Abstract
Brain ANG II plays an important role in modulating sympathetic function and homeostasis. The generation and degradation of ANG II are carried out, to a large extent, through the angiotensin-converting enzyme (ACE) and ACE2, respectively. In disease states, such as hypertension and chronic heart failure, central expression of ACE is upregulated and ACE2 is decreased in central sympathoregulatory neurons. In this study, we determined the expression of ACE and ACE2 in response to ANG II in a neuronal cell culture and the subsequent signaling mechanism(s) involved. A mouse catecholaminergic neuronal cell line (CATH.a) was treated with ANG II (30, 100, and 300 nM) for 24 h, and protein expression was determined by Western blot analysis. ANG II induced a significant dose-dependent increase in ACE and decrease in ACE2 mRNA and protein expression in CATH.a neurons. This effect was abolished by pretreatment of the cells with the p38 MAPK inhibitor SB-203580 (10 μM) 30 min before administration of ANG II or the ERK1/2 inhibitor U-0126 (10 μM). These data suggest that ANG II increases ACE and attenuates ACE2 expression in neurons via the ANG II type 1 receptor, p38 MAPK, and ERK1/2 signaling pathways.
Keywords: angiotensin, converting enzyme, signaling
the renin-angiotensin system (RAS) in the brain plays an important role in the regulation of blood pressure, water balance, and endocrine secretion. Functional studies have clearly established that augmented ANG II and ANG II type 1 receptor (AT1R) signaling in the central nervous system plays an essential role in the sympathoexcitation in disease states such as hypertension and chronic heart failure in various animal models (43). Elevated plasma ANG II can directly activate neurons in the circumventricular organs, which lack a tight blood-brain barrier (6). On the other hand, other brain regions, such as the presympathetic neurons in the paraventricular nucleus (PVN) and rostral ventrolateral medulla, do not have direct access to systemic ANG II but have also been associated with an upregulation of local ANG II signaling in disease states (44).
The conversion of bioactive angiotensin peptides is mainly carried out by two angiotensin-converting enzymes: angiotensin-converting enzyme (ACE) and angiotensin-converting enzyme 2 (ACE2). ACE catalyzes the conversion from ANG I to the major sympathoexcitatory peptide ANG II, while ACE2 primarily metabolizes ANG II to form ANG-(1–7). ANG-(1–7) exhibits effects that are counter to the effects of ANG II through activation of the Mas receptor (30, 32). ACE also mediates the degradation of ANG-(1–7) to form the nonactive peptide ANG-(1–5) (7). Therefore, a balance between ACE and ACE2 expression and activity may contribute to the control of sympathetic nerve activity. Reciprocal changes in ACE and ACE2 expression in brain autonomic regions have been shown in studies using different models of heart failure (13, 34). Similarly, an increase in the ratio of ACE to ACE2 has been observed in the brain (1) and kidneys (36) in hypertensive patients and animal models. ANG II has been implicated in the reduction of ACE2 activity in the brain and in baroreflex dysfunction in hypertensive mice (39). Experimental evidence suggests that common regulatory mechanisms, such as increased ANG II signaling, are involved in these diseases. It has been shown that ANG II increases the ACE-to-ACE2 ratio via the AT1R, p38 MAPK, and ERK1/2 (also known as p42/44 MAPK) in a human kidney tubular epithelial cell line (14). It is not clear if this mechanism also applies to neurons.
In current study, we tested the hypothesis that ANG II upregulates ACE and downregulates ACE2 in a neuronal cell line (CATH.a) through AT1R, p38 MAPK, and ERK1/2 signaling.
MATERIALS AND METHODS
Cell culture.
CATH.a neurons were purchased from American Type Culture Collection (Manassas, VA). The neuronal cells were grown in RPMI 1640 medium containing 8% horse serum, 4% fetal bovine serum, and 100 IU/l penicillin at 37°C in 5% CO2 in a humidified atmosphere. Before treatment, the neuronal cells were allowed to differentiate in serum-free medium for 48 h. For immunofluorescence, neurons were grown on 12-mm poly-l-lysine-coated coverslips in 24-well plates at a density of 1 × 105 cells/well. For real-time RT-PCR and Western blotting, the neurons were plated at a density of 1.5 × 106 cells/well in six-well culture plates. The neurons were treated with ANG II (30, 100, and 300 nM) for 4 h for real-time RT-PCR or 24 h for immunofluorescence and Western blot analysis. An AT1R antagonist (losartan, 10 μmol/l), an AT2R antagonist (PD-123319, 10 μmol/l), a p38 MAPK inhibitor (SB-203580, 10 μmol/l), or an ERK1/2 inhibitor (U-0126, 10 μmol/l) was given 30 min before ANG II treatment.
Immunofluorescence confocal microscopy.
