Introduction
Human birth defects remain a major public health burden, with CDC estimates showing that 1 of every 33 US newborns present with a birth defect. Worldwide, the estimate approaches 6% of all births—some 7.9 million infants. Among the most common and debilitating of human birth defects are those affecting the formation of the neural tube, the precursor to the central nervous system. While some birth defects can be repaired surgically with little long-term effect on the patient, neural tube defects (NTDs) are debilitating, even after surgical repair. Despite the fact that NTDs pose life-long challenges for patients, their families, and societies as a whole, research into their etiologies has lagged far behind that of other diseases.
NTDs are a multifactorial disorder, arising from a complex combination of genetic and environmental interactions, and while we are only now beginning to understand the etiologies of NTDs, two significant advances have been made in their prevention and treament. First, it is widely known that taking folic acid (FA) during child-bearing years can significantly reduce a woman’s risk of having a baby with an NTD (1). Second, recent studies have demonstrated that in utero repair of spina bifida significantly improves patient outcomes (2). In spite of these advances, we have only just begun to tackle the problem of NTDs. For example, 20 years after mandated folate fortification in the US, NTD rates remain unacceptably high, and in fact, we understand little about the mechanism by which folate acts on NTDs. Likewise, even after successful repair, NTD patients still suffer wide-ranging neurological problems. Thus, despite these widely reported advances, NTDs continue to present a major public health burden afflicting 1 in 2000 births in the US, and significantly more births in developing areas such as China and Latin America.
Here, we review the process of neural tube development and how defects in this process lead to NTDs. We discuss both human NTDs and the ways in which animal studies are providing new insights. We focus on recent important findings and highlight unanswered questions: How effective is FA in preventing NTDs? What is the molecular basis of FA action? What genes determine susceptibility to NTDs? Finally, we discuss strategies that could build upon our current understanding to lessen the personal and societal burdens that accompany these serious malformations.
NTDs are a problem of embryology
NTDs arise as a defect in embryonic development. During embryogenesis, the central nervous system normally develops first as a flat sheet of cells that subsequently rolls up and fuses shut to form the hollow central nervous system (Fig. 1; Fig. 2A). NTDs arise when this process of neural tube closure (NTC) is disrupted (Fig. 2B). As discussed below, cellular changes that drive NTC and the molecular mechanisms that control them are largely conserved from humans and mice to birds and amphibians. Indeed, decades of work in animal models have established a comprehensive framework for the developmental processes that underlie NTC (3–6).
Figure 1.
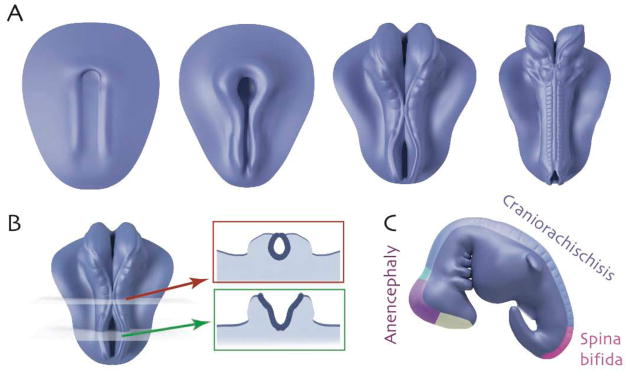
A. Successive images showing the time course of neural tube closure in a stylized vertebrate embryo (rostral = up). Initially, the CNS is a flat sheet; paired neural folds elevate along the rostrocaudal axis and move medially, eventually fusing to enclose the neural tube. B. Cross-sections illustrate closed (red) and open (green) regions of the neural tube. C. Region-specific neural tube defects.
Figure 2.
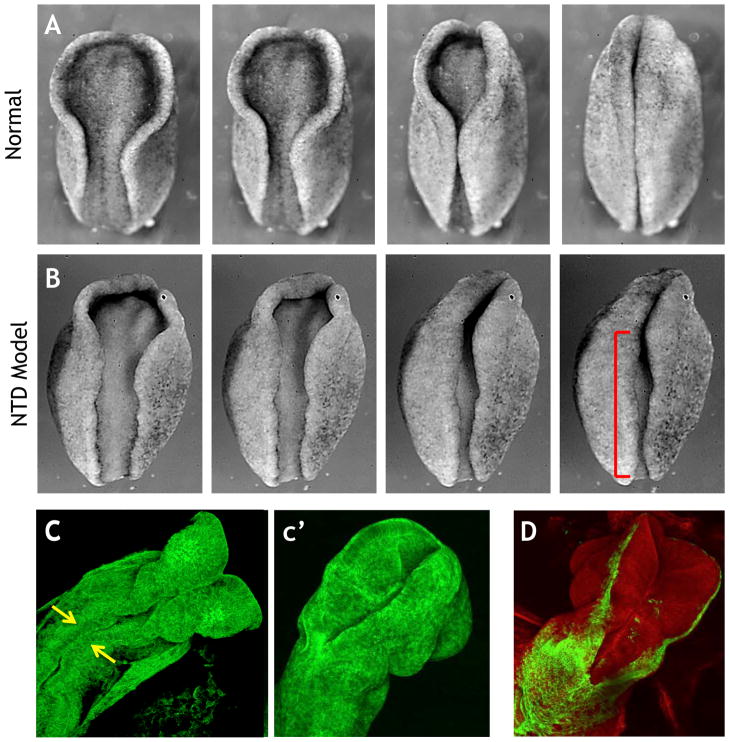
A. Still frames from a time lapse movie showing neural tube closure in an amphibian embryo (rostral = up). B. Disruption of PCP signaling results in disrupted neural tube closure (red bracket). C. Still frame from a movie of mouse neural tube closure; arrows indicate initial meeting of neural folds (47). c′. Neural tube closure has progressed in a later time point. D. Genetically-inducible fluorescent reporters allow visualization of specific tissues (green), in this case the ectoderm that borders the neural tissue.
