Abstract
HIV-infected individuals are significantly more susceptible to tuberculosis (TB) than uninfected individuals. Although it is established that HIV reduces Mycobacterium tuberculosis–specific T cell responses, the causes of this dysfunction are not known. We used the cynomolgus macaque model of TB to demonstrate that ex vivo SIV reduces the frequency of M. tuberculosis–specific TNF and IFN-γ–producing T cells within 24 h after infection. In vivo, T cell IFN-γ responses in granulomas from animals with SIV/M. tuberculosis coinfection were lower than SIV-negative animals with active TB. The SIV effects on the inhibition of T cell responses were primarily on APCs and not the T cells directly. Specifically, reductions in the frequency of TNF-producing M. tuberculosis–specific CD4 T cells were caused, at least in part, by SIV-induced production of monocyte derived IL-5.
Introduction
Tuberculosis (TB) is one of the most common causes of death in HIV-infected persons worldwide, and HIV is a significant risk factor for development of TB (1, 2). The mechanisms responsible for the increased susceptibility of HIV-infected persons to Mycobacterium tuberculosis are not currently known, but multiple hypotheses have been proposed (3–6), with the most obvious being loss of M. tuberculosis–specific CD4 T cells. HIV-induced manipulation of M. tuberculosis–specific effector T cell function and inhibition of the ability of macrophages to kill M. tuberculosis are two possible additional contributors to this increased susceptibility to TB (5, 6).
Coinfected individuals have significantly fewer proliferating or cytokine-producing peripheral M. tuberculosis–specific T cells compared with individuals with active TB alone (7–10). HIV decreases IFN-γ mRNA production and proliferation by M. tuberculosis–specific T cells in the airways of patients with AIDS and TB compared with those with active TB without AIDS (11, 12). HIV also reduces IFN-γ+TNF+IL-2+ polyfunctional bacillus Calmette–Guérin (BCG)-specific CD4 T cells within the airways of BCG-vaccinated individuals relative to HIV-uninfected controls (13). These studies demonstrate that HIV can reduce protection against M. tuberculosis by impairing T cell functions, but they do not address the timing of these events, mechanisms of inhibition, or whether the inhibition occurs within granulomas.
Previous studies have demonstrated that SIV infects cells in the granulomas of coinfected macaques (3), and HIV is present at sites of active TB in humans (14–16). Granulomas contain many potential targets for manipulation by HIV, including M. tuberculosis–specific T cells and macrophages; therefore, causes for dysfunction in HIV-coinfected individuals could include HIV-induced T cell exhaustion and death (17) or disruption of macrophage function (18). To elucidate possible mechanisms for the HIV-induced increased susceptibility to TB, we developed a model of SIV/M. tuberculosis coinfection using latently infected cynomolgus macaques (3, 19). We found that changes in peripheral T cell counts during acute SIV infection correlated with the reactivation of latent TB (3), suggesting that reactivation is strongly influenced by events during the acute phase of HIV infection. Based on these findings, we hypothesize that HIV disrupts M. tuberculosis–specific T cell function within granulomas shortly after HIV infection, diminishing immune pressure on the bacteria and enabling reactivation.
We developed in vitro and ex vivo systems using cynomolgus macaques with TB as an extension to these studies to investigate mechanisms of M. tuberculosis–specific T cell dysfunction following HIV infection. In this work, we used T cells and monocytes from SIV-uninfected monkeys with TB to demonstrate that exposing monocytes to SIV significantly decreases TNF and IFN-γ production by M. tuberculosis–specific T cells in an IL-5–dependent manner. Moreover, we identified SIV-stimulated monocytes as the source of the IL-5. These data provide evidence for a novel role for IL-5 in inhibition of M. tuberculosis–specific T cell function, and provide additional insight into the complexities of M. tuberculosis/HIV coinfection.
Materials and Methods
Ethics statement
All experimental manipulations and protocols were approved by the University of Pittsburgh School of Medicine Institutional Animal Care and Use Committee. The animals were housed and maintained in accordance with standards established in the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals.
M. tuberculosis infection
Cynomolgus macaques were infected with ∼25 or ∼200 CFU of Erdman strain M. tuberculosis via intrabronchial instillation as described previously (20) for other ongoing studies. The infection status of the monkeys (active versus latent disease) used as sources for cells did not lead to measurable differences in the outcomes of these experiments. PBMCs and thoracic lymph node cells from of monkeys with active or latent TB were used for in vitro studies. PBMCs were isolated via Percoll gradient centrifugation as described previously (20–22). Animals were humanely euthanized, and necropsies were performed as previously described (20–22). Thoracic lymph nodes from M. tuberculosis–infected monkeys were homogenized into single-cell suspensions with Medimachines (BD Biosciences, San Jose, CA) as described previously (20–22).
Flow cytometry
All Abs used for flow cytometry were direct conjugates against human proteins and obtained from BD Biosciences unless otherwise noted. Staining procedure was described previously (19). Approximately 1 × 106 PBMCs or tissue cells were stained using combinations of the following Abs to identify T cell phenotypes: CD3 (clone SP34-2), CD4 (clone L200), CD8 (clone DK25 [Dako; Carpinteria, CA], clone SK-1, clone OKT8 [eBioscience; San Diego, CA]), IFN-γ (clone B27; BD Bioscience), IL-2 (clone MQ1- 17H12; eBioscience), IL-5-FITC (clone JES1-3910), and TNF (clone mab11; eBioscience). Monocytes were identified using CD14-Pacific Blue (clone M5E2; BD Pharmingen). HLA-ABC-PE (clone G46-2.6; BD Pharmingen), HLA-DP, DR, DQ-FITC (clone Tu-39; BD Pharmingen), CD154-APC (clone 24.31; eBioscience), CD40-PE-cy7 (clone 5C3; BD Pharmingen) and CD80-Alexa Fluor 700 (clone L307.4; BD Pharmingen) were measured on the CD14+ monocytes. Cell phenotypes were read with an LSR II flow cytometer (BD Biosciences), and positively stained populations were gated using fluorochrome-matched isotype Abs as negative controls using the FlowJo software package (Tree Star, Ashland, OR). Gating strategies are presented in Supplemental Fig. 1.
Magnetic separation
Anti-CD3 and anti-CD14 magnetic beads (Miltenyi Biotec, Auburn, CA) were used to separate CD3+ T cells and CD14+ monocytes from PBMCs, according to the manufacturer’s instructions. Isolated monocytes were cultured at a density of 0.5–1.2 × 106 cells/ml in RPMI 1640 media supplemented with 10% human AB serum (Gemini Bio-Products, West Sacramento, CA), 1% l-glutamine (Sigma-Aldrich, St. Louis, MO), and 1% HEPES (Sigma-Aldrich; R-10 media). Cultures were established in a 5-ml polypropylene round-bottom tube (Becton Dickinson), 24-well flat bottom plate or 96-well round-bottom plate (Becton Dickson), depending on the assay. Isolated CD3 T cells were resuspended in supplemented RPMI 1640 plus 10% human AB serum at a density of 5 × 106 cells/ml in a 24-well flat-bottom plate. Cells were incubated overnight at 37°C with 5% CO2 before adding back to the CD3-depleted PBMCs or monocytes (see below).
PBMC stimulations
Freshly isolated PBMCs were resuspended in supplemented R-10 media at a density of 5 × 105 to 1 × 106 cells/ml. Half of the PBMCs were inoculated with SIVmac251 (MOI 1, based on monocyte and CD4 T cell counts in PBMCs), and the other half were incubated with media. After an overnight incubation, the cells were washed and transferred to FACS tubes for stimulation with M. tuberculosis culture filtrate protein-10 (CFP10; overlapping peptide pools; 10 μg/ml), Pneumocystis jiroveci kexin (overlapping peptide pools; 10 μg/ml) or PHA (10 μg/ml) for 5–6 h at 37°C at 5% CO2 (Supplemental Fig. 2A). Brefeldin A was added to the cells after 1 h stimulation, and the cells were incubated for an additional 4–5 h. Cells were washed and stained after stimulation.