After appropriate treatment and incubation with ANG II, the media were aspirated and the cells were washed with ice-cold PBS. The neurons were fixed with 4% paraformaldhyde for 10 min. The cells were washed five times with PBS at room temperature and blocked using 10% normal donkey serum (NDS), 1% BSA, and 1% Triton in PBS for ≥1 h at room temperature. Primary antibody-directed anti-ACE or anti-ACE2 (catalog nos. sc-20791 and sc-20998, Santa Cruz Biotechnology) was diluted (1:200) in 1% NDS, 1% BSA, and 1% Triton in PBS and applied to the cells, which were incubated overnight at 4°C. The cells were washed with PBS and then incubated with anti-rabbit fluorescence-labeled secondary antibody in 1% NDS, 1% BSA, and 1% Triton in PBS for 1 h at room temperature in darkness. The coverslips were mounted using mounting medium with 4′,6′-diamidino-2-phenylindole. A confocal microscope (model TCS SP5, Leica) was used to examine at least five to six fields on the same slide under an oil immersion objective (×63, 1.4 numerical aperture). As a negative control, we stained the cells with primary or secondary antibody alone to determine the specificity of the fluorescence signal.
Western blot analysis.
The cells were scraped off the culture plate and suspended in ice-cold PBS. Total protein was extracted with radioimmunoprecipitation assay buffer, and equal amounts of sample were loaded and separated by SDS-PAGE and then electrophoretically transferred to polyvinylidene difluoride membranes. The membranes were blocked in Tris-buffered saline solution containing 1% Tween 20 and 5% nonfat dry milk, washed, and incubated with primary antibodies. Primary antibodies were purchased from Santa Cruz Biotechnology [rabbit polyclonal antibodies anti-ACE2, anti-ACE, anti-total p38 MAPK (sc-7972), anti-phosphorylated p38 MAPK (sc-7973), anti-total ERK1/2 (sc-94), and mouse monoclonal anti-GAPDH (sc-32233)] and Cell Signaling Technology [anti-phosphorylated ERK1/2 (catalog no. 4370)]. Membranes were thoroughly washed in Tris-buffered saline solution containing 1% Tween 20 and incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (Thermo Scientific). Bands were visualized using an enhanced chemiluminescence system and quantified using ImageJ software.
Real-time RT-PCR.
Total RNA was isolated from CATH.a neurons after appropriate treatment using the RNeasy RNA isolation kit (Qiagen, Valencia, CA) according to the manufacturer's guidelines. For each RT-PCR, 1 μg of total RNA was used, and the purity of the RNA was determined by the ratio of the optical density reading at 260 nm to the optical density reading at 280 nm. The ratio of the RNA used for RT-PCR was 1.8–2.0. RT-PCR was carried out in a programmable thermal controller (model PTC-100, Bio-Rad) with the following oligonucleotide primers: ACE [AAC AAA CAT GAT GGC CAC ATC CCG (forward) and CGT GTA GCC ATT GAG CTT GGC AAT (reverse)], ACE2 [AGG GTT CCA TGA AGC TGT TGG AGA (forward) and ATC GGA TGG CAG AAG ACC AAT GGA (reverse)], and GAPDH [TCA ACA GCA ACT CCC ACT CTT CCA (forward) and ACC CTG TTG CTG TAG CCG TAT TCA (reverse)]. cDNA synthesis was performed using an iScript kit (Bio-Rad) with the following parameters: 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C. For amplification of the DNA template, SsoFast EvaGreen Supermix (Bio-Rad) was used according to the manufacturer's guidelines using the specific primer pairs shown above. The reaction was carried out in a thermal cycler (model PTC-200, Bio-Rad) with a continuous fluorescence detector (Chromo4, Bio-Rad) with the following parameters: 95°C for 5 min, 95°C for 5 s, and 60°C for 20 s, with steps 2 and 3 repeated for 40 cycles. Target genes were normalized to GAPDH levels, expressed as (1 + E)Ct[target]/(1 + E)Ct[GAPDH] (where E is amplification efficiency). A value of 1 was attributed to the average of the vehicle-treated group, and values are expressed as a ratio to the vehicle-treated control group.
Statistical analysis.
Values are means ± SE. A two-way ANOVA with Bonferroni's post hoc test was used to analyze the differences between multiple groups. Prism 5 software (GraphPad Software, San Diego, CA) was used for statistical analysis. P < 0.05 was taken as indicative of statistical significance.
RESULTS
ANG II modulates ACE and ACE2 expression in CATH.a neurons.