NTDs: Not one disease, but many
While often lumped into a single category, NTDs actually encompass a wide array of morphologically distinct malformations, and this heterogeneity is a central but generally misunderstood aspect of the phenotype. In general, failure of NTC is associated with defects in the overlying bony structures (i.e., cranial vault and neural arches) such that neural tissue is exposed. Consequently, most defects of primary NTC are often referred to as open NTDs. There are also a number of closed or skin-covered, conditions that involve the neural tube, though essentially nothing is known about their etiology. Among the open NTDs, further finer classifications must be made based upon vertebral location and extent of the defect.
NTDs restricted to the cranial regions are referred to as anencephaly (Fig. 1C). This invariably lethal condition is characterized by absence of the cranial vault and severe defects in the cerebral hemispheres. The cerebellum is usually absent and the brain stem may be hypoplastic. NTDs restricted to the caudal portion of the neural tube are referred to generally as spina bifida (meningomyelocele)(Fig. 1C). More prevalent than anencephaly, this condition is associated with defects in the neural arches, through which meninges and spinal cord tissue protrude. The majority of fetuses with meningomyeloceles are live born, and with proper treatment, survival to adulthood is common. Failure of NTC over the entire body axis, called craniorachischisis (Fig. 1C), is also lethal but is relatively rare.
Epidemiology of human NTDs
Prevalence of open NTDs has varied over time and geography. Prevalence in the US is ~1/2000, but in neighboring Mexico is significantly higher (7). NTDs are etiologically complex, and both genetic and environmental risk factors have been proposed. In terms of genetic underpinnings, monozygotic twinning and single gene disorders have long been associated with NTDs (8). Numerous studies have explored a variety of candidate gene pathways (reviewed in (9)), such as the folate/1-methyl carbon metabolic pathway (10, 11), glucose metabolism/transport (9), DNA repair (12), oxidative stress pathway (9), retinoic acid receptors (13), and the WNT/Planar Cell Polarity (PCP) signaling network (14). In a following section, we discuss animal studies that are shedding new light on genetic factors that govern NTC. However, it is crucial to note that the population burden of human NTDs thus far explained by known genetic polymorphisms remains quite small, a fact that almost certainly reflects the relatively modest amount of research effort in this area, rather than the importance of genetic factors in NTDs. Indeed, no large-scale NTD-focused genomic discovery project (GWAS, exome sequencing, etc.) has yet been published.
The key role that environment plays in NTD etiology is highlighted by the important impact of maternal nutrition, specifically folate intake. Both observational studies and randomized trials have provided evidence that FA reduces the risk of NTD-affected pregnancies (1, 15). However, 30–50 % of NTDs are not folate preventable, and other environmental factors must also be considered (1). Amongst the most notable environmental risk factors for NTDs are maternal pregestational insulin-dependent diabetes (16) and maternal prepregnant obesity (17), as well as maternal use of specific anticonvulsant drugs, including valproic acid (18).
Diagnosis, Treatment and Outcome
Individuals with spina bifida can survive with appropriate medical treatment, generally surgical repair soon after birth (19). Screening to identify pregnant women at risk for carrying NTD-affected fetuses can be achieved by evaluation of maternal serum alpha-fetoprotein levels, amniocentesis, and ultrasound imaging (20). Such screening is particualry important because a recent randomized trial showed that in utero repair of spina bifida improved patient outcomes in terms of improved motor function/ambulation and mental development compared to those receiving surgery post-partum (2). Moreover, only 40% of infants receiving in utero surgery required a ventriculoperitoneal shunt at 12 months of age, compared with 82% of infants receiving postnatal surgery (2).
However, even after treatment (in utero or postnatally), individuals with spina bifida remain at elevated risk for nervous system malformations, including hydrocephalus and skull malformations that press the brain downward into the spinal canal. Lower extremity weakness and paralysis, sensory loss, and bowel and bladder dysfunction are also common. Individuals with spina bifida are also at risk for a range of orthopedic abnormalities including clubfoot, contractures, hip dislocation, scoliosis, and kyphosis. Moreover, while most individuals with spina bifida have normal intelligence, specific cognitive and language difficulties are common (21).
While the recent advances in surgical treatment should have a major impact, it is important to note that even perfect surgical repairs would represent only a partial solution. Because NTC occurs so early in development (~3 weeks post-conception), an NTD will leave the delicate neural tissue, normally encased within bone, exposed and subjected to trauma in utero until the much later time point when surgical intervention might be applied. As such, substantial effort should be focused on understanding the mechanisms of NTC and the biological basis of NTDs, with an eye toward developing preventive strategies.
