Abstract
Electromechanical coupling via membrane depolarization-mediated activation of voltage-dependent Ca2+ channels (VDCC) is an important mechanism in regulating pulmonary vascular tone, while mouse is an animal model often used to study pathogenic mechanisms of pulmonary vascular disease. The function of VDCC in mouse pulmonary artery (PA) smooth muscle cells (PASMC), however, has not been characterized, and their functional role in reactive oxygen species (ROS)-mediated regulation of vascular function remains unclear. In this study, we characterized the electrophysiological and pharmacological properties of VDCC in PASMC and the divergent effects of ROS produced by xanthine oxidase (XO) and hypoxanthine (HX) on VDCC in PA and mesenteric artery (MA). Our data show that removal of extracellular Ca2+ or application of nifedipine, a dihydropyridine VDCC blocker, both significantly inhibited 80 mM K+-mediated PA contraction. In freshly dissociated PASMC, the maximum inward Ca2+ currents were −2.6 ± 0.2 pA/pF at +10 mV (with a holding potential of −70 mV). Window currents were between −40 and +10 mV with a peak at −15.4 mV. Nifedipine inhibited currents with an IC50 of 0.023 μM, and 1 μM Bay K8644, a dihydropyridine VDCC agonist, increased the inward currents by 61%. XO/HX attenuated 60 mM K+-mediated increase in cytosolic free Ca2+ concentration ([Ca2+]cyt) due to Ca2+ influx through VDCC in PASMC. Exposure to XO/HX caused relaxation in PA preconstricted by 80 mM K+ but not in aorta and MA. In contrast, H2O2 inhibited high K+-mediated increase in [Ca2+]cyt and caused relaxation in both PA and MA. Indeed, RT-PCR and Western blot analysis revealed significantly lower expression of CaV1.3 in MA compared with PA. Thus our study characterized the properties of VDCC and demonstrates that ROS differentially regulate vascular contraction by regulating VDCC in PA and systemic arteries.
Keywords: vascular contraction, reactive oxygen species, pulmonary arterial smooth muscle
intracellular free calcium (Ca2+) ions play a major role in the regulation of arterial smooth muscle tone under physiological and pathological conditions (36, 38). In vascular smooth muscle cells, membrane depolarization serves as a fundamental regulator of the open state probability of voltage-dependent Ca2+ channels (VDCC) in the plasma membrane. Alterations in membrane potential regulate cytosolic free Ca2+ concentration ([Ca2+]cyt) via Ca2+ entry through VDCC and consequently modulate vascular muscle cell excitability and vascular contraction and relaxation (37). Abnormal voltage-dependent Ca2+ influx has been implicated as a mechanism associated with the pathogenesis of idiopathic pulmonary arterial hypertension (IPAH) and hypoxia-induced pulmonary hypertension (5, 19). An experimental approach by Hirensallulr et al. (19) corroborates the findings that the activation of VDCC by high K+ solution or Bay K8644 elicited greater contractions in the pulmonary artery (PA) isolated from animals with pulmonary hypertension. Thus targeting VDCC channels in the plasma membrane of PA smooth muscle cells (PASMC) is an efficient approach for the development of novel therapies for pulmonary hypertension.
Animal models have long since been used to study the disease development and progression, since the investigations in human specimens are mainly restricted to end-stage pulmonary hypertension. Several animal models in different species have been used, among which rats and mice were commonly used for most studies. Although the rat has been a preferred animal model for mechanistic studies of pulmonary hypertension for more than 50 yr, recently there is an understandable desire among the investigators to use mice, as they offer a variety of tools often lacking for other species. Approaches ranging from generating a transgenic or knock-out models have strongly influenced the way we think of the pathogenesis of pulmonary hypertension and helped shift our understanding on the mechanisms such as cell-cell interactions, metabolic changes, cell phenotypic alterations, and immune dysregulation (16, 22, 27, 45). In recent years, a significant number of knockout and transgenic mice models has been used to investigate pathogenic mechanisms associated with pulmonary hypertension (1, 8, 34). However, using mouse models in investigating the electrophysiological properties of VDCC has been limited due to technical difficulties in isolating the PA and single smooth muscle cells from mouse arteries. With this in mind, we sought to examine the electrophysiological and pharmacological characteristics of VDCC in freshly dispersed mouse PASMC.
In parallel, the ability of reactive oxygen species (ROS) to modify vascular tone has been a topic of great interest for the past several decades (3, 13). ROS-mediated vasoconstriction, smooth muscle cell proliferation, and vascular remodeling are likely to play a critical role in many forms of pulmonary hypertension. Several studies proposed that hypoxia paradoxically initiates an increase in ROS signaling in the PA. It was also shown that the resulting shift in the redox status to a more oxidized state triggers the release of intracellular Ca2+ stores and recruitment of Ca2+ channels in the plasma membrane and thereby mediates contraction associated with the pathogenesis of systemic or pulmonary hypertension (2, 4, 10, 23, 24, 50). It is therefore of particular interest to determine the response of Ca2+ current and the vascular response to ROS in mouse PA (and PASMC) compared with systemic arteries. To facilitate our study of ROS effects, we used xanthine oxidase (XO) and hypoxanthine (HX) as well as H2O2 (35). Herein, we examined the effects of ROS on VDCC and [Ca2+]cyt in mouse PASMC. Data presented here also show evidence of the divergent effect of ROS on arterial contraction in mouse PA compared with systemic arteries such as the aorta and mesenteric artery (MA).
METHODS AND MATERIALS
Cell isolation.
Freshly dissociated mouse PASMC were used in this study to functionally characterize VDCC. Male C57BL/6 mice (7–8 wk) were killed by cervical dislocation in accordance with the approved protocol by the University of Illinois at Chicago Institutional Animal Care and Use Committee. Lungs were removed from the chest cavity and washed in a normal Tyrode solution composed of the following (mM): 143 NaCl, 5.4 KCl, 0.33 NaH2PO4, 0.5 MgCl2, 1.8 CaCl2, 5 HEPES, and 16.6 glucose (adjusted with NaOH to pH 7.4). The second- or third-order branches of intrapulmonary arteries (<400-μm external diameter) were isolated by dissecting the surrounding connective tissues from lung in a normal Tyrode solution. The isolated arteries were then cut open longitudinally, and smooth muscle cells were isolated from the PA segment by treatment of enzyme with collagenase type XI (1 mg/ml), protease type XXIV (0.45 mg/ml), bovine serum albumin (2 mg/ml), and trypsin inhibitor (2 mg/ml) at 4°C for 30 min, followed by 37°C for 8 min. The digested arteries were washed with Ca2+ free solution for several times and then placed in the storage solution containing the following (mM): 70 KOH, 50 l-glutamate, 55 KCl, 20 taurine, 20 KH2PO4, 3 MgCl2, 20 glucose, 10 HEPES, and 0.5 EGTA (pH 7.4). Cells were dispersed by trituration with a fire-polished glass pipette to make a suspension of asingle PASMC. All experiments were carried at room temperature (22–24°C). Cells were used within 6 h and stored at 4°C until use.
Whole cell patch clamp.
Ca2+ currents were recorded with whole cell patch-clamp technique using an Axopatch-1D amplifier and a DigiData 1322 interface (Molecular Devices, Sunnyvale, CA). Borosilicate patch pipettes (3–4 MΩ) were fabricated on a model P-97 electrode puller (Sutter Instrument, Novato, CA) and polished with a MF-63 microforge (Narashige Scientific Instruments Laboratories) for whole cell current recording. Command voltage pulse protocols and data acquisition were performed with pCLAMP 8.1 software (Molecular Devices). Currents were filtered at 1–2 kHz and digitized at 2–4 kHz. The extracellular solution for the Ca2+ currents recording contained the following (in mM): 100 NaCl, 10 BaCl2, 5 CsCl, 0.5 MgCl2, 10 HEPES, 5.0 TEA-Cl, and 10 glucose (adjusted with NaOH to pH 7.4). The pipette solution for the Ca2+ currents recording contained the following (in mM): 130 CsCl, 10 EGTA, 5 Mg-ATP, and 10 HEPES (adjusted with CsOH to pH 7.2). The P/4 subtraction protocol in the pCLAMP software was used for the subtraction of leakage currents.
Measurement of [Ca2+]cyt.
Cells on coverslips were incubated at room temperature for 30 min in normal Tyrode solution containing the membrane-permeable Ca2+-sensitive fluorescent indicator fura-2 acetoxymethyl ester (fura-2 AM; 4 μM) to load cells with dye. Cells on coverslips were then placed in a perfusion chamber on the stage of an inverted Nikon Eclipse/TE 200 microscope equipped with the TE-FM epifluorescence. The fura-2 AM-loaded cells were then washed with normal Tyrode solution for 20 min to remove excess extracellular dye before the experiments started. The fluorescence for fura-2 was recorded as 510 nm wavelength light emission with excitation wavelengths of 340 and 380 nm by use of the digital fluorescence imaging system from Intracellular Imaging. [Ca2+]cyt was measured as the ratio of fluorescence intensities (F340/F380) in PASMC loaded with fura-2 AM.
Tension measurement.
The PA ring was cut and two tungsten hooks were carefully passed through the lumen of ring in an organ bath. One hook was connected on the bottom of a perfusion chamber, and the other was attached to an isometric force transducer (Harvard Apparatus). The resting passive tension was maintained at 300 mg, and the rings were allowed to stabilize at resting tension for 1 h. Isometric tension was continuously recorded, and data were acquired using DATAQ software (DATAQ Instruments). The PA rings were then applied with 40 mM K+ for three times to obtain a stable contractile response before challenge drugs. Isolated PA rings were perfused with modified Krebs solution (MKS; at 37°C) consisting of the following (in mM): 138 NaCl, 1.8 CaCl2, 4.7 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 5 HEPES, and 10 glucose (pH 7.4). The absolute amount of force relative to the basal tension was measured, which was expressed as the net increase in tension (mg). The endothelium was removed for all experiments except in experiments assessing endothelium function.
Quantitative real-time RT-PCR.