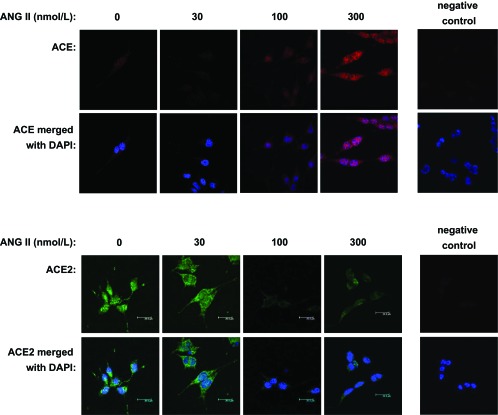
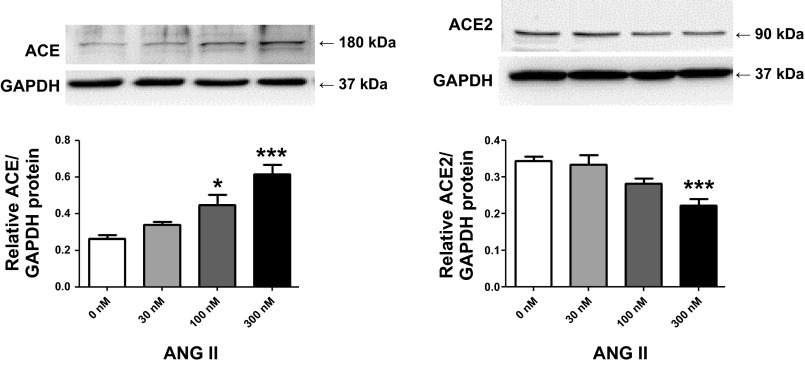
ACE and ACE2 were detected in the membrane and cytoplasm of CATH.a neurons by laser confocal immunofluorescence. ACE and ACE2 were found in the cell membrane and cytoplasm. After 24 h of treatment with 30, 100, and 300 nM ANG II, ACE expression was increased in a dose-dependent manner (Fig. 1, top). In contrast, ACE2 expression was decreased after the same ANG II treatments (Fig. 1, bottom). The changes in protein expression were quantified by Western blot analysis (Fig. 2). ACE protein was significantly increased after 100 nM (0.45 ± 0.06) and 300 nM (0.62 ± 0.05) ANG II compared with the control group (0.27 ± 0.02). ACE2 protein level was significantly reduced with 300 nM ANG II (0.22 ± 0.01) compared with the control group (0.34 ± 0.01). ACE and ACE2 gene transcription was also determined by real-time RT-PCR, as shown in Fig. 3. ACE mRNA was increased twofold at 100 nM ANG II. ACE2 mRNA levels were decreased by ∼60% by 100 nM ANG II. ACE mRNA was augmented by 3.7-fold and ACE2 was reduced by ∼75% by 300 nM ANG II.
Fig. 1.
Immunofluorescence images showing angiotensin-converting enzyme (ACE, top) and ACE2 (bottom) expression in CATH.a neurons treated with increasing doses of ANG II. Blue, 4′,6′-diamidino-2-phenylindole (DAPI); red, ACE; green, ACE2.
Fig. 2.
ANG II increases ACE (left) and decreases ACE2 (right) protein expression in CATH.a neurons in a dose-dependent manner. CATH.a neurons were treated with 30, 100, and 300 nM ANG II. Values are means ± SE [n = 4–5 (ACE) and n = 6 (ACE2) in each group]. *P < 0.05, ***P < 0.001 vs. control (0 nM ANG II).
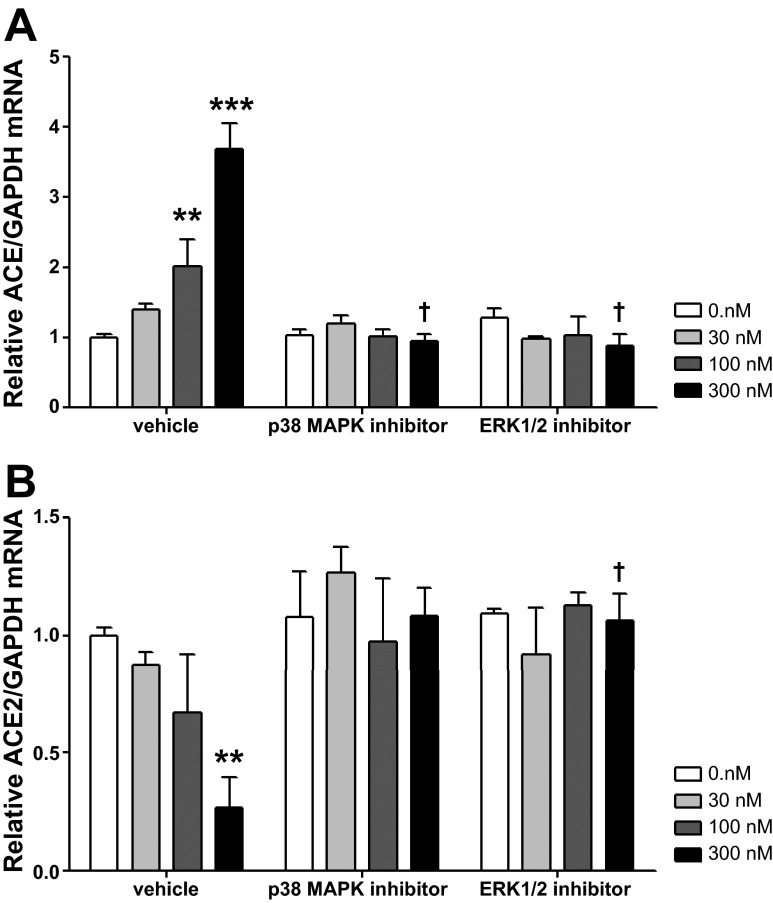
Fig. 3.