Primary prevention of NTDs by folic acid
NTDs stand out as one of few birth defects for which primary prevention strategies are available. Research spanning decades, including randomized and community-based trials demonstrate that maternal, periconceptional supplementation with FA alone, or multivitamins containing FA can reduce risk of NTDs in offspring (1, 22, 23). How FA acts to prevent NTDs is a major outstanding question, and the answer will be complex because folate is central to numerous cellular reactions. These include production of purines and thymidylate, the building blocks for DNA and RNA biosynthesis, and production of the universal methyl donor S-adenosyl- methionine (SAM), utilized in methylation of DNA, histones, proteins, and lipids. Therefore, deficits in FA metabolism could affect cell proliferation, cell survival, transcriptional regulation, or a host of other cellular reactions; defects in any of these processes could disrupt NTC. To bring insight into the mechanisms by which FA acts during NTC, studies have turned to animal models, in particular mouse NTD models, which are thought to be representative of human neurulation anatomically and molecularly and in which folate levels can be altered prior to or during pregnancy.
Curiously, even though targeted mutations have been made in numerous mouse genes required for FA metabolism or utilization, only three (Folr1, RFC, Mthfd1l) have overt NTD phenotypes (24–26). Even under conditions of folate deficiency, there is relatively little evidence of altered NTD incidence for mouse FA pathway mutations (27). Moreover little compelling association has been established between Folr1 or RFC and human NTDs and despite a long-standing focus on this pathway, only a few polymorphisms have been identified as possible risk factors for human NTDs, such as the 677C > T SNP in the MTHFR gene (11). Currently, in the folate-replete population in the US, the majority of human pregnancies are within the normal range of FA levels and recent studies found little association between NTD risk and maternal FA intake, perhaps suggesting that FA-sensitive NTDs have largely been prevented (28). Therefore, data to date suggest that deficits in the FA pathway likely represent only a modest fraction of NTD risk.
Looking beyond the folate pathway to elucidate gene–environment interactions
A lack of evidence between FA pathway mutants and NTD risk indicates the need for novel approaches to elucidate how FA impacts NTC. The large number of mouse NTD models with no apparent link to the FA pathway provide enormous potential to explore how genetics impact responsiveness to FA and to define mechanisms by which FA influences NTC. This potential has been only minimally realized, as only 23 of the >200 mouse NTD mutants have been tested for FA responsiveness (27, 29, 30). FA treatment has some preventive effect in 11 mouse NTD models and in a few cases this correlates with compromised FA utilization. Splotch2H (Pax3) and Mthfd2l mutants show a deficit in FA metabolism, but Cited2, Fkbp8, Fuz, and curly tail (Grhl3) mutants do not, indicating that disrupted FA metabolism is unlikely to be the full explanation for FA-mediated effects. The requirement for FA in DNA synthesis might be expected to impact cell proliferation or survival, but to date there is no evidence that these processes are normalized in FA-preventable mouse NTD models. We therefore currently have little that ties together NTD models that are FA-responsive or FA-resistant at a mechanistic level. Testing a much larger set of NTD models will expand this dataset and may reveal common pathways or targets and should lead to better predictions as to whether FA, or perhaps another treatment, would be most effective in preventing NTDs.
Contrary to expectations, in a few mouse NTD models FA treatment resulted in detrimental effects, including an increased risk for NTDs and embryo loss prior to the time of NTC (29, 30). Although it is largely assumed that FA prevents NTDs by correcting the embryological defect, these findings of early embryo loss are consistent with the possibility raised back in 1997 based on miscarriage risk that embryo loss may explain some of the decrease in human NTD occurrence upon FA supplementation (31). If these unexpected findings are relevant to human NTDs, it could suggest that, for certain mutations, FA may not be protective or even neutral in its action, although the consensus is that in humans, at a population-scale, FA has a preventive effect. Additional studies in animal models will be required to determine the basis for the observed detrimental effect and whether there are particular gene or pathway mutations that are more susceptible, either positively or negatively to FA effects.
Possible epigenetic changes induced by FA
A striking but understudied aspect of FA is the potential to cause epigenomic changes. Changes in SAM levels could impact DNA methylation and histone modification, both of which can influence gene transcription. Indeed, there is evidence that methyl donor-enriched diets can induce alterations in gene expression and long-term generational exposure can result in increasing variation in DNA methylation even in wildtype mice (32). Moreover, questions have been raised as to whether the increase in FA intake acting through the methylation cycle may predispose to allergic airway disease, although the current evidence is conflicting (33–35). With respect to NTD risk, some mutant mice showed a beneficial response to increased FA over a single gestation period but a detrimental response over multiple generations (30). This contradictory response depending on the length of FA exposure highlights the difficulties in considering how best to model human exposure to FA as currently implemented. Moreover, it raises the important but little studied question of whether there may be unexpected effects of long-term FA fortification and supplementation in humans or potential effects due to increased levels of metabolized and unmetabolized FA.