Total RNA was prepared from the isolated PA and MA from C57BL mice using TRIzol reagent (Invitrogen) and quantified with NanoDrop 2000c (Thermo Scientific). The equal amount of RNA from each sample was reversely transcribed to cDNA with High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The quantitative real-time PCR analysis was performed for the following gene-specific primers: Cav1.1 (forward, 5′-aat gcc aca cta ttt gcc ctg gtg-3′; reverse, 5′-ttg gtc caa gag ctt cat gct ggt-3′), Cav 1.2 (forward, 5′-ttg agc aac ctt gtg gca tcc ttg-3′; reverse, 5′-acg ggt ctg cat ctc atc gaa gtt-3′), Cav 1.3 (forward, 5′-tgc aag atg acg agc cag aag act-3′; reverse, 5′-gtg gca ttg aag gcc ttt gga cat-3′), and Cav 1.4 (forward, 5′-tca tgt tcg cct gca ttg gtg ttc-3′; reverse, 5′-acc aaa ggt cgt gac aca tct cca-3′). Quantitative RT-PCR was performed in a Real-Time Cycler (Bio-Rad CFX384, C1000 Thermal Cycler; Roche) using 1 μg of RNA, which was added to a final volume of 10 μl, which contained 5 μl of iTaq Universal SYBR Green Supermix (Bio-Rad, Qiagen, Hilden, Germany) and 500 nmol/l of each primer. The reaction was initiated at 95°C for 15 min, followed by 40 cycles of denaturation at 95°C for 10 s, annealing for 30 s, and extension at 72°C for 15 s. After amplification, a melting curve analysis from 50 to 95°C with a heating rate of 0.2°C/s with continuous fluorescence acquisition was performed to assure correct PCR amplification. The relative quantification method was used whereby the change in expression of the target genes relative to the housekeeping gene (GAPDH) was calculated.
Western blotting.
Protein samples were prepared from isolated PA and MA of C57BL/6 mice in 1× RIPA lysis buffer (Milipore), supplemented with 2% n-dodecyl-β-d-maltoside (Thermo Scientific) and protease inhibitor cocktail (Roche). Tissues were homogenized on ice with a glass Dounce homogenizer, followed by brief pulses of sonication until completely uniform. The crude lysate was briefly centrifuged, and protein concentrations were determined using a BCA Protein Assay Kit (Pierce). Equal amounts (30 μg) of total protein from each sample were loaded onto 4–20% SDS gradient gels (Mini-PROTEAN TGX; Bio-Rad) and separated by gel electrophoresis. Proteins were transferred onto PVDF membranes and stained with Ponceau S. Blots were probed with polyclonal rabbit anti-CaV1.2 and anti-CaV1.3 (1:200; Alomone) and monoclonal β-actin (1:1,000; Santa Cruz Biotechnology). Bands were visualized using ImageJ software.
Chemicals.
Nifedipine, HX, XO, Bay K8644, phenylephrine (PE), and acetylcholine (ACh) were obtained from Sigma (St. Louis, MO), and fura-2 AM was obtained from Molecular Probes. A stock solution (10 mM) of nifedipine and fura-2 AM was dissolved in DMSO and kept away from light to avoid photodegradation. The stock solution of 250 mM HX was prepared by dissolving in distilled water and adding a few drops of NaOH to adjust to pH 7.4: aliquots of the stock solution were diluted 1:1,000 into the bath solution to a final concentration of 250 μM.
Data analysis and statistics.
Data analysis and curve fitting were presented with pCLAMP 8.1 and SigmaPlot 2000 software. Activation and inactivation curves, including half activation and inactivation potentials, were determined after normalization to maximal current and fit to Boltzmann equations. Time constants were derived from single exponential curves. The results are expressed as means ± SE. Statistical comparison was performed with a Student's t-test, with values of P < 0.05 being considered significant.
RESULTS
Functional role of VDCC in pulmonary vasoconstriction.
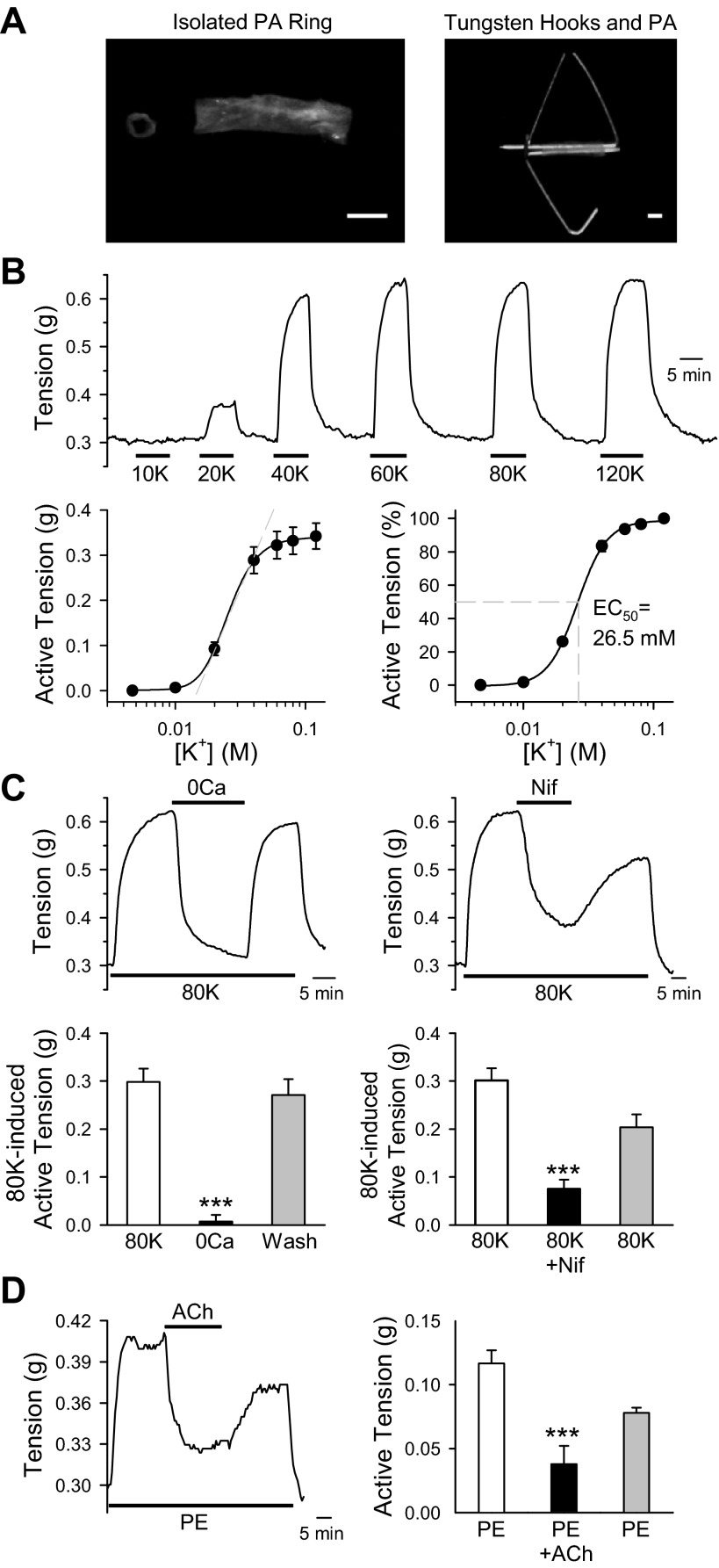
The PA rings isolated from intrapulmonary arteries of mice were used in this study. For the measurements of vascular tension, two tungsten hooks were carefully passed through the lumen of PA rings (Fig. 1A). To evaluate the contribution of VDCC-mediated Ca2+ influx to mediating vasoconstriction, PA rings were exposed to high K+-solutions ranging from 10 to 120 mM. Raising extracellular K+ concentration ([K+]o) from 4.7 to 120 mM shifts the K+ equilibrium potential and resulting membrane depolarization. This subsequently opens the VDCC, which in turn enhances Ca2+ influx, increasing [Ca2+]cyt and causing vasoconstriction. With this available knowledge, we exposed PA rings, for which the basal tension was set at 300 mg, to increasing [K+]o from 4.7 to 20, 40, 60, 80, and 120 mM, respectively. We observed a significant increase in the amplitude of active tension (Fig. 1B, top). The dose-response curve showed an almost linear relationship at [K+]o between 15 and 40 mM and arrived at a plateau phase when [K+]o reached 60–80 mM (EC50 = 26.5 mM; Fig. 1B, bottom). These data suggest that high K+-mediated VDCC activation plays a significant role in pulmonary vasoconstriction.
Fig. 1.
Ca2+ influx through voltage-dependent Ca2+ channels (VDCC) plays an important role in pulmonary vasoconstriction. A: image shows intrapulmonary artery isolated from mice (left). Two tungsten hooks were carefully passed through the lumen of pulmonary artery (PA) rings (right) to measure isometric tension. B: PA rings were exposed to modified Krebs solution (MKS) containing extracellular K+ concentrations of 10, 20, 40, 60, 80, and 120 mM. Representative tension tracings are shown at top. Summarized absolute active tension and active tension normalized to the maximum tension generated by 120 mM K+ are shown at bottom. C: mouse PA were treated with Ca2+ free (left) or 300 nM nifedipine (Nif; right) on the 80 mM K+ (80K)-induced contraction. After peak contraction was achieved, arteries were exposed to either Ca2+ free (0Ca) or 300 nM Nif to attenuate Ca2+ influx. Summarized data showed active tension induced by 80K before (80K), during (0Ca), and after (Wash) exposure to 0Ca (bottom left) or Nif-containing solution (bottom right). D: representative tension record showing phenylephrine (PE; 100 nM)-induced active tension before, during, and after application of acetylcholine (ACh; 10 μM). After peak contraction with PE was achieved, arteries were exposed to ACh (left). Summarized data showed absolute tension after application of PE, ACh, and washout (right). Data are expressed as means ± SE. Rings were isolated from 5–7 mice. ***P < 0.001, statistical difference from the control value.
Removal of extracellular Ca2+ almost abolished the 80 mM K+ (80K)-mediated PA contraction (Fig. 1C, left). In addition, extracellular application of the VDCC blocker nifedipine (300 nM) also significantly and reversibly inhibited 80K-induced active tension (Fig. 1C, right). The 80K-induced active tension was 0.2981 ± 0.278 g in the PA rings superfused with 1.8 mM Ca2+-containing MKS, 0.0073 ± 0.0137 g (a 99% inhibition) in PA rings superfused with Ca2+-free MKS, and 0.2710 ± 0.332 g when extracellular Ca2+ was restored. Furthermore, nifedipine caused a marked inhibition of tension (from 0.301 ± 0.025 to 0.075 ± 0.019 g; Fig. 1C, right). These data indicate that 80K-induced PA contraction is mainly due to membrane depolarization-mediated Ca2+ influx through nifedipine-sensitive VDCC in PASMC.