Relative ACE (A) and ACE2 (B) mRNA levels in CATH.a neurons treated with p38 MAPK and ERK1/2 inhibitors. Values are means ± SE (n = 3 in each group). **P < 0.01, ***P < 0.001 vs. 0 nM ANG II. †P < 0.05 vs. vehicle.
Effect of p38 MAPK and ERK1/2 inhibition on ACE and ACE2 expression.
Because ANG II increases phosphorylation of p38 MAPK and ERK1/2, it was of interest to determine the influence of these proteins on ACE and ACE2. ACE and ACE2 gene transcription was measured following p38 MAPK or ERK1/2 inhibition. Significant interaction (P < 0.0001) was observed between MAPK inhibitor pretreatments and ANG II treatments (Fig. 3). Although baseline ACE and ACE2 mRNA levels were not affected by the p38 MAPK inhibitor SB-203580 or the ERK1/2 inhibitor U-0126, both abolished the ANG II modulation of ACE and ACE2 gene transcription. Post hoc analysis showed a significant difference in relative ACE mRNA levels for 300 nM ANG II (3.68 ± 0.37 with vehicle vs. 0.95 ± 0.11 and 0.89 ± 0.16 with SB-203580 and U-0126, respectively, both P < 0.05). Significant differences in ACE2 mRNA were also found between the vehicle-treated group (0.27 ± 0.13) and neurons treated with the p38 MAPK inhibitor (1.08 ± 0.12) or the ERK1/2 inhibitor (1.07 ± 0.11) at 300 nM ANG II.
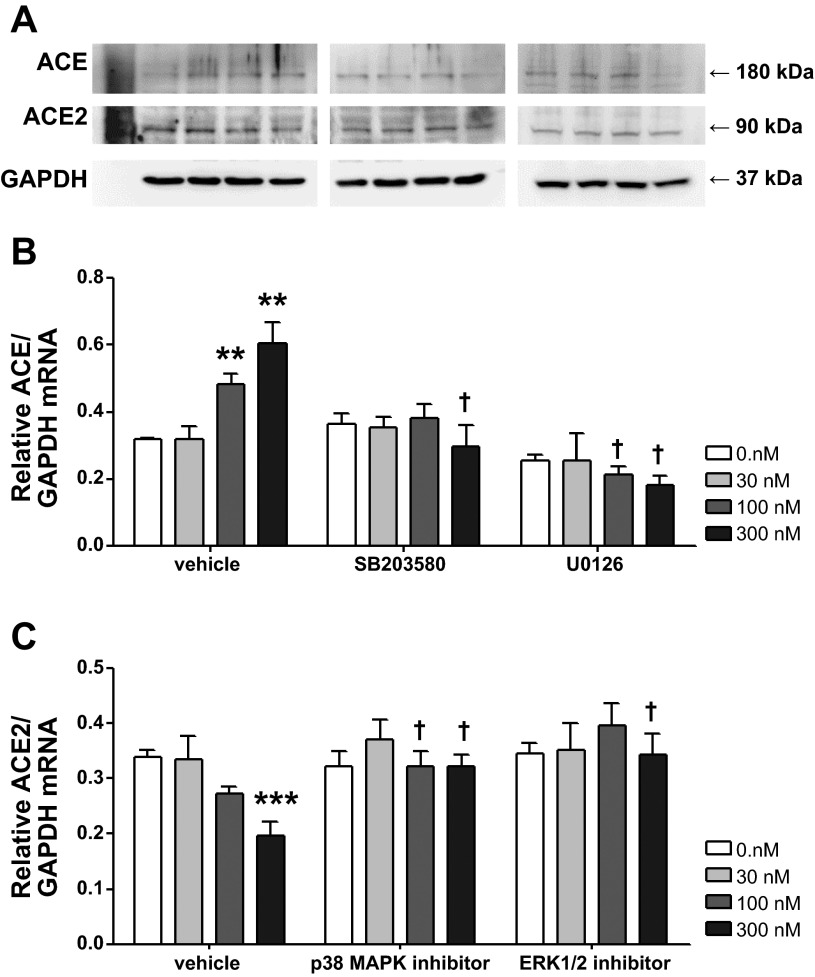
Protein expression following MAPK inhibition was measured by Western blot analysis. As shown in Fig. 4, similar to the data for real-time RT-PCR, the p38 MAPK inhibitor or the ERK1/2 inhibitor normalized the dose-dependent upregulation of ACE and downregulation of ACE2 without affecting the baseline expression of the two enzymes.
Fig. 4.
Effects of ERK1/2 and p38 MAPK inhibition on ACE and ACE2 protein expression. CATH.a neurons were treated with 30, 100, and 100 nM ANG II for 24 h. SB-203580 (a p38 MAPK inhibitor) and U-0126 (an ERK1/2 inhibitor) were administered 30 min before ANG II treatment. A: representative blots. B and C: quantitative data. Values are means ± SE (n = 4–8 in each group). **P < 0.01, ***P < 0.001 vs. 0 nM ANG II. †P < 0.05 vs. vehicle.