In a subset of experiments, T cells were removed from PBMCs to determine whether SIV was affecting the T cells or APCs directly (Supplemental Fig. 2B). T cells were magnetically separated from PBMCs. Half of the T cells and T cell depleted–PBMCs were inoculated with SIV (multiplicity of infection [1.0], based on monocyte counts) and incubated overnight. These cells were used to establish three treatment groups: SIV-infected T cells added to uninfected T cell–depleted PBMCs, uninfected T cells added to SIV-infected T cell–depleted PBMCs, and uninfected T cells added to uninfected T cell–depleted PBMC. Cells were subsequently stimulated with CFP10 and stained as above.
T cell–depleted PBMCs and T cell stimulation
T cell–depleted PBMCs were incubated for 24 h with M. tuberculosis (MOI 0.5) in 500 μl supplemented R-10 media, then washed and resuspended in 500 μl fresh media (Supplemental Fig. 2C). Aliquots of M. tuberculosis–infected cells were incubated with SIVmac251 (MOI 1.0) for 12–16 h. Other aliquots of T cell–depleted PBMCs were used as controls and were infected only with SIV or M. tuberculosis. Trypan blue exclusion indicated there was not a difference in viability between SIV-infected and SIV-uninfected cells during this incubation. The T cells were added back to the T cell–depleted PBMCs and resuspended in 250 μl of R-10 media containing brefeldin A (GolgiPlug; BD Bioscience) for 5–6 h. After stimulation, cells were washed and stained for flow cytometry.
M. tuberculosis infection of monocytes and T cell stimulation
Isolated CD14+ monocytes isolated from PBMCs were incubated overnight in R-10 media in 5-ml round-bottom tubes overnight (Supplemental Fig. 2C). Monocytes were aliquoted into four treatment groups: media, SIV, M. tuberculosis, and M. tuberculosis plus SIV. T cells were also isolated from PBMCs and incubated in R-10 media until monocyte infections were complete and T cell stimulation could commence. Two groups of monocytes were infected with M. tuberculosis (MOI 0.5) in 500 μl R-10 media for 4 h, and then 1 ml of media was added to each tube and the cells were incubated overnight. Cells were washed with warm R-10 media after overnight incubation to remove M. tuberculosis from media. Cells were incubated with SIVmac251 (MOI 1.0) or with media alone overnight (12–16 h). The next morning, the cells were washed with RPMI 1640 and autologous T cells were added to the monocytes at a 3:1 T cell:monocyte ratio in 250 μl of media with brefeldin and incubated for 5–6 h. After stimulation, cells were washed and stained for flow cytometry.
To compare the effects of inactivated and live virus, we added ∼0.1 pg p28 equivalents of inactivated SIVmac251 Aldrithiol-2 per 1 × 106 monocytes (courtesy Dr. Jeff Lifson, AIDS and Cancer Virus Program, Frederick, MD) to M. tuberculosis–infected monocytes.
Monocyte-derived macrophages
Macrophages were differentiated from PBMC-isolated monocytes plated in a concentration of 1 × 106 monocytes per well in R-10 media supplemented with 1% w/v l-pyruvate and 1000 U/ml GM-CSF. Media was changed every 2–3 d for a total 7 d of culture.
Lymphocyte proliferation assay
PBMCs from M. tuberculosis–infected monkeys were isolated and suspended in AIM V media (Life Technologies, Grand Island, NY) at 200,000 cells/well in 200 μl. Cells were stimulated with PHA (5 μg/ml), CFP10, or media in triplicate for 60 h at 37°C, 5% CO2 with or without SIV; for the final 18 h, [3H]-thymidine 1 μCi/well (GE Healthcare, Piscataway, NJ) was added. Cells were harvested onto filters, and thymidine incorporation into proliferating cells was measured. Data were reported as a stimulation index (SI) calculated as fold increase in counts per minute (cpm) over unstimulated controls.
Multiplex analysis of cytokines
Isolated monocytes (4 × 106 to 6 × 106 cells) were divided into four groups: media only, infected with SIV, infected with M. tuberculosis, infected with M. tuberculosis plus SIV (Supplemental Fig. 3A). The media was removed after 20–24 h, filtered with a 0.45 μM syringe filter (Millipore, Billerica, MA) and frozen until analysis. A 23-plex nonhuman primate Luminex assay (Millipore) was performed on the filtered supernatants following manufacturer’s instructions. Proteins analyzed were soluble CD40L, G-CSF, GM-CSF, IFN-γ, IL-12/23 (p40), IL-13, IL-15, IL-17, IL-18, IL-1ra, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, MCP-1, MIP-1α, MIP-1β, TGF-α, TNF, and VEGF. Samples were read with a Luminex 100 IS Bio-Plex System machine (Luminex Corporation, Austin, TX).
Neutralization of IL-5 and IL-13
Monocytes were infected with M. tuberculosis and then SIV, as above. Neutralizing Abs to IL-5 (40 pg/ml per 1 × 106 cells; eBioscience), IL-13 (64 pg/ml per 1 × 106 cells; Miltenyi Biotec) or control isotype Abs were added during SIV infection (Supplemental Fig. 3B). Monocytes were incubated for 12–16 h at 37°C in 5% CO2 then washed with warm RPMI 1640. Autologous T cells were added to the monocytes and analyzed as above.
Addition of recombinant IL-5 and IL-13
Recombinant human IL-5 (PeproTech, Rocky Hill, NJ; 40 pg/ml per 1 × 106 cells), human IL-13 (Miltenyi Biotec; 60 pg/ml per 1 × 106 cells) or media were added to the M. tuberculosis–infected monocytes in 50 μl of R-10 media and incubated overnight at 37°C in 5% CO2 (Supplemental Fig. 3C). Autologous T cells were added to the monocytes and analyzed as above. The amount of recombinant protein added to the wells was equivalent to the highest amount of IL-5 or IL-13 produced by coinfected monocytes.
IFN-γ ELISPOT assay
Cells from granuloma-containing thoracic lymph nodes from SIVmac251 coinfected macaques and macaques with active TB without SIV that were obtained in previously published studies (3, 19) were used in IFN-γ ELISPOT assays as described previously (3, 23, 24). Duplicate wells containing 100,000 cells per well were stimulated with peptide pools (overlapping 20-mers; Sigma-Genosys, Woodlands, TX) including CFP10 (10 μg/ml) and ESAT-6 (10 μg/ml) or phorbol 12,13-dibutyrate with ionomycin (50 nM and 10 μM final concentration, respectively; Sigma-Aldrich) as positive controls. Media-only wells were used as negative controls. Cells incubated with Ag for two days at 37°C/5% CO2 and developed (3, 23) and read with an ELISPOT plate reader (Cellular Technology LTD, Cleveland, OH). ELISPOT data were normalized and expressed as spot-forming units per 106 cells.
RNA isolation and IL-5 detection in monocytes
RNA was prepared using Trizol Reagent (Life Technologies, Grand Island, NY) from isolated monocytes (4 × 106 cells) from four groups as above (Supplemental Fig. 3). RT-PCR was performed using primers for IL-5 (forward: 5′-GAGACCTTGGCACTGCTTTC-3′; and reverse: 5′-ACTCTCCGCCTTTCTTCTCC-3′) or β-actin (forward: 5′-CGACAGGATGCAGAAGGAGA-3′; and reverse: 5′-GAAGGGCCAGACTCGTCATA-3′; generated from rhesus macaque cDNA sequence) with AMV RT (Promega, Madison WI) in the following program: 42°C (30 min); 94°C (5 min); 94°C (30 s), 55°C (30 s), 72°C (30 s) for 30 cycles; and 72°C (5 min). RNA isolated from PBMCs stimulated with phorbol 12,13-dibutyrate and ionomycin for 8 h was used as the positive control. PCR products were run on a 2% agarose gel with 5× loading buffer (Bio-Rad, Hercules, CA) along with a 100 bp m.w. ladder (Bio-Rad) and then imaged. PCR product identity was confirmed by sequencing (data not shown).