The variation in NTD risk depending on the length of FA exposure (30) points toward the possibility of epigenetic changes. Consistent with this idea, mutations in genes that affect DNA methylation, histone modification (in particular acetylation) or chromatin remodeling result in NTDs in mice (4, 36, 37). Furthermore, the anti-epileptic drug valproic acid is a histone deacetylase inhibitor and it is a well-known risk factor for NTDs in humans (18). Interestingly, NTDs in mice bearing mutations in the histone acetyltransferases Gcn5 or Cited2 can be prevented with FA supplementation (38, 39). Epigenetic influence has also been suggested to help explain the predominance of cranial NTDs in females versus males. X chromosome inactivation is maintained by DNA methylation and hence there is more demand on the methylation cycle in female cells after every division relative to male cells (40). Epigenomic studies as outlined below should bring new insights into how FA may affect transcriptional programs during NTC.
In summary, we have a relatively poor understanding of FA action on NTC and little insight into why some genetic mutants respond, positively or negatively, to this environmental factor. As described below, efforts to understand the cellular mechanisms governing NTC in animal models are providing important new tools for NTD research. Investigation of FA action in a much larger set of mouse NTD models will help to reveal whether particular developmental processes or molecular pathways can be related in terms of FA responsiveness and to gain molecular insights into optimal interventions to prevent NTDs.
The cell biology of NTDs
One complication that has hindered our understanding of NTDs generally, and of FA action specifically, is the generally underappreciated complexity of NTC. While it can be described simply and succinctly as a sheet rolling up to form a tube, NTC is actually the sum of several autonomous and region-specific changes in cell behavior (3–6). For example, NTC begins not with rolling, but instead with a thickening of the neural ectoderm, only after which do the neural folds elevate and begin moving toward the midline. This medial movement of the folds is facilitated by bending of the epithelial sheet and by narrowing and lengthening of the neural tissue. In addition, some studies suggest that the epidermis generates a pushing force that aids the movement of neural folds towards the midline. Finally, a poorly defined process of epithelial fusion links the two neural folds into a sheet of epidermal cells covering the hollow neural tube.
Adding to this complexity is the discontinuity of the NTC process along the rostrocaudal axis. Rather than progressing continuously from one end or the other, NTC initiates at multiple sites along the axis and progresses rostrally and/or caudally from those sites. Interestingly, the cellular machines that drive the rolling up of the neural tube differ regionally along the length of the neural tube. This point is important in light of the heterogeneity observed in human NTDs (e.g., spina bifida vs. anencephaly), as studies in animals now make clear that these spatially restricted sub-types of NTD stem from regional differences in the underlying cell behaviors.
One example is the process of convergent extension, whereby cells interdigitate mediolaterally in order to elongate the tissue perpendicularly along the rostrocaudal axis (Fig. 3A). This process acts specifically in the hindbrain and spinal cord, and so disruption of genes governing convergent extension results in craniorachischisis (5, 14). In contrast, another cellular process called apical constriction converts columnar cells into wedge shaped cells, leading to localized bending of the neural epithelium and facilitating NTC (Fig 3B). Apical constriction is most important in the future brain and the caudal-most spinal cord, as disruption of genes controlling apical constriction is associated most strongly with anencephaly and caudally restricted spina bifida (5).
Figure 3.
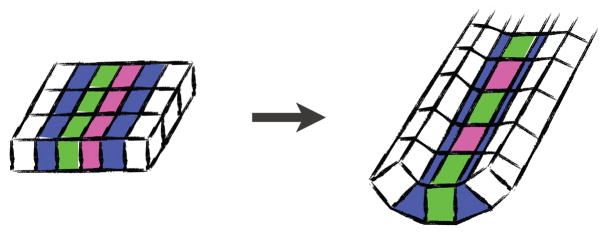
A. Convergent extension cell behavior elongates a tissue (the cell sheet is viewed en face). B. Apical constriction cell behavior transforms a columnar cell into a wedge shape. b′ Localized apical constriction bends cell sheets (viewed here in cross section).
Finally, much of what is known about the dynamic events of vertebrate NTC comes from live imaging studies of amphibian and chick embryos (Fig. 2A)(41–44), but important advances have recently been made in dynamic imaging of NTC in the living mouse embryo (Fig. 2C, D)(45–47). The emerging ability to combine prolonged live imaging with the wealth of mouse genetic mutants will provide a deeper understanding of how morphogenesis is disrupted at a cellular, tissue, and spatio-temporal level and how this results in NTDs.
The genetics of NTDs in animal models
In parallel to the studies exposing the cell biological basis of NTC, other animal studies have led the way to identification of hundreds of genes as candidates for human genetic studies. In the mouse, over 200 genes are causative for NTDs (37, 48), and many others have been provided by studies in amphibians (5). Given the large number of genes associated with NTDs, it is unsurprising that the genes fall into diverse functional classes, ranging from predictable regulators of actin dynamics or cell adhesion to less predictable genes regulating electron transport and DNA damage repair (37, 48). Regrettably, most studies reporting NTDs in mutant animals were focused primarily on other biological questions, and so most remain only cursorily characterized. Nonetheless, some themes are emerging.
