Abstract
Background
Depression is relatively common in patients with coronary heart disease (CHD) and is associated with worse prognosis. Recently there has been interest in evaluating the impact of treating depression on clinical outcomes. Anti-depressant medications have been shown to be safe and efficacious for many patients; exercise also may be effective for treating depression and may also improve cardiopulmonary functioning. However, methodological limitations of previous studies have raised questions about the value of exercise, and no study has compared the effects of exercise with standard anti-depressant medication in depressed cardiac patients.
Purpose
UPBEAT is a randomized clinical trial (RCT) funded by NHLBI to evaluate the effects of sertraline or exercise compared to placebo on depression and biomarkers of cardiovascular risk in patients with CHD and elevated depressive symptoms.
Methods
The UPBEAT study includes 200 stable CHD patients with scores on the Beck Depression Inventory (BDI) ≥ 9 randomized to 4 months of treatment with aerobic exercise, sertraline, or placebo. The primary outcomes include depressive symptoms determined by clinical ratings on the Hamilton Rating Scale for Depression (HAM-D) and measures of heart rate variability (HRV), baroreflex control (BRC), vascular function (i.e., flow-mediated dilation (FMD)), and measures of inflammation and platelet aggregation.
Results
This article reviews the rationale and design of UPBEAT and addresses several key methodologic issues that were carefully considered in the development of this protocol: the use of a placebo control condition in depressed cardiac patients, study design, and selection of intermediate endpoints or biomarkers of cardiovascular risk.
Limitations
This study is not powered to assess treatment group differences in CHD morbidity and mortality. Intermediate endpoints are not equivalent to ‘hard’ clinical events and further studies are needed to determine the clinical significance of these biomarkers.
Conclusions
The UPBEAT study is designed to assess the efficacy of exercise in treating depression in cardiac patients and evaluates the impact of treating depression on important biomarkers of cardiovascular risk.
Introduction
Coronary heart disease (CHD) is the leading cause of death in the United States [1]; one million people experience a cardiac event (acute coronary syndrome or sudden cardiac death) each year. The term ‘cardiovascular vulnerable patient’ has been used to describe patients susceptible to acute coronary events based upon plaque, blood, or myocardial characteristics [2,3]. Recent evidence has suggested that depression is a significant and independent risk factor for patients with CHD, and also may be associated with increased cardiovascular vulnerability [4–10].
Approximately 15–20% of patients are diagnosed with Major Depressive Disorder (MDD) following an acute myocardial infarction (MI) or revascularization procedure, and an additional 20% experience either minor depression or elevated levels of depressive symptoms [6,10]. In the past decade, a number of studies have documented the relationship of depression and CHD risk. Most studies have reported large effect sizes, suggesting that the presence of depression confers about 2.5 times the risk for mortality or non-fatal cardiac events [8,9,11–15]. Davidson [10] has noted that even minor elevations in depressive symptoms significantly increase the risk of CHD in patients with established CHD. Thus, the high prevalence of elevated depressive symptoms in cardiac populations and the increased risk for adverse clinical events associated with depression provide a strong rationale for identifying and treating depression in cardiac patients.
Mechanisms of depression-related risk
A number of biobehavioral mechanisms have been hypothesized to underlie the relationship between depression and CHD. Most evidence is derived from cross-sectional studies and suggest that depression is associated with traditional risk factors for CHD including hypertension, diabetes, and insulin resistance [16,17], In addition, there are a number of ‘biomarkers’ of risk that have been shown to be associated with depression and also are prognostic of cardiac events in patients with CHD, including lower heart rate variability [18–23], abnormally low baroreceptor-mediated heart rate control [24–29], endothelial dysfunction [30–35], inflammation [36–39], and enhanced platelet activation [40–45]. Because of the prognostic significance of these variables, and their apparent association with clinical depression, they are appropriate surrogate markers of disease activity and may be clinically relevant intermediate endpoints in treatment studies of depressed cardiac patients (Figure 1).