Statistics
Wilcoxon ranked paired test was used to compare the results of in vitro experiments, with p < 0.05 considered significant. Mann–Whitney analysis was used to compare two different groups with significance set at p < 0.05. Kruskal–Wallis (ANOVA) multiple comparison test with Dunn’s posttest were used to compare differences among three unpaired groups. Freidman test was used with Dunn’s multiple comparison test to determine significance among three or more paired groups, with significance set at p < 0.05.
Results
SIV impairs TNF production in M. tuberculosis–specific T cells indirectly by manipulating APCs
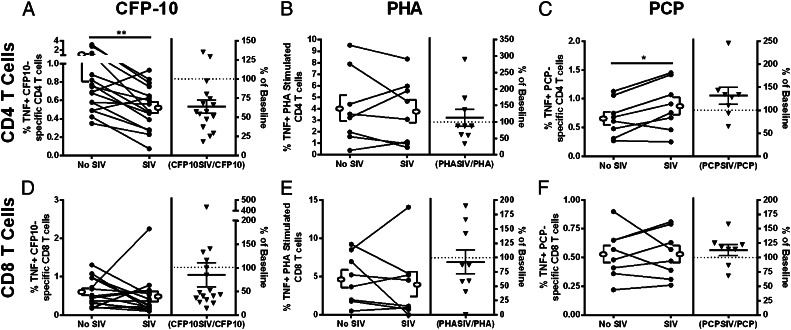
HIV can reduce TNF production in BCG- and M. tuberculosis–specific T cells in coinfected individuals (10, 13, 25). To determine whether SIV has a similar effect on M. tuberculosis–specific macaque T cells, we performed experiments in which we added SIV to isolated PBMCs or thoracic lymph node cells and measured TNF production after stimulation in vitro. The addition of SIVmac251 to macaque PBMCs did not modify T cell proliferation (data not shown), but it significantly reduced the percentage of TNF producing CFP10-stimulated CD4 T cells (Fig. 1A; Supplemental Figs. 1, 2A). SIV increased TNF production in Pneumocystis-stimulated CD4 T cells (Fig. 1C). but did not significantly reduce TNF production in CFP10-specific CD8 T cells (Fig. 1D), PHA-stimulated (Fig. 1B, 1E) T cells or Pneumocystis-specific (Fig. 1F) CD8 T cells.
FIGURE 1.
SIV reduces the frequency of TNF-producing CFP10-specific CD4 T cells. PBMCs were stimulated with M. tuberculosis–specific Ags (CFP10; A and D), with PHA (B and E) or Pneumocystis jirovecii kexin (PCP; C and F). TNF expression in CD4 (A–C) and CD8 (D–F) T cells was measured by flow cytometry. SIV caused significant decreases in the frequency of TNF-expressing CFP10-specific CD4 T cells [n = 16 animals; (A)] and increases in P. jirovecii–specific CD4 T cells (n = 8 animals; C). No change was observed in PHA-stimulated CD4 T cells [n = 9 animals; (B)] or CD8 T cells (D–F). Open ellipse and bracket to the left and right of the line graphs represent mean ± SEM. The column on the right side of each graph indicates the percent difference in responses from PBMCs incubated with SIV compared with PBMCs without SIV (triangles; line represents mean ± SEM percentage). The dotted line represents the baseline cytokine response in T cells from PBMCs without SIV. Wilcoxon matched-pairs signed rank test was used with significance set at p < 0.05. *p < 0.05, **p < 0.01.
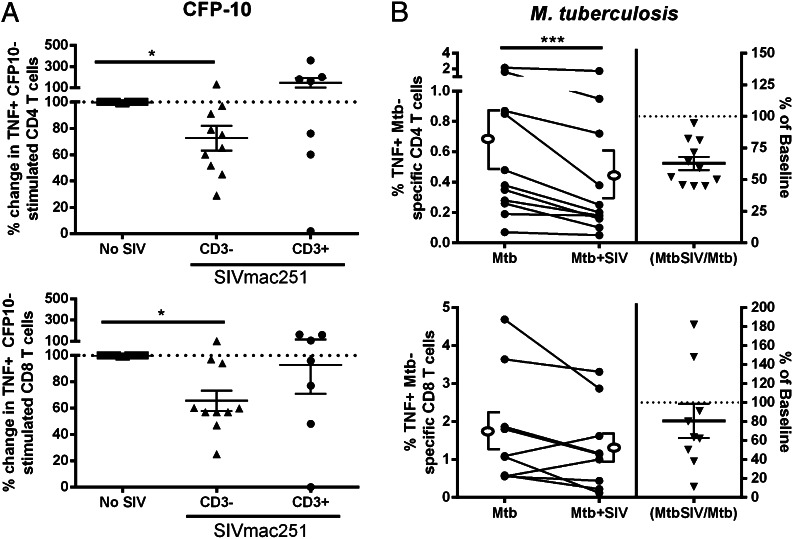
To address whether reductions in TNF production were caused by SIV manipulation of APCs or T cells, we performed the same assay on PBMCs depleted of T cells prior to SIV infection (Supplemental Fig. 2B). We found that adding the SIV-infected fraction (APCs) to autologous T cells impaired CFP10-specific TNF production (Fig. 2A). Exposing T cells to SIV did not lead to reductions in frequencies of TNF-producing M. tuberculosis–specific CD4 or CD8 T cells when these cells were incubated with SIV-uninfected APCs (Fig. 2A). This finding suggested that SIV was not directly affecting the T cell population, but instead was manipulating cells in the APC fraction, resulting in altered cytokine production by M. tuberculosis–specific CD4 T cells. To more closely approximate the situation in vivo, we assessed whether SIV could inhibit T cell responses to M. tuberculosis–infected cells, rather than peptide pulsed cells (Supplemental Fig. 2C). In results that were similar to our peptide-based findings (Fig. 1A) both in the trend and the magnitude of change, we found that incubating T cell–depleted PBMCs with SIV reduced the frequency of autologous TNF-producing CD4 T cells, but not CD8 T cells (Fig. 2B). There was not a reduction in T cell or APC numbers in cultures incubated with SIV relative to SIV-uninfected cells; therefore, the impaired cytokine responses do not appear to be related to SIV-induced cell death (data not shown). Interestingly, we found similar production of TNF by CD4 T cells in both CFP10-stimulated (Fig. 1A) and M. tuberculosis–infected monocytes (Fig. 2B). The similar number of M. tuberculosis– and CFP10-specific T cell responses might also result from peptides being more easily presented by macrophages than whole bugs, which must be engulfed and processed by the APC prior to presentation.
FIGURE 2.
Reductions in the frequency of TNF-producing peripheral T cells is caused by SIV-manipulating, T cell–depleted-PBMCs. (A) SIV-incubated, T cell–depleted PBMCs (CD3−) had lower frequencies of TNF-producing CD4 (CD3−; n = 10 animals) and CD8 (CD3−; n = 10 animals) T cells. Incubation of T cells with SIV did not reduce frequencies of TNF+ CD4 (CD3+, n = 7 animals) or CD8 (CD3−; n = 7 animals) T cells. The dotted line represents baseline TNF production by T cells without added SIV (No SIV; n = 10 animals). Mean ± SEM are included in each of the columns. Kruskal–Wallis with Dunn’s multiple comparison test with significance set at p < 0.05. (B) TNF expression by CD4 and CD8 T cells incubated with CD3-depleted PBMC that has been infected with M. tuberculosis and SIV (Mtb+SIV) without SIVmac251 (Mtb). SIV reduced TNF production in CD4 T cells (n = 11 animals). Open ellipse and bracket to the left and right of the line graphs represent mean ± SEM. Data presented in (B) are the same as Fig. 1. Wilcoxon matched-pairs signed rank test was used with significance set at p < 0.05. The p values that indicate significance are represented. *p < 0.05, ***p < 0.005.
