Abstract
In cerebral microvascular endothelial cells (CMVEC) of newborn pigs, glutamate at excitotoxic concentrations (mM) causes apoptosis mediated by reactive oxygen species (ROS). Carbon monoxide (CO) produced by CMVEC or delivered by a CO-releasing molecule, CORM-A1, has antioxidant properties. We tested the hypothesis that CORM-A1 prevents cerebrovascular endothelial barrier dysfunction caused by glutamate excitotoxicity. First, we identified the glutamate receptors (GluRs) and enzymatic sources of ROS involved in the mechanism of endothelial apoptosis. In glutamate-exposed CMVEC, ROS formation and apoptosis were blocked by rotenone, 2-thenoyltrifluoroacetone (TTFA), and antimycin, indicating that mitochondrial complexes I, II, and III are the major sources of oxidative stress. Agonists of ionotropic GluRs (iGluRs) N-methyl-D-aspartate (NMDA), cis-ACPD, AMPA, and kainate increased ROS production and apoptosis, whereas iGluR antagonists exhibited antiapoptotic properties, suggesting that iGluRs mediate glutamate-induced endothelial apoptosis. The functional consequences of endothelial injury were tested in the model of blood-brain barrier (BBB) composed of CMVEC monolayer on semipermeable membranes. Glutamate and iGluR agonists reduced transendothelial electrical resistance and increased endothelial paracellular permeability to 3-kDa dextran. CORM-A1 exhibited potent antioxidant and antiapoptotic properties in CMVEC and completely prevented BBB dysfunction caused by glutamate and iGluR agonists. Overall, the endothelial component of the BBB is a cellular target for excitotoxic glutamate that, via a mechanism involving a iGluR-mediated activation of mitochondrial ROS production and apoptosis, leads to BBB opening that may be prevented by the antioxidant and antiapoptotic actions of CORMs. Antioxidant CORMs therapy may help preserve BBB functional integrity in neonatal cerebrovascular disease.
Keywords: cerebral vascular endothelium, mitochondria, carbon monoxide, vascular injury, glutamate excitotoxicity, NMDA, AMPA, kainate
glutamate, the major excitatory neurotransmitter in the central nervous system, is excessively released by neurons during numerous pathological conditions, including seizures, hypoxia, and stroke (23). In the millimolar concentration range (excitotoxic levels), glutamate produces neuronal death. In the cerebral circulation of neonatal pigs, glutamate also targets nonneuronal cell types, including endothelial cells (14, 33) and astrocytes (37, 45). Physiological cerebrovascular functions of glutamate include endothelial- and astrocyte-dependent dilation of pial arterioles, leading to cerebral blood flow increase (14, 21, 37). At excitotoxic levels, glutamate causes endothelial cell death by apoptosis in the cerebral circulation of newborn pigs (33). Glutamate-induced apoptosis in the neonatal cerebral vascular bed may contribute to the loss of blood flow regulation, leading to aggravated neurotoxicity. Superoxide radicals are major contributors to apoptosis in glutamate-exposed cerebral vascular endothelial cells (33). The intracellular enzymatic sources of superoxide may include NADPH oxidase, nitric oxide (NO) synthase, xanthine oxidase, and the mitochondrial respiratory chain. However, the sources of superoxide evoked by glutamate, the mechanisms leading to excitotoxic endothelial cell death, and functional consequences of cerebral vascular endothelial damage are not known.
Glutamate receptors (GluRs) are important mediators of biological effects of glutamate. CMVEC from newborn piglets express various types of glutamate receptors (GluRs), including ionotropic glutamate receptors (iGluRs; Ref. 34). iGluRs contribute to the glutamate-evoked dilation of cerebral arterioles that involves activation of endothelial production of carbon monoxide (CO), a gaseous vasodilator messenger (14, 34, 41). The involvement of iGluRs in mediating oxidative stress and apoptosis caused by excitotoxic levels of glutamate in the cerebral circulation of newborn pigs has not yet been investigated.
There is compelling evidence that CO also has antioxidant properties (2, 20, 36). In the cerebral circulation of newborn pigs in vivo, CO delivered in a pharmacologically active form as a CO-releasing molecule, CORM-A1, reduced oxidative stress and prevented loss of cerebral vascular dilator function caused by glutamatergic seizures (35, 37, 46). Furthermore, CORM-A1 prevented the appearance of brain-derived circulating endothelial cells in peripheral blood as early indicators of cerebral vascular endothelial damage caused by seizures (35).
We have now tested, in vitro, the hypothesis that CORM-A1 prevents cerebrovascular endothelial barrier dysfunction caused by glutamate excitotoxicity. To test this hypothesis, we used primary cultures of cerebral microvascular endothelial cells (CMVEC) from newborn piglets. First, to uncover the mechanisms leading to glutamate-induced endothelial damage, we determined the following: 1) intracellular enzymatic sources of reactive oxygen species (ROS) leading to apoptosis; 2) involvement of N-methyl-D-aspartate (NMDA) and non-NMDA types of iGluRs in the mechanism of glutamate-induced oxidative stress and apoptosis; and 3) downstream mechanisms linking iGluR stimulation to apoptosis. Second, we tested whether glutamate- and iGluR-induced cerebral vascular endothelial oxidative stress injury leads to blood-brain barrier (BBB) dysfunction. Third, we investigated the effects of antioxidant CORM-A1 on iGluR-mediated endothelial damage and BBB dysfunction caused by glutamate excitotoxicity.
METHODS
All protocols and procedures involving animals were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center, Memphis.
Cerebral microvessels and endothelial cell cultures.
Brain cortex was obtained from ketamine/xylaxine-anesthetized newborn pigs (1–5 days of age, either sex). The experiments were conducted in primary cultures of CMVEC. We emphasize the importance of primary cultures for physiological studies because they maintain tissue-specific properties and physiological responsiveness. Specifically, our data show that piglet brain in vivo, isolated cerebral microvessels, and CMVEC in vitro respond to glutamate and iGluR agonists by increasing CO production (20, 34, 41). To ensure the quality of endothelial preparations, the cells were isolated and cultured under standardized conditions and controlled by phenotyping and endothelial antigen profiling. CMVEC were isolated by collagenase-dispase digestion of cerebral cortical microvessels (60–300 μm) followed by Percoll density gradient centrifugation (33). Cells were plated on Matrigel-coated plates or 0.4 μm collagen-coated polytetrafluoroethylene membranes in Costar Transwell inserts. CMVEC were cultured in DMEM containing 20% FBS, 30 μg/ml endothelial cell growth supplement, 1 U/ml heparin, and antibiotic/antimycotic mixture (DMEM-E medium) for 5–6 days. CMVEC identified by von Willebrand factor accounted for >90% of the cell population. All experiments were performed on confluent cells in primary cultures brought to quiescence by overnight starvation in glutamine- and serum-depleted DMEM.
Detection of ROS.
We used the oxidant-sensitive fluorescent probe dihydroethidium (DHE) to detect ROS generation in CMVEC as we described earlier (3, 4). The products of DHE oxidation (ox-DHE), including superoxide, intercalate with nuclear DNA, forming a red fluorescent complex. CMVEC were incubated with glutamate and ligands of iGluRs for 1 h at 37°C. DHE (20 μM) was added to the incubation media for the last 20 min of the incubation. For spectroscopic measurements of ROS, CMVEC were sonicated to extract the DNA-bound ox-DHE; the fluorescence (excitation/emission, 485/590 nm) was measured with a Synergy HT microplate reader (BioTek Instruments, Winooski, VT).
Detection of apoptosis by DNA fragmentation.
Accumulation of cytoplasmic histone-associated-DNA-fragments was measured as described elsewhere (33). Quiescent CMVEC were exposed to glutamate (0.5–2 mM) or iGluR ligands (0.1–1.0 mM) in glutamine-free DMEM-0% FBS for 3 h at 37°C. At the end of the incubation period, cells were lysed, and apoptotic cytoplasmic DNA fragments were immunodetected by ELISA kit (Roche Applied Science, Indianapolis, IN) and visualized with 2,2′-azino-di(3-ethylbenzthiazolin-sulfonate). The absorbance at 405 nm was measured by the Synergy HT microplate reader and normalized to the protein amount detected by the bicinchoninic acid assay (Pierce, Rockford, IL). The number of detached floating cells in the aspirated incubation media was counted using a microscope counting chamber.
Cytochrome c immunodetection.
Cytochrome c release from mitochondria to the cytoplasm indicative of the mitochondrial pathway of apoptosis (16, 18, 22, 30, 38) was detected by immunostaining and immunoblotting. For immunostaining, cells were permeabilized with 0.1% Triton X-100 in DPBS (20 min, room temperature), blocked in 5% BSA-DPBS, and immunostained by Alexa Fluor 647-conjugated mouse anti-cytochrome c (BD Biosciences, Bedford, MA) according to the manufacturer's protocol. For immunoblotting, mitochondria-free cytoplasmic fraction (20 μg protein/lane) separated by 10–20% gradient SDS-PAGE was transferred to Amersham's Hybond-P polyvinylidene difluoride membranes (GE Healthcare Biosciences, Pittsburg, PA), blocked with 5% milk-0.1% Tween-20, and probed with cytochrome c polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were reprobed with monoclonal anti-actin (Roche Molecular Biochemicals, Indianapolis, IN) to ensure equal loading. The immunocomplexes were visualized with a Western Lightning Enhanced Chemiluminescence Kit (Perkin Elmer; Waltham, MA).
Measurements of cytosolic Ca2+.
