:167–176. doi: 10.1242/dev.02701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Fei T, Zhang J, Zhu G, Wang L, Lu D, Chi X, Teng Y, Hou N, Yang X, et al. BMP4 signaling acts via dual-specificity phosphatase 9 to control ERK activity in mouse embryonic stem cells. Cell stem cell. 2012;10:171–182. doi: 10.1016/j.stem.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- Morikawa M, Koinuma D, Tsutsumi S, Vasilaki E, Kanki Y, Heldin C-H, Aburatani H, Miyazono K. ChIP-seq reveals cell type-specific binding patterns of BMP-specific Smads and a novel binding motif. Nucleic Acids Research. 2011 doi: 10.1093/nar/gkr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern JM, Li X, Shoemaker SE, Zhou J, Zhong J, Wu Y, Bonder D, Hollenback S, Coppola G, Geschwind DH, et al. Specific functions for ERK/MAPK signaling during PNS development. Neuron. 2011;69:91–105. doi: 10.1016/j.neuron.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor R, Tessier-Lavigne M. Identification of maxillary factor, a maxillary process-derived chemoattractant for developing trigeminal sensory axons. Neuron. 1999;24:165–178. doi: 10.1016/s0896-6273(00)80830-2. [DOI] [PubMed] [Google Scholar]
- Parikh P, Hao Y, Hosseinkhani M, Patil SB, Huntley GW, Tessier-Lavigne M, Zou H. Regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proceedings of the National Academy of Sciences. 2011;108:E99–E107. doi: 10.1073/pnas.1100426108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TD, Jackman A, Rice FL, Kucera J, Snider WD. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo . Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003;17:3023–3028. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3:1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Silberschmidt D, Rodriguez-Mallon A, Mithboakar P, Cali G, Amendola E, Sanges R, Zannini M, Scarfo M, De Luca P, Nitsch L, et al. In vivo role of different domains and of phosphorylation in the transcription factor Nkx2-1. BMC Developmental Biology. 2011;11:9. doi: 10.1186/1471-213X-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin C-S, William C, Tanabe Y, Jessell T, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Developmental Biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A. In: The cranial sensory nervous system: specification of sensory progenitors and placodes. StemBook Girard L., editor. Harvard Stem cell Institute; 2011. [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- White FA, Silos-Santiago I, Molliver DC, Nishimura M, Phillips H, Barbacid M, Snider WD. Synchronous onset of NGF and TrkA survival dependence in developing dorsal root ganglia. The Journal of Neuroscience. 1996;16:4662–4672. doi: 10.1523/JNEUROSCI.16-15-04662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhoo A, Alonso MBD, Droggiti A, Turmaine M, D'Antonio M, Parkinson DB, Wilton DK, Al-Shawi R, Simons P, Shen J, et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci. 2009;12:839–847. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt S, Middleton G, Doxakis E, Davies AM. Selective regulation of trkC expression by NT3 in the developing peripheral nervous system. The Journal of Neuroscience. 1999;19:6559–6570. doi: 10.1523/JNEUROSCI.19-15-06559.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Mizuno M, Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sciences. 2002;70:735–744. doi: 10.1016/s0024-3205(01)01461-8. [DOI] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Li X, McNamee C, Chen AP, Baccarini M, Snider WD. Raf kinase signaling functions in sensory neuron differentiation and axon growth in vivo. Nat Neurosci. 2007;10:598–607. doi: 10.1038/nn1898. [DOI] [PubMed] [Google Scholar]
- Zou H, Ho C, Wong K, Tessier-Lavigne M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci. 2009;29:7116–7123. doi: 10.1523/JNEUROSCI.5397-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.