Interaction between SIV and M. tuberculosis–infected monocytes decreases the frequency of TNF+ and IFN-γ+ CD4 T cells
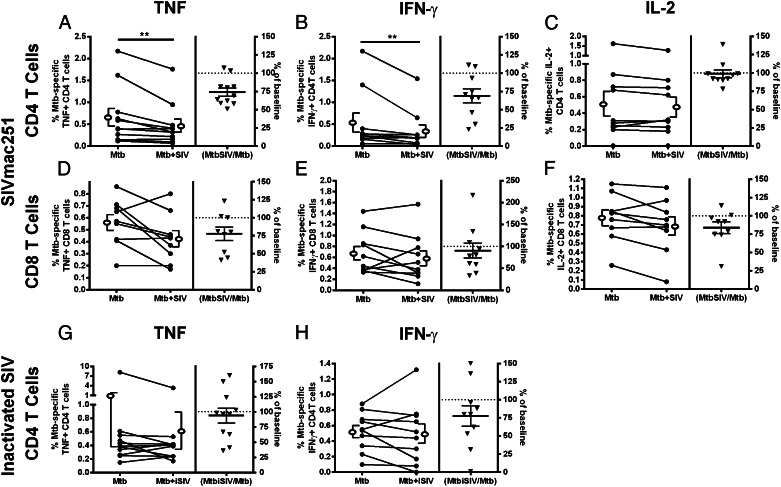
To determine whether monocytes were the cells affected by SIV, we separated CD14+ monocytes and CD3+ T cells from PBMCs, infected the monocytes with M. tuberculosis alone or with M. tuberculosis and SIVmac251, and measured the responses of M. tuberculosis–specific T cells (Supplemental Fig. 2C). Coinfecting monocytes for the time period we used for these studies did not result in decreased viability relative to monocytes infected with M. tuberculosis alone (data not shown). SIV infection did not change the expression of costimulatory molecules (CD40, CD40L, and CD80) or MHC (HLA-A, -B, -C or HLA-DR, -DQ, -DP) molecules on monocytes (data not shown), indicating that any observed changes in T cell cytokine expression were not attributable to deficits in costimulatory molecule or MHC expression. Coculturing T cells with M. tuberculosis/SIV coinfected monocytes led to reduced frequencies of TNF and IFN-γ producing CD4 T cells relative to T cells cultured with M. tuberculosis–infected monocytes without SIV (Fig. 3A, 3B). Adding SIV to M. tuberculosis–infected monocytes did not significantly change CD8 T cell function (Fig. 3D–F); however, a trend toward reduced TNF (Fig. 3D; p = 0.0781) production was observed. We used monocyte-derived macrophages to confirm the effects of SIV on macrophages as APCs (n = 4 animals). SIV modestly reduced TNF (84% ± 24.5 of SIV-negative control) and IFN-γ (87% ± 9.3 of SIV-negative control) production by M. tuberculosis–specific CD4 T cells.
FIGURE 3.
SIV reduces frequencies of TNF and IFN-γ–producing CD4 T cells by manipulating M. tuberculosis–infected monocytes. SIV/M. tuberculosis–infected monocytes (Mtb+SIV) led to a decrease in TNF [n = 11 animals; (A)] and IFN-γ [n = 10 animals; (B)] production by CD4 T cells without inducing significant changes in the frequency of IL-2+ CD4 T cells (n = 10 animals; C) when compared with T cells incubated with M. tuberculosis–infected monocytes (Mtb). SIV led to a trend of decreased frequencies of TNF+ [n = 9 animals; p = 0.0781; (D)] and IL-2+ (n = 9 animals; p = 0.0547; F) CD8 T cells with no change in IFN-γ [n = 10 animals; (E)] production. Inactivated aldrithiol-2 Tx SIV (iSIV) did not reduce TNF+ [n = 11 animals; (G)] or IFN-γ [n = 10 animals; (H)] frequencies of M. tuberculosis–specific CD4 T cells. Open ellipse and bracket to the left and right of the line graphs represent mean ± SEM. Data are presented in the same way as Fig. 1. Wilcoxon matched-pairs signed rank test was used with significance set at p < 0.05. The p values that indicate significance are represented. **p < 0.01.
We performed the monocyte–T cell experiments with inactivated SIVmac251 (iSIV) to determine whether viral infection is responsible for diminished TNF and IFN-γ production in CD4 T cells. Incubation with iSIV did not cause significant changes in TNF (Fig. 3G) or IFN-γ (Fig. 3H) production by M. tuberculosis–specific CD4 T cells. Inactivated SIV did not reduce TNF or IFN-γ production in CD8 T cells (data not shown). SIV and iSIV did not cause an apparent change in cytokine production per cell (as measured by mean fluorescent intensity) for any of the cytokines we measured in these assays (data not shown).
T cells from granulomas of SIV-negative and SIV-infected macaques replicate the responses seen in peripheral blood
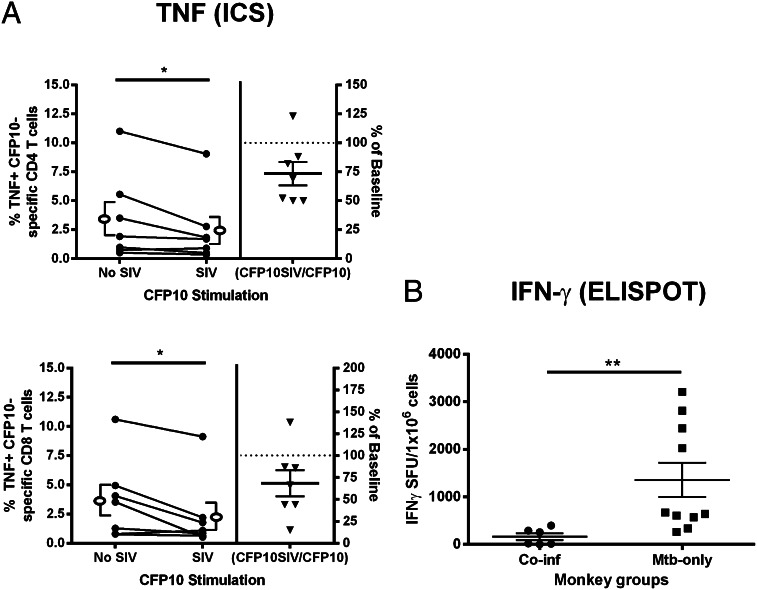
We performed similar assays with T cells from granuloma-containing thoracic lymph nodes to determine whether our observations of peripheral T cell and monocyte interactions are reflective of the behavior of T cells from M. tuberculosis–involved tissues (Supplemental Fig. 2A). Similar to peripheral blood, ex vivo SIV infection of homogenized granulomas from thoracic draining lymph nodes significantly reduced the frequency of TNF-producing CFP10-specific CD4 and CD8 T cells (Fig. 4A). To determine whether cytokine production is reduced in vivo, we examined IFN-γ expression by granuloma-containing thoracic lymph nodes from M. tuberculosis–SIV coinfected and M. tuberculosis–only monkeys by ELISPOT assay (Fig. 4B). Thoracic lymph node IFN-γ responses against M. tuberculosis Ags from coinfected monkeys were lower than SIV-negative monkeys with active TB (p = 0.0017; Fig. 4B). T cell numbers in the coinfected lymph nodes were not statistically different, although there was a trend toward fewer T cells in coinfected animals (3).
FIGURE 4.
SIV reduces frequencies of TNF+ T cells and numbers of IFN-γ+ T cells within thoracic lymph nodes. (A) Intracellular cytokine staining (ICS) performed on CD4 (n = 7 animals) and CD8 (n = 7 animals) T cells from thoracic lymph nodes stimulated with CFP10 and incubated with SIV had decreased TNF production when compared with cells not incubated with SIV; p values indicating statistical significance are displayed. Open ellipse and bracket to the left and right of the line graphs represent mean ± SEM. (B) Thoracic lymph nodes of monkeys with SIV-induced reactivation of latent TB (n = 6 animals; Coinf) have fewer IFN-γ secreting cells (spot-forming units) than cells from monkeys with active TB without SIV (n = 10 animals; Mtb-only). Line represents mean ± SEM. IFN-γ ELISPOT assays were performed on cells stimulated with M. tuberculosis–specific Ags (CFP-10 and ESAT-6). Mann–Whitney U test was used with significance set at *p < 0.05, **p < 0.01. Wilcoxon matched-pairs signed rank test.