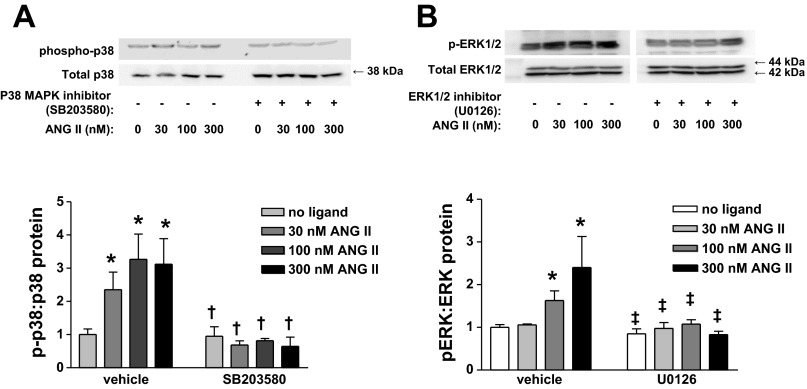
The effects of SB-203580 and U-0126 pretreatments were confirmed by immunoblotting the phosphorylated and total proteins for p38 and ERK. As shown in Fig. 5, dose-dependent phosphorylation of p38 MAPK was prevented by pretreatment with SB-203580 (Fig. 5A), while U-0126 inhibited ERK1 and ERK2 activation (Fig. 5B).
Fig. 5.
Top: representative Western blots for p38 MAPK and ERK1/2 phosphorylation in CATH.a neurons with p38 MAPK or ERK1/2 inhibition. Bottom: quantitative data. Values are means ± SE (n = 3–4 in each group). *P < 0.05 vs. no ligand. †P < 0.05 vs. all ANG II treatments. ‡P < 0.05 vs. 100 and 300 nM ANG II.
Effect of angiotensin receptor blockade on ACE and ACE2 expression.
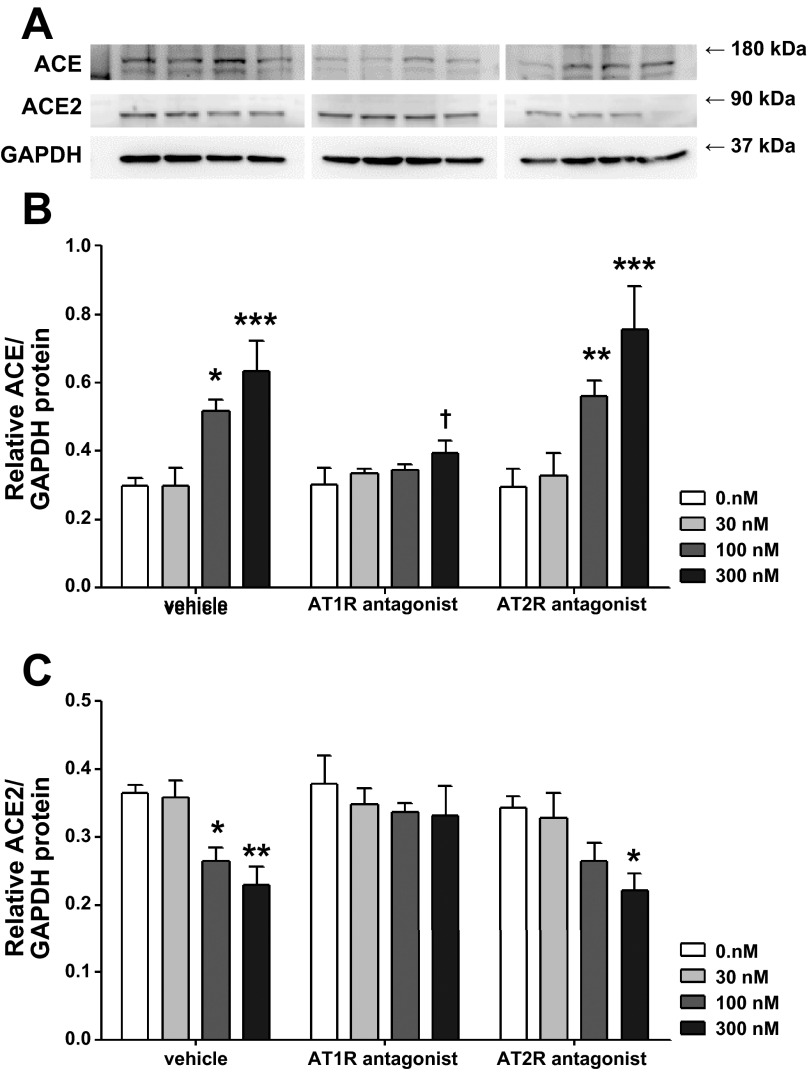
Since both major subtypes of the ANG II receptors have been associated with MAPK signaling activation in different cell types or tissues (12, 28), AT1R and AT2R antagonists were used to determine which subtype is involved in the modulation of ACE and ACE2. As shown in Fig. 6, significant interactions were observed between ANG II and its receptor antagonist treatments for ACE expression. The AT1R antagonist losartan normalized the dose-dependent upregulation of ACE (0.66 ± 0.01 and 0.40 ± 0.04 with vehicle and losartan, respectively, at 300 nM ANG II, P < 0.05), while AT2R antagonism with PD-123319 had no effect. ACE2 expression was significantly decreased in neurons treated with vehicle + 300 nM ANG II (0.37 ± 0.01 and 0.23 ± 0.03 at 0 and 300 nM ANG II, respectively, P < 0.05) and neurons treated with PD-123319 + 300 nM ANG II (0.34 ± 0.02 and 0.22 ± 0.03 at 0 and 300 nM ANG II, respectively, P < 0.05). Losartan completely blocked the effects of ANG II on ACE2. These data suggest a dominant role of the AT1R in the regulation of ACE and ACE2 expression in CATH.a neurons.