Confluent CMVEC on 96-well plates were exposed to the glutamine-free starvation media (0.1% FBS-DMEM) for 6–8 h. Cytosolic Ca2+ concentrations ([Ca2+]c) were measured in fura-2 AM-loaded cells using the Flexstation II scanning fluorometer (Molecular Devices, Sunnyvale, CA) as described elsewhere (13). The system incorporates a fluid transfer workstation for addition of test compounds from a source plate to the cell plate during data acquisition. CMVEC were loaded with fura-2 AM (4 μM) in the presence of 0.01% pluronic acid in modified Krebs solution (120 mM NaCl, 5 mM KCl, 0.62 mM MgSO4, 1.8 mM CaCl2, 10 mM HEPES, and 6 mM glucose, pH 7.4) for 30 min at 38°C in the dark. The loading medium was replaced with modified Krebs solution before analysis. Fura-loaded CMVEC were stimulated with glutamate (1–20 μM), and [Ca2+]c tracings were monitored for 80–120 s by the ratio of emitted light intensity at 520 nm elicited by excitation at a 340- or 380-nm wavelength lights, respectively. Ca2+ transients were automatically quantified by the SoftMax Pro software (Molecular Devices, Sunnyvale, CA) based on the difference between maximum and baseline ratio values for each well. As positive controls, we used ionomycin (10 μM) and ATP (20 μM). [Ca2+]c was expressed as a percentage of maximal ionomycin response.
Detection of BBB permeability.
Confluent CMVEC on the collagen-coated Transwell inserts were exposed overnight to glutamine- and serum-depleted DMEM. CMVEC in monolayer were incubated for 1–5 h with glutamate or iGluR ligands applied to the upper chamber (luminal side).
CORM-A1 (50 μM) was also applied to the luminal side of the endothelial monolayer. Transendothelial electrical resistance (TEER) was measured using the Millicell electrical resistance system (Millicell-ERS, Millipore; Billerica, MA) and calculated as ohms per centimeters squared (42). To measure BBB paracellular permeability, 3-kDa dextran-conjugated Alexa Fluor 488 (1 μg/ml) was applied to the luminal side of CMVEC. Following the 5-h exposure to glutamate or iGluR ligands as above, aliquots of media from the upper (luminal side) and lower (abluminal side) chambers were collected for measurements of endothelial paracellular permeability to dextran-Alexa Fluor 488. Alexa Fluor 488 fluorescence (excitation/emission maxima of 495/519 nm) was detected by a Synergy HT microplate reader.
Statistical analysis.
Data are presented as means ± SE of absolute values or percent of control. ANOVA with repeated measures and the Tukey-Kramer multiple comparisons test were used to confirm differences among and then between groups, respectively. A level of P < 0.05 was considered significant.
Materials.
Cell culture reagents were purchased from Life Technologies (Gaithersburg, MD), Hyclone (South Logan, UT), Roche Diagnostics (Indianapolis, IN), and GE Healthcare Biosciences. Matrigel was from BD Biosciences (Bedford, MA). Dihydroethidium was from Invitrogen (Life Technologies, Grand Island, NY). Glutamate receptor ligands were from Tocris (R&D Systems, Minneapolis, MN). CORM-A1 was from Dalton Pharma Services (Toronto, Canada). All other reagents were from Sigma (St. Louis, MO).
RESULTS
Endogenous enzymatic sources of ROS activated by glutamate in CMVEC.
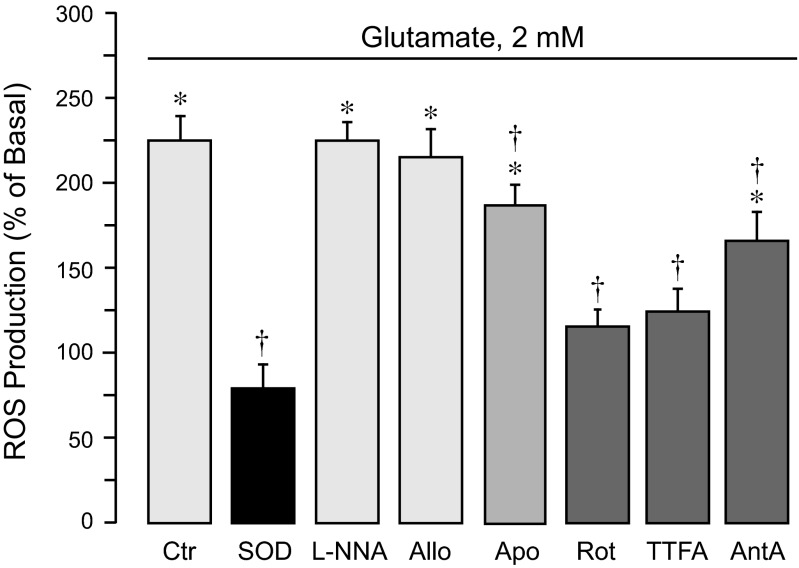
Glutamate (0.1–2 mM) increased ROS formation in CMVEC (median effective concentration, EC50 ∼0.3 mM; maximal ROS increase, 240 ± 20% of control). ROS production is provided by numerous enzyme complexes, including NADPH oxidase, NO synthase, xanthine oxidase, and the mitochondrial respiratory chain. Using selective pharmacological inhibitors of these complexes, we investigated the contributions of distinct enzymatic sources to oxidative stress caused by excitotoxic glutamate in CMVEC (Fig. 1). The superoxide scavenger superoxide dismutase completely blocked glutamate-stimulated ROS, suggesting that superoxide is the major component of endothelial ROS (Fig. 1). Mitochondrial respiratory chain inhibitors, rotenone (a complex I NADH dehydrogenase inhibitor; 1–5 μM), 2-thenoyltrifluoroacetone (TTFA, a complex II inhibitor; 5–10 μM), and antimycin A (a complex III inhibitor; 1–5 μM), greatly reduced glutamate-evoked ROS (50–90% reduction). Apocynin (0.5 mM), a NADPH oxidase inhibitor, reduced the ROS response by ∼20%. The NOS inhibitor NG-nitro-l-arginine (l-NNA; 1 mM) and xanthine oxidase inhibitor allopurinol (50 μM) did not alter the glutamate-induced ROS formation. These data suggest that mitochondrial complexes I, II, and III are the major endogenous producers of ROS evoked by glutamate in CMVEC.
Fig. 1.
Intracellular sources of reactive oxygen species (ROS) activated by glutamate in cerebral microvascular endothelial cells (CMVEC) from newborn piglets. Confluent quiescent CMVEC were untreated (baseline) or exposed to 2 mM glutamate for 1 h in the absence (Ctr) or presence of ROS inhibitors: superoxide dismutase (SOD from bovine erythrocytes; 1,000 U/ml); a nitric oxide synthase inhibitor, NG-nitro-l-arginine (l-NNA; 1 mM); a xanthine oxidase inhibitor, allopurinol (Allo; 50 μM); a NADPH oxidase inhibitor, apocynin (Apo; 0.5 mM); a mitochondrial complex I inhibitor, rotenone (Rot; 1 μM); a complex II inhibitor, thenoyltrifluoroacetone (TTFA; 5 μM); or a complex III inhibitor antimycin A (AntA; 1 μM). ROS production was measured by accumulation of oxidized dihydroethidium (Ox-DHE) fluorescent products and expressed as %baseline values. Data represent the average of 4 experiments. Values are means ± SE. *P < 0.05, compared with the basal value. †P < 0.05, compared with glutamate alone.
Effects of ROS inhibitors on glutamate-induced endothelial apoptosis.
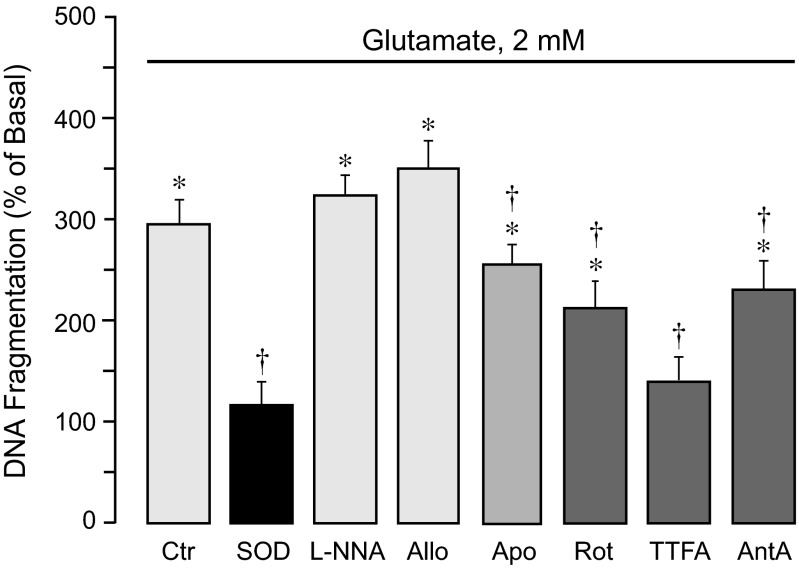
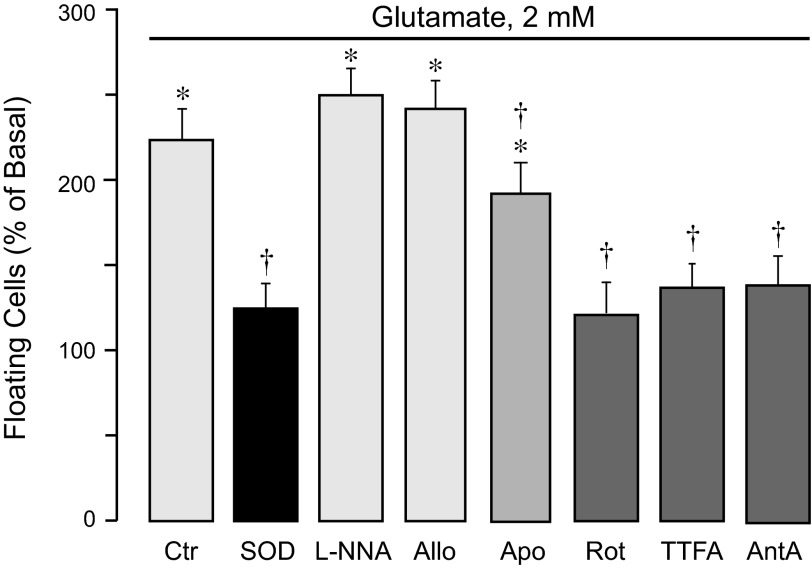
Glutamate at excitotoxic concentrations (1–2 mM, 3 h) causes apoptosis of CMVEC as determined by early apoptotic DNA fragmentation (Fig. 2). Apoptosis in cell types that require extracellular matrix for growth and differentiation is intimately associated with loss of cell-cell and cell-matrix interactions (15, 24). In CMVEC, glutamate-induced DNA fragmentation is accompanied by simultaneous cell detachment from the substrate (Fig. 3). Glutamate-induced apoptosis and cell detachment are completely blocked by superoxide dismutase (Figs. 2 and 3), suggesting that superoxide is a major proapoptotic mediator. l-NNA (1 mM) and allopurinol (50 μM) had no effect on apoptosis, whereas apocynin (0.5 mM) reduced the DNA fragmentation and cell detachment caused by glutamate by 20–25% (Figs. 2 and 3). The inhibitors of the mitochondrial respiratory chain at concentrations that reduced the ROS response (5 μM rotenone, 5 μM TTFA, and 5 μM antimycin A) reduced glutamate-evoked DNA fragmentation and cell detachment by 50–80% (Figs. 2 and 3). These data suggest that mitochondrial oxidative stress is a leading factor in cerebrovascular endothelial cell apoptosis caused by glutamate excitotoxicity.