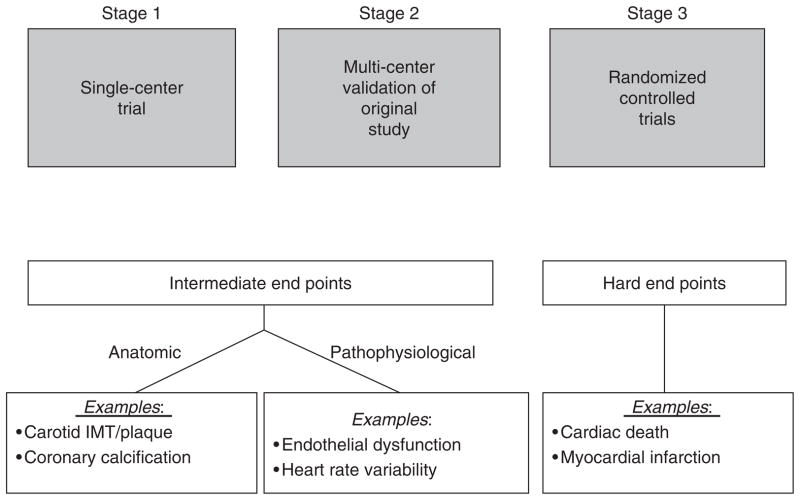
Figure 1.
Stages of Randomized Controlled Trials. Stage 1 consists of a single-center evaluation of a specific behavioral intervention. If successful, this intervention would be repeated at multiple centers, to assess the reproducibility of findings (stage 2). In both stages, intermediate endpoints, such as change in vascular function or plaque size during carotid ultrasonography, would be used to minimize necessary sample size and follow-up time. If reproducible results are obtained during stage 2, a multi-center intervention trial would be performed in stage 3, during which subjects would be observed for the occurrence of hard cardiac events such as cardiovascular mortality and morbidity. Adapted from Rozanski et al. [81]
Treatment of depression in cardiac patients
Treatment of depression has focused on reduction of symptoms and restoration of functioning. Antidepressant medication has been particularly effective in this regard, having become the treatment of choice [46]. Selective serotonin reuptake inhibitors (SSRIs) are especially effective, particularly for cardiac patients. For example, in the CREATE trial [47], citalopram was superior to placebo in reducing HAM-D scores in 284 CHD patients with HAM-D rating scores ≥20. Similarly, in the SADHART trial [48], sertraline was superior to placebo, but only in a subset of patients with more severe depression. However, neither CREATE nor SADHART examined the impact of treating depression on morbidity or mortality. Thus, despite the compelling reasons for treating depression in patients with heart disease, the clinical significance of treating depression in cardiac patients remains uncertain.
To date, only the ENRICHD trial has examined the impact of treating depression in post-MI patients on ‘hard’ clinical endpoints [49]. Although more than 2400 patients were enrolled in the trial, results were disappointing because there were only modest (i.e., 2-points on the HAM-D) reductions in depressive symptoms in the group receiving cognitive behavior therapy (CBT) relative to usual care controls, and there were no treatment group differences in the primary outcome – all cause mortality and nonfatal cardiac events. Although a subsequent reanalysis of the ENRICHD study revealed that anti-depressant use was associated with improved clinical outcomes [50], because patients were not randomized to medication treatment it could not be concluded that SSRI use was responsible for the improved outcomes. In fact, a recent study of patients with heart failure [51] revealed that medication use was associated with increased risk of mortality or hospitalization. It was suggested that medication use may have been a marker for individuals with more severe depression, which might have contributed to their higher risk, or identified a subset of depressed, treatment-resistant patients. Indeed, data from ENRICHD showed that patients receiving CBT (and, in some cases, anti-depressant medication) who failed to improve with treatment had higher mortality rates compared to patients who exhibited a positive response to treatment [52]. Taken together, these data illustrate that for many patients medication may not adequately relieve depressive symptoms and that there continues to be a need to identify alternative approaches for treating depression, particularly in cardiac patients. There is now reason to believe that exercise may be one such approach.
Exercise and depression
An increasing number of studies have shown beneficial effects of exercise on psychological functioning, including improvements in mood and cognitive performance [53–57]. If proven effective as a treatment for depression, exercise would hold a number of potential advantages over traditional medical therapies: it is relatively inexpensive, improves cardiovascular functioning, as well as mood, and avoids the side effects sometimes associated with medication use. To date, however, the therapeutic potential of exercise has remained unfulfilled due to a paucity of data from well-designed clinical studies of depressed patients, and virtually no randomized controlled trials of exercise training in depressed CHD patients. In a meta-analysis evaluating 11 randomized controlled trials, Lawlor and Hopker [57] noted that studies were limited because of self-selection bias, absence of adequate control groups, and inadequate assessment of exercise training effects, and they concluded that ‘the effectiveness of exercise in reducing symptoms of depression cannot be determined because of a lack of good quality research on clinical populations with adequate follow up.’