Fig. 6.
Effects of blockade of ANG II receptor types 1 and 2 (AT1R and AT2R) on ACE and ACE2 protein expression. CATH.a neurons were treated with 30, 100, and 100 nM ANG II for 24 h. AT1R and AT2R antagonists (losartan and PD-123319, respectively) were administered 30 min before ANG II treatment. A: representative blots. B and C: effects of AT1R and AT2R antagonists (losartan and PD-123319) on ACE and ACE2 protein expression. Values are means ± SE (n = 4 in each group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. 0 nM ANG II.
DISCUSSION
In the current study we hypothesized that ANG II upregulates ACE and downregulates ACE2 expression in CATH.a neurons. These cellular data support the putative effects of ANG II on ACE and ACE2 gene transcription through an AT1R-p38 MAPK and ERK1/2 signaling pathway.
The localization of ACE and ACE2 has been mapped in various sites in the brain in humans and experimental animals (40, 42). ACE in the brain is associated with the endothelium of cerebral blood vessels (4, 13), epithelial cells of the choroid plexus, and plasma membranes of astrocytes in the circumventricular organs (42). ACE is also expressed at moderate levels in neurons in the PVN, subfornical organ, and dorsal vagal complex (3, 4), and its distribution coincides with ANG II immunoreactivity and AT1R expression (18, 19, 42). Because of the presence of the blood-brain barrier, ANG II and ACE from the endothelial side cannot directly influence neuronal function (except for neurons in the circumventricular organs). For this reason, ACE and ACE2 expression in neurons and glial cells inside the blood-brain barrier is important. On the other hand, ACE2 is also present in brain centers that control cardiovascular function and is associated with AT1R expression (8, 16). In the present study, we demonstrated that ACE and ACE2 are expressed in the cell membrane and cytoplasm of CATH.a neurons and that ANG II modulates these two enzymes in opposite directions. The subcellular localization of ACE2 agrees with previous in vitro studies (8, 13); however, it is not clear if ACE is localized in the cytoplasm as well as in the membrane. The increase in the ratio of ACE to ACE2 correlates with results in animal models of heart failure or hypertension (1, 13). This mechanism may serve a positive-feedback function in hyperactivated central ANG II signaling and exacerbation of the diseases through augmented sympathoexcitation. It has previously been shown that knocking down the expression of the AT1aR causes downregulation of ACE2 in the brain stem of normal mice without affecting ACE expression (16). We speculate that the effects of the AT1R on ACE and ACE2 expression may be different in the basal state and following ANG II stimulation, since some of our findings are statistically different only at high doses of ANG II, which may trigger a series of signaling pathways.
In the current study we elucidated an AT1R-p38 MAPK and ERK1/2 signaling pathway in the modulation of ACE and ACE2 expression in neurons. Central AT1R-dependent phosphorylation of p38 MAPK or ERK1/2 in the subfornical organ, PVN, and rostral ventrolateral medulla has been reported previously and has been associated with increased oxidative stress and hyperactivated ANG II-AT1R signaling in hypertension and heart failure (5, 33, 38). Activation of p38 MAPK and ERK1/2 by ANG II has also been observed in other tissues, including heart (24, 31), vascular smooth muscle (25, 35), and kidney (14, 26). Several different upstream signaling pathways may link AT1R activation to phosphorylation of p38 MAPK or ERK1/2. The Src family of protein tyrosine kinases has been shown to play a key role in the coupling of cell surface receptors with MAPK activation (14, 27) and NADPH oxidase-derived O2·−, which also serves as a second messenger downstream of the AT1R for MAPK activation (5, 14). Our laboratory has shown that ANG II induces O2·− production through AT1R and NADPH oxidase activation in CATH.a neurons (21). Therefore, O2·− may also participate in the regulation of ACE and ACE2 expression. In the current study we used a relatively high concentration of ANG II (300 nM), and the changes in ACE and ACE2 proteins occurred at 24 h after administration of ANG II. This suggests that this effect may not occur under physiological conditions but may reflect disease states such as heart failure or hypertension, where ANG II levels in the brain may be high. It is highly possible that ANG II concentration in the medium was not sustained at the given dose during the treatment. We speculate that ANG II may trigger a series of downstream effects, including oxidative stress, that modulate cell function for a longer period of time. We observed changes in ACE and ACE2 mRNA levels within 4 h. However, changes in protein expression were not detected until 8 h after ANG II was added (data not shown) and were still observed after 24 h.