1. Discrete genetic modules govern closure in discrete regions of the CNS
NTDs in mutant mice fall into heterogeneous sub-types similar to those seen in humans. Interestingly, genes with common cell-biological functions tend to associate with the same sub-type. For example, disruption of genes encoding Shroom3, Abl, or Mena results in highly penetrant exencephaly and caudally-restricted spina bifida (49–51), and each of these genes has been implicated in the control of apical constriction (52–54). As described below, exencephaly also consistently results from disruption of any of a large number of genes associated with the assembly or function of cilia, while mutations in genes associated with the PCP signaling network associate with craniorachischisis (14). These linkages of genetic modules to particular NTD sub-types is important, as they suggest that more accurate and more specific diagnosis of human NTD sub-types will facilitate studies of how specific genetic polymorphisms confer NTD risk in humans.
2. Cilia and NTDs
One genetic module that is well defined and has emerged as a critical regulator of NTC is that governing ciliogenesis (55). Cilia are small microtubule-based cellular protrusions that are essential for cell–cell signaling (56), a discovery first made in the course of mouse genetic screens focused on neural tube morphogenesis (57, 58). Roughly two dozen genes associated with ciliogenesis have now been implicated in NTC in mice (55). The cell biological mechanism linking cilia to NTDs remains unclear, but there is evidence to suggest defects in apical constriction. First, like mutation of genes associated with apical constriction, mutation of cilia-related genes elicits exencephaly specifically (55). Second, cilia are crucial organelles for Hedgehog signal transduction (56), and Hedgehog signals have been linked directly to neural patterning and to bending of the neural epithelium, a process facilitated by apical constriction (59).
This work in animals has paralleled work in humans revealing a link between cilia and an array of human diseases, and this “ciliopathy” disease spectrum includes both severe and milder forms of NTDs (60–62). For example, the fuz gene is crucial for ciliogenesis and NTC in animal models (63, 64), and mutations in fuz have also been associated with human NTDs (65). Moreover, a number of genes associated with Meckel-Gruber syndrome in which occipital encephaloceles are frequently observed, are also known to compromise cilia formation (61, 62).
3. Planar cell polarity and NTDs
Another genetic module for which a link to NTDs is emerging is the PCP network, which was discovered in Drosophila and has now been shown to govern a wide array of polarized cell behaviors (66). Beginning with studies of the mouse mutant loop-tail, researchers have found that mutations in many PCP genes (Fz, Dvl, Vangl, or Celsr) lead to NTDs (14, 66, 67). In mice, most manipulations of PCP function result in craniorachischisis, and studies in frogs initially revealed that craniorachischisis was in fact a secondary phenotype stemming from an essential role for PCP genes in convergent extension (41, 68). In the absence of PCP function, cells fail to interdigitate mediolaterally, and the resulting—overly wide—neural tissue cannot roll closed (41, 68). Subsequent studies demonstrated the same mechanism in mice with craniorachischisis (69, 70). Importantly, human fetuses with craniorachischisis frequently display shortened and widened axes, consistent with a failure of convergent extension (71, 72). Taking cues from these animal studies, concerted efforts have now identified mutations in several PCP genes in patients with NTDs (14, 67).
While the association of PCP gene variants with human NTDs is exciting, these data also highlight the difficulty in predicting strong genotype/phenotype relationships in human or in animal models. The NTDs in patients with PCP gene mutations range from the expected craniorachischisis to open spina bifida and even to closed NTDs (14). This outcome likely reflects the hypomorphic nature of the particular PCP gene mutations in these patients, because strong homozygous mutation of PCP genes in mice often leads to craniorachischisis, whereas trans-heterozygosity for many of these mouse mutations results in other types of NTDs (14).
The growing abundance of genotype/phenotype data for genes with a shared biological function serves to demonstrate the importance of cilia-related genes and PCP genes in determining susceptibility to NTDs, and studies are now needed to determine the proportion of human NTDs accounted for by variation in these genetic modules and how these variants place embryos at risk of failed NTC. As the first of what is likely to be many such genetic modules to be discerned, these data provide a conceptual framework for gene identification.
Future Directions
Identification of NTD-associated mutations in humans
A major advance in the past ten years has been the discovery in animal models of over 200 genes whose function is required for NTC, and many new NTD models continue to be discovered (37). This insight should now be translated into medical re-sequencing efforts in human NTD patients and the existing large cohorts of NTD patient DNA. Going forward it will be important to move beyond the analysis of single candidate genes to genome-wide sequencing efforts of large sets of patient samples in order to have the power to reveal significant associations and to begin to understand the multi-factorial nature of NTDs in humans. Polymorphisms and possible genetic interactions identified in human NTD cases can then be validated in animal models where both specific mutations as well as multiple genetic changes can be tested.