SIV-induces the expression of IL-5 and IL-13 by monocytes
To better understand how SIV effects monocytes, and to determine whether SIV infection causes monocytes to produce cytokines that may downregulate T cell responses, we subjected supernatant from cultures of isolated CD14+ monocytes that had been incubated with media only, SIV, M. tuberculosis, or SIV plus M. tuberculosis to a 23-plex Luminex assay (Supplemental Table I). M. tuberculosis infection upregulated TNF and IL-1β expression (Fig. 5A), which is consistent with published results from human studies (26–28). In contrast, coinfected monocyte cultures had reduced TNF and IL-1β expression relative to monocyte cultures infected with M. tuberculosis alone (Fig. 5A). SIV-infected monocyte cultures displayed upregulated IL-5 and IL-13 expression (Fig. 5A).
FIGURE 5.
SIV/M. tuberculosis coincubated monocytes produce more IL-5 and IL-13 than do monocytes infected with only M. tuberculosis. (A) M. tuberculosis and SIV coinfection (Mtb+SIV) reduces TNF and IL-1β and increases IL-5 and IL-13 production relative to M. tuberculosis–infected monocytes (Mtb). SIV alone (SIV) decreases IL-1β and increases IL-5 and IL-13 production relative to monocytes incubated in media alone (Media). M. tuberculosis (Mtb) increased TNF and IL-1β production compared with monocytes incubated with media alone (Media). Open ellipse and bracket to the left and right of the line graphs represent mean ± SEM; p values indicating statistical significance are indicated (n = 10 animals). (B) Purity of the CD14 monocyte separation from PBMCs is represented. CD3 T cell and CD14 monocyte frequencies out of total event populations are presented. IL-5 production in both CD3 T cells and CD14 monocytes are presented in a histogram from monocytes incubated with or without SIV. Images are representative of four separate experiments. (C) RT-PCR was used to generate IL-5 (249 bp) or β-actin (174 bp) mRNA from monocytes incubated with media, M. tuberculosis, SIV, or both M. tuberculosis and SIV. This experiment is representative of four different macaques. *p < 0.05, **p < 0.01, Wilcoxon matched-pairs signed rank test
There was an approximately 1–3% CD3+ T cell contamination following isolation of monocytes (Fig. 5B). Because of this minimal cellular contamination, we measured IL-5 production in the isolated fraction of cells by flow cytometry and determined that monocytes and not T cells were producing IL-5 (Fig. 5B). IL-5 mRNA was observed in SIV-infected monocytes by reverse transcription PCR (Fig. 5C). The RT-PCR product contained 98% sequence homology with Homosapiens IL-5 (data not shown).
IL-5 production by SIV-infected monocytes reduces TNF expression in M. tuberculosis–specific CD4 T cells
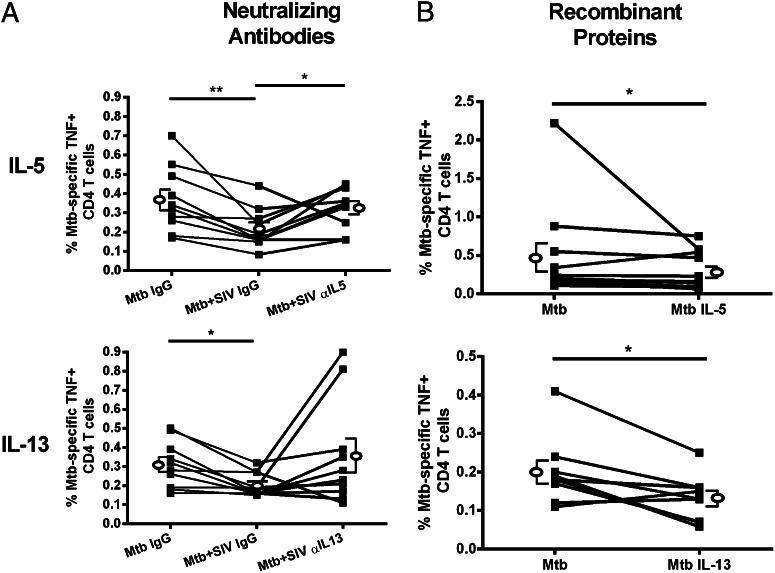
Neutralizing Abs against IL-5 or IL-13 were added to monocytes during SIV and M. tuberculosis infection (Supplemental Fig. 3B) and M. tuberculosis–specific T cell responses were assessed. Neutralizing IL-5 increased TNF-expression by M. tuberculosis–specific CD4 T cells (Fig. 6A). Responses to IL-13 neutralization were variable, and although there was an increase in the mean frequency of TNF-expressing CD4 T cells, this increase was not statistically significant (Fig. 6A).
FIGURE 6.
IL-5 neutralization restores TNF production, whereas adding recombinant IL-5 recapitulates the effect SIV-infected monocytes have on frequencies of M. tuberculosis–specific TNF+CD4 T cells. (A) SIV added to M. tuberculosis–infected monocytes with IgG control Abs (Mtb+SIV IgG) causes a significant reduction in frequencies of TNF+ CD4 T cells compared with M. tuberculosis–infected monocytes incubated with IgG (Mtb IgG). The addition of IL-5–neutralizing Ab to SIV/M. tuberculosis–coinfected cultures (Mtb+SIV αIL-5) significantly increased CD4 T cell TNF production [n = 10 animals; (A)] compared with coinfected monocytes incubated with an isotype control (Mtb+SIV IgG). The addition of neutralizing IL-13 Ab did not affect the frequency of TNF+CD4 T cells [n = 10 animals; (A)]. (B) The addition of recombinant IL-5 or IL-13 to M. tuberculosis–infected monocytes reduced the frequency of TNF+CD4 T cells (Mtb IL-5: n = 11 animals; Mtb IL-13: n = 9 animals) relative to CD4 T cells incubated with M. tuberculosis–infected monocytes without IL-5 or IL-13 (Mtb). Open ellipse and brackets to the left and right and overlapping line graphs represent mean ± SEM. The p values indicating statistical significance are displayed. *p < 0.05, **p < 0.01, Wilcoxon matched-pairs signed rank test.
To further confirm that monocyte-produced IL-5 and IL-13 are involved in inhibition of T cell cytokine production during SIV infection, we added recombinant IL-5 or IL-13 to M. tuberculosis–infected monocytes, washed the monocytes to remove exogenous cytokines, added T cells, and assayed for T cell TNF production (Supplemental Fig. 3C). We observed significantly reduced TNF production by CD4 T cells (Fig. 6B) when recombinant cytokines were added to M. tuberculosis–infected monocytes, thus complementing the neutralization experiments and recapitulating the results of experiments using SIV-infected monocytes.
Discussion
Impaired M. tuberculosis–specific T cell responses in HIV-coinfected individuals have been described in several studies (7, 8, 10, 13, 25, 29), yet the mechanisms responsible are not well understood. HIV (16, 30, 31) and SIV (3) are present in M. tuberculosis–infected tissues and fluids and can manipulate macrophage and T cell function within granulomas. Unfortunately, our ability to identify the influence of HIV on macrophages and T cells in granulomas is complicated by the difficulty associated with sampling this unique microenvironment. To address these issues, we developed a model system using T cells from blood and lung-draining thoracic lymph nodes of macaques with TB and used SIV-infected monocytes to elucidate how SIV, as a surrogate for HIV, influences granuloma T cell responses. Our study identifies a previously unappreciated aspect of lymphoid-myeloid cell biology associated with lentiviral infection, namely monocyte production of cytokines that inhibit Th1 cytokine responses. Specifically, we demonstrated that SIV-infected monocytes can inhibit TNF and IFN-γ production by M. tuberculosis–specific T cells, and the TNF inhibition is dependent, in part, on expression of IL-5 by monocytes.