Fig. 2.
Contributions of endogenous ROS sources to glutamate-induced apoptosis. Confluent quiescent CMVEC from newborn pigs were incubated with 2 mM glutamate in the absence or presence of various ROS inhibitors: SOD (1,000 U/ml); a nitric oxide synthase inhibitor, l-NNA (1 mM); a xanthine oxidase inhibitor, Allo (50 μM); a NADPH oxidase inhibitor, Apo (0.5 mM); or mitochondrial inhibitors: Rot (5 μM), TTFA (5 μM), or AntA (5 μM) for 3 h at 37°C. Apoptosis was detected by DNA fragmentation and expressed as %baseline values. Data represent the average of 4 experiments. Values are means ± SE. *P < 0.05, compared with the basal value. †P < 0.05, compared with glutamate alone.
Fig. 3.
Contributions of endogenous ROS sources to glutamate-induced loss of cell contacts. Confluent quiescent CMVEC from newborn pigs were incubated with 2 mM glutamate in the absence or presence of various ROS inhibitors: SOD (1,000 U/ml); a nitric oxide synthase inhibitor, l-NNA (1 mM); a xanthine oxidase inhibitor, Allo (50 μM); a NADPH oxidase inhibitor, Apo (0.5 mM); or mitochondrial inhibitors: Rot (5 μM), TTFA (5 μM), and AntA (5 μM) for 3 h at 37°C. Loss of cell contacts was detected by the number of floating cells detached from the matrix and expressed as %baseline values. Data represent the average of 5 experiments. Values are means ± SE. *P < 0.05, compared with the basal control value. †P < 0.05, compared with glutamate alone.
Glutamate elevates cytosolic Ca2+ in CMVEC.
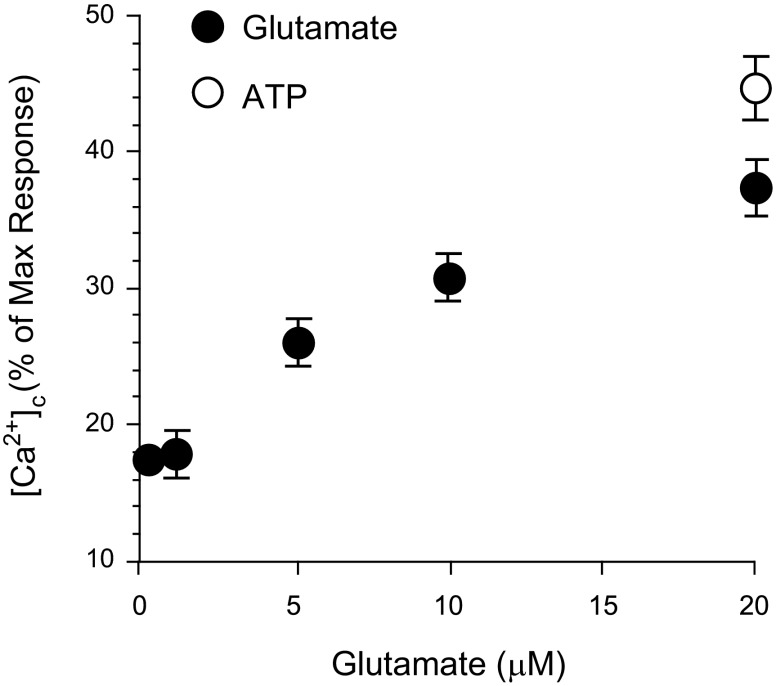
Glutamate (10−6-2 × 10−5 M) dose dependently increased cytosolic Ca2+ in CMVEC (Fig. 4). This finding suggests that glutamate stimulation causes Ca2+ overload that may trigger apoptosis in the cerebral vascular endothelium.
Fig. 4.
Effects of glutamate on cytosolic Ca2+ ([Ca2+]c) in CMVEC. CMVEC loaded with fura-2 AM were stimulated with glutamate, and [Ca2+]c was measured by a Flexstation II scanning fluorometer. Ionomycin (10 μM) and ATP (20 μM) were used as positive controls. [Ca2+]c was expressed as %ionomycin response.
iGluR agonists increase ROS production and apoptosis in CMVEC.
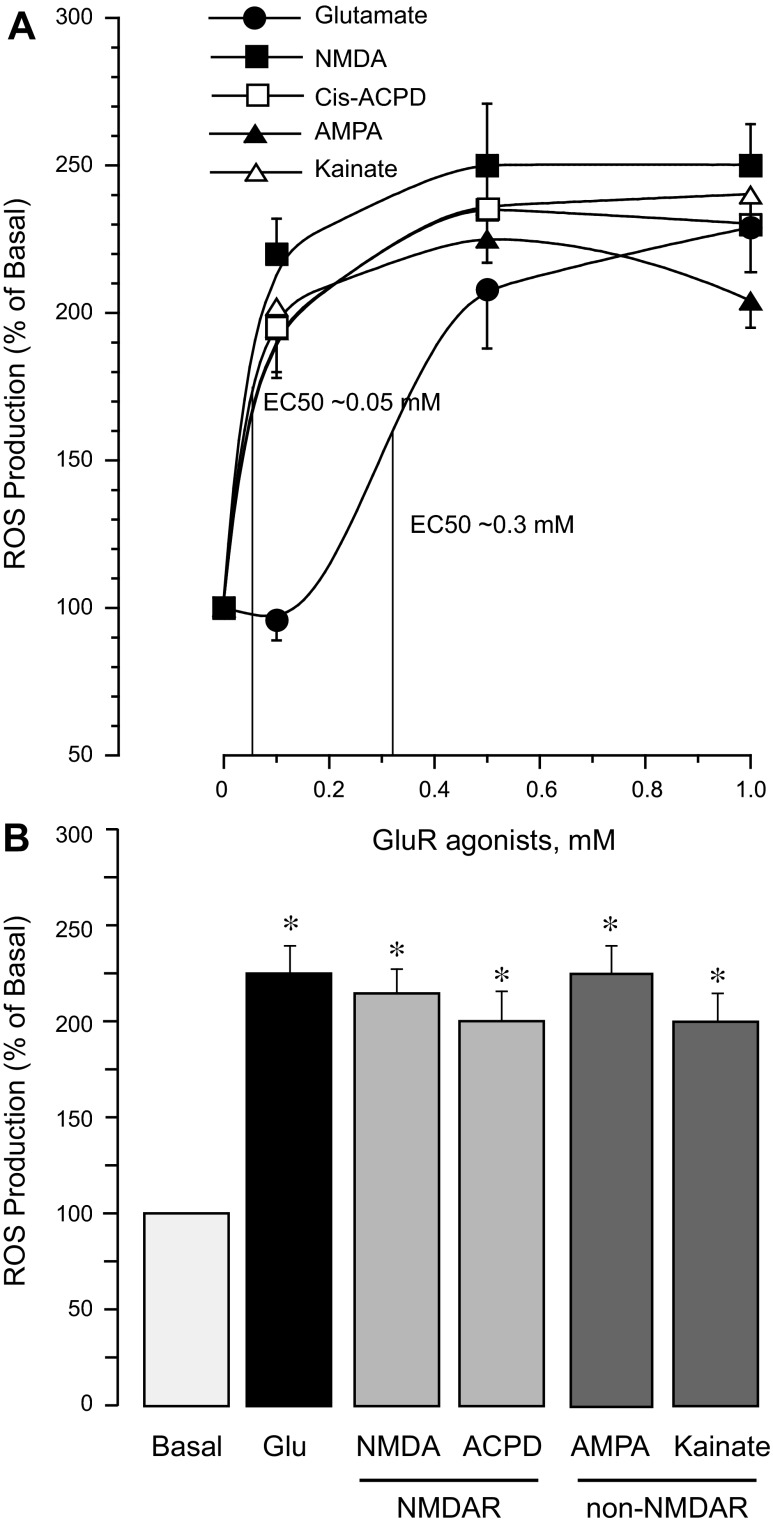
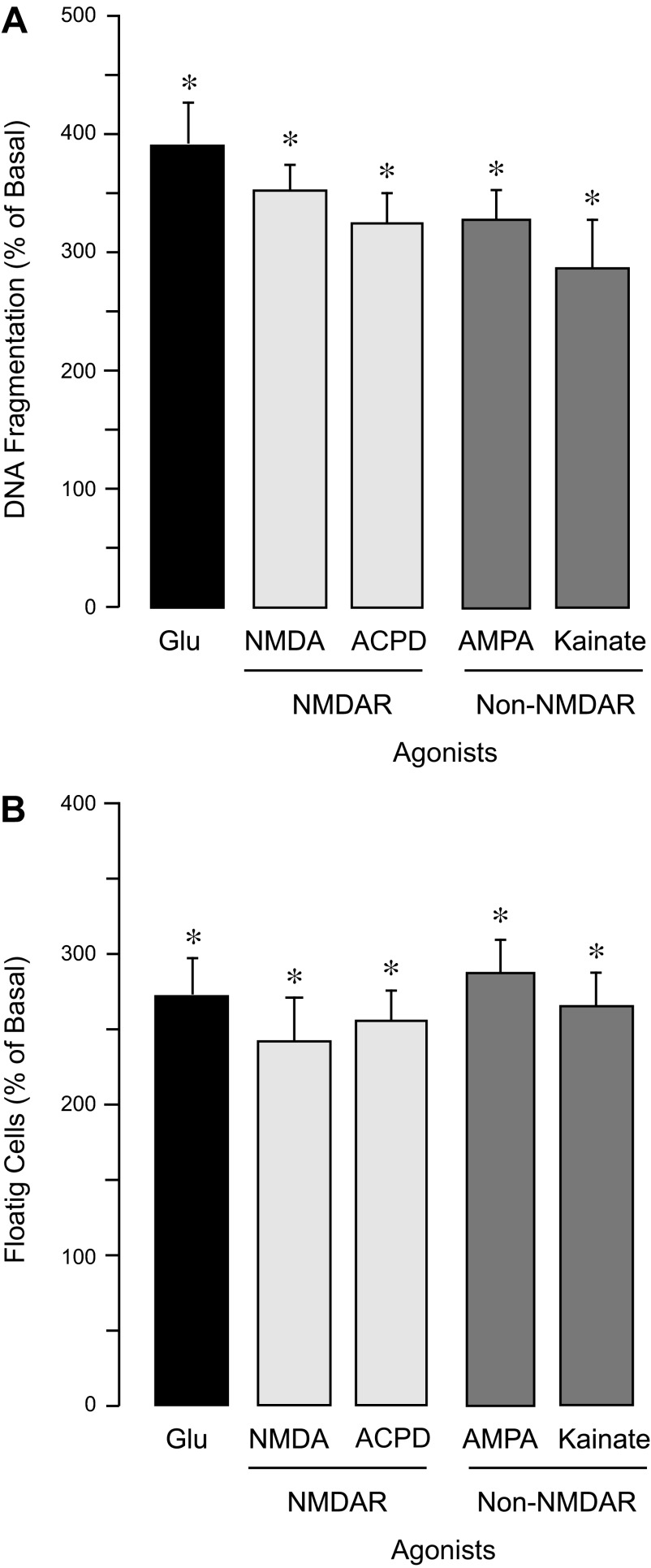
We determined the dose-dependent effects of agonists of iGluRs on endothelial ROS. Agonists of NMDA receptors (NMDAR), NMDA and cis-ACPD (0.1–1.0 mM), as well as agonists of AMPA/kainate receptors, AMPA and kainic acid, efficiently increased ROS production by CMVEC (Fig. 5A). Concentrations that produced half-maximal ROS stimulation (EC50) were 0.05 and 0.3 mM for iGluR agonists and glutamate, respectively (Fig. 5A). However, the maximal stimulation of endothelial ROS production achieved by glutamate (1 mM) or iGluR agonists (0.5 mM) was nearly identical (220 ± 25% of the baseline value; Fig. 5, A and B). A 3-h exposure of CMVEC to NMDA, cis-ACPD, AMPA, and kainate caused apoptosis and cell detachment (Fig. 6, A and B). The iGluR agonists were more dose efficient than glutamate in their proapoptotic effects (a 2- to 4- fold increase in DNA fragmentation and cell floating was achieved either by 0.1 mM iGluR agonists or by 2 mM glutamate; Fig. 6, A and B).
Fig. 5.
iGluR agonists increase ROS production by CMVEC. Confluent quiescent CMVEC were incubated with: glutamate, agonists of N-methyl-D-aspartate (NMDA) receptors (NMDAR) NMDA and cis-ACPD; or agonists of AMPA/kainate receptors (non-NMDAR), AMPA, and kainic acid, for 1 h at 37°C. A: dose-dependent effects of glutamate and iGluR agonists (0.1–1.0 mM) on ROS production. B: comparison of maximal ROS-increasing potencies of glutamate (1.0 mM) and iGluR agonists (0.5 mM). ROS production was detected by accumulation of Ox-DHE fluorescent products and expressed as %control baseline values. Data represent the average of 4 experiments. Values are means ± SE. *P < 0.05, compared with the basal value.
Fig. 6.
Effects of iGluR agonists on DNA fragmentation and loss of cell contacts in CMVEC. Confluent quiescent CMVEC were incubated with 2 mM glutamate or 0.1 mM of iGluR agonists (NMDA, cis-ACPD, AMPA, and kainic acid) for 3 h at 37°C. Apoptosis was detected by DNA fragmentation (A) and expressed as %baseline values. Loss of cell contacts was detected by the number of detached floating cells and expressed as %baseline values. Data represent the average of 3 experiments. Values are means ± SE. *P < 0.05, compared with the basal values.
Contribution of iGluRs to glutamate-induced apoptosis in CMVEC.
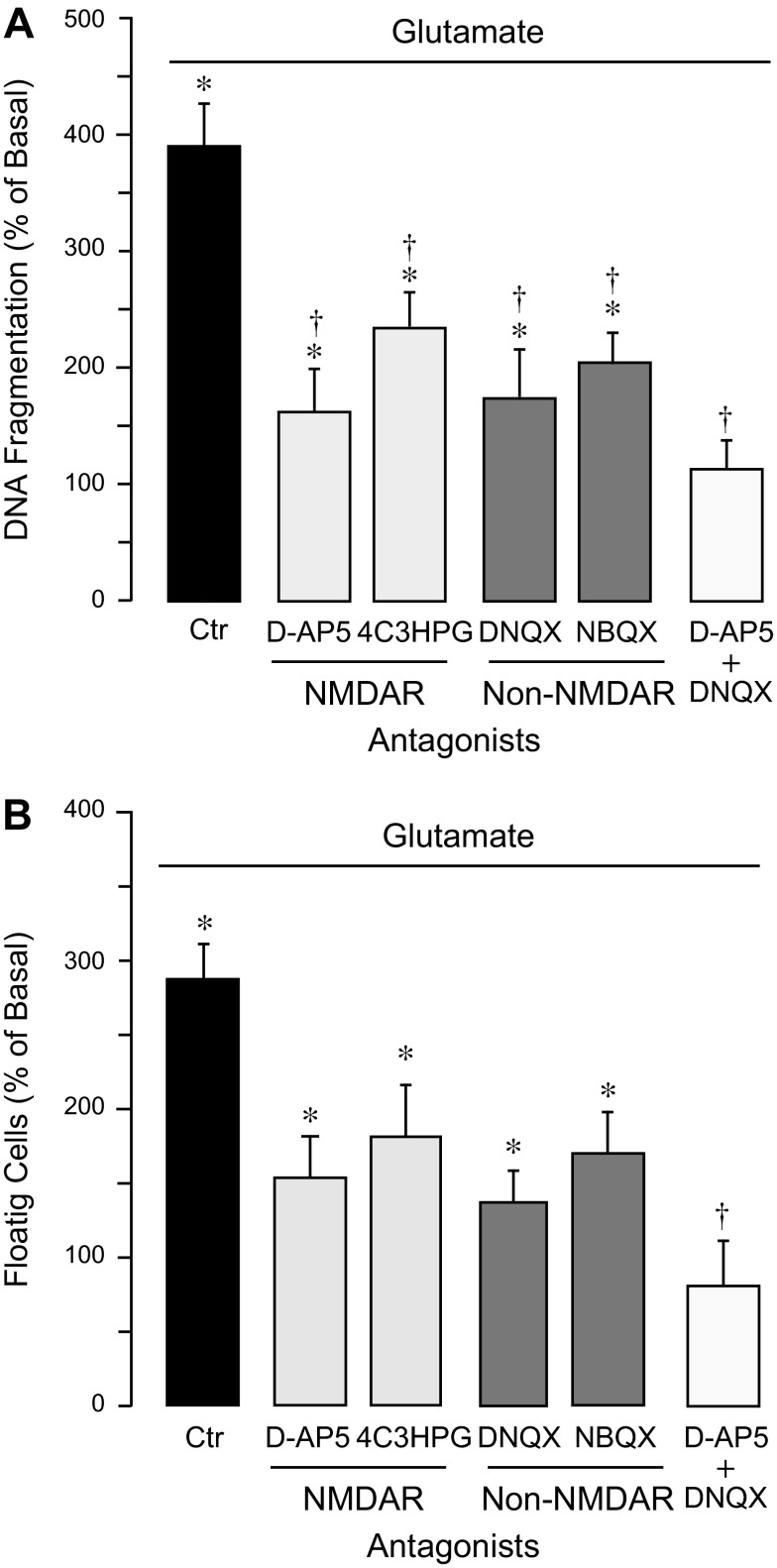
To investigate the relative contributions of iGluRs to proapoptotic effects of glutamate, we applied a pharmacological competitive displacement approach using NMDAR antagonists, D-AP5 and 4C3HPG, and AMPA/kainate antagonists, DNQX and NBQX. A 15-min preincubation of CMVEC with either antagonist (0.1 mM) greatly reduced DNA fragmentation and loss of cell-cell contacts caused by 2 mM glutamate (Fig. 7, A and B). Combining the antagonists of NMDAR and non-NMDARs, D-AP5 and DNQX (0.1 mM), completely prevented glutamate-induced apoptosis and cell detachment (Fig. 7, A and B). These data suggest that apoptotic effects of glutamate are largely mediated via NMDAR and AMPA/kainate types of GluRs.