Epigenomic studies
Epigenetic mechanisms can underlie human disease, as is becoming evident for neurological diseases and cancer (73–75). Based on the findings that epigenetic regulators play key roles in mouse NTC and that such factors may be affected by FA (36, 38, 39), it is likely that genome-wide analyses will reveal epigenomic changes associated with NTDs. In animal models, advanced technologies can evaluate the transcriptional program of specific cell types and correlate this with changes in DNA methylation, histone marks, and higher-order chromatin states. These technologies can also be utilized in human tissues, with the caveat that transcriptional and epigenetic signatures may be vastly different between the available tissue (generally collected at the time of delivery or in infancy) and the early embryonic tissues that mediate NTC. Nonetheless, significant efforts have begun to define the DNA methylation and histone states in numerous control and diseased tissues from human patients, including some preliminary studies of DNA methylation from human NTD tissue (76). Future studies incorporating information on FA status should help to define potential FA-mediated changes, with a particular focus on the genes known to be necessary for NTC. In the near future, animal models provide the clearest means to explore the convergence between genes and environmental factors at the level of the epigenome during the process of NTC.
Developing new therapies for FA-resistant NTD based on knowledge of molecular pathways
Clearly, not all NTDs are preventable with FA treatment in human or in animal models (28, 37, 48), underscoring the need to consider alternative therapies for FA non-responsive NTDs. Mouse NTD models provide a substantial but underutilized resource to develop alternative strategies based on biochemical and mechanistic information. For example, there is evidence for some preventive effect of inositol in curly tail (Grhl3) mutants; inositol deficiency increases NTD incidence in mice and rats, and mutations in genes associated with inositol metabolism and utilization can lead to NTDs in mice (4, 77). This evidence has led to a small PONTI Study (Prevention of NTDs by Inositol), in conjunction with FA for women with a previous NTD-affected pregnancy. Expansion of studies to additional micronutrients—for instance vitamin B12 or zinc, low levels of which appear associated with NTD risk in humans (78, 79)—carry the possibility of defining which genetic risk factors may be best targeted by therapies beyond FA. Human epidemiological studies have uncovered a number of risk factors and animal models provide a largely untapped resource for defining which gene mutations may be most susceptible to these environmental influences and the mechanistic basis for their interaction.
Stem cell alternatives to animal models
While animal models clearly serve as important tools, work with iPS cells now allows direct study of basic biological processes in cells derived from human patients. With the recent demonstration that complex 3-dimensional morphogenetic events can be recapitulated in vitro using stem cells (80, 81), it is now conceivable that NTC could also be modeled in this way. The promise of such an approach would be three-fold. First, it would allow for the first time direct, dynamic studies of neural morphogenesis in human cells. Second, by using cells derived from human NTD patients, this approach would allow direct comparison of normal and affected tissues as they engage in NTC; this should increase the efficacy of transcriptomic and epigenomic studies proposed above. Finally, a stem cell approach should provide more abundant material and a relatively fast time frame to analysis; such increased efficiency could provide a tractable platform with which to screen small molecules for therapeutic/preventive potential.
In conclusion, NTD etiologies remain undefined, NTD population risk remains stubbornly high, and the occurrence of NTDs translates to a great cost in terms of physical, emotional, and financial burden placed on the affected child and their families. The time is ripe for a burst of research in this area. New approaches can now overcome the technical barriers that have for decades made the search for causes, especially genetic causes, elusive. Important and rich human data sources have now become available such as the National Birth Defects Prevention Study, which has collected environmental and lifestyle data and DNA samples on thousands of women and their infants. Furthermore, the rapid advances being made in animal models are contributing significantly to understanding the genetic basis of human NTD and the intersection between genes and environmental factors. Increased attention to this problem is essential for the development of alternative therapies to help to prevent NTDs.
Acknowledgments
This review grew from conversations at the 7th International Conference on Neural Tube Defects, sponsored by the NICHD. We thank Victoria Grier and Elizabeth Stauber for help with the manuscript. This work was supported by grants from the NIH: R01NS058979 (LAN), R01NS050249 (GMS), R01NS076465 (RHF), P01HD067244 (RHF & GMS), 5R01GM074104 (JBW). JBW and LAN are members of the Howard Hughes Medical Institute.