The inhibition of T cell responses appears to be specific to M. tuberculosis–specific T cells. Previous studies have shown that HIV preferentially infects and kills peripheral M. tuberculosis–specific CD4 T cells without affecting CMV-specific T cells (7) and reduces M. tuberculosis–specific T cell responses without manipulating mitogen-stimulated or Candida albicans Ag-stimulated cells in coinfected individuals compared with HIV-negative individuals with pulmonary TB (10). Our findings are consistent with these reports, considering we found that SIV did not significantly reduce Pneumocystis-specific (P. jirovecii, kexin-stimulated) or cells nonspecifically activated with PHA. Instead, SIV increased TNF production in P. jirovecii–specific CD4 T cells. One possible reason is that the Pneumocystis-specific T cells in the monkeys are likely memory cells, because these monkeys were serologically positive for Pneumocystis (data not shown) but had no signs of active infection, although it remains possible that they were colonized. In contrast, the persistence and replication of M. tuberculosis bacilli, even in clinically latent animals, could result in a higher percentage of effector T cells, rather than memory T cells, and these could be affected differently by SIV interactions with the monocytes.
TNF expression is necessary for controlling M. tuberculosis infection in vivo (22), and TNF neutralization in monkeys (22) and humans (32) correlates with an increased risk of reactivation of latent tuberculosis. We found that M. tuberculosis–SIV coinfected monocytes produced less TNF than monocytes infected with M. tuberculosis alone (Fig. 5A), as was previously reported for HIV in coinfected macrophages (26, 27). Similarly, SIV reduced the frequency of TNF-producing M. tuberculosis–specific T cells from thoracic lymph nodes of monkeys with active TB, demonstrating that SIV can dampen the responses of T cells from granulomas. We speculate that SIV infection reduced TNF production by monocytes and CD4 T cells, and the combination of these factors could lead to levels of TNF in granulomas that are below the threshold necessary for control of M. tuberculosis (33, 34).
IFN-γ is also necessary for the control of TB (35, 36) and has been the focus of multiple coinfection studies (7, 8, 11, 12). These studies conclude that HIV reduces IFN-γ production by M. tuberculosis–specific CD4 T cells in the periphery and airway. Our study expands on these data by determining that SIV can disrupt these cytokine responses within the first 24 h of infection and examining changes in granulomatous T cells. We demonstrated that coinfecting monocytes with SIV and M. tuberculosis reduced the frequency of IFN-γ–producing CD4 T cells in vitro and that coinfected monkeys have fewer IFN-γ–producing T cells than SIV-negative monkeys with active TB. Considered with the TNF data, these experiments indicate that SIV can manipulate T cell responses in the peripheral blood and granuloma in this particular instance. Inactivated SIV appeared to affect IFN-γ and TNF production in some animals, but there was substantial variability in the response to inactivated virus, and no statistically significant difference was measured. Further study on the effects of live and inactivated virus is warranted to determine the mechanisms by which SIV influences cytokine production by CD4 T cells.
Several factors have been implicated in how HIV can manipulate macrophages in ways that negatively affect outcomes in TB. These mechanisms can include inhibition of macrophage TNF production, decreases in lysosomal acidification (18) to capacity to kill M. tuberculosis (27, 37), and increased macrophage apoptosis (26, 27). Our study provides further evidence that HIV manipulates antimycobacterial responses, this time through monocyte-produced IL-5 and possibly IL-13. Although CD4 T cells are often considered the source of IL-5 (38, 39), the literature is not definitive on this topic and there is some evidence suggesting macrophages (40–42) and non–T cells (43) can also produce IL-5. A variety of anti-inflammatory functions have been ascribed to IL-5 including eosinophil activation, B cell growth and promotion of Ab production (44, 45). IL-5 is detected in CFP- and purified protein derivative–stimulated PBMC (46–48) and plasma (49) of HIV/M. tuberculosis coinfected individuals. However, these studies have not addressed whether IL-5 manipulates immune responses in coinfected individuals. In this study, we attempt to identify the role of IL-5 during coinfection.
The results of this study present a novel role for IL-5 and suggest this cytokine may be a factor in the reduction of M. tuberculosis–specific T cell responses within coinfected individuals. We found that neutralizing IL-5 in coinfected monocytes partially restored normal T cell TNF production while adding IL-5 to M. tuberculosis–infected monocytes replicated the inhibition of TNF production observed during coincubation with M. tuberculosis/SIV–infected monocytes. Interestingly, we observed that IL-5 (and SIV) inhibits TNF production but not always IFN-γ. One possible reason for this discrepancy is that TNF production occurs before IFN-γ upon mycobacterial stimulation of CD4 and CD8 T cells (50); therefore, IL-5 or SIV might need more time to modulate IFN-γ production.
IL-5 and IL-13 have also been correlated with alternative activation of macrophages (51, 52), decreased ability to kill phagocytosed bacteria, and reduced IFN-γ and IL-1β expression in nasal mucosa tissue homogenates (52). In addition, IL-5Rα, the unique heterodimer subunit composing the IL-5 receptor, is present on human monocytes (53), supporting our hypothesis that IL-5 can manipulate monocytes through autocrine stimulation. It is possible that increased IL-5 disrupts the cytokine balance that is required for proper activation of M. tuberculosis–infected macrophages and M. tuberculosis–specific T cells. There are several lines of evidence suggesting that overexpression of Th2 cytokines increases the severity of TB, including observations that virulent M. tuberculosis strains preferentially induce Th2 cytokine expression, whereas less virulent strains induce Th1 cytokines, including IFN-γ and TNF (41, 54, 55). Appropriate macrophage activation is likely to be important for controlling TB, and previous studies have shown that classically activated macrophages induced by Th1 cytokines reduce M. tuberculosis growth more readily than alternatively activated macrophages (56).
If the hypothesis that IL-5 is inducing alternatively activated macrophages is incorrect, a more speculative hypothesis is that IL-5 might reduce T cell activity by inducing macrophage NO synthase expression, as has been observed in myeloid-lineage microglial cells (57). Ancillary effects could include NO-mediated downregulation of T cell activity (58) and macrophage-expressed proinflammatory cytokines, including IL-1β and TNF (59), both of which were observed in the current study.
The granuloma is a highly complex cellular environment with a poorly understood cytokine milieu; therefore, it is difficult to ascertain how IL-5 manipulates immune response to M. tuberculosis, but this study suggests a novel role for this cytokine in HIV–M. tuberculosis coinfected individuals. These findings provide evidence of a role for virus-induced expression of monocyte-derived IL-5 and possibly IL-13, with downstream effects including inhibition of IFN-γ and TNF production by M. tuberculosis–specific T cells. Our results have implications for controlling M. tuberculosis infection in HIV-coinfected individuals, the group most at risk for tuberculosis.
Acknowledgments
We thank Keith A. Reimann (Beth Israel Deaconess Medical Center, Boston, MA) for supplying the SIVmac251; Julian Bess and Jeffery Lifson for the Aldrithiol-2 Tx SIVmac251viral stocks, which were provided by the AIDS and Cancer Virus Program (Science Applications International Corporation Frederick and the National Cancer Institute, Frederick, MD) supported with federal funds from the National Cancer Institute–National Institutes of Health, under Contract HHSN261200800001E; Karen Norris for providing kexin peptides and assessing anti-kexin Ab responses in our animals; and Kyle Rohde, Dawn O’Dee, Leslie Milk, Olabisi Ojo, Matt Bigbee, and our research and veterinary technical staff for assistance.
This work was supported by National Institutes of Health Grants RO1 HL075845 (to J.L.F.), RO1 AI50732 (to J.L.F.), and T32 AI060525 (to C.R.D.).
The online version of this article contains supplemental material.