Fig. 7.
Contributions of iGluRs to glutamate-induced apoptosis in CMVEC. Confluent quiescent CMVEC were incubated with 2 mM glutamate alone (Ctr) or supplemented with antagonists of NMDARs (D-AP5, 4C3HPG, 0. 1 mM) and non-NMDARs (DNQX, NBQX, 0.1 mM) for 3 h at 37°C. Apoptosis was detected by DNA fragmentation (A) or by cell detachment from the substrate (B) and expressed as %baseline values. Data represent the average of 4 experiments. Values are means ± SE. *P < 0.05, compared with the basal values. †P < 0.05, compared with glutamate alone.
Glutamate and iGluR agonists cause intracellular redistribution of cytochrome c.
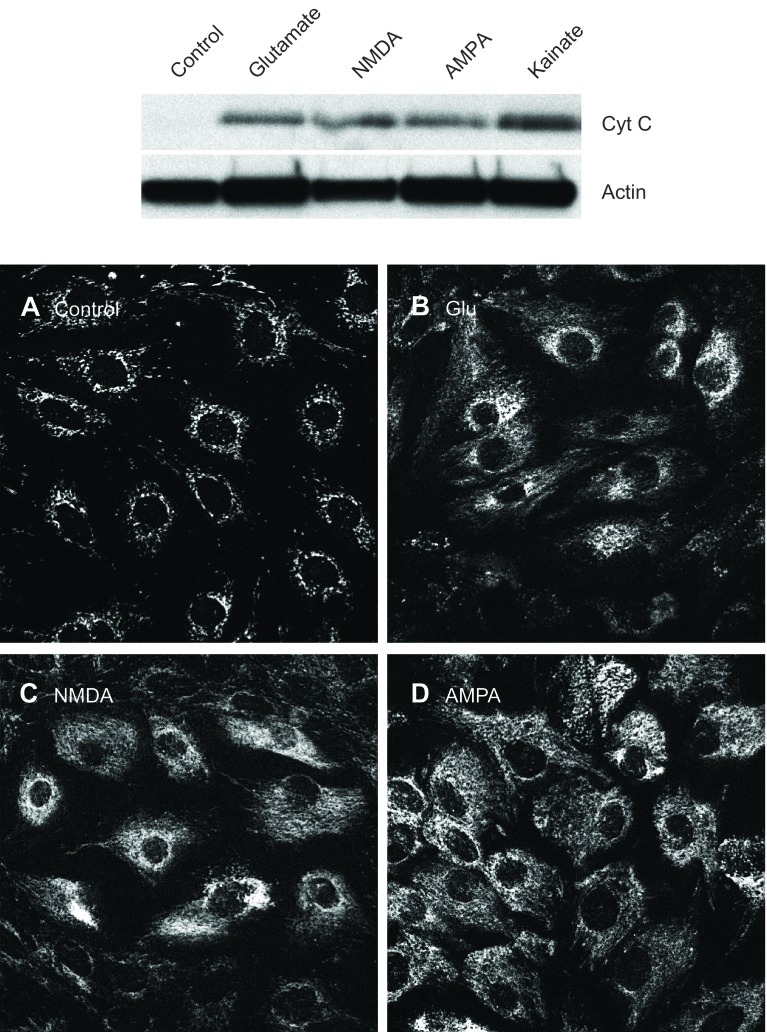
In control CMVEC, cytochrome c is localized perinuclearly coincident with mitochondria; no cytochrome c is detectable by immunofluorescence and Western immunoblotting in the cytoplasm (Fig. 8). Cytochrome c release from the mitochondrial compartment to the cytoplasm initiates the proteolytic caspase cascade leading to apoptosis (16, 18, 22, 30, 38). In cells exposed for 3 h to glutamate (1 mM), NMDA (0.1 mM), or AMPA (0.1 mM), cytochrome c is relocated to the cytoplasmic area (Fig. 8), indicating that glutamate activates the mitochondrial pathway of apoptosis.
Fig. 8.
Detection of cytochrome c. Confluent quiescent CMVEC were exposed to glutamate (1 mM), NMDA (0.1 mM), AMPA (0.1 mM), or kainate (0.1 mM) for 3 h. Cytochrome c in control and treated cells was detected by immunoblotting in mitochondria-free cytosolic fraction (top) and by immunofluorescence in paraformaldehyde-fixed cells (A–D).
Glutamate and iGluR agonists increase BBB permeability.
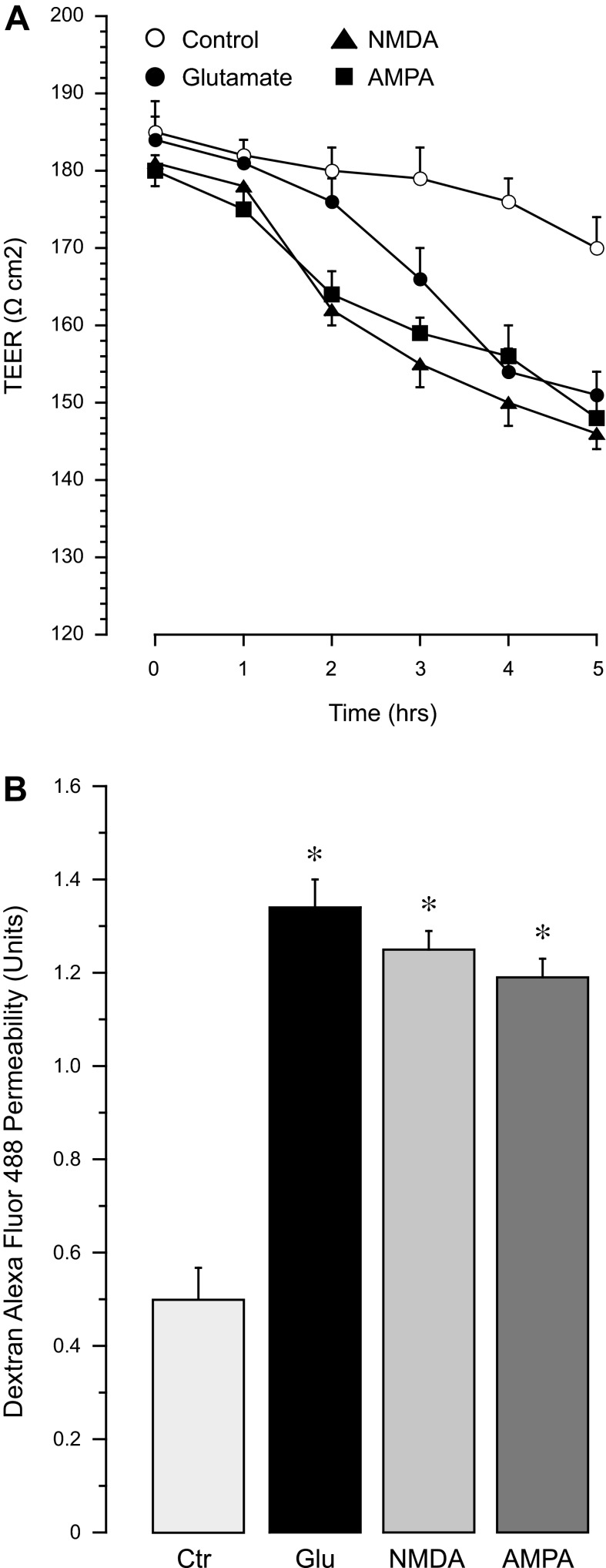
To investigate functional implications of glutamate excitotoxicity in cerebral vascular endothelial cells, we detected the effects of iGuR agonists on BBB permeability. the BBB properties of endothelial monolayers were measured by TEER and paracellular permeability to 3-kDa dextran-conjugated Alexa Fluor 488. CMVEC monolayers on semipermeable membranes responded to 2 mM glutamate by a time-dependent reduction in TEER in 3–5 h (Fig. 9A) and by a approximately threefold increase in permeability to 3-kDa dextran (Fig. 9B). NMDA and AMPA, at a 10-fold lower concentration (0.2 mM), caused a faster onset of TEER reduction that was observed after a 2-h exposure (Fig. 9A). The maximal loss of BBB properties (TEER reduction and increased paracellular permeability) in the presence of 0.2 mM iGluR agonists was comparable to the effects of 2 mM glutamate (Fig. 9, A and B). Glutamate-induced BBB dysfunction was concomitant with high sensitivity of CMVEC to prooxidant and proapoptotic effects of iGluR agonists (Figs. 5 and 6).
Fig. 9.
Effects of glutamate and iGluR agonists on blood-brain barrier (BBB) properties of endothelial monolayers. Confluent quiescent CMVEC grown on semipermeable membranes in Costar Transwells were exposed to glutamate (Glu; 2 mM), NMDA (0.2 mM), or AMPA (0.2 mM) for 1–5 h. A: transendothelial electrical resistance (TEER) was measured using a Millicell electrical resistance system. B: paracellular permeability of the endothelial monolayer to 3-kDa dextran-conjugated Alexa Fluor 488 was detected after 5 h incubation with glutamate and iGluR agonists and expressed as the ratio of abluminal to luminal fluorescence values. Data represent the average of 3 experiments. Values are means ± SE. *P < 0.05, compared with the control values.
Cytoprotective effects of CORM-A1 against endothelial injury and loss of BBB integrity caused by iGluR stimulation.