To access the function of endothelium, ACh was applied to the PA precontracted with PE, a selective α1-adrenergic receptor agonist. The PE-induced vasoconstriction was significantly inhibited by exposure of the ring to ACh, an organic, polyatomic ion that activates muscarinic receptors in the endothelial cells and causes synthesis and release of nitric oxide and endothelium-derived hyperpolarizing factors. These findings suggest that the endothelium was intact in the PA (Fig. 1D) we used to test the functional role of VDCC.
Passive membrane properties of mouse PASMC.
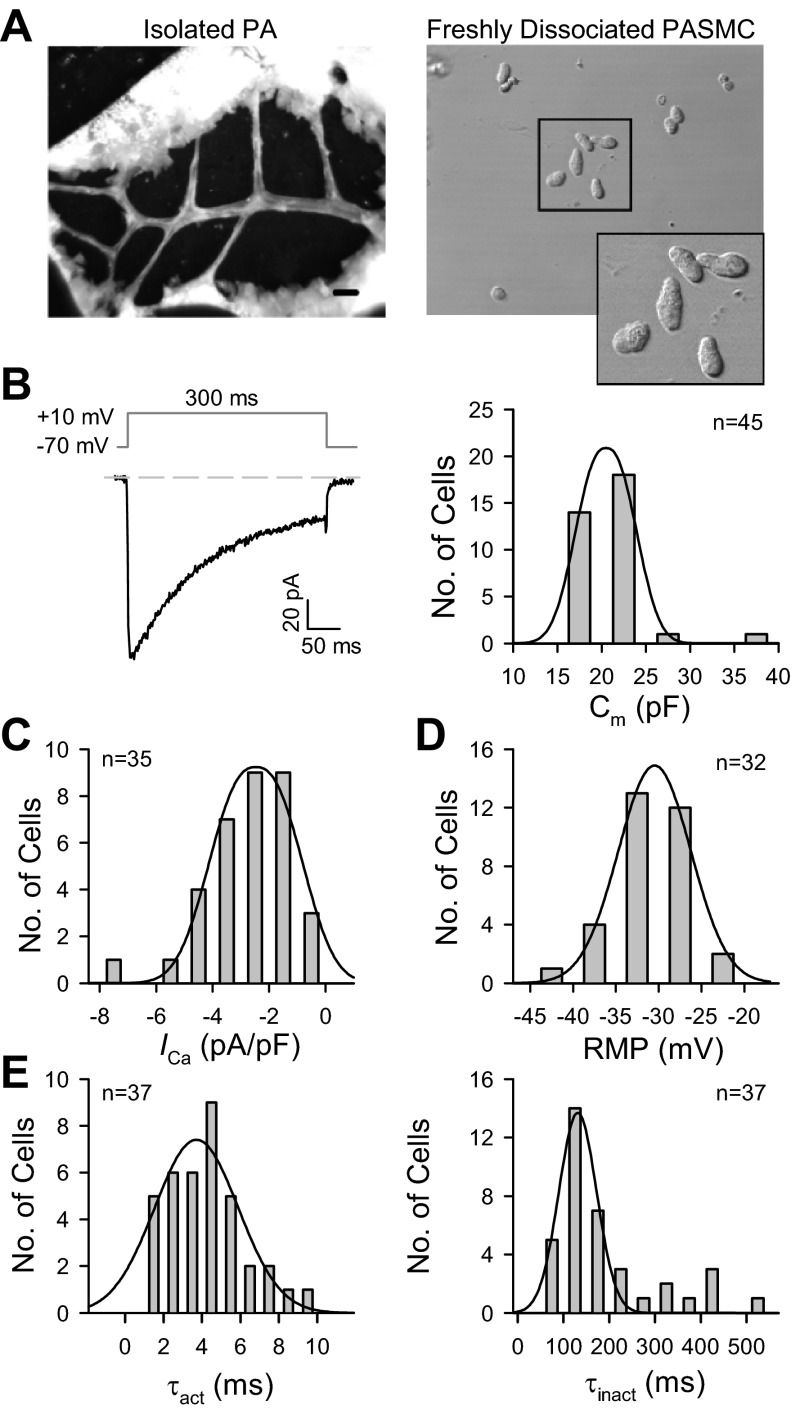
Freshly dissociated PASMC from mouse PA isolated from second- to third-order intrapulmonary arterial branches (100- to 400-μm diameters) were used to measure the electrophysiological properties of VDCC. As previously reported (28), the freshly dissociated mouse PASMC from intrapulmonary arteries were oblong, unlike the long spindle-shaped cells isolated from other species (Fig. 2A). To measure the basic biophysical properties of VDCC, the maximum inward current was evoked by a +10-mV depolarizing pulse from a holding potential of −70 mV (Fig. 2B, left). The membrane capacitance (Cm), in mouse PASMC (Fig. 2B, right) was 21.6 ± 0.6 pF (n = 45). Peak currents were determined at +10 mV and normalized to cell capacitance (current density) were −2.6 ± 0.2 pA/pF (n = 35; Fig. 2C), and the averaged resting membrane potential was −31.5 ± 0.8 mV (n = 32; Fig. 2D). The activation time constant of maximum currents, τact, was measured by fitting a monoexponential curve to the rising phase of each type of current. The currents were maximally activated at 4.2 ± 0.3 ms (n = 37) after depolarization, with peak activation occurring at +10 mV (Fig. 2E, left). The current then decayed exponentially with a mean time constant of 195.5 ± 18.6 ms (n = 37; Fig. 2E, right).
Fig. 2.
Passive membrane properties of freshly dissociated mouse PA smooth muscle cells (PASMC). A: images show the isolated pulmonary artery from the left lobe in a 7- to 8-wk-old male mouse (left) and single PASMC dissociated from the 2nd-3rd orders of intrapulmonary arterial branches isolated from mice (right). Scale bar = 800 μm. B: representative currents were evoked by a depolarizing pulse to +10 mV from a −70-mV holding potential (left). Histogram shows the distribution of membrane capacitance (Cm, n = 45 cells; right). C: peak currents (or current density) were determined at +10 mV, normalized to cell capacitance (n = 35). D: averaged resting membrane potential (RMP; n = 32) in freshly dissociated mouse PASMC. E: activation and inactivation time constants, τact (left) and τinact (right), were measured by fitting a monoexponential curve to the rising phase (n = 37) and decaying phase (n = 37) of each type of current. Cells were isolated from 30–42 mice.
Biophysical properties of VDCC.
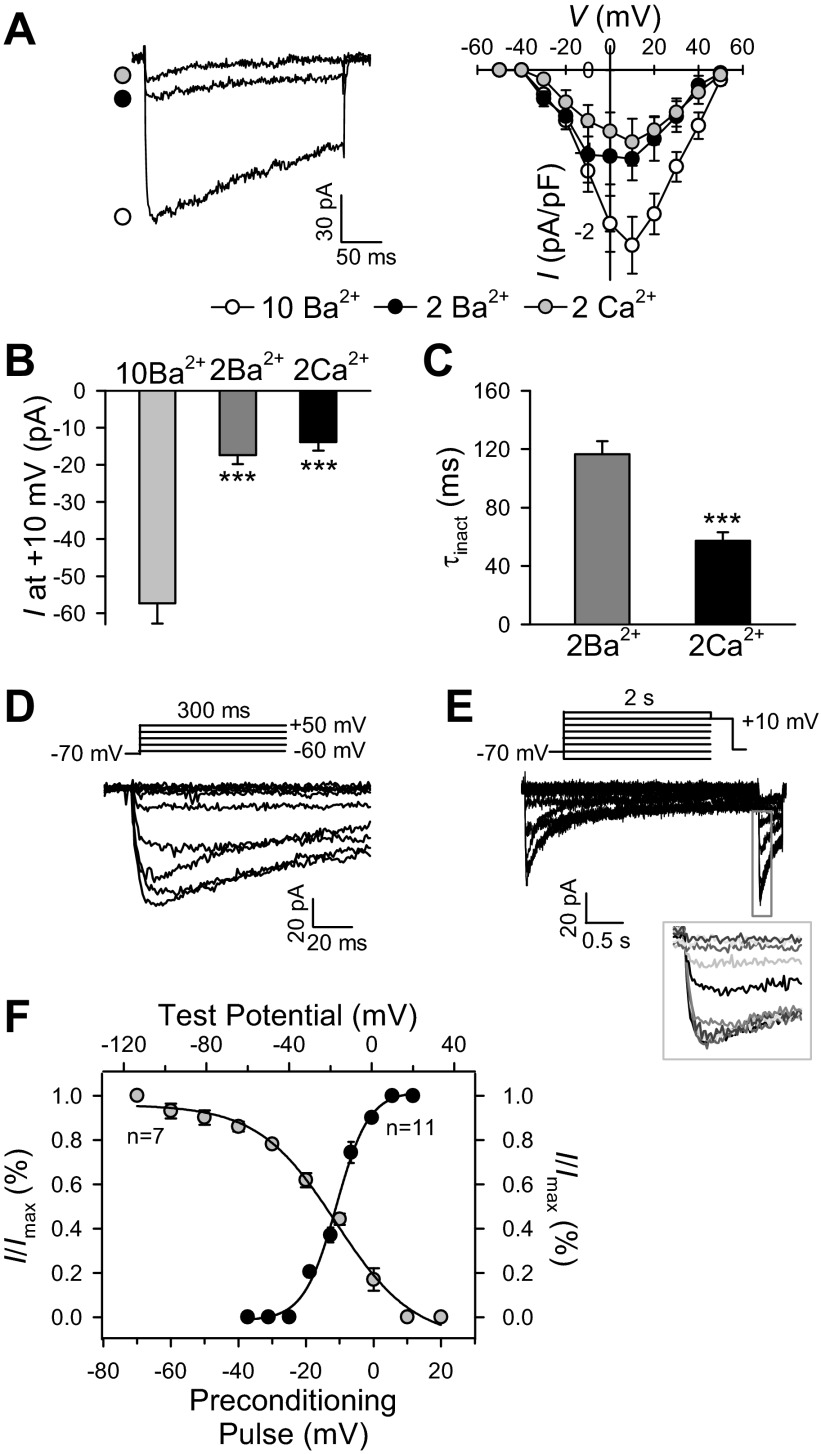
Figure 3A shows representative traces of currents recorded to examine the currents of Ca2+ (ICa) and Ba2+ (IBa) through VDCC channels. When cells were superfused with 2 mM Ca2+-containing solution, depolarization from a holding potential of −70 to +10 mV induced a relative rapidly inactivating inward current (Fig. 3A). Substitution of 2 mM Ca2+ with 2 mM Ba2+ slightly increased the amplitude, which was followed by a slower inactivation phase of the inward currents. Increasing extracellular Ba2+ concentration from 2 to 10 mM significantly increased the amplitude of the currents (Fig. 3A). When the external solution with 2 mM Ba2+ was substituted with 10 mM Ba2+, the amplitude of the peak currents increased by almost threefold (from −17.3 ± 2.5 to −57.3 ± 5.5 pA; Fig. 3B). Inactivation time constants (τinact) were calculated via a monoexponential fit on the decaying phase of the inactivation curves for 2 mM Ba2+ and 2 mM Ca2+; τinact decreased from 116.5 ± 8.9 ms (2 mM Ba2+) to 57.2 ± 6.0 ms (2 mM Ca2+; Fig. 3C). This difference in the current-voltage (I-V) relationship profile between Ba2+ and Ca2+ solutions is consistent with the contention that the amplitude of Ba2+ currents is much higher than that of Ca2+ currents through VDCC, while the inactivation time constant of Ba2+ currents is greater than that of Ca2+ currents.