The influences of the AT2R on MAPK activation are different from those for the AT1R. AT2R activation induces sustained activation of ERK1/2 in the NG-108 neuronal cell line (28). However, it has been shown to play an inhibitory role in ERK1/2 activation in cardiomyocytes during ischemia-reperfusion injury (37). We did not measure AT1R and AT2R expression in the current study. Our previous work clearly showed that ANG II upregulates AT1R transcription and protein expression at 100 nM within 4 h in this neuronal cell line (21, 22). Recent work by Herrera et al. (11) has called into question the validity of AT1R measurements, at least in mice, because of nonspecificity of commercial antibodies. While it is certainly true that many AT1R antibodies may not be completely specific, data from Gao et al. (9) clearly show that the use of a blocking peptide abolished the AT1R band in the Western blot in the brain. However, the effect of ANG II on AT2R expression is not as clear in CATH.a neurons. Functional AT2R effects have been observed, in that AT2R-specific agonists increased potassium current in these neurons (10). In the current study the AT2R antagonist PD-123319 did not significantly alter the effects of ANG II on ACE and ACE2 expression, whereas losartan reversed the effects of ANG II, strongly suggesting a dominant AT1R mechanism.
One of the limitations of the current study is that it is still unclear which transcriptional mechanisms downstream of p38 MAPK and ERK1/2 participate in the modulation of ACE and ACE2. Our laboratory has shown that several transcription factors, including activator protein-1, NF-κB, and cAMP responsive element-binding protein, are involved in the regulation of AT1R expression in response to ANG II (20, 22; unpublished observations). Similar factors may also be involved in the regulation of both converting enzymes. Hypoxia-inducible factor 1α has been shown to inhibit ACE2 and promote ACE expression in pulmonary artery smooth muscle cells during hypoxic pulmonary hypertension (41). A study by Cardinale et al. (2) showed that chronic subcutaneous infusion of ANG II resulted in an increase in ACE and a decrease in ACE2 expression in the PVN, which were normalized by inhibition of NF-κB. Another recent study found that an upregulation of ACE in local tissues (heart, kidney, and aorta) of spontaneously hypertensive rats via histone code modifications, which was normalized by the AT1R antagonist valsartan (15). In addition, although mRNA and protein levels were measured in these experiments, the enzyme activities need to be determined. Finally, whether this mechanism is operative in vivo is not known. Because of the complex interaction among RAS components, it is technically challenging to dissect and test one of the RAS components in vivo without affecting the others.
In conclusion, the current findings suggest that ACE and ACE2 expression is regulated by ANG II via AT1R and p38 MAPK and ERK1/2 signaling pathways in CATH.a neurons. This may help explain the bidirectional ACE and ACE2 expression in the central nervous system in heart failure and hypertension.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants P01 HL-62222 and F32 HL-116172-01 to K. K. V. Haack.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.X. and K.K.V.H. performed the experiments; L.X. and K.K.V.H. analyzed the data; L.X. and I.H.Z. interpreted the results of the experiments; L.X. and K.K.V.H. prepared the figures; L.X. drafted the manuscript; L.X. and I.H.Z. edited and revised the manuscript; L.X., K.K.V.H., and I.H.Z. approved the final version of the manuscript; I.H.Z. is responsible for conception and design of the research.
REFERENCES
- 1. Agarwal D, Welsch MA, Keller JN, Francis J. Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res Cardiol 106: 1069–1085, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-κB in the paraventricular nucleus. Hypertension 59: 113–121, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chai SY, McKenzie JS, McKinley MJ, Mendelsohn FA. Angiotensin converting enzyme in the human basal forebrain and midbrain visualized by in vitro autoradiography. J Comp Neurol 291: 179–194, 1990 [DOI] [PubMed] [Google Scholar]
- 4. Chai SY, Mendelsohn FA, Paxinos G. Angiotensin converting enzyme in rat brain visualized by quantitative in vitro autoradiography. Neuroscience 20: 615–627, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res 97: 772–780, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Dilauro M, Burns KD. Angiotensin-(1–7) and its effects in the kidney. Sci World J 9: 522–535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 292: R373–R381, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao J, Chao J, Parbhu KJ, Yu L, Xiao L, Gao F, Gao L. Ontogeny of angiotensin type 2 and type 1 receptor expression in mice. J Renin Angiotensin Aldosterone Syst 13: 341–352, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao J, Zhang H, Le KD, Chao J, Gao L. Activation of central angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am J Hypertens 24: 724–730, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension 61: 253–258, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol 20: 953–970, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Kar S, Gao L, Zucker IH. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J Appl Physiol 108: 923–932, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koka V, Huang XR, Chung AC, Wang W, Truong LD, Lan HY. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol 172: 1174–1183, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee HA, Cho HM, Lee DY, Kim KC, Han HS, Kim IK. Tissue-specific upregulation of angiotensin-converting enzyme 1 in spontaneously hypertensive rats through histone code modifications. Hypertension 59: 621–626, 2012 [DOI] [PubMed] [Google Scholar]