Previously we demonstrated that exercise was as effective as anti-depressant medication in reducing depressive symptoms in 156 older patients with MDD [58]. Patients were randomly assigned to exercise, medication, or a combination of exercise and medication. However, because of the absence of a no-treatment control group, we could not rule out the possibility that the treatment benefits were due to the non-specific effects of being involved in a research study with close patient monitoring, attention and support, or regression to the mean. Although a small randomized controlled trial [59] recently demonstrated that exercise was associated with reduced depression independent of group support, only 53 out of 80 patients actually completed the 12-week trial, including only 5 of 13 no-treatment controls.
To our knowledge, there have been no RCTS that have examined the impact of exercise training in depressed cardiac patients. A recent analysis of post-MI patients in the ENRICHD trial [60] revealed that those patients who exercised had greater reductions in depressive symptoms and achieved a 50% reduction in all cause mortality compared to their sedentary counterparts. However, because patients were not randomly assigned to exercise, and the ‘dose’ of exercise was not quantified, it was not possible to attribute the survival benefits to exercise. Clearly, a randomized trial is necessary to (1) examine the efficacy of exercise in treating depressive symptoms in cardiac patients; (2) compare the effects of exercise with pharmacotherapy; and (3) examine the impact of treating depression on important biomedical endpoints.
The UPBEAT study
The Understanding Prognostic Benefits of Exercise and Antidepressant Therapy for Persons with Depression and Heart Disease (UPBEAT) Study is a 5-year, single-site randomized clinical trial examining the effects of aerobic exercise and sertraline therapy on depression and biomarkers of cardiovascular risk. UPBEAT is sponsored by the National Heart, Lung, and Blood Institute. It is our expectation that this study will enhance existing knowledge about (a) the role of exercise in the management of depression in clinical populations, and (b) the effects of exercise and sertraline on important cardiovascular biomarkers.
The UPBEAT study was designed to improve upon recognized methodological flaws in previous studies; for example, the design includes a placebo control group of depressed persons with CHD and minimizes the social support attributable to patients’ participation in the treatment groups. In addition, we have selected outcomes that currently represent the most plausible explanations for the increased risk of adverse events in cardiac patients with clinical depression, including elevated blood biomarkers of platelet function and inflammation, reduced heart rate variability, and impaired vascular function. Thus, the UPBEAT study will examine the effects of exercise on depression and key intermediate cardiac endpoints compared to medication and placebo controls in a population of CHD patients with depressive symptoms. We will examine the following hypotheses: (a) exercise training and/or medication will reduce depressive symptoms to a greater extent than placebo control; (b) exercise training and/or medication will improve heart rate variability, endothelial function, inflammation, and platelet function to a greater extent than placebo control; (c) exercise training will improve endothelial function and heart rate variability to a greater extent than medication, but medication will reduce platelet activation to a greater extent than exercise training; and (d) improvements in functional capacity will mediate the relationship between treatment group and changes in biomarkers of CHD risk.
Design and methods
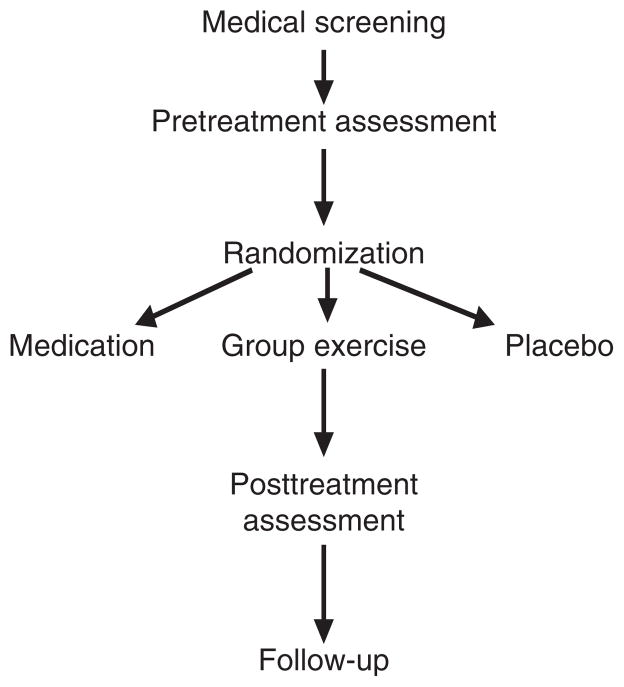
Two hundred depressed men and women with CHD who meet eligibility criteria will be randomized to one of three groups for the 16-week intervention to include (a) supervised aerobic exercise, (b) sertraline therapy, or (c) placebo. The study design is depicted in Figure 2. Persons complete written informed consent, are initially screened for depression and history of CHD and, if eligible, undergo a physical examination by the study physician to determine eligibility and safety of participation, and a psychiatric interview with the study psychologist to determine the presence of clinical depression and the severity of depressive symptoms. Upon completion of the medical and psychiatric screening, eligible participants undergo an assessment of cardiovascular biomarkers at baseline and again after 4 months of treatment; patients also undergo another depression evaluation after 4 months and 10 months (6 months posttreatment) by an examiner blind to patient group and baseline ratings. Our protocol was approved by the National Institutes of Mental Health and by the Institutional Review Board at Duke University Medical Center.