Fig. 3.
Ca2+ permeability and steady-state voltage dependence of inactivation and activation of VDCC in freshly dissociated mouse PASMC. A: representative recording of inward currents through VDCC elicited by a depolarizing pulse of +10 mV from a holding potential of −70 mV (left). Maximum currents were superimposed in the presence of 10 mM Ba2+, 2 mM Ba2+, and 2 mM Ca2+. Right: mean current-voltage (I-V) curves for 10 mM Ba2+, 2 mM Ba2+, and 2 mM Ca2+. Peak currents were determined at each voltage, normalized to cell capacitance. Voltage-clamp steps were applied from a holding potential of −70 mV to test potentials ranging from −60 to +50 mV with increments of 10 mV (300-ms steps). B: bar graph shows the peak currents in the presence of 10 mM Ba2+, 2 mM Ba2+, and 2 mM Ca2+. C: inactivation time constant, τinact, was measured by fitting a monoexponential curve to the decaying phase in the presence of 2 mM Ba2+ and 2 mM Ca2+, respectively. D: representative family of superimposed currents, elicited by depolarization from a holding potential of −70 mV to test potentials (300 ms) ranging from −60 to +50 mV (in 10-mV increments). E: representative family of currents, elicited by a double-pulse protocol, to construct steady-state inactivation curve. The cell was held at −70 mV and stepped to a series of conditioning pulses (2 s) ranging from −90 to +20 mV before depolarization to +10 mV for 200 ms. Inset: magnified inactivation currents. F: averaged data showing the activation curve (closed circle, n = 11 cells) and the inactivation curve (open circle, n = 7 cells). Smooth curves through inactivation and activation data points are the best fit generated by the computer. Peak current was determined at each voltage, normalized to cell capacitance. Data are expressed as means ± SE. Cells were isolated from 5–9 mice. ***P < 0.001, statistical difference from the control value.
We also determined the steady-state activation and inactivation kinetics of VDCC. Steady-state activation data were derived from the I-V relationship, measured by depolarization from a holding potential of −70 mV to a series of test potentials ranging from −60 to +50 mV in 10-mV increments. A representative record of superimposed currents is shown in Fig. 3D. The normalized activation curve indicates that the channels were activated at −30 mV, and the channel activation maximized at approximately +10 mV. To examine the inactivation of VDCC in more detail, a standard two-pulse protocol was applied. Figure 3F shows a typical experiment in which a cell was held at conditioning potentials ranging from −90 to +20 mV for 2 s before depolarizing to the test potential of +10 mV for 200 ms to elicit the peak inward currents through VDCC. Figure 3F, filled circles, shows steady-state activation and inactivation curves plotted from the data shown in Fig. 3, D and E, respectively. The normalized inactivation curve implies that the channels were inactivated at potentials from −60 mV and the maximal inactivation occurred at +10 mV. The window currents of VDCC fell within a voltage range of −40 to +10 mV (half activation and inactivation of ICa in mouse PASMC was −11.2 ± 2.0 and −17.3 ± 1.3 mV, respectively), with a peak window current of −15.4 mV.
Pharmacological properties of VDCC.
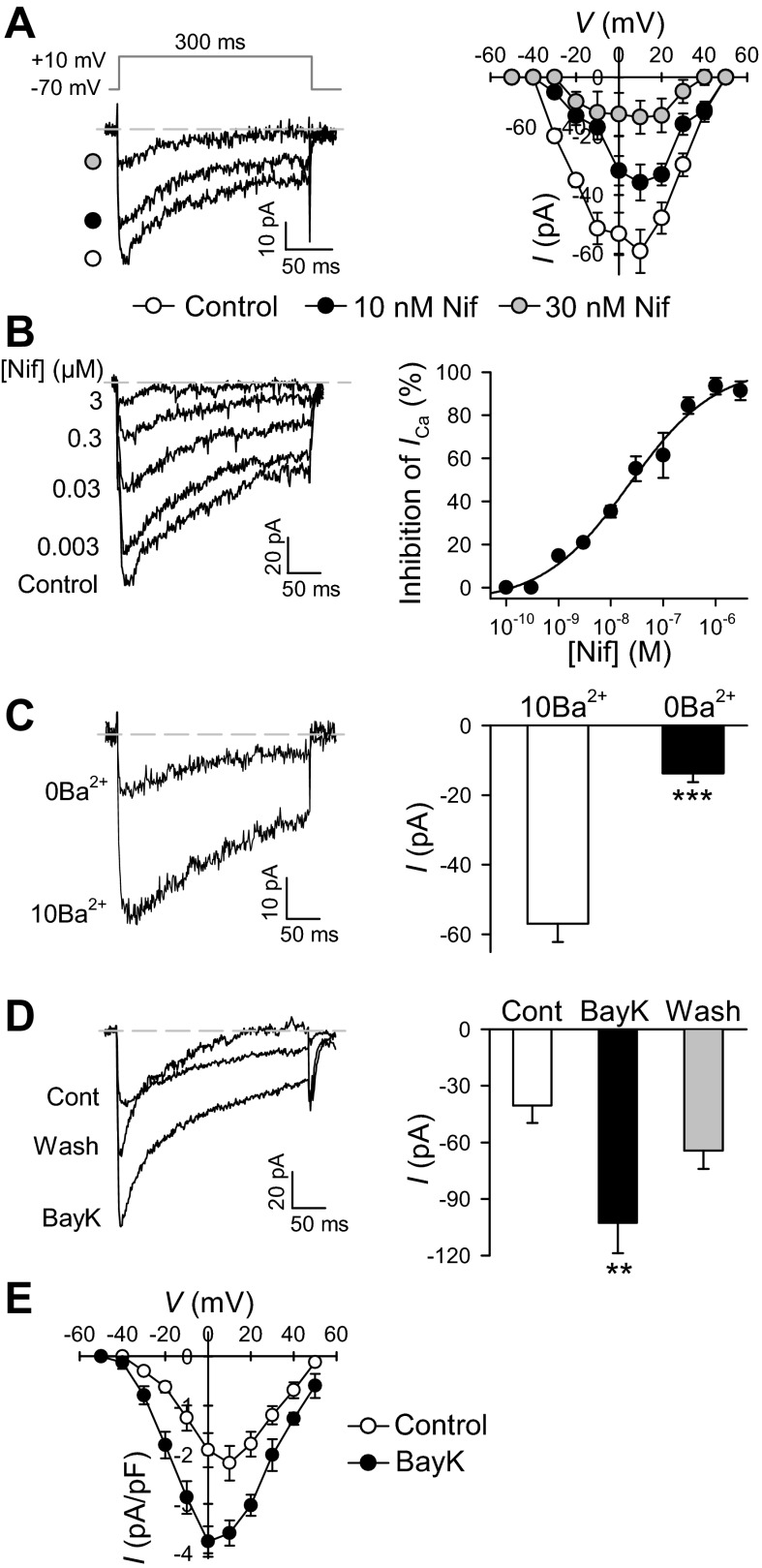
Next, we used nifedipine (a known dihydropyridine blocker of VDCC) to characterize the channels function in PASMC. Cells treated with nifedipine (10 or 30 nM) showed a rapid attenuation of peak inward currents (Fig. 4A). Furthermore, a dose-dependent inhibition of inward current at the concentrations ranging from 0.001 to 3 μM of nifedipine was observed (Fig. 4B, left). There was progressive inhibition of the peak, with increasing concentrations of nifedipine. Blockade was not complete (on average it was 91.3 ± 4.3% with nifedipine, 3 μM) but showed a tendency towards saturation at the concentration of 3 μM. The concentration-inhibition curve illustrating the effects of nifedipine on VDCC was constructed, and IC50 value for nifedipine was ∼0.023 μM (Fig. 4B, right). We next observed the effect of Ba2+ removal on the amplitude of the peak inward currents (Fig. 4C). Following removal of external Ba2+, the amplitude of the VDCC was significantly reduced from −56.9 ± 5.3 to −13.7 ± 2.6 pA (Fig. 4C). The currents in Ba2+-free conditions could imply that the remaining currents were not carried by Ba2+ (potentially carried by other monovalent cations). Several studies have shown that in the absence of extracellular Ca2+, Ca2+ channels are permeable to monovalent cations and that Mg2+ blocks this current.
Fig. 4.
Pharmacological properties of VDCC in freshly dissociated mouse PASMC. A: Superimposed representative currents were elicited by a depolarizing pulse of +10 mV from a membrane holding potential of −70 mV before (control) and during application of 10 and 30 nM nifedipine. Right: mean I-V curves for control in the presence of 10 and 30 nM nifedipine, respectively. Voltage-clamp steps were applied from a holding potential of −70 mV to test potentials of −60 to +50 mV with increments of 10-mV, 300-ms steps. Peak current was determined at each voltage. B: superimposed currents obtained before and during subsequent application of various concentrations of nifedipine (from 0.003 to 3 μM). Right: concentration-inhibition curves for nifedipine from 0.0001 to 3 μM. C: representative recordings of inward currents through VDCC elicited by a depolarizing pulse to +10 mV from a holding potential of −70 mV in PASMC superfused with 10 mM Ba2+ (10Ba) or Ba2+-free (0Ba) solutions. Right: bar graph shows current amplitudes in the absence (0Ba) or presence of 10 mM Ba2+ (10Ba). D: representative recordings of inward currents through VDCC elicited by a depolarizing pulse of 10 mV from a holding potential of −70 mV before (Cont), during (BayK), and after (Wash) exposure to 1 μM Bay K8644. Bar graph shows current amplitudes in PASMC before, during and after application of Bay K8644. Data are expressed as means ± SE. Cells were isolated from 7–10 mice. **P < 0.01 and ***P < 0.001, statistical difference from the control value. E: mean I-V curves in PASMC before (control) and during (BayK) application of BayK 8644.