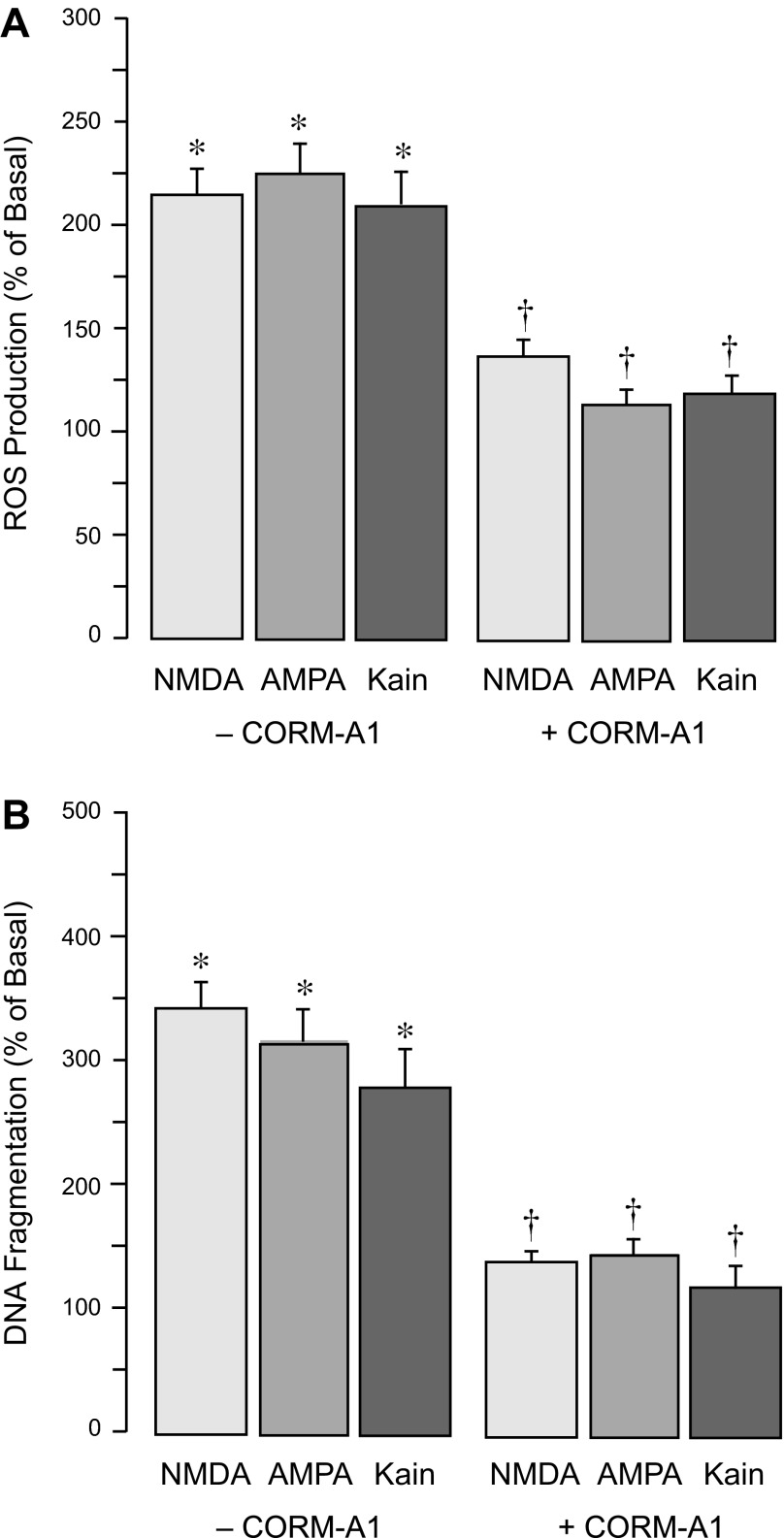
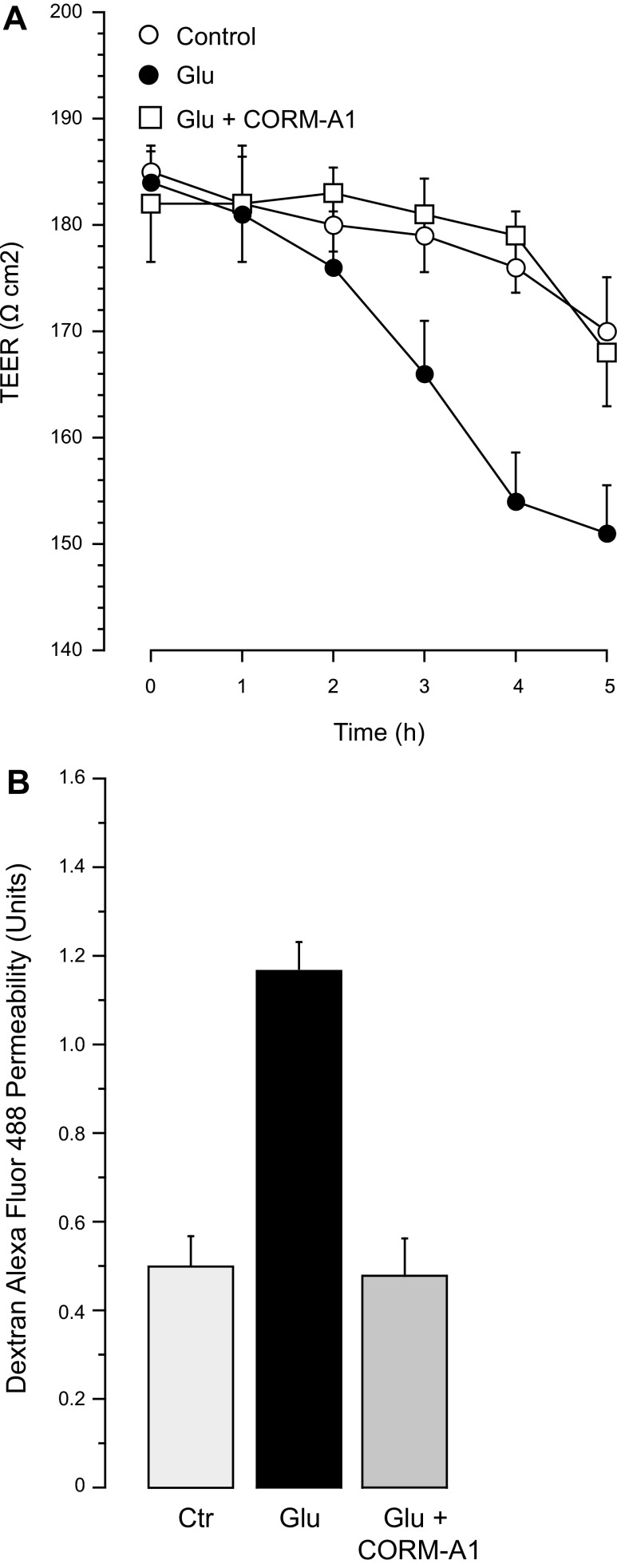
A CO-releasing molecule, CORM-A1, exhibits strong antioxidant and antiapoptotic properties against cerebrovascular endothelial damage caused by seizures in newborn pigs (35, 46). We have now used CORM-A1 (50 μM) to model the cytoprotective effect of gaseous CO in the in vitro model of cerebral vascular endothelial injury. CORM-A1 in physiological solutions provides a concentration- and time-dependent release of gaseous CO, averaging 2 nmol CO/μM CORMA1/10 min in a 2-ml closed space during the first hour, with a half-life of 3.5 h (46). We estimate that 50 μM CORM-A1 produces a physiologically relevant level of gaseous CO (<10−5 M). CORM-A1 (50 μM) effectively blocked ROS production and apoptosis in CMVEC caused by glutamate (2 mM) or the iGluR agonists NMDA, AMPA, and kainate (0.1 mM; Fig. 10). CORM-A1 also completely prevented loss of BBB integrity caused by excitotoxic levels of glutamate (Fig. 11). In the presence of 50 μM CORM-A1, TEER and paracellular permeability were maintained at the baseline levels in spite of the continuous presence of 2 mM glutamate in the incubation media (Fig. 11, A and B).
Fig. 10.
Antioxidant and antiapoptotic effects of CORM-A1 against endothelial injury caused iGluR agonists. Confluent quiescent CMVEC were incubated with NMDA, AMPA, or kainic acid (0.1 mM) in the absence or presence of 50 μM CORM-A1 at 37°C for 1 h (A) or 3 h (B). A: ROS production was detected by accumulation of Ox-DHE fluorescent products and expressed as %baseline values. B: apoptosis was detected by DNA fragmentation and expressed as %baseline values. Data represent the average of 3 experiments. Values are means ± SE. *P < 0.05, compared with basal values. †P < 0.05, compared with the corresponding values in the absence of CORM-A1.
Fig. 11.
Cytoprotective effects of CORM-A1 against BBB dysfunction caused by glutamate and iGluR agonists. Confluent quiescent CMVEC grown on semipermeable membranes in Costar Transwells were exposed to glutamate (2 mM), in the absence or presence of CORM-A1 (50 μM) for 5 h. A: TEER was detected using a Millicel electrical resistance system. B: paracellular permeability of endothelial monolayers to 3-kDa dextran-conjugated Alexa Fluor 488 was detected after 5 h incubation with glutamate and expressed as the ratio of abluminal to luminal fluorescence values. Data represent the average of 6 experiments. Values are means ± SE. *P < 0.05, compared with the baseline control values. †P < 0.05, compared with glutamate alone.
DISCUSSION
Glutamatergic epileptic seizures cause oxidative stress and long-term loss of endothelial vasodilator function in the cerebral circulation of newborn piglets (21, 35, 46). We investigated the mechanisms and functional consequences of glutamate-induced oxidative stress and apoptosis in cerebral vascular endothelium. Our major findings in CMVEC from newborn piglets are as follows: 1) glutamate causes endothelial apoptosis associated with oxidative stress; 2) mitochondria are a major intracellular source of apoptosis-inducing ROS; 3) iGluRs mediate glutamate-induced endothelial oxidative damage; 4) iGluR-mediated apoptosis and disruption of endothelial contacts result in increased BBB permeability; and 5) a CO-releasing molecule, CORM-A1, prevents oxidative stress, apoptosis, loss of endothelial contacts, and BBB dysfunction caused by glutamate.