Figure 2.
Study design
Participant eligibility
Eligible participants include persons aged 35 and older who have a history of heart disease, including a previous (>60 days) myocardial infarction, revascularization procedure, such as a PTCA or CABG, or a cardiac catheterization demonstrating significant coronary artery stenosis. Participants must also be experiencing depressive symptoms, as reflected in a score of at least 9 on the BDI-II. Participants cannot be currently exercising regularly, taking anti-depressant medication, or engaging in psychotherapy. Persons are also ineligible for the study if they have medical conditions that preclude exercise or have conditions for which sertraline is contraindicated (such as bipolar disorder).
Interventions
All eligible subjects are randomly assigned to one of the three treatment conditions. We employ a conditional randomization procedure such that equal numbers of mild and moderate-severely depressed men and women will be assigned to the respective treatment groups. We also stratify by disease severity, defined by history of a prior myocardial infarction (MI), and by age. In order to have adequate power to detect between group differences, 75 patients will be randomized to aerobic exercise, 75 patients to medication, and 50 patients to placebo control.
Supervised aerobic exercise
The primary mode of exercise is walking, supplemented by stationary bicycle. Exercise progression is gradual; during the first 4 weeks, participants exercise three times per week at an intensity 50–70% heart rate reserve (HRR) established at the time of the baseline treadmill test and at 70–85% HRR during the subsequent 12 weeks. Trained exercise physiologists monitor the exercise and exercise logs are used to track participants’ attendance and adherence to the program’s protocol. Supervising exercise physiologists monitor participants’ radial pulses and rating of perceived exertion at least three times per session to ensure that the participants are exercising within the prescribed exercise training ranges.
Pill groups
Participants in the pill conditions are blinded to treatment group. Participants are reassessed by the study psychiatrist at regular intervals to insure patient safety and adjust dosages as needed. Participants’ medication adherence is calculated by pill count at each clinic visit. Pill users are encouraged to maintain their usual level of physical activity while enrolled in the study. Following the 16-week intervention, pill participants are unblinded and, if necessary, referred for further treatment.
Drug dispensing is performed by licensed pharmacists. Medication is dispensed as tablets of sertraline (50 mg) or placebo in individually coded bottles. A new bottle of medications is dispensed at each medication dispensing visit. Patients are seen by the study psychiatrist (who is blinded to pill condition) at baseline and at the end of weeks 1, 2, 4, 6, 8, and 12 of the 16-week intervention. Patients are contacted weekly by telephone to monitor their depressive symptoms. It is important for safety reasons that study staff regularly assesses depression but in order to minimize the therapeutic benefits of interacting with the study psychiatrist, only 6 face-to-face visits are scheduled during the study. The psychiatrist makes all medication adjustments based primarily upon the weekly HAM-D score and presence of side effects. Sertraline (or placebo) doses begin at 50 mg and are titrated to 100 mg after week 2 and to 150 mg or placebo equivalent at week 4 or 6 if patients show no change or only minimal improvement. After week 8, if patients show no change or only minimal improvement, they receive a maximum daily dose of 200 mg, or placebo equivalent. Also, if patients have achieved satisfactory response (remission or near remission) at any given dose level, then the dose is not increased further.