Extracellular application of 1 μM Bay K8644, a VDCC activator, significantly and reversibly increased the amplitude of inward currents in freshly dissociated mouse PASMC (Fig. 4, D and E). The peak amplitude increased from 40.5 ± 9.1 to −102.8 ± 16.1 pA in the presence of Bay K8644 at +10-mV depolarizing pulse (Fig. 4D, right). In addition, the augmenting effect of Bay K8644 was fairly rapid, taking <3 min for maximal changes, and the I-V for the peak of Bay K8644-mediated currents was shifted 10 mV in the hyperpolarizing direction (Fig. 4E).
Divergent effect of ROS on VDCC, [Ca2+]cyt, and vascular contraction.
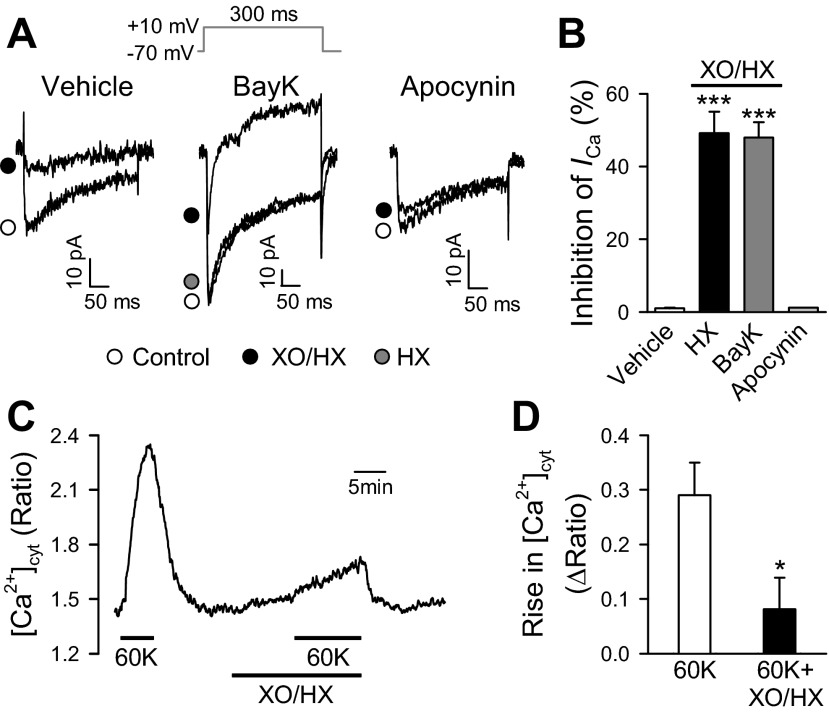
VDCC on the plasma membrane are affected by exogenous and endogenous redox compounds (21). A study in isolated guinea pig ventricular myocytes demonstrated that oxidation of thiol groups in L-type VDCC channels inhibited Ca2+ entry (30). To examine the effects of ROS on PASMC, we exposed the cells to XO (0.2 mU/ml) and HX (250 μM). The inward currents through VDCC, elicited by a depolarizing pulse of +10 mV, were inhibited to 49.2 ± 5.9% by XO/HX in cells superfused with vehicle (Fig. 5, A, left, and B). In addition, the inward currents through VDCC in the presence of 1 μM Bay K8644 were inhibited to 48.0 ± 4.2% with exposure to XO/HX (Fig. 5, A, middle, and B). However, the challenge of HX alone had no effect on the inward currents through VDCC (Fig. 5A, middle). Furthermore, the inhibitory effect of XO/HX on VDCC was prevented following treatment with apocynin, used as an inhibitor of NADPH oxidase (Fig. 5, A, right, and B). These results suggested that ROS produced by HO/HX (in the presence of NADPH oxidase) significantly inhibited VDCC in freshly dissociated mouse PASMC. Indeed, treatment of PASMC with XO/HX, although it slightly increased the basal [Ca2+]cyt, significantly inhibited 60 mM K+-induced increase in [Ca2+]cyt (Fig. 5, C and D).
Fig. 5.
Effect of reactive oxygen species (ROS) produced by xanthine oxidase (XO) and hypoxanthine (HX) on VDCC and cytosolic free Ca2+ concentration ([Ca2+]cyt) in mouse PASMC. A: superimposed representative currents were recorded in PASMC before (control, open circle) and during (XO/HX, closed circle) application of XO (0.2 mU) and HX (250 μM; left; with vehicle in the bath solution); before (control, open circle) and during application of XO/HX (closed circle) or HX alone (HX, grey circle; middle; with 1 μM Bay K8644 in the bath solution); and before (control, open circle) and during application of XO/HX (closed circle; right; with 10 μM apocynin in the bath solution). Internal pipette solutions were concocted with 5 mM ATP and 10 mM EGTA to minimize ICa run down. Additionally, to exclude possible artifacts of VDCC run down, only freshly dissociated PASMCs with stable current amplitudes during a 2- to 3-min depolarizing pulse of 10 mV were tested. B: summarized data (means ± SE) showing XO/HX-mediated inhibition of inward currents in PASMC perfused with the bath solution containing vehicle, Bay K8644 or apocynin. ***P < 0.001 vs. vehicle control and apocynin. C: representative record showing 60 mM K+ (60K)-mediated increases in [Ca2+]cyt in the absence and presence of XO/HX. D: summarized data showing averaged increases of [Ca2+]cyt induced by 60K with and without XO/HX. *P < 0.05 vs. 60K alone. Cells were isolated from 4–6 mice.
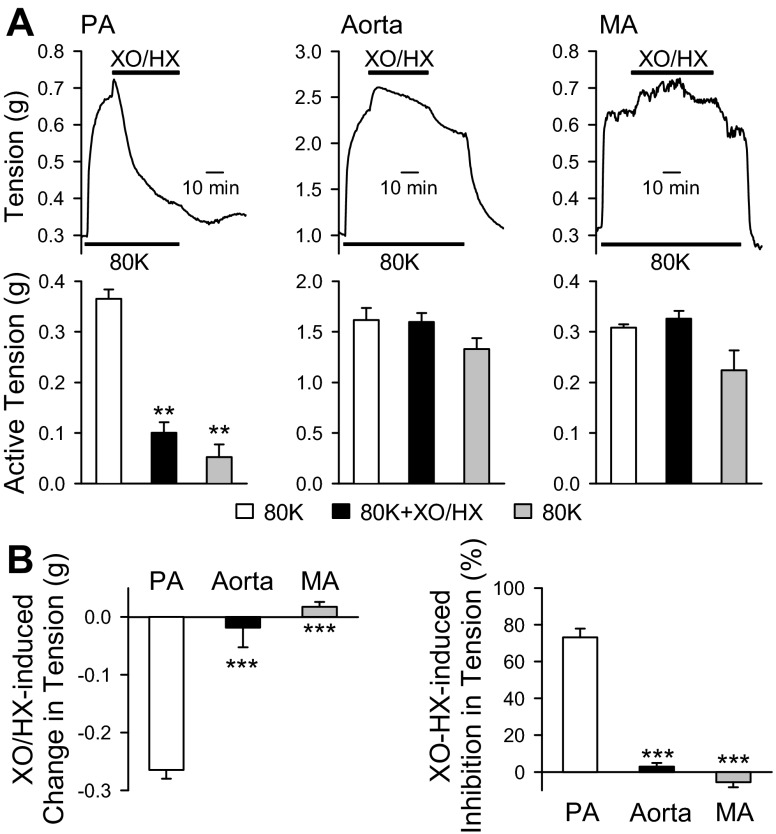
Next in the tension measurements, we set out to investigate the differences in vascular contraction among mouse PA, aorta, and MA in response to XO/HX. The XO/HX challenged to PA was the same in the same concentration as that used in the electrophysiological study. To activate VDCC, 80K was applied to the PA to elicit a contraction as demonstrated in Fig. 6A. An additional transient contraction was observed following addition of XO/HX when 80K-mediated active tension reached plateau; however, it caused gradual relaxation to 73.2 ± 4.8% of 80K-induced vasoconstriction (Fig. 6, A, left, and B). These data are consistent with the observation that XO/HX inhibits VDCC and decreases Ca2+ influx through VDCC in PASMC. In addition, we determined the effect of XO/HX on the vascular contraction in both aorta and MA. In contrast to the findings observed in PA, aorta and MA exposed to XO/HX did not show relaxation and returned to the baseline upon a washout with MKS solution (Fig. 6, A, middle and right, and B).
Fig. 6.
ROS produced by XO/HX inhibits 80 mM K+ (80K)-induced contraction in mouse PA but not in aorta and mesenteric artery (MA). A: representative tension traces (top) and summarized data (bottom) showing 80K-mediated active tension in PA (left), aorta (middle), and MA (right) in the absence of presence of XO (0.2 mU) and HX (250 μM). XO/HX was applied to the arterial rings when the 80K-mediated contractions reached plateau. **P < 0.01 vs. open bars (80K). B: bar graph showing the changes in 80K-mediated active tension (actual tension in g, left, and %maximal active tension, right) in PA (open bars), aorta (solid bars), and MA (grey bars). Data are expressed as means ± SE. Arteries were isolated from 4–6 mice. ***P < 0.001 vs. open bars (PA).