Glutamate excitotoxicity is a pathological condition when the extracellular level of this main excitatory neurotransmitter in the brain is elevated to 10−4-10−3 M. This level is achieved during seizures that are associated with massive release of glutamate thus recruiting neurons into epileptic discharges (7, 18, 23). Excitotoxic glutamate causes neuronal death by apoptosis and necrosis (8, 22, 29). The neonatal brain is the most susceptible for seizure-induced injury that leads to sustained neurological problems in children (12, 44).
Cerebrovascular endothelial cells are nonneuronal targets of excitotoxic glutamate in the neonatal brain. In cultured CMVEC from newborn piglets, glutamate (≥10−4 M) increased ROS formation. In piglet endothelial cells, the prooxidant potency of glutamate is comparable to that of such strong oxidants as arachidonic acid, peroxynitrite, and hydrogen peroxide (33). However, only neurotoxic concentrations of glutamate (1–2 mM) lead to cerebrovascular endothelial cell death by oxidative stress-mediated apoptosis. Of note, CMVEC from neonatal piglets have much greater sensitivity to glutamate excitotoxicity than cells from the adult mouse brain (33). Glutamate-induced disruption of cell contacts leading to detachment of cerebral vascular endothelial cells from the substrate highly correlates with DNA fragmentation and caspase-3 activation, the hallmarks of apoptosis. Apoptosis in cell types that require extracellular matrix for proliferation and differentiation is intimately associated with inadequate cell-cell and cell-matrix interactions; vice versa, disruption of cell anchorage may induce apoptosis (15, 24). The loss of functional integrity of adherens junction proteins, such as focal adhesion kinase, VE-cadherin, and β-catenin, is the key component in the mechanism linking apoptosis and cell detachment in anchorage-dependent cells. In CMVEC from newborn piglets and adult mice, DNA fragmentation and apoptosis induced by TNF-α are accompanied by simultaneous disassembly of adherens junctions, leading to loss of cell anchorage and to cell detachment from the matrix (2). CMVEC detached from the extracellular matrix following a 3-h exposure to TNF-α, glutamate, or iGluR agonists have little ability to reattach and grow after reseeding (data not shown), indicating that these cells are irreversibly damaged. This mechanism may account for the appearance of circulating endothelial cells of brain origin in the peripheral blood of newborn piglets during glutamatergic epileptic seizures (35). Brain-derived circulating endothelial cells that appear 2–4 h after onset of seizures are early indicators of cerebral vascular damage and loss of endothelium-mediated vasodilator functions (35).
What is the physiological relevance of cerebral vascular endothelial damage by glutamate? Brain endothelial cells are exposed to glutamate from both luminal (blood) and abluminal (brain interstitial space) surfaces. Blood glutamate level (10−5 M) is maintained steadily in healthy people even after excessive dietary intake and in patients with epilepsy and stroke (8, 18, 40). Therefore, the blood glutamate is below the level of cytotoxicity for brain endothelium. In contrast, concentrations of glutamate in the brain interstitial space may reach mM levels during seizures and stroke (5, 23, 28). Local concentrations of glutamate released by neurons and astrocytes in proximity to the endothelial component of the neurovascular unit during seizures could be even higher. In vivo experimental evidence suggests that glutamatergic seizures in neonatal piglets cause damage in small resistance vessels (20–300 μm), including increased ROS and the number of terminal transferase-mediated dUTP nick end-labeling-positive cells (35). Furthermore, the loss of endothelial-dependent vasodilator function and the appearance of brain-derived endothelial cells in peripheral blood clearly suggest that neonatal glutamatergic seizures produce cerebral vascular endothelial damage (35). Overall, during seizures, the excitotoxic level of glutamate in the brain interstitial space poses a substantial risk factor to the cerebral vascular endothelial lining. These findings are in agreement with our data in cultured CMVEC demonstrating that excitotoxic glutamate causes oxidative stress and apoptosis, and produces endothelial dysfunction.
Mitochondria are the major intracellular sources of ROS in glutamate-challenged cerebrovascular endothelial cells. The mitochondrial respiratory chain, a major cellular source of superoxide, consists of electron transport carriers that include complexes I (NADH/CoQ oxidoreductase); II (succinate dehydrogenase); III (ubiquinol/cytochrome c oxidoreductase, or cytochrome bc1), a central component of the respiratory electron transport chain involved in oxidative phosphorylation; IV (cytochrome c oxidase); and V (ATP synthase) (16, 19). Electron transfer to molecular oxygen during oxidative phosphorylation generates superoxide mainly via complexes I and III (16, 19, 29). During normal conditions, only 1–2% of electrons leak out to form superoxide, whereas under certain pathophysiological conditions, the electron transport chain may become uncoupled, thus causing a surge in superoxide that, in turn, may impair mitochondrial functions (19, 29). Mitochondrial dysfunction is linked to seizures and epilepsy (43). In cerebral vascular endothelial cells, superoxide anion is a leading factor in glutamate-induced oxidative stress, as suggested by the potent cytoprotective effects of superoxide dismutase against glutamate excitotoxicity. Our data suggest that mitochondria account for ∼80% of glutamate-evoked ROS in the cerebral vascular endothelium. We identified mitochondrial complexes I, II, and III as the major sites responsible for elevation of superoxide production in cerebral vascular endothelium exposed to glutamate.
NADPH oxidase also contributes to glutamate-induced oxidative stress and cerebral vascular endothelial injury. Constitutively active Nox-1, Nox-2, and Nox-4 isoforms of NADPH oxidase are expressed in piglet CMVEC (3, 4). The Nox-4 isoform is a major source of oxidative stress, leading to apoptosis in CMVEC exposed to the proinflammatory cytokine TNF-α (3). Our new data in glutamate-challenged CMVEC demonstrate that the contribution of NADPH oxidase to ROS and apoptosis is moderate but significant (∼20%). The contribution of various Nox isoforms to the endothelial response to glutamate remains to be elucidated. It also cannot be excluded that NADPH oxidase activation in glutamate-challenged CMVEC may result from cross talk with mitochondrial ROS, as has been described in certain experimental situations (11).
iGluRs are involved in the mechanism of glutamate-induced oxidative stress injury of cerebral vascular endothelium. In neurons, excitotoxic effects of glutamate are mediated via GluRs. The most rapid neuronal injury results from activation of NMDAR and AMPA/kainate receptors and massive Ca2+ entry (7, 23, 38). Cerebral vascular endothelium expresses NMDA- and non-NMDA (AMPA and kainate) types of iGluRs, as well as metabotropic glutamate receptors (mGluRs; Ref. 34). Agonists of iGluRs (NMDA, cis-ACPD, AMPA, and kainate) increased ROS and apoptosis in CMVEC. Endothelial cells exhibited ∼10-fold higher sensitivity to prooxidant activities of the specific ligands of iGluRs than to glutamate itself. Addition of antagonists of NMDA- and AMPA/kainate receptors, in a synergistic manner, greatly reduced ROS formation and apoptosis in response to excitotoxic glutamate. These data suggest that iGluR antagonists exhibit potent cerebroprotective effects against glutamate-induced endothelial injury in the neonatal cerebral circulation.
Disruption of Ca2+ homeostasis is the key mechanism in mitochondrial dysfunction linked to glutamate excitotoxicity. Mitochondrial uncoupling has been implicated in excitotoxic neuronal damage (29, 38) and in pathophysiology of epilepsy (43) and neurodegenerative disease. In neurons, activation of iGluRs by glutamate leads to opening of Ca2+ channels and increasing intracellular Ca2+ followed by mitochondrial oxidative stress, and mitochondrial permeabilization that results in release of cytochrome c to the cytoplasm (16, 18, 22, 30, 38). Cytochrome c release from the mitochondrial compartment to the cytoplasm is the key event in the mitochondrial pathway of apoptosis (16, 18, 22, 30, 38). Cytochrome c is normally sequestered in the inner mitochondrial membrane and participates in the electron transport chain between complexes III and IV. When released from the mitochondrial compartment to the cytoplasm, cytochrome c initiates the proteolytic caspase cascade leading to apoptosis (mitochondrial, or “intrinsic,” pathway of apoptosis).
We, for the first time, demonstrate that glutamate-induced mitochondrial dysfunction is the key event leading to apoptosis of cerebrovascular endothelial cells. Our data suggest that in cerebral vascular endothelium, similar to neuronal events, the mechanism of iGluR-mediated glutamate-induced apoptosis is associated with the increase in cytoplasmic Ca2+, excessive generation of mitochondrial ROS, and release of cytochrome c to the cytoplasm. As our data demonstrate, selective inhibitors of mitochondrial complexes I, II, and III prevented glutamate-induced excessive ROS formation and protected the cerebral vascular endothelium from apoptosis.