Participant adherence
Supervising exercise physiologists document exercisers’ attendance at each exercise session as well as the modality used during training (i.e., walking on a treadmill, stationary bicycle, etc.). In addition, they record exercisers’ heart rate ranges during training and ratings of perceived exertion three times during the exercise session.
Pill participants are required to bring their pill bottles to each scheduled clinic visit and the research staff counts any remaining pills to determine compliance with their prescribed medication regimen.
All participants complete a monthly tracking form that inquires about changes in health status, medication use, or lifestyle habits (e.g., diet, exercise, etc.). This procedure is designed to determine the occurrence of ‘unplanned crossover’, e.g., exercisers who initiate antidepressant medication or pill participants who initiate exercise, and any participant who enrolls in psychotherapy.
Participant safety
Because we employ a placebo control condition, which in theory does not provide optimal treatment for study participants, we follow several procedures to ensure patient safety. Patients are carefully evaluated before being enrolled in the study such that persons who are at high risk for suicide or have substance abuse disorders are excluded from participating in the trial. Once enrolled, participants are closely and regularly monitored. Each participant receives a weekly phone call from a study psychologist who uses a modified version of the HAM-D to determine depression severity. In the event that patients exhibit a significant increase in their depression (increase in HAM-D scores >25% from the preceding week) they may be withdrawn from the protocol and referred for immediate psychiatric treatment. In addition, at the conclusion of the 4-month program patients who remain depressed are counseled and referred for treatment.
Assessment of intermediate endpoints
In this study, we will examine changes in important biomarkers of risk (i.e., ‘intermediate endpoints’) in ‘vulnerable’ patients with stable CHD. The concept of looking at markers of ‘vulnerable plaque’ (e.g., endothelial dysfunction), ‘vulnerable (thombogenic) blood’ (e.g., markers of platelet activation and inflammation), and ‘vulnerable myocardium’ (e.g., heart rate variability and baroreflex control) in combination to identify the ‘vulnerable patient’ has been advanced by a panel of prominent cardiovascular scientists [2,3]. We assess changes in heart rate variability (HRV) and baroreflex control (BRC), flow mediated dilation (FMD), C-reactive protein (CRP) and platelet activation before and after treatment. These intermediate endpoints tap key dimensions of the ‘vulnerable patient’ (2,3) and were selected because of: (a) their association with depression; (b) their association with adverse prognosis; and (c) their potential to be modified by treatment, including exercise and/or anti-depressant medication.
Heart rate variability
For the 24-hour HRV measurement, patients will be instrumented with a DelMar Holter ambulatory ECG monitor. A Laser scanner (DelMar Medical, Irvine, California, USA) will be used to scan the tapes using standard Holter analysis procedures. The labeled beat-to-beat file will then be processed using the DelMar time domain HRV analysis software and the DelMar enhanced 24-hour spectral heart rate variability analysis software. Heart rate variability will be estimated from the standard deviation of all normal R-R intervals (SDNN) and from spectral power summed across each of the following bands: high frequency (0.14–0.5 Hz), low frequency (0.05–0.139 Hz), very low frequency (0.003–0.049 Hz), and ultra low frequency (0.000015–0029 Hz). The primary hypotheses will be tested using the SDNN measure of HRV because it is less affected by artifact and nonstationarities found in the ambulatory environment and is the most strongly predictive of increased risk of mortality [19,61,62].
Baroreflex control
For the BRC measurement, beat-to-beat blood pressure and R-R interval data will be collected from patients while they are lying comfortably in a supine position on a hospital bed using the Finapres noninvasive blood pressure monitor (Ohmeda, Madison, WI). Beat-to-beat R-R interval will be collected from an electrocardiogram (ECG) recorded digitally at 1000 Hz. Following 5 min of quiet rest, beat-by-beat blood pressure and R-R interval will be recorded for 10 min; during the data acquisition period, patients will be asked to relax and to refrain from speaking. To derive BRS, beat-by-beat systolic blood pressure (SBP) and R-R interval will be edited for artifacts, interpolated, and sampled at a frequency of 4 Hz to obtain equally spaced events. Power spectra will be estimated using the Welch algorithm [63].