- BCG
- bacillus Calmette–Guérin
- iSIV
- inactivated SIVmac251
- MOI
- multiplicity of infection
- TB
- tuberculosis.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Corbett E. L., Watt C. J., Walker N., Maher D., Williams B. G., Raviglione M. C., Dye C. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163: 1009–1021 [DOI] [PubMed] [Google Scholar]
- 2.Harries A. D., Zachariah R., Corbett E. L., Lawn S. D., Santos-Filho E. T., Chimzizi R., Harrington M., Maher D., Williams B. G., De Cock K. M. 2010. The HIV-associated tuberculosis epidemic—when will we act? Lancet 375: 1906–1919 [DOI] [PubMed] [Google Scholar]
- 3.Diedrich C. R., Mattila J. T., Klein E., Janssen C., Phuah J., Sturgeon T. J., Montelaro R. C., Lin P. L., Flynn J. L. 2010. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS ONE 5: e9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn S. D., Butera S. T., Shinnick T. M. 2002. Tuberculosis unleashed: the impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect. 4: 635–646 [DOI] [PubMed] [Google Scholar]
- 5.Kwan C. K., Ernst J. D. 2011. HIV and tuberculosis: a deadly human syndemic. Clin. Microbiol. Rev. 24: 351–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diedrich C. R., Flynn J. L. 2011. HIV-1/Mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect. Immun. 79: 1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geldmacher C., Ngwenyama N., Schuetz A., Petrovas C., Reither K., Heeregrave E. J., Casazza J. P., Ambrozak D. R., Louder M., Ampofo W., et al. 2010. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J. Exp. Med. 207: 2869–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geldmacher C., Schuetz A., Ngwenyama N., Casazza J. P., Sanga E., Saathoff E., Boehme C., Geis S., Maboko L., Singh M., et al. 2008. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J. Infect. Dis. 198: 1590–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M., Gong J., Iyer D. V., Jones B. E., Modlin R. L., Barnes P. F. 1994. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J. Clin. Invest. 94: 2435–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hertoghe T., Wajja A., Ntambi L., Okwera A., Aziz M. A., Hirsch C., Johnson J., Toossi Z., Mugerwa R., Mugyenyi P., et al. 2000. T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB). Clin. Exp. Immunol. 122: 350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Condos R., Rom W. N., Weiden M. 2000. Lung-specific immune response in tuberculosis. Int. J. Tuberc. Lung Dis. 4(2, Suppl 1): S11–S17 [PubMed] [Google Scholar]
- 12.Bonecini-Almeida Mda. G., Werneck-Barroso E., Carvalho P. B., de Moura C. P., Andrade E. F., Hafner A., Carvalho C. E., Ho J. L., Kritski A. L., Morgado M. G. 1998. Functional activity of alveolar and peripheral cells in patients with human acquired immunodeficiency syndrome and pulmonary tuberculosis. Cell. Immunol. 190: 112–120 [DOI] [PubMed] [Google Scholar]
- 13.Kalsdorf B., Scriba T. J., Wood K., Day C. L., Dheda K., Dawson R., Hanekom W. A., Lange C., Wilkinson R. J. 2009. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am. J. Respir. Crit. Care Med. 180: 1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch C. S., Toossi Z., Johnson J. L., Luzze H., Ntambi L., Peters P., McHugh M., Okwera A., Joloba M., Mugyenyi P., et al. 2001. Augmentation of apoptosis and interferon-gamma production at sites of active Mycobacterium tuberculosis infection in human tuberculosis. J. Infect. Dis. 183: 779–788 [DOI] [PubMed] [Google Scholar]
- 15.Nakata K., Rom W. N., Honda Y., Condos R., Kanegasaki S., Cao Y., Weiden M. 1997. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am. J. Respir. Crit. Care Med. 155: 996–1003 [DOI] [PubMed] [Google Scholar]
- 16.Toossi Z., Johnson J. L., Kanost R. A., Wu M., Luzze H., Peters P., Okwera A., Joloba M., Mugyenyi P., Mugerwa R. D., et al. 2001. Increased replication of HIV-1 at sites of Mycobacterium tuberculosis infection: potential mechanisms of viral activation. J. Acquir. Immune Defic. Syndr. 28: 1–8 [DOI] [PubMed] [Google Scholar]
- 17.Nakayama K., Nakamura H., Koga M., Koibuchi T., Fujii T., Miura T., Iwamoto A., Kawana-Tachikawa A. 2012. Imbalanced production of cytokines by T cells associates with the activation/exhaustion status of memory T cells in chronic HIV type 1 infection. AIDS Res. Hum. Retroviruses. 28: 702–714 [DOI] [PubMed] [Google Scholar]
- 18.Mwandumba H. C., Russell D. G., Nyirenda M. H., Anderson J., White S. A., Molyneux M. E., Squire S. B. 2004. Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J. Immunol. 172: 4592–4598 [DOI] [PubMed] [Google Scholar]
- 19.Mattila J. T., Diedrich C. R., Lin P. L., Phuah J., Flynn J. L. 2011. Simian immunodeficiency virus-induced changes in T cell cytokine responses in cynomolgus macaques with latent Mycobacterium tuberculosis infection are associated with timing of reactivation. J. Immunol. 186: 3527–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin P. L., Rodgers M., Smith L., Bigbee M., Myers A., Bigbee C., Chiosea I., Capuano S. V., Fuhrman C., Klein E., Flynn J. L. 2009. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect. Immun. 77: 4631–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin P. L., Pawar S., Myers A., Pegu A., Fuhrman C., Reinhart T. A., Capuano S. V., Klein E., Flynn J. L. 2006. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect. Immun. 74: 3790–3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin P. L., Myers A., Smith L., Bigbee C., Bigbee M., Fuhrman C., Grieser H., Chiosea I., Voitenek N. N., Capuano S. V., et al. 2010. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 62: 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawar S. N., Mattila J. T., Sturgeon T. J., Lin P. L., Narayan O., Montelaro R. C., Flynn J. L. 2008. Comparison of the effects of pathogenic simian human immunodeficiency virus strains SHIV-89.6P and SHIV-KU2 in cynomolgus macaques. AIDS Res. Hum. Retroviruses 24: 643–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capuano S. V., III, Croix D. A., Pawar S., Zinovik A., Myers A., Lin P. L., Bissel S., Fuhrman C., Klein E., Flynn J. L. 2003. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 71: 5831–5844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wanchu A., Bhatnagar A., Talreja J., Sapra S., Suryanarayana B. S., Suresh P. 2009. Immunophenotypic and intracellular cytokine profile of Indian patients with tuberculosis with and without human immunodeficiency virus co-infection. Indian J. Chest Dis. Allied Sci. 51: 207–211 [PubMed] [Google Scholar]
- 26.Patel N. R., Swan K., Li X., Tachado S. D., Koziel H. 2009. Impaired M. tuberculosis-mediated apoptosis in alveolar macrophages from HIV+ persons: potential role of IL-10 and BCL-3. J. Leukoc. Biol. 86: 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel N. R., Zhu J., Tachado S. D., Zhang J., Wan Z., Saukkonen J., Koziel H. 2007. HIV impairs TNF-alpha mediated macrophage apoptotic response to Mycobacterium tuberculosis. J. Immunol. 179: 6973–6980 [DOI] [PubMed] [Google Scholar]
- 28.Maddocks S., Scandurra G. M., Nourse C., Bye C., Williams R. B., Slobedman B., Cunningham A. L., Britton W. J. 2009. Gene expression in HIV-1/Mycobacterium tuberculosis co-infected macrophages is dominated by M. tuberculosis. Tuberculosis (Edinb.) 89: 285–293 [DOI] [PubMed] [Google Scholar]