Heme oxygenase, constitutively expressed in cerebral vascular endothelium as the HO-2 isoform, provides endogenous cytoprotection against vascular damage resulting from glutamatergic seizures in newborn piglets (21, 35). In cerebral vascular endothelium, HO-2 is posttranslationally activated by glutamate via iGluR-mediated mechanism (21, 33, 34). The cytoprotective properties of HO are linked to antioxidant capacities of CO and bilirubin, the by-products of HO-mediated heme degradation. CO, long perceived as a toxic gas, has emerged as an endogenously produced messenger that has vasodilator and antioxidant functions in the cerebrovascular system (21). The biological effects of CO are caused by its high-affinity binding to heme. The antioxidant properties of low physiologically relevant concentrations of CO (200–500 ppm) result from the ability of this heme-binding gaseous mediator to inhibit various intracellular sources of ROS. Conversely, high concentrations of CO may exaggerate mitochondrial ROS production and cause cell death (39). Using a water-soluble CO-releasing molecule (CORM-A1) that provides a sustained slow release of CO, we demonstrated the ability of CO to inhibit Nox4 NADPH oxidase, thus preventing TNF-α-induced apoptosis of CMVEC (3, 4). In glutamate-stimulated CMVEC, the role of NADPH oxidase as a cellular course of ROS is less significant, whereas mitochondrial complexes I, II, and III are major contributors to oxidative stress and apoptosis. CORM-A1 acutely blocked endothelial ROS production in response to glutamate, unveiling the ability of CO to prevent mitochondrial uncoupling caused by glutamate excitotoxicity and to promote endothelial survival. In contrast, prolonged (24 h) exposure to CO increased mitochondrial ROS in airway vascular smooth muscle cells (10). High-affinity CO binding to the heme prosthetic groups may explain the diverse effects of CO on numerous heme-containing key signaling proteins. In mitochondria, hemes are used as protein-bound prosthetic groups in succinate dehydrogenase (complex II), in bc1 complex (complex III), and in cytochrome c oxidase (complex IV) to mediate the sequential flow of electrons in the respiratory chain (19). Cytochrome c oxidase and other mitochondrial heme proteins of complexes II, III, and IV are potential targets for CO regulation. The detailed mechanism of CO interaction with mitochondrial heme-containing proteins in relation to the redox potential of the heme has not yet been elucidated.
To elucidate the functional implications of glutamate excitotoxicity, we investigated the barrier functions of cerebral vascular endothelium. Endothelial cells are critical components defining the permeability of the BBB via specially organized cellular junctions, whereas pericytes and astrocytes have been reported as essential functional BBB components (1, 6, 9, 17, 25–27, 31, 32). In the present study, we focused on the endothelial component of the BBB that is a target for glutamate excitotoxicity. The baseline characteristics of the endothelial monolayer has TEER parameters comparable to that of reported by other investigators in various models of BBB (1, 6, 9, 26). This finding suggesting that primary cultures of newborn piglet cerebrovascular cells preserve physiological BBB properties. Glutamate and iGluR agonists caused BBB dysfunction as reflected by TEER reduction and increased paracellular permeability to large molecules. CORM-A1 at a concentration that blocks endothelial oxidative stress and apoptosis, completely prevented BBB dysfunction caused by glutamate.
Overall, the cerebral vascular endothelial component of the neurovascular unit and BBB in newborn piglets is vulnerable to glutamate excitotoxicity. The mechanism of this glutamate-induced cerebral vascular injury involves iGluR-mediated disruption of Ca2+ homeostasis, mitochondrial oxidative stress leading to cytochrome c release, and initiation of apoptotic pathway of cell death. Loss of functional integrity of the BBB resulting from glutamate excitotoxicity can be prevented by the antioxidant and antiapoptotic CO-releasing compound CORM-A1. This approach may be of great importance in preventing BBB dysfunction in neonatal cerebrovascular disease.
GRANTS
This study was supported by National Institutes of Health Grants R01-HL-99655 and R01-NS-63936 (to H. Parfenova) and R01-HL034059 (to C. W. Leffler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Author contributions: S.B. performed experiments; S.B. and H.P. analyzed data; S.B. and H.P. interpreted results of experiments; S.B., C.W.L., and H.P. edited and revised manuscript; S.B., C.W.L., and H.P. approved final version of manuscript; C.W.L. and H.P. conception and design of research; H.P. prepared figures; H.P. drafted manuscript.
REFERENCES
- 1. Abbott NJ, Dolman DE, Drndarski S, Fredriksson SM. An improved in vitro blood-brain barrier model: rat brain endothelial cells co-cultured with astrocytes. Methods Mol Biol 814: 415–430, 2012 [DOI] [PubMed] [Google Scholar]
- 2. Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H. Heme oxygenase-2 provides endogenous protection against oxidative stress and apoptosis caused by TNFa in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 291: C897–C908, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 296: C422–C432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basuroy S, Tcheranova D, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase-derived reactive oxygen species, via endogenous carbon monoxide, promote survival of brain endothelial cells during TNF-α-induced apoptosis. Am J Physiol Cell Physiol 300: C256–C265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berg-Johnsen J, Haugstad TS, Langmoen IA. Glutamate in the human brain: possible role in synaptic transmission and ischemia. Progr Brain Res 116: 287–302, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Cecchelli R, Berezowski V, Lundquist S, Culot M, Renftel M, Dehouck MP, Fenart L. Modelling of the blood-brain barrier in drug discovery and development. Nat Rev Drug Discov 6: 650–661, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Chapman AG. Glutamate and epilepsy. J Nutr 130: 1043S–1045S, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 262: 689–695, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Cucullo L, Marchi N, Hossain M, Janigro D. A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J Cereb Blood Flow Metab 31: 767–777, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desmard M, Boczkowski J, Poderoso J, Motterlini R. Mitochondrial and cellular heme-dependent proteins as targets for the bioactive function of the heme oxygenase/carbon monoxide system. Antioxid Redox Signal 9: 2139–2155, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du Plessis AJ, Volpe JJ. Perinatal brain injury in preterm and term newborn. Curr Opin Neurol 15: 151–157, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Durgam GG, Tsukahara R, Makarova N, Walker MD, Fujiwara Y, Pigg KR, Baker DL, Sardar VM, Parrill AL, Tigyi G, Miller DD. Synthesis, structure - activity relationships, and biological evaluation of fatty alcohol phosphates as lysophosphatidic acid receptor ligands, activators of PPRgamma, and inhibitors of autotaxin. J Med Chem 48: 4919–4930, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Fiumana E, Parfenova H, Jaggar JH, Leffler CW. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol Heart Circ Physiol 284: H1073–H1079, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124: 619–626, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans 34: 232–237, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57: 173–185, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Henshall DC, Simon RP. Epilepsy and apoptosis pathways. J Cereb Blood Flow Metab 25: 1557–1572, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kim HJ, Khalimonchuk O, Smith PM, Winge DR. Structure, function, and assembly of heme centers in mitochondrial respiratory complexes. Biochim Biophys Acta 1823: 1604–1616, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leffler CW, Balabanova L, Sullivan D, Wang X, Fedinec AL, Parfenova H. Regulation of CO production in cerebral microvessels of newborn pigs. Am J Physiol Heart Circ Physiol 285: H292–H297, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Leffler CW, Parfenova H, Jaggar J. Carbon monoxide as an endogenous cerebrovascular modulator. Am J Physiol Heart Circ Physiol 301: H1–H11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res Bull 46: 281–309, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 130: 1007S–S1015S, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell 4: 953–961, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 160: 985–1000, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naik P, Cucullo L. In vitro blood-brain barrier models: Current and perspective technologies. J Pharm Sci 101: 1337–1354, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel A, Tanaka K, Niwa M. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int 54: 253–263, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci 3: 748–755, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 47: 143–183, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol 9: 532–452, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 222: 218–227, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Paolinelli R, Corada M, Orsenigo F, Dejana E. The molecular basis of the blood brain barrier differentiation and maintenance. Is it still a mystery? Pharmacol Res 63: 165–171, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Parfenova H, Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection. Am J Physiol Cell Physiol 290: C1399–C1410, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Parfenova H, Fedinec A, Leffler CW. Ionotropic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase. J Cereb Blood Flow Metab 23: 190–197, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Parfenova H, Leffler CW, Tcheranova D, Basuroy S, Zimmermann A. Epileptic seizures increase circulating endothelial cells in peripheral blood as early indicators of cerebral vascular damage. Am J Physiol Heart Circ Physiol 298: H1687–H1698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parfenova H, Leffler CW, Basuroy S, Liu J, Fedinec AL. Antioxidant roles of heme oxygenase, carbon monoxide, and bilirubin in cerebral circulation during seizures. J Cereb Blood Flow Metab 32: 1024–1034, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parfenova H, Tcheranova D, Basuroy S, Fedinec AL, Liu J, Leffler CW. Functional role of astrocyte glutamate receptors and carbon monoxide in cerebral vasodilation response to glutamate. Am J Physiol Heart Circ Physiol 302: H2257–H2266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med 37: 1951–1962, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Piantadosi CA, Carrawy MS, Suliman HB. Carbon monoxide, oxidative stress, and mitochondrial permeability pore transition. Free Radic Biol Med 40: 132–139, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Rainesalo S, Keranen T, Palmio J, Peltola J, Oja SS, Saransaari P. Plasma and cerebrospinal fluid amino acids in epileptic patients. Neurochem Res 29: 319–324, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Robinson JS, Fedinec AL, Leffler CW. Role of carbon monoxide in glutamate receptor-induced dilation of newborn pig pial arterioles. Am J Physiol Heart Circ Physiol 282: H2371–H2376, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Seth A, Basuroy S, Sheth P, Rao RK. l-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol 287: G510–G517, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Waldbaum S, Patel M. Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res 88: 23–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wirrell EC. Neonatal seizures: to treat or not to treat? Semin Pediatr Neurol 12: 97–105, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Xi Q, Tcheranova D, Basuroy S, Parfenova H, Jaggar J, Leffler CW. Role of calcium signaling in glutamate-stimulated CO production in newborn piglet astrocytes. Am J Physiol Heart Circ Physiol 301: H428–H433, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zimmermann A, Leffler CW, Tcheranova D, Fedinec AL, Parfenova H. Cerebroprotective effects of the CO-releasing molecule CORM-A1 against seizure-induced neonatal vascular injury. Am J Physiol Heart Circ Physiol 293: H2501–H2507, 2007 [DOI] [PubMed] [Google Scholar]