References
- 1.Blom HJ, Shaw GM, den Heijer M, Finnell RH. Nat Rev Neurosci. 2006;7:724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adzick NS, et al. N Engl J Med. 2011;364:993–1004. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi Y, Miura M. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Copp AJ, Greene NDE. J Pathol. 2010;220:217–230. doi: 10.1002/path.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallingford JB. Am J Med Genet. 2005;135C:59–68. doi: 10.1002/ajmg.c.30054. [DOI] [PubMed] [Google Scholar]
- 6.Colas JF, Schoenwolf GC. Dev Dyn. 2001;221:117–45. doi: 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
- 7.CDC . Morbidity and Mortality Weekly Report. 2000;49:1–4. [PubMed] [Google Scholar]
- 8.Hall JL, Harris MJ, Juriloff DM. Teratology. 1997;55:306–313. doi: 10.1002/(SICI)1096-9926(199705)55:5<306::AID-TERA2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 9.Au KS, Ashley-Koch A, Northrup H. Dev Disabil Res Rev. 2010;16:6–15. doi: 10.1002/ddrr.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw GM, et al. BMC Med Genet. 2009;10:49. doi: 10.1186/1471-2350-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molloy AM, Brody LC, Mills JL, Scott JM, Kirke PN, Molloy AM, Brody LC, Mills JL, Scott JM, Kirke PN, editors. Birth Defects Res Part A Clin Mol Teratol. 2009;85:285–294. doi: 10.1002/bdra.20566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olshan AF, Shaw GM, Millikan RC, Laurent C, Finnell RH. Am J Med Genet A. 2005;135:268–273. doi: 10.1002/ajmg.a.30713. [DOI] [PubMed] [Google Scholar]
- 13.Tran PX, et al. Birth Defects Res Part A Clin Mol Teratol. 2011;91:39–43. doi: 10.1002/bdra.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juriloff DM, Harris MJ. Birth Defects Res A Clin Mol Teratol. 2012;94:824–840. doi: 10.1002/bdra.23079. [DOI] [PubMed] [Google Scholar]
- 15.Obican SG, Finnell RH, Mills JL, Shaw GM, Scialli AR. FASEB J. 2010;24:4167–4174. doi: 10.1096/fj.10-165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheffield JS, Butler-Koster EL, Casey BM, McIntire DD, Leveno KJ. Obstetrics & Gynecology. 2002;100:925. doi: 10.1016/s0029-7844(02)02242-1. [DOI] [PubMed] [Google Scholar]
- 17.Shaw GM, Velie EM, Schaffer D. JAMA. 1996;275:1093–1096. doi: 10.1001/jama.1996.03530380035028. [DOI] [PubMed] [Google Scholar]
- 18.Lammer EJ, Sever LE, Oakley GP. Teratology. 1987;35:465–473. doi: 10.1002/tera.1420350319. [DOI] [PubMed] [Google Scholar]
- 19.Bowman RM, McLone DG, Grant JA, Tomita T, Ito JA. Pediatr Neurosurg. 2001;34:114–120. doi: 10.1159/000056005. [DOI] [PubMed] [Google Scholar]
- 20.Drugan A, Weissman A, Evans MI. Clinics in Perinatology. 2001;28:279–287. doi: 10.1016/s0095-5108(05)70083-x. [DOI] [PubMed] [Google Scholar]
- 21.Dennis M, Barnes MA. Dev Disabil Res Rev. 2010;16:31–39. doi: 10.1002/ddrr.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Group MVS. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 23.Berry RJ, et al. N Engl J Med. 1999;341:1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 24.Finnell RH, et al. Nat Genet. 1999;23:228–232. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- 25.Gelineau-van Waes J, et al. Birth Defects Res Part A Clin Mol Teratol. 2008;82:494–507. doi: 10.1002/bdra.20453. [DOI] [PubMed] [Google Scholar]
- 26.Momb J, et al. Proc Natl Acad Sci USA. 2013;110:549–554. doi: 10.1073/pnas.1211199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris MJ. Birth Defects Res Part A Clin Mol Teratol. 2009;85:331–339. doi: 10.1002/bdra.20552. [DOI] [PubMed] [Google Scholar]
- 28.Mosley BS, et al. Am J Epidemiol. 2009;169:9–17. doi: 10.1093/aje/kwn331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray JD, et al. Hum Mol Genet. 2010;19:4560–4572. doi: 10.1093/hmg/ddq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marean A, Graf A, Zhang Y, Niswander L. Hum Mol Genet. 2011;20:3678–3683. doi: 10.1093/hmg/ddr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hook EB, Czeizel AE. Lancet. 1997;350:513–515. doi: 10.1016/S0140-6736(97)01342-1. [DOI] [PubMed] [Google Scholar]
- 32.Li CCY, et al. PLoS Genet. 2011;7:e1001380. doi: 10.1371/journal.pgen.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollingsworth JW, et al. J Clin Invest. 2008;118:3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Am J Epidemiol. 2009;170:1486–1493. doi: 10.1093/aje/kwp315. [DOI] [PubMed] [Google Scholar]
- 35.Magdelijns FJH, Mommers M, Penders J, Smits L, Thijs C. Pediatrics. 2011;128:e135–44. doi: 10.1542/peds.2010-1690. [DOI] [PubMed] [Google Scholar]
- 36.Greene N, Stanier P, Moore GE. Epigenetics. 2011;6:875–883. doi: 10.4161/epi.6.7.16400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris MJ, Juriloff DM, Harris MJ, Juriloff DM, editors. Birth Defects Res Part A Clin Mol Teratol. 2010;88:653–669. doi: 10.1002/bdra.20676. [DOI] [PubMed] [Google Scholar]
- 38.Lin W, et al. Dev Dyn. 2008;237:928–940. doi: 10.1002/dvdy.21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Q, Behringer RR, de Crombrugghe B. Nat Genet. 1996;13:275–283. doi: 10.1038/ng0796-275. [DOI] [PubMed] [Google Scholar]