- 29.Matthews K., Ntsekhe M., Syed F., Scriba T., Russell J., Tibazarwa K., Deffur A., Hanekom W., Mayosi B. M., Wilkinson R. J., Wilkinson K. A. 2012. HIV-1 infection alters CD4+ memory T-cell phenotype at the site of disease in extrapulmonary tuberculosis. Eur. J. Immunol. 42: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoshino Y., Nakata K., Hoshino S., Honda Y., Tse D. B., Shioda T., Rom W. N., Weiden M. 2002. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J. Exp. Med. 195: 495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawn S. D., Pisell T. L., Hirsch C. S., Wu M., Butera S. T., Toossi Z. 2001. Anatomically compartmentalized human immunodeficiency virus replication in HLA-DR+ cells and CD14+ macrophages at the site of pleural tuberculosis coinfection. J. Infect. Dis. 184: 1127–1133 [DOI] [PubMed] [Google Scholar]
- 32.Keane J., Gershon S., Wise R. P., Mirabile-Levens E., Kasznica J., Schwieterman W. D., Siegel J. N., Braun M. M. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345: 1098–1104 [DOI] [PubMed] [Google Scholar]
- 33.Marino S., Myers A., Flynn J. L., Kirschner D. E. 2010. TNF and IL-10 are major factors in modulation of the phagocytic cell environment in lung and lymph node in tuberculosis: a next-generation two-compartmental model. J. Theor. Biol. 265: 586–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marino S., Sud D., Plessner H., Lin P. L., Chan J., Flynn J. L., Kirschner D. E. 2007. Differences in reactivation of tuberculosis induced from anti-TNF treatments are based on bioavailability in granulomatous tissue. PLOS Comput. Biol. 3: 1909–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., Bloom B. R. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178: 2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onwubalili J. K., Scott G. M., Robinson J. A. 1985. Deficient immune interferon production in tuberculosis. Clin. Exp. Immunol. 59: 405–413 [PMC free article] [PubMed] [Google Scholar]
- 37.Kumawat K., Pathak S. K., Spetz A. L., Kundu M., Basu J. 2010. Exogenous Nef is an inhibitor of Mycobacterium tuberculosis-induced tumor necrosis factor-alpha production and macrophage apoptosis. J. Biol. Chem. 285: 12629–12637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsui K., Tanaka N., Nishikawa A. 2001. Lipopolysaccharide of Haemophilus influenzae induces interleukin-5 mRNA expression in human peripheral blood mononuclear cells. J. Interferon Cytokine Res. 21: 439–443 [DOI] [PubMed] [Google Scholar]
- 39.Takatsu K., Tominaga A. 1991. Interleukin 5 and its receptor. Prog. Growth Factor Res. 3: 87–102 [DOI] [PubMed] [Google Scholar]
- 40.Cozzi-Lepri A., French M. A., Baxter J., Okhuysen P., Plana M., Neuhaus J., Landay A., INSIGHT SMART study group 2011. Resumption of HIV replication is associated with monocyte/macrophage derived cytokine and chemokine changes: results from a large international clinical trial. AIDS 25: 1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman S., Post F. A., Bekker L. G., Harbacheuski R., Steyn L. M., Ryffel B., Connell N. D., Kreiswirth B. N., Kaplan G. 2006. Mycobacterium tuberculosis H37Ra and H37Rv differential growth and cytokine/chemokine induction in murine macrophages in vitro. J. Interferon Cytokine Res. 26: 27–33 [DOI] [PubMed] [Google Scholar]
- 42.Smith A. M., Rahman F. Z., Hayee B., Graham S. J., Marks D. J., Sewell G. W., Palmer C. D., Wilde J., Foxwell B. M., Gloger I. S., et al. 2009. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J. Exp. Med. 206: 1883–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castro A. G., Silva R. A., Minóprio P., Appelberg R. 1995. In vivo evidence for a non-T cell origin of interleukin-5. Scand. J. Immunol. 41: 288–292 [DOI] [PubMed] [Google Scholar]
- 44.Huffnagle G. B., Boyd M. B., Street N. E., Lipscomb M. F. 1998. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 160: 2393–2400 [PubMed] [Google Scholar]
- 45.Morikawa K., Oseko F., Morikawa S., Imai K., Sawada M. 1993. Recombinant human IL-5 augments immunoglobulin generation by human B lymphocytes in the presence of IL-2. Cell. Immunol. 149: 390–401 [DOI] [PubMed] [Google Scholar]
- 46.Elliott A. M., Hodsdon W. S., Kyosiimire J., Quigley M. A., Nakiyingi J. S., Namujju P. B., Watera C., French N., Gilks C. F., Dockrell H. M., Whitworth J. A. 2004. Cytokine responses and progression to active tuberculosis in HIV-1-infected Ugandans: a prospective study. Trans. R. Soc. Trop. Med. Hyg. 98: 660–670 [DOI] [PubMed] [Google Scholar]
- 47.Giampietro F., de Waard J. H., Rivas-Santiago B., Enciso-Moreno J. A., Salgado A., Araujo Z. 2010. In vitro levels of cytokines in response to purified protein derivative (PPD) antigen in a population with high prevalence of pulmonary tuberculosis. Hum. Immunol. 71: 1099–1104 [DOI] [PubMed] [Google Scholar]
- 48.Mawa P. A., Pickering J. M., Miiro G., Namujju P. B., Watera C., Anyaegani G., Whitworth J. A., Elliott A. M. 2004. The effect of tuberculin skin testing on viral load and anti-mycobacterial immune responses in HIV-1-infected Ugandan adults. Int. J. Tuberc. Lung Dis. 8: 586–592 [PubMed] [Google Scholar]
- 49.Oliver B. G., Elliott J. H., Saphonn V., Vun M. C., French M. A., Price P. 2010. Interferon-γ and IL-5 production correlate directly in HIV patients co-infected with mycobacterium tuberculosis with or without immune restoration disease. AIDS Res. Hum. Retroviruses 26: 1287–1289 [DOI] [PubMed] [Google Scholar]
- 50.Antas P. R., Sales J. S., Pereira K. C., Oliveira E. B., Cunha K. S., Sarno E. N., Sampaio E. P. 2004. Patterns of intracellular cytokines in CD4 and CD8 T cells from patients with mycobacterial infections. Braz. J. Med. Biol. Res. 37: 1119–1129 [DOI] [PubMed] [Google Scholar]
- 51.Wynn T. A., Barron L., Thompson R. W., Madala S. K., Wilson M. S., Cheever A. W., Ramalingam T. 2011. Quantitative assessment of macrophage functions in repair and fibrosis. Curr. Protoc. Immunol. Chapter 14: Unit14.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krysko O., Holtappels G., Zhang N., Kubica M., Deswarte K., Derycke L., Claeys S., Hammad H., Brusselle G. G., Vandenabeele P., et al. 2011. Alternatively activated macrophages and impaired phagocytosis of S. aureus in chronic rhinosinusitis. Allergy 66: 396–403 [DOI] [PubMed] [Google Scholar]
- 53.Linch S. N., Danielson E. T., Kelly A. M., Tamakawa R. A., Lee J. J., Gold J. A. 2012. Interleukin 5 is protective during sepsis in an eosinophil-independent manner. Am. J. Respir. Crit. Care Med. 186: 246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manca C., Reed M. B., Freeman S., Mathema B., Kreiswirth B., Barry C. E., III, Kaplan G. 2004. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 72: 5511–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Y. J., Lim T. K., Ong A. K., Ho B. C., Seah G. T., Paton N. I. 2006. Tuberculosis associated with Mycobacterium tuberculosis Beijing and non-Beijing genotypes: a clinical and immunological comparison. BMC Infect. Dis. 6: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kahnert A., Seiler P., Stein M., Bandermann S., Hahnke K., Mollenkopf H., Kaufmann S. H. 2006. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur. J. Immunol. 36: 631–647 [DOI] [PubMed] [Google Scholar]
- 57.Liva S. M., de Vellis J. 2001. IL-5 induces proliferation and activation of microglia via an unknown receptor. Neurochem. Res. 26: 629–637 [DOI] [PubMed] [Google Scholar]
- 58.van der Veen R. C., Dietlin T. A., Dixon Gray J., Gilmore W. 2000. Macrophage-derived nitric oxide inhibits the proliferation of activated T helper cells and is induced during antigenic stimulation of resting T cells. Cell. Immunol. 199: 43–49 [DOI] [PubMed] [Google Scholar]
- 59.Meldrum D. R., Shames B. D., Meng X., Fullerton D. A., McIntyre R. C., Jr., Grover F. L., Harken A. H. 1998. Nitric oxide downregulates lung macrophage inflammatory cytokine production. Ann. Thorac. Surg. 66: 313–317 [DOI] [PubMed] [Google Scholar]