Endothelial function
Our technique for assessing endothelial function is based upon the arterial ultrasound procedures originally described by Celermajer and colleagues in 1992 [64] and conforms to the recently published guidelines for assessment of flow-mediated arterial vasodilatation [65]. Under resting conditions (following 10 min of relaxation), high resolution end-diastolic (ECG R-wave synchronized) longitudinal B-mode images of the brachial artery, in the region 4–6 cm proximal to the antecubital fossa, will be captured and stored digitally using an Acuson (Mountain View, California) ultrasound imaging system (7–11 MHz linear-array transducer and Aspen system). Reactive hyperemia is then induced secondary to forearm ischemia, achieved by inflation of an appropriate-sized pneumatic occlusion cuff, located around the forearm, to supra-systolic pressure for 5 min. A second scan spans the period 30 s prior to cuff-deflation until 120 s following cuff-deflation, and involves beat-by-beat storage of end-diastolic B-mode images of the brachial artery, with repeated pulsed-Doppler flow velocity measurement during the first 10-s postdeflation.
After a 15-min rest period, to permit recovery of the artery to normal resting function, a second set of resting baseline ultrasound images of the same segment of the brachial artery is acquired, followed by administration of a sublingual glyceryl trinitrate (GTN) tablet (400 mg), used to induce non-endothelium-dependent arterial dilatation. Similarly, a second extended scan is used to store beat-by-beat end-diastolic B-mode images from 3 to 6 min after the administration of GTN.
Image processing is performed using PC-based software (Brachial Analyzer, Medical Imaging Applications, Iowa City, IA). All diameter measurements are made between the media-adventitia interfaces (M-line) of the two arterial walls. Our primary indices of endothelium-dependent FMD and endothelium-nondependent GTN dilation will be expressed as percent increase in arterial diameter, which is the index of FMD that has been adopted most widely [66].
Measures of platelet activation and chronic inflammation
Specimens for biomarkers of inflammation and platelet activation will be collected after 10 min of supine rest. Because Platelet Factor 4 (PF 4) and β-thromboglobulin (β-TG) have been shown to be elevated in depressed patients [43,67], are associated with increased risk for CHD events [68], and may be improved with treatment for depression [69], we selected β-TG and PF 4 as our primary markers of platelet activation. We will also explore the functional capacity of platelets in response to therapeutic interventions using platelet aggregometry. Assays for PF 4 and β-TG (by ELISA) and platelet aggregation studies are performed in the Duke Clinical Coagulation Laboratory under the direction of Dr Thomas Ortel. High sensitivity C-reactive protein (hs CRP) assays are performed by latex-enhanced immunonephelometry in the Duke Clinical Immunology Laboratory.
The assessment schedule is displayed in Table 1.
Table 1.
Schedule of assessments
| Measure | Initial screen | Pretreatment assessment | Posttreatment assessment | 6 months posttreatment | 1 year posttreatment |
|---|---|---|---|---|---|
| Physical exam | X | ||||
| Exercise treadmill test | X | X | |||
| Depression assessments | |||||
| BDI-II | X | X | X | X | X |
| DIS | X | X | |||
| HAM-D | X | X | |||
| CGI | X | X | |||
| Intermediate endpoints | |||||
| HRV | X | X | |||
| BRC | X | X | |||
| FMD | X | X | |||
| C-Reactive protein | X | X | |||
| Platelet aggregation | X | X | |||
| Other measures | |||||
| Ancillary questionnaires | X | X | |||
| Medical status/utilization costs | X | X | X | ||
Statistical analyses
The basic analytic strategy consists of maximum likelihood models [70], with treatment type as the factor of primary interest, and ethnicity, age, gender, and pre-treatment measurement of the outcome variable as the adjustment covariables. The three primary outcomes, HAM-D, HRV, and FMD, will be analyzed in this same manner. In the case of depression as the outcome, however, the serial measurements will allow us to use latent growth model methodology, with the posttreatment HAM-D scores at weeks 2, 4, 6, 8, 12, and 16 as the response, and two orthogonal contrast variables (two active treatments versus control and exercise versus medication) as the primary predictors. Covariates will include ethnicity, gender, age, and pretreatment level of the HAM-D as the adjustment covariables. The primary test will be the orthogonal contrasts at 16 weeks, which will be examined directly, i.e., no omnibus or ‘gateway’ test will be required before interpreting them. The two remaining outcomes, HRV and FMD will also be modeled using maximum likelihood estimation; the posttreatment level for these variables is assessed only at 16 weeks, and therefore models will be akin to the analysis of covariance form rather than a latent growth model. All analyses will follow the intent-to-treat principle using the full information maximum likelihood method for missing data.