- 40.Juriloff DM, Harris MJ, Kappen C, Molloy AM, editors. Birth Defects Res Part A Clin Mol Teratol. 2012;94:849–855. doi: 10.1002/bdra.23036. [DOI] [PubMed] [Google Scholar]
- 41.Wallingford JB, Harland RM. Development. 2002;129:5815–25. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- 42.Morita H, et al. Development. 2012;139:1417–1426. doi: 10.1242/dev.073239. [DOI] [PubMed] [Google Scholar]
- 43.Van Straaten HW, Janssen HC, Peeters MC, Copp AJ, Hekking JW. Dev Dyn. 1996;207:309–18. doi: 10.1002/(SICI)1097-0177(199611)207:3<309::AID-AJA8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 44.Van Straaten HW, Sieben I, Hekking JW. Dev Dyn. 2002;224:103–8. doi: 10.1002/dvdy.10078. [DOI] [PubMed] [Google Scholar]
- 45.Jones EA, et al. Genesis. 2002;34:228–235. doi: 10.1002/gene.10162. [DOI] [PubMed] [Google Scholar]
- 46.Pyrgaki C, Trainor P, Hadjantonakis AK, Niswander L. Dev Biol. 2010;344:941–947. doi: 10.1016/j.ydbio.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Massarwa R, Niswander L. Development. 2013;140:226–236. doi: 10.1242/dev.085001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris MJ, Juriloff DM. Birth Defects Res A Clin Mol Teratol. 2007;79:187–210. doi: 10.1002/bdra.20333. [DOI] [PubMed] [Google Scholar]
- 49.Hildebrand JD, Soriano P. Cell. 1999;99:485–497. doi: 10.1016/s0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- 50.Lanier LM, et al. Neuron. 1999;22:313–325. doi: 10.1016/s0896-6273(00)81092-2. [DOI] [PubMed] [Google Scholar]
- 51.Koleske AJ, et al. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- 52.Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Curr Biol. 2003;13:2125–2137. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- 53.Roffers-Agarwal J, Xanthos JB, Kragtorp KA, Miller JR. Dev Biol. 2008;314:393–403. doi: 10.1016/j.ydbio.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fox DT, Peifer M. Development. 2007;134:567–578. doi: 10.1242/dev.02748. [DOI] [PubMed] [Google Scholar]
- 55.Murdoch JN, Copp AJ. Birth Defects Res A Clin Mol Teratol. 2010;88:633–652. doi: 10.1002/bdra.20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goetz SC, Anderson KV. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huangfu D, et al. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 58.Zohn IE, Anderson KV, Niswander L. Birth Defects Res A Clin Mol Teratol. 2005;73:583–590. doi: 10.1002/bdra.20164. [DOI] [PubMed] [Google Scholar]
- 59.Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ. Development. 2002;129:2507–2517. doi: 10.1242/dev.129.10.2507. [DOI] [PubMed] [Google Scholar]
- 60.Oh EC, Katsanis N. Development. 2012;139:443–448. doi: 10.1242/dev.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Logan CV, Abdel-Hamed Z, Johnson CA. Mol Neurobiol. 2011;43:12–26. doi: 10.1007/s12035-010-8154-0. [DOI] [PubMed] [Google Scholar]
- 62.Vogel TW, Carter CS, Abode-Iyamah K, Zhang Q, Robinson S. Neurosurg Focus. 2012;33:E2. doi: 10.3171/2012.6.FOCUS12222. [DOI] [PubMed] [Google Scholar]
- 63.Park TJ, Haigo SL, Wallingford JB. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 64.Gray RS, et al. Nat Cell Biol. 2009;11:1225–1232. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seo JH, et al. Hum Mol Genet. 2011;20:4324–4333. doi: 10.1093/hmg/ddr359. [DOI] [PubMed] [Google Scholar]
- 66.Wallingford JB. Annu Rev Cell Dev Biol. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 67.Kibar Z, et al. N Engl J Med. 2007;356:1432–1437. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- 68.Wallingford JB, Harland RM. Development. 2001;128:2581–2592. doi: 10.1242/dev.128.13.2581. [DOI] [PubMed] [Google Scholar]
- 69.Wang J, et al. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ybot-Gonzalez P, et al. Development. 2007;134:789–799. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marin-Padilla M. Acta Anat. 1966;63:32–48. doi: 10.1159/000142778. [DOI] [PubMed] [Google Scholar]
- 72.Kirillova I, et al. Teratology. 2000;61:347–54. doi: 10.1002/(SICI)1096-9926(200005)61:5<347::AID-TERA6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 73.Santen GWE, et al. Nat Genet. 2012;44:379–380. doi: 10.1038/ng.2217. [DOI] [PubMed] [Google Scholar]
- 74.Tsurusaki Y, et al. Nat Genet. 2012;44:376–378. doi: 10.1038/ng.2219. [DOI] [PubMed] [Google Scholar]
- 75.Hargreaves DC, Crabtree GR. Cell Res. 2011;21:396–420. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang L, et al. Am J Clin Nutr. 2010 [Google Scholar]
- 77.Greene ND, Copp AJ. Nat Med. 1997;3:60–66. doi: 10.1038/nm0197-60. [DOI] [PubMed] [Google Scholar]
- 78.Ray JG, et al. Epidemiology. 2007;18:362–366. doi: 10.1097/01.ede.0000257063.77411.e9. [DOI] [PubMed] [Google Scholar]
- 79.Dey AC, et al. J Health Popul Nutr. 2010;28:343–350. doi: 10.3329/jhpn.v28i4.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eiraku M, et al. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 81.Suga H, et al. Nature. 2011;480:57–62. doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]