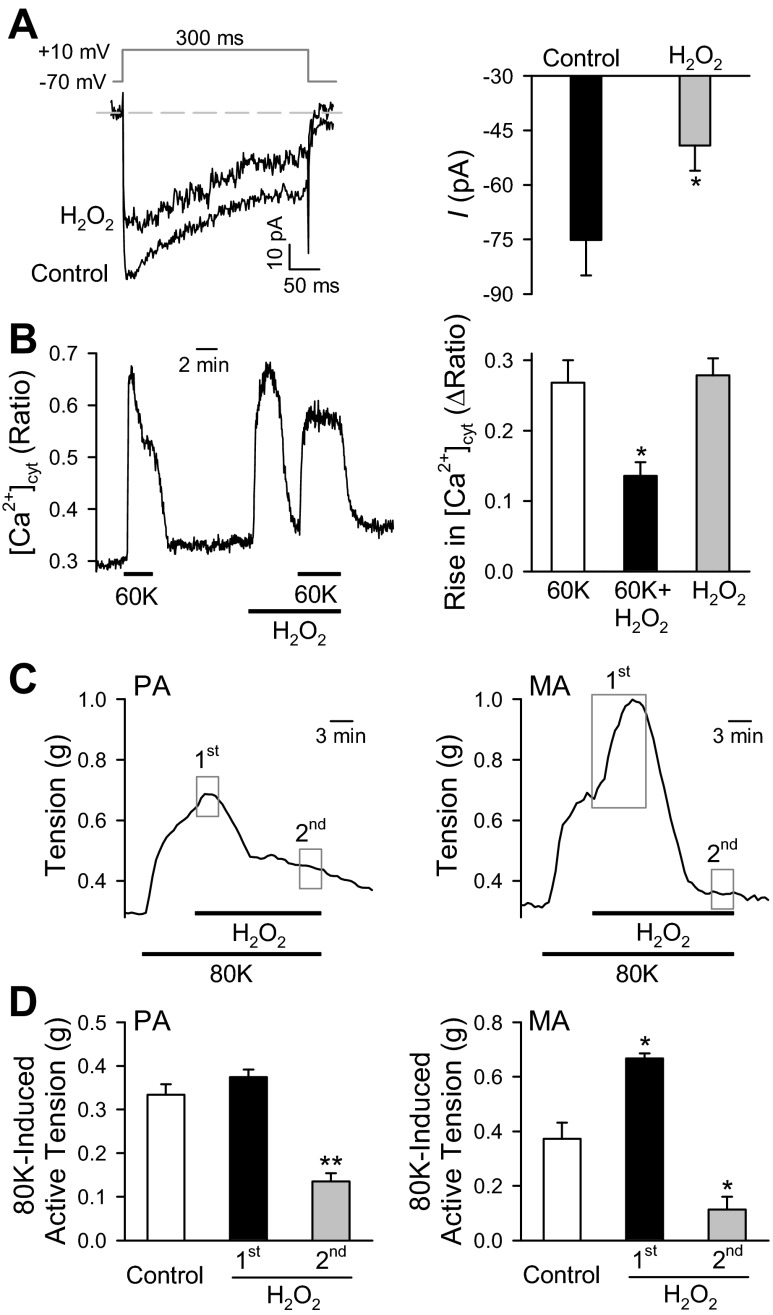
We also examined the effect of H2O2 on VDCC, [Ca2+]cyt, and vascular contraction. Addition of 0.3 μM H2O2 to the bath solution inhibited inward currents through VDCC to 34.6% of control (Fig. 7A). In PASMC loaded with fura-2, H2O2 (0.3 μM) elicited a transient increase in [Ca2+]cyt which declined to the basal level within 3–5 min. In the presence of H2O2, the 60 mM K+-mediated increase in [Ca2+]cyt was, however, significantly inhibited compared with [Ca2+]cyt in the absence of H2O2 (Fig. 7B). As for tension measurement experiments, H2O2 (0.3 μM) caused relaxation in 80K-induced contractions for PA preparations (Fig. 7C). However, exogenous H2O2 exerted a differential effect on the 80K-induced contractions in MA preparations, where application of H2O2 led to a significant enhancement of the initial contraction followed by vasorelaxation (Fig. 7D).
Fig. 7.
Effects of hydrogen peroxide (H2O2) on ICa, [Ca2+]i and contractility in the mouse vasculature. A and B: inhibitory effects of H2O2 on inward currents through VDCC and [Ca2+]cyt in mouse PASMC. Superimposed representative currents (A, left), elicited by a 300-ms test potential of +10 mV (with a holding potential of −70 mV), are recorded in PASMC before (control) and during (H2O2) extracellular application of 0.3 μM H2O2. Summarized data (means ± SE; A, right) showing the averaged amplitudes of inward currents in the absence (control) or presence of H2O2. Representative trace (B, left) showed increases of [Ca2+]cyt induced by 60 mM KCl in the absence and presence of H2O2. Bar graph (B, right) shows averaged increases of [Ca2+]cyt induced by 60 mM KCl with and without H2O2. Data are expressed as means ± SE. Cells were isolated from 4–6 mice. *P < 0.05 vs. control (60K). C and D: divergent effects of H2O2 on 80 mM K+ (80K)-induced contraction in mouse PA and MA. Representative tension records (C) in PA (left) and MA (right) before, during, and after application of 80K-containing solution with or without H2O2 (0.3 μM). H2O2 was applied to the arterial rings when the maximal tension was reached. Summarized data (means ± SE; D) showing 80K-induced active tension (g) in PA (left) and MA (right) before (control) and during the beginning (1st, closed bars) and end (2nd, grey bars) application of H2O2. The times when the 1st and 2nd tension measurements were obtained are shown in C (grey boxes). Arteries were isolated from 4–6 mice. *P < 0.05 and **P < 0.01 vs. control (open bars).
Expression of VDCC subtype in PA and MA.
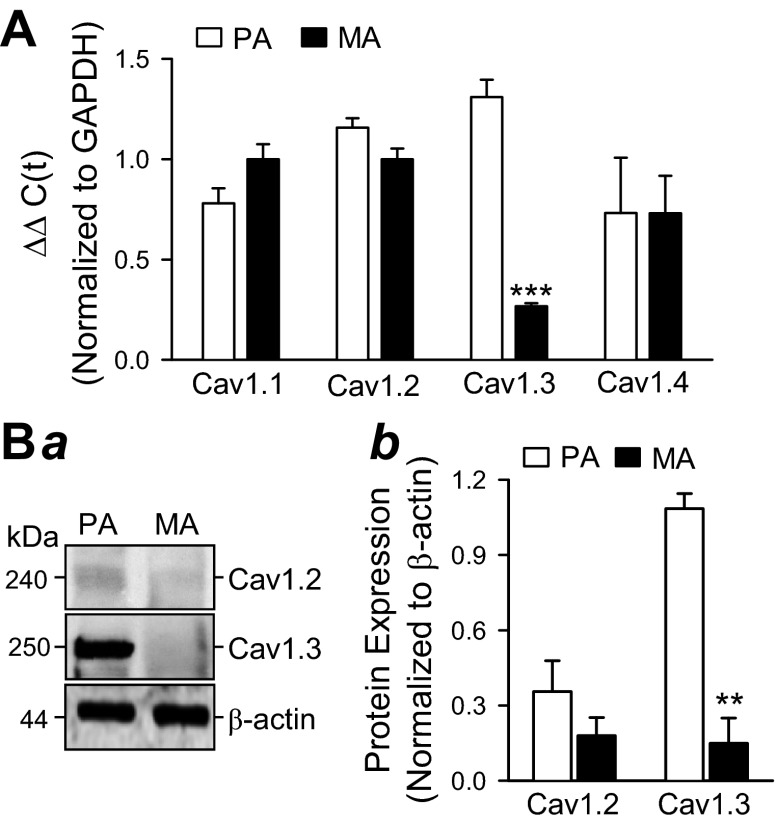
Real-time RT-PCR experiments were performed to compare the mRNA expression level of various VDCC channels in PA and MA. As shown in Fig. 9, both PA and MA express multiple VDCC including Cav1.1, Cav1.2, Cav1.3, and Cav1.4. The expression of Cav1.1, Cav1.2, and Cav1.4 channels was similar between PA and MA in mice, whereas the expression level of Cav1.3 was significantly higher in PA than in MA (Fig. 8A).
Fig. 8.
Differential expression of VDCC channels in mouse pulmonary and mesenteric arteries. A: real-time RT-PCR analyses on Cav1.1, Cav1.2, Cav1.3, and Cav1.4 in isolated PA and MA from normal mice. PA and MA were dissected from normal mice and used for RNA isolation, followed by real-time RT-PCR analysis. Quantitation of mRNA expression level of each VDCC subunit was determined by the ΔΔCT method, as described in materials and methods. Bar graph depicts the expression of Cav1.1, Cav1.2, Cav1.3, and Cav1.4 in PA (open bars) or MA (solid bars), normalized with respect to GAPDH. Data are expressed as means ± SE (n = 3 animals, each sample was measured in triplicate). ***P < 0.001 vs. PA (open bar). B: Western blot analysis of Cav1.2 and Cav1.3 protein expression in mouse pulmonary and mesenteric arteries. Normal PA and MA mouse arteries were dissected, homogenized and subjected to SDS-PAGE analysis as described in materials and methods. Band intensities were quantified with ImageJ software and normalized with respect to β-actin as a loading control. Image depicts a representative Western blot (a) and a bar graph (b) of average band intensities (means ± SE) from 3 independent experiments (n = 3; **P < 0.01).
Western blot analysis was performed to examine whether increased mRNA levels of Cav1.3 in PA of mice, as measured by RT-PCR, correspond to enhanced Cav1.3 protein expression. Consistent with the RT-PCR experiments, the protein expression of Cav1.2 was not significantly different in PA and MA preparations, whereas Cav1.3 expression increased approximately sevenfold in mouse PA (Fig. 8B). These data indicate that Cav1.3, an L-type VDCC α-subunit, is differentially expressed in PA and MA and the diverging effects of ROS on high K+-mediated contraction in PA and MA may be related to this variation in expression of Cav1.3.
DISCUSSION
The expression and appropriate functioning of VDCC channels in PASMC play an indispensable role in regulating pulmonary vascular tone and contributes significantly to the pathological states. Previously, it has been reported that dysfunction and upregulation of Ca2+ channels in animal and human PASMC are linked to the development of sustained pulmonary vasoconstriction and vascular remodeling in pulmonary hypertension (6, 19). While mice are commonly used as models for various disease processes, very little is known about the biophysical properties and molecular basis of VDCC in mouse PASMC. This is due in part to the technical difficulties in isolating PA from mouse lungs and dispersing single smooth muscle cells from small PA. Therefore, we have characterized the electrophysiological and pharmacological properties of VDCC in freshly dissociated mouse PASMC and their contribution to vascular contraction in mouse PA.