We powered the study such that the treatment effect for depression (as expressed by HAM-D scores) serves as the primary hypothesis test. We assumed that there will be about 20% attrition and that analytic models would reflect the intent-to-treat principle, imputing missing values with the full-information maximum likelihood method. We also based our estimate assuming the lowest correlation between the adjustment covariates and the outcome.
Based on our previous work with clinically depressed patients [49], the standard deviation of the HAM-D is about 6 and the R2 between the aforementioned covariates and the posttreatment HAM-D is (conservatively) about 0.10. A 3-point (0.5 SD) difference in the HAM-D is usually considered a small but clinically significant effect for a treatment. Using simulation techniques, we estimated that with an initial sample size of 200, the power to detect an effect of this size at the 16 week endpoint will be 0.95 at an α of 0.05, and 0.84 if we use the more conservative α of 0.01. With respect to HRV, our prior work showed that the R2 between the baseline covariates (age, gender, ethnicity, and baseline HRV) and HRV is about 0.73 [71]. For the primary comparison, active treatments versus placebo, we will have a power of 0.98 to detect a 0.3 SD effect for this variable if α is 0.05 and 0.88 if α is 0.01. Finally, our earlier work shows that for FMD, the multiple R2 between the baseline covariates (age, gender, ethnicity, and baseline FMD) and FMD after 16 weeks is about 0.50. We will have a power of 0.90 to detect a 0.25 SD effect for this variable if α is 0.05 and 0.80 if α is 0.01. Following the tests of these primary hypotheses, we will then proceed to examine further mechanisms, such as CRP, BRC, and platelet activity, using the Benjamini-Hochberg False Discovery Correction [72]. Thus, the planned sample size will provide ample power for the primary tests even if we were to take the more conservative tack of using an α of 0.01.
Key methodological issues
For the purpose of this manuscript, we focus on several key methodological issues that we faced in the development of this protocol: (a) inclusion of a placebo control group; (b) consideration of alternative study designs; and (c) the use of intermediate endpoints.
The use of a placebo control group
The question of whether to include a placebo-drug condition in the present design has been the focus of considerable debate and much deliberation by members of our research group. In a previous grant application, we proposed a two (exercise versus no exercise) by 2 (medication versus placebo) randomized trial. However, the NIH Review Panel argued that the use of a placebo condition was unethical, and suggested a 3-group design (Exercise, Medication, or the Combination), which we adopted and the application was subsequently approved and funded. However, when we attempted to publish our results, our study was sharply criticized because of the absence of a no-treatment control condition. Ultimately, the study was published [58], but we have revisited the value of placebo controls in treatment studies of depression. We recognize that the employment of placebo control groups has been questioned on ethical grounds [73,74], and we believe that the burden of proof rests with the research team to demonstrate the scientific necessity of subjecting clinical research subjects to a treatment condition known to be of inferior effectiveness. In developing the current protocol, it was only after careful consideration of the relevant scientific, ethical, and safety issues that we concluded that a placebo drug condition is crucial to accomplishing the aims of the study, and that appropriate safeguards could be implemented to assure the safety of patients randomized to receive this treatment.
The fact that sertraline already has been proven superior to placebo in double blind clinical trials could lead one to argue that a positive study finding that exercise therapy is equivalent in clinical effectiveness to sertraline would be tantamount to demonstrating that exercise therapy is superior to placebo. Unfortunately, it is not so simple. The problem is that this argument assumes the rate of placebo responding to be relatively low and fairly consistent across study situations and patient populations [75]; in fact, however, placebo response rates in pharmacological studies of MDD are high and variable, ranging from 15 to 67% [76]. This variability greatly limited our freedom to make assumptions about the placebo response rate in any new study context. It is for this reason that Klein [77] concluded that any investigation comparing psychosocial interventions to an established antidepressant medication (representing the ‘standard of care’) must include a pill-placebo condition in order to assure that the patient sample is actually one that is responsive to the medication beyond the effects of a placebo. Unless a superiority of medication to pill-placebo can be demonstrated in the current study, it could be maintained that these patients were not medication-responsive and that any observed treatment effects would be open to parsimonious explanation as a placebo response. This viewpoint is quite compelling, and suggests that it is not adequate merely to compare exercise intervention to an FDA-approved and widely-utilized antidepressant (e.g., sertraline), but that all such randomized controlled trials must include a pill-placebo condition if a claim of therapeutic equivalence is to carry any weight. Klerman [78] has identified several scientific reasons for the use of placebo controls, and alternatives to placebo-controlled studies have significant limitations [79] that cannot be adequately addressed by even the most sophisticated statistical procedures [80].