- 16. Lin Z, Chen Y, Zhang W, Chen AF, Lin S, Morris M. RNA interference shows interactions between mouse brainstem angiotensin AT1 receptors and angiotensin-converting enzyme 2. Exp Physiol 93: 676–684, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Lind RW, Swanson LW, Ganten D. Angiotensin II immunoreactivity in the neural afferents and efferents of the subfornical organ of the rat. Brain Res 321: 209–215, 1984 [DOI] [PubMed] [Google Scholar]
- 19. Lind RW, Swanson LW, Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. An immunohistochemical study. Neuroendocrinology 40: 2–24, 1985 [DOI] [PubMed] [Google Scholar]
- 20. Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Neuronal angiotensin II type 1 receptor upregulation in heart failure: activation of activator protein 1 and Jun N-terminal kinase. Circ Res 99: 1004–1011, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Role of oxidant stress on AT1 receptor expression in neurons of rabbits with heart failure and in cultured neurons. Circ Res 103: 186–193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mitra AK, Gao L, Zucker IH. Angiotensin II-induced upregulation of AT1 receptor expression: sequential activation of NF-κB and Elk-1 in neurons. Am J Physiol Cell Physiol 299: C561–C569, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Molavi B, Chen J, Mehta JL. Cardioprotective effects of rosiglitazone are associated with selective overexpression of type 2 angiotensin receptors and inhibition of p42/44 MAPK. Am J Physiol Heart Circ Physiol 291: H687–H693, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Morrell NW, Upton PD, Kotecha S, Huntley A, Yacoub MH, Polak JM, Wharton J. Angiotensin II activates MAPK and stimulates growth of human pulmonary artery smooth muscle via AT1 receptors. Am J Physiol Lung Cell Mol Physiol 277: L440–L448, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y. Effects of AT1 receptor blockade on renal injury and mitogen-activated protein activity in Dahl salt-sensitive rats. Kidney Int 65: 972–981, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene 23: 7906–7909, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Plouffe B, Guimond MO, Beaudry H, Gallo-Payet N. Role of tyrosine kinase receptors in angiotensin II AT2 receptor signaling: involvement in neurite outgrowth and in p42/p44mapk activation in NG108-15 cells. Endocrinology 147: 4646–4654, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Sampaio WO, Henrique de CC, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1–7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 50: 1093–1098, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Sano M, Fukuda K, Sato T, Kawaguchi H, Suematsu M, Matsuda S, Koyasu S, Matsui H, Yamauchi-Takihara K, Harada M, Saito Y, Ogawa S. ERK and p38 MAPK, but not NF-κB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res 89: 661–669, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100: 8258–8263, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seyedabadi M, Goodchild AK, Pilowsky PM. Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension 38: 1087–1092, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Tan J, Wang H, Leenen FH. Increases in brain and cardiac AT1 receptor and ACE densities after myocardial infarct in rats. Am J Physiol Heart Circ Physiol 286: H1665–H1671, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Touyz RM, He G, El MM, Diep Q, Mardigyan V, Schiffrin EL. Differential activation of extracellular signal-regulated protein kinase 1/2 and p38 mitogen activated-protein kinase by AT1 receptors in vascular smooth muscle cells from Wistar-Kyoto rats and spontaneously hypertensive rats. J Hypertens 19: 553–559, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Wakahara S, Konoshita T, Mizuno S, Motomura M, Aoyama C, Makino Y, Kato N, Koni I, Miyamori I. Synergistic expression of angiotensin-converting enzyme (ACE) and ACE2 in human renal tissue and confounding effects of hypertension on the ACE to ACE2 ratio. Endocrinology 148: 2453–2457, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Wang LX, Ideishi M, Yahiro E, Urata H, Arakawa K, Saku K. Mechanism of the cardioprotective effect of inhibition of the renin-angiotensin system on ischemia/reperfusion-induced myocardial injury. Hypertens Res 24: 179–187, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Wei SG, Yu Y, Zhang ZH, Felder RB. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol 296: H1425–H1433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension 53: 210–216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem 107: 1482–1494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang R, Wu Y, Zhao M, Liu C, Zhou L, Shen S, Liao S, Yang K, Li Q, Wan H. Role of HIF-1α in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 297: L631–L640, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Zhuo J, Moeller I, Jenkins T, Chai SY, Allen AM, Ohishi M, Mendelsohn FA. Mapping tissue angiotensin-converting enzyme and angiotensin AT1, AT2 and AT4 receptors. J Hypertens 16: 2027–2037, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Zucker IH, Schultz HD, Patel KP, Wang W, Gao L. Regulation of central angiotensin type 1 receptors and sympathetic outflow in heart failure. Am J Physiol Heart Circ Physiol 297: H1557–H1566, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zucker IH, Wang W, Pliquett RU, Liu JL, Patel KP. The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide, and exercise training. Ann NY Acad Sci 940: 431–443, 2001 [PubMed] [Google Scholar]