The maximum inward currents with extracellular Ba2+ as the charge carrier were recorded as −57 pA (−2.6 pA/pF) in freshly dissociated mouse PASMC. The maximum Ca2+ currents recorded vary among different species of smooth muscle cells. From PASMC, the peak currents were less than −5 pA with 10 mM Ba2+ in piglet (19), −60 pA (−4 pA/pF) with 1.8 mM Ca2+ in rabbit (29), and 230 pA (5 pA/pF) with 20 mM Ba2+ in rat (47). In addition, other types of smooth muscle cells showed a mean current density of −90 pA (−6 pA/pF) in rat MA smooth muscle cells (51) and −100 pA (−4 pA/pF) in dog basilar arteries (39). In mouse aortic smooth muscle cells, the maximum Ba2+ currents were 25 pA (4 pA/pF) (43). In our results, the half activation and inactivation of the currents were recorded (−11.2 ± 2.0 and −17.3 ± 1.3 mV) at a more negative potential in mouse PASMC compared with other types of arteries. For example, in rat mesenteric smooth muscle cells, the half activation and inactivation of the currents were −0.2 and −2.8 mV (40), and +12.2 and −9.3 mV (51), respectively. In rabbit ear arteries and coronary arteries, the half activation and inactivation of the currents were +5 and −17 mV (55), and −4 and −28 mV (33), respectively. These data suggest that the open probability of VDCC from freshly dissociated mouse PASMC is enhanced at more negative potentials, which could indicate that differential expression of VDCC subtypes alters the gating kinetics of Ca2+ channels in mouse PASMC compared with other forms of arterial smooth muscle cells.
Nifedipine relaxes high K+-induced contraction of mouse PA, supporting the role of VDCC in pulmonary vascular contraction. In the present study, electrophysiological data clearly demonstrate that nifedipine caused the inhibition of VDCC in mouse PASMC (IC50 = 23 nM) more effectively than that observed in single rabbit ear artery cells (17), where nifedipine produced a 50% reduction in current. In rat MA, nifedipine caused half inhibition of VDCC and vascular contraction at a concentration of 100 nM. In addition, nifedipine caused the inhibition of Ca2+ current with a half-maximal effective dose, 159 ± 54 nM in human urethra smooth muscle cells (20). The dihydropyridine agonist Bay K8644 increases VDCC and shifts the peak I-V relationship to more hyperpolarized potentials. Similar results were observed in other smooth muscle cells (18, 33). These results support the view that the recorded inward currents in mouse PASMC were mostly VDCC and contributed to PA contraction.
The opposite effects of ROS on vascular contraction in different types of vasculature have been reported. For example, superoxide and H2O2 caused vasoconstriction and attenuated endothelium-dependent dilation (2, 25), whereas in vessels preconstricted with agonists H2O2 caused a relaxation response in rat and rabbit aorta (23, 58), porcine and canine coronary arteries (4, 46), cat and canine cerebral arteries (12, 56), and rabbit MA (14). Even in the same artery, either constriction or relaxation can be induced depending on the concentration of ROS. For example, in the MA, a biphasic response is caused: a transient contraction followed by a persistent relaxation at high concentrations of H2O2, whereas the MA only caused contraction at low concentrations of H2O2 (15). In our study, we observed the reciprocal effect of ROS on vascular contraction in the PA and systemic (aortic and MA) arteries. Both aorta and MA slightly contracted further from the elicited contraction induced by 80K followed by the gradual return to the contraction elicited by 80K with exposure of exogenous ROS generated by XO/HX. However, in PA, exposure to XO/HX caused a relaxation of 80K-induced vasoconstriction after slightly further contraction. These responses appear to be mediated via the impaired Ca2+ influx due to VDCC inhibition. Increases in [Ca2+]cyt by high K+ in PASMC were also inhibited by exposure to XO/HX. When XO/HX was removed, the effects of relaxation were sustained or accelerated in mouse PA, and also the inhibitory effect on VDCC increased. However, the arterial contraction evoked by 80K returned to basal tension when the arteries were washed with MKS solution in the aorta and MA. These findings suggest a fundamental difference in the way that basic signaling pathways interact with VDCC, which ultimately have a greater influence on the vascular tone of PA. There are several reports that suggest a direct correlation between ROS and VDCC activation. Amberg et al. (2) reported that XO/HX elicit the activation of VDCC in rat cerebral arterial smooth muscle cell. H2O2 can activate Ca2+ channels in guinea pig ventricular myocytes when using a perforated patch configuration (52), whereas, in mouse cerebral cortical neurons, the hydroxyl ion suppressed Ca2+ influx through VDCC (49). These differential responses may relate to the concentration and type of ROS, vessel heterogeneity, artery size, species, and experimental conditions.
We also characterized the effect of H2O2 on VDCC, [Ca2+]cyt and vascular contraction to identify whether exogenous H2O2 elicits a response similar to that of XO/HX. Similar to the patch-clamp data from PASMCs treated with XO/HX, VDCC were inhibited by exposure to H2O2. This effect was unlikely an artifact of ICa run down as all cells were intracellularlly perfused with 5 mM ATP. It has been previously shown that ATP sustains the current amplitude of VDCC in guinea pig ventricular (7, 26) and MA cells (41), bovine chromaffin cells (11), and rat MA (57). In addition, our patch pipette solutions were made with 10 mM EGTA, which is reported to prolong the survival of ICa in ventricular myocytes (7).
Increases in [Ca2+]cyt induced by high K+ were impaired in the presence of H2O2 after shown increases of basal [Ca2+]cyt. Exogenous application of H2O2 to PA preparations precontracted with 80K led to slightly enhanced constriction followed by relaxation. Consistent with the results of XO/HX treatment, impaired increases of [Ca2+]cyt through VDCC caused inhibition of 80K-induced contraction in PA. Similar to our results, others investigators have reported that H2O2 caused relaxation in other types of arteries (4, 9, 54). However, it should be noted that other studies have divergently reported exogenous H2O2 can significantly enhance [Ca2+]cyt and induce vasoconstriction in PA (32, 42, 44, 48). It is possible that the observed vasorelaxation of mouse PA in our studies is a result of H2O2 inhibition on VDCC and/or ryanodine receptors (RyR). Functional evidence of RyR1 in Ca2+ release and contraction in mouse PA has been previously reported (31). In this study, local and global Ca2+ increases and high K+-induced contractions are significantly reduced in RyR1 homozygous and heterozygous mice (31). As VDCC are known to be physically coupled to RyR1 in skeletal muscle (53), where membrane depolarization of VDCC subsequently opens RyR1 leading to SR Ca2+ release, it is possible that H2O2 attenuates extracellular Ca2+ flux by inhibiting VDCC, which prevents the opening of RyR1 ultimately leading to vasorelaxation. Further studies are required to elucidate the effects of H2O2 on vasomotor tone and Ca2+ mobilization. Additionally, we aim to determine which ion channels contribute to further contraction, followed by relaxation induced by H2O2, which shows a different response compared with the treatment with XO/HX in MA.
As for ROS generation, XO/HX and H2O2 are different sources of ROS; this resulted in different responses of vascular contraction in MA. However, in PA, the effect of XO/HX and H2O2 showed consistent vascular response with relaxation, but MA exhibited vascular relaxation only with exposure to H2O2. These results imply the existence of different susceptibilities, depending on the type of ROS generation, especially in MA.
In the present study, we also demonstrated that MA expresses lower levels of Cav1.3 channels compared with PA. Another report suggested that the lack of Cav1.3 Ca2+ channels in null mutant mice resulted in a depolarizing shift in the voltage-dependent activation in atrial myocytes (59). Therefore, it is possible that the differential expression of the Cav1.3 channel in PA and MA causes the changes in whole cell VDCC function and offer different reactivity to ROS. However, the physiological role of Cav1.3 in vascular contraction in PA must be determined, and the existence of a direct interaction with ROS remains unclear.
In summary, our study provides an extensive functional characterization of VDCC channels in mouse PA and PASMC. The electrophysiological and pharmacological properties demonstrated in the present study provide an important basis for using mice as a disease model to study the pathogenic roles of VDCC in pulmonary vascular disease. In addition, our investigation also demonstrates the novel finding that ROS differentially regulates vascular contraction between PA and systemic arteries. The mechanisms underlying these different responses may relate to the inhibition of different subtype of VDCC by ROS in PASMC and systemic arterial smooth muscle cells.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-115014, HL-066012, and HL-098053 and National Natural Science Foundation of China Grants 81228001, 81270117, and 30810103904.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.A.K., K.A.S., A.M., and J.X.-J.Y. conception and design of research; E.A.K., J.W., A.Y., A.M.Z., H.Y., H.Y.Y., and H.T. performed experiments; E.A.K., J.W., A.Y., A.M.Z., H.Y., H.Y.Y., H.T., K.A.S., P.C.S., A.Z., R.J.A., A.M., and J.X.-J.Y. analyzed data; E.A.K., J.W., A.Y., A.M.Z., H.Y., H.T., K.A.S., P.C.S., R.J.A., A.M., and J.X.-J.Y. interpreted results of experiments; E.A.K., J.W., A.Y., H.Y., H.T., K.A.S., P.C.S., A.Z., R.J.A., A.M., and J.X.-J.Y. prepared figures; E.A.K., P.C.S., and J.X.-J.Y. drafted manuscript; E.A.K., A.M.Z., H.Y., H.Y.Y., K.A.S., P.C.S., A.Z., R.J.A., A.M., and J.X.-J.Y. edited and revised manuscript; E.A.K., J.W., A.Y., A.M.Z., H.Y., H.Y.Y., H.T., K.A.S., P.C.S., A.Z., A.M., and J.X.-J.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of A. Yamamura: Kinjo Gakuin University School of Pharmacy, Nagoya, Japan.
Present address of H. Yamamura: Nagoya City University Graduate School of Pharmaceutical Sciences, Nagoya, Japan.
REFERENCES
- 1. Ambartsumian N, Klingelhofer J, Grigorian M, Karlstrom O, Sidenius N, Georgiev G, Lukanidin E. Tissue-specific posttranscriptional downregulation of expression of the S100A4(mts1) gene in transgenic animals. Invasion Metastasis 18: 96–104, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Amberg GC, Earley S, Glapa SA. Local regulation of arterial L-type calcium channels by reactive oxygen species. Circ Res 107: 1002–1010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 231: 237–251, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol Heart Circ Physiol 275: H1283–H1289, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Barst RJ. Recent advances in the treatment of pediatric pulmonary artery hypertension. Pediatr Clin North Am 46: 331–345, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation 99: 1197–1208, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Belles B, Malécot C, Hescheler J, Trautwein W. “Run-down” of the Ca current during long whole-cell recordings in guinea pig heart cells: role of phosphorylation and intracellular calcium. Pflügers Arch 411: 353–360, 1988 [DOI] [PubMed] [Google Scholar]
- 8. Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol 221: 249–258, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Burke T, Wolin M. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol Heart Circ Physiol 252: H721–H732, 1987 [DOI] [PubMed] [Google Scholar]
- 10. Crosswhite P, Sun Z. Nitric oxide, oxidative stress and inflammation in pulmonary arterial hypertension. J Hypertens 28: 201–212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elhamdani A, Bossu J, Feltz A. ADP exerts a protective effect against rundown of the Ca2+ current in bovine chromaffin cells. Pflügers Arch 430: 401–409, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Fraile ML, Conde MV, Sanz L, Moreno MJ, Marco EJ, Lopez de Pablo AL. Different influence of superoxide anions and hydrogen peroxide on endothelial function of isolated cat cerebral and pulmonary arteries. Gen Pharmacol 25: 1197–1205, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Fresquet F, Pourageaud F, Leblais V, Brandes RP, Savineau JP, Marthan R, Muller B. Role of reactive oxygen species and gp91phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. Br J Pharmacol 148: 714–723, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujimoto S, Asano T, Sakai M, Sakurai K, Takagi D, Yoshimoto N, Itoh T. Mechanisms of hydrogen peroxide-induced relaxation in rabbit mesenteric small artery. Eur J Pharmacol 412: 291–300, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Gao YJ, Hirota S, Zhang DW, Janssen LJ, Lee RM. Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol 138: 1085–1092, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaultier C, Matrot B, Gallego J. Transgenic models to study disorders of respiratory control in newborn mice. ILAR J 47: 15–21, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hering S, Beech DJ, Bolton TB, Lim SP. Action of nifedipine or BAY K8644 is dependent on calcium channel state in single smooth muscle cells from rabbit ear artery. Pflügers Arch 411: 590–592, 1988 [DOI] [PubMed] [Google Scholar]
- 18. Hering S, Hughes AD, Timin EN, Bolton TB. Modulation of calcium channels in arterial smooth muscle cells by dihydropyridine enantiomers. J Gen Physiol 101: 393–410, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirenallur SD, Haworth ST, Leming JT, Chang J, Hernandez G, Gordon JB, Rusch NJ. Upregulation of vascular calcium channels in neonatal piglets with hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L915–L924, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hollywood MA, Woolsey S, Walsh IK, Keane PF, McHale NG, Thornbury KD. T- and L-type Ca2+ currents in freshly dispersed smooth muscle cells from the human proximal urethra. J Physiol 550: 753–764, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hool LC, Corry B. Redox control of calcium channels: from mechanisms to therapeutic opportunities. Antioxid Redox Signal 9: 409–435, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Ichinose F, Ullrich R, Sapirstein A, Jones RC, Bonventre JV, Serhan CN, Bloch KD, Zapol WM. Cytosolic phospholipase A2 in hypoxic pulmonary vasoconstriction. J Clin Invest 109: 1493–1500, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iesaki T, Okada T, Yamaguchi H, Ochi R. Inhibition of vasoactive amine induced contractions of vascular smooth muscle by hydrogen peroxide in rabbit aorta. Cardiovasc Res 28: 963–968, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, Tanimoto A, Okazaki M, Sasaguri Y, Adachi T, Otsuji Y. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med 177: 219–226, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol Heart Circ Physiol 257: H33–H37, 1989 [DOI] [PubMed] [Google Scholar]
- 26. Keung E, Karliner J. Complex regulation of calcium current in cardiac cells. Dependence on a pertussis toxin-sensitive substrate, adenosine triphosphate, and an alpha 1-adrenoceptor. J Clin Invest 85: 950–954, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim DY, Ryu SY, Lim JE, Lee YS, Ro JY. Anti-inflammatory mechanism of simvastatin in mouse allergic asthma model. Eur J Pharmacol 557: 76–86, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Ko EA, Burg ED, Platoshyn O, Msefya J, Firth AL, Yuan JX. Functional characterization of voltage-gated K+ channels in mouse pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 293: C928–C937, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Ko EA, Park WS, Ko JH, Han J, Kim N, Earm YE. Endothelin-1 increases intracellular Ca2+ in rabbit pulmonary artery smooth muscle cells through phospholipase C. Am J Physiol Heart Circ Physiol 289: H1551–H1559, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Lacampagne A, Duittoz A, Bolanos P, Peineau N, Argibay JA. Effect of sulfhydryl oxidation on ionic and gating currents associated with L-type calcium channels in isolated guinea-pig ventricular myocytes. Cardiovasc Res 30: 799–806, 1995 [PubMed] [Google Scholar]
- 31. Li XQ, Zheng YM, Rathore R, Ma J, Takeshima H, Wang YX. Genetic evidence for functional role of ryanodine receptor 1 in pulmonary artery smooth muscle cells. Pflügers Arch 457: 771–783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin MJ, Yang XR, Cao YN, Sham JS. Hydrogen peroxide-induced Ca2+ mobilization in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292: L1598–L1608, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Matsuda JJ, Volk KA, Shibata EF. Calcium currents in isolated rabbit coronary arterial smooth muscle myocytes. J Physiol 427: 657–680, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maurer B, Reich N, Juengel A, Kriegsmann J, Gay RE, Schett G, Michel BA, Gay S, Distler JH, Distler O. Fra-2 transgenic mice as a novel model of pulmonary hypertension associated with systemic sclerosis. Ann Rheum Dis 71: 1382–1387, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol 25: 274–278, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol Cell Physiol 259: C3–C18, 1990 [DOI] [PubMed] [Google Scholar]
- 37. Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Ng LC, Gurney AM. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res 89: 923–929, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Nikitina E, Zhang ZD, Kawashima A, Jahromi BS, Bouryi VA, Takahashi M, Xie A, Macdonald RL. Voltage-dependent calcium channels of dog basilar artery. J Physiol 580: 523–541, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ohya Y, Abe I, Fujii K, Takata Y, Fujishima M. Voltage-dependent Ca2+ channels in resistance arteries from spontaneously hypertensive rats. Circ Res 73: 1090–1099, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Ohya Y, Sperelakis N. ATP regulation of the slow calcium channels in vascular smooth muscle cells of guinea pig mesenteric artery. Circ Res 64: 145–154, 1989 [DOI] [PubMed] [Google Scholar]
- 42. Pelaez NJ, Braun TR, Paul RJ, Meiss RA, Packer CS. H2O2 mediates Ca2+- and MLC20 phosphorylation-independent contraction in intact and permeabilized vascular muscle. Am J Physiol Heart Circ Physiol 279: H1185–H1193, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Pinho JF, Medeiros MA, Capettini LS, Rezende BA, Campos PP, Andrade SP, Cortes SF, Cruz JS, Lemos VS. Phosphatidylinositol 3-kinase-delta up-regulates L-type Ca2+ currents and increases vascular contractility in a mouse model of type 1 diabetes. Br J Pharmacol 161: 1458–1471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pourmahram GE, Snetkov VA, Shaifta Y, Drndarski S, Knock GA, Aaronson PI, Ward JP. Constriction of pulmonary artery by peroxide: role of Ca2+ release and PKC. Free Radic Biol Med 45: 1468–1476, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Ramanantsoa N, Vaubourg V, Dauger S, Matrot B, Vardon G, Chettouh Z, Gaultier C, Goridis C, Gallego J. Ventilatory response to hyperoxia in newborn mice heterozygous for the transcription factor Phox2b. Am J Physiol Regul Integr Comp Physiol 290: R1691–R1696, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Rubanyi GM, Vanhoutte PM. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol Heart Circ Physiol 250: H815–H821, 1986 [DOI] [PubMed] [Google Scholar]
- 47. Sen L, Bialecki RA, Smith E, Smith TW, Colucci WS. Cholesterol increases the L-type voltage-sensitive calcium channel current in arterial smooth muscle cells. Circ Res 71: 1008–1014, 1992 [DOI] [PubMed] [Google Scholar]
- 48. Sheehan DW, Giese EC, Gugino SF, Russell JA. Characterization and mechanisms of H2O2-induced contractions of pulmonary arteries. Am J Physiol Heart Circ Physiol 264: H1542–H1547, 1993 [DOI] [PubMed] [Google Scholar]
- 49. Shirotani K, Katsura M, Higo A, Takesue M, Mohri Y, Shuto K, Tarumi C, Ohkuma S. Suppression of Ca2+ influx through L-type voltage-dependent calcium channels by hydroxyl radical in mouse cerebral cortical neurons. Brain Res Mol Brain Res 92: 12–18, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Shuvaev VV, Christofidou-Solomidou M, Bhora F, Laude K, Cai H, Dikalov S, Arguiri E, Solomides CC, Albelda SM, Harrison DG, Muzykantov VR. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther 331: 404–411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thakali KM, Kharade SV, Sonkusare SK, Rhee SW, Stimers JR, Rusch NJ. Intracellular Ca2+ silences L-type Ca2+ channels in mesenteric veins: mechanism of venous smooth muscle resistance to calcium channel blockers. Circ Res 106: 739–747, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thomas GP, Sims SM, Cook MA, Karmazyn M. Hydrogen peroxide-induced stimulation of L-type calcium current in guinea pig ventricular myocytes and its inhibition by adenosine A1 receptor activation. J Pharmacol Exp Ther 286: 1208–1214, 1998 [PubMed] [Google Scholar]
- 53. Van Petegem F. Ryanodine receptors: structure and function. J Biol Chem 287: 31624–31632, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wei EP, Kontos HA, Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol Heart Circ Physiol 271: H1262–H1266, 1996 [DOI] [PubMed] [Google Scholar]
- 55. Wijetunge S, Lymn JS, Hughes AD. Effects of protein tyrosine kinase inhibitors on voltage-operated calcium channel currents in vascular smooth muscle cells and pp60(c-src) kinase activity. Br J Pharmacol 129: 1347–1354, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang ZW, Zhang A, Altura BT, Altura BM. Endothelium-dependent relaxation to hydrogen peroxide in canine basilar artery: a potential new cerebral dilator mechanism. Brain Res Bull 47: 257–263, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Yokoshiki H, Katsube Y, Sperelakis N. Regulation of Ca2+ channel currents by intracellular ATP in smooth muscle cells of rat mesenteric artery. Am J Physiol Heart Circ Physiol 272: H814–H819, 1997 [DOI] [PubMed] [Google Scholar]
- 58. Zembowicz A, Hatchett RJ, Jakubowski AM, Gryglewski RJ. Involvement of nitric oxide in the endothelium-dependent relaxation induced by hydrogen peroxide in the rabbit aorta. Br J Pharmacol 110: 151–158, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Z, He Y, Tuteja D, Xu D, Timofeyev V, Zhang Q, Glatter KA, Xu Y, Shin HS, Low R, Chiamvimonvat N. Functional roles of Cav1.3(alpha1D) calcium channels in atria: insights gained from gene-targeted null mutant mice. Circulation 112: 1936–1944, 2005 [DOI] [PubMed] [Google Scholar]