Consideration of alternative designs
We carefully considered several alternative experimental designs for the proposed study. Because the proposed biomarker assessments are relatively time-and labor-intensive, the an important consideration in selecting a design was to identify an approach that was feasible with respect to cost and participant burden, but that still allowed for scientifically useful conclusions. A two group design (i.e., medication versus exercise) was not adopted because it did not allow for a rigorous examination of the separate effects of exercise and medication. If both groups improved, it would be impossible to determine if this were true improvement or a ‘placebo’ response. We recognize that the 3-group design which we adopted created an imbalance with respect to patients’ knowledge of their treatment condition. That is, subjects in the medication and the placebo conditions will not know if they are receiving an active antidepressant or a placebo, whereas subjects in the exercise condition will know for certain which treatment they are receiving. We considered adding a fourth group in which patients would be unblinded to their receiving the active drug. This option was attractive because we could examine the extent to which certainty of receiving the medication could affect the magnitude of changes in depressive symptoms. However, we rejected this option because of the additional expense of adding another group that would not provide any new insights into the effects of the medication on the biomarkers, and would also require more patients than we would be able to recruit at a single site over a 3-year time period. Moreover, this design would not introduce uncertainty into the exercise condition. We also considered adding a low dose exercise condition or an ‘attention control’ condition, in addition to aerobic exercise. However, we did not implement this for a number of reasons: (i) this intervention is difficult to deliver convincingly and presumably also would be less effective, which raises the same ethical issues presented by a drug-placebo; (ii) the present design already contains a ‘social support’ control by virtue of the placebo-medication conditions meeting regularly with the psychiatrist and study staff; and (iii) the inclusion of an attention control condition would not resolve the issue of uncertainty, as unlike a pill condition, patients would be aware of what treatment they were receiving. Unlike medication trials, it is impossible to conduct a ‘double blind’ study that involves patients’ active participation in lifestyle change. We also considered including additional control groups such as the inclusion of nondepressed CHD patients and/non-CHD controls. However, because depression is associated with increased risk, particularly in cardiac patients, examination of the effects of treatment on depressed cardiac patients represents the most informative analysis, and one that we believe best contributes to our understanding of why depression moderates the risk of events in patients with CHD and how treating depression might reduce that risk.
In summary, we believe that a placebo controlled trial which we adopted is essential to fulfill the primary objectives of this study, and that the three group design for UPBEAT makes the most sense. Coupled with this, however, will be the careful implementation of procedures to monitor and safeguard the well-being of subjects in the placebo-drug condition, as well as the exercise condition.
Selection of end-points
We have argued that the use of intermediate endpoints have considerable value for a variety of reasons. Multi-center trials that focus only on clinical end-points such as MI or cardiac death require a longer follow-up period and greater number of patients, as these ‘hard’ end-points occur relatively infrequently in a stable CHD population. Moreover, these measures are not sensitive to change. As summarized in Table 2, each of the intermediate endpoints selected for the UPBEAT study is clinically relevant, has been shown to be related to depression, and may be modifiable with treatment. HRV and FMD will serve as our primary intermediate endpoints because there are the most data to indicate that they are associated with (a) worse prognosis, (b) impairment in depressed patients, and (c) may be improved with exercise. Because experts have advocated a comprehensive assessment of vulnerable patients [2,3] we will assess other intermediate endpoints including BRC, chronic inflammation, and platelet activation.
Table 2.
Strength of evidence of intermediate endpoints
| Intermediate endpoint | Associated with depression | Associated with CHD outcomes | Improved with exercise | Improved with SSRIs |
|---|---|---|---|---|
| Heart rate variability | + + + | + + + | + | −/? |
| Baroreflex control | + | ++ | + | + |
| Flow mediated dilation | + | + + | + + | ? |
| Inflammation | + | + ++ | + | ? |
| Platelet activity | + | + + + | + | ++ |
−=not related;? = few studies have addressed; + = preliminary evidence; + + = some evidence; + + + = strong evidence.
Conclusions
There is considerable evidence that the presence of depressive symptoms is associated with increased risk for adverse clinical events in patients with established CHD. However, there is little evidence that treating depression in cardiac patients improves clinical outcomes. The UPBEAT study is designed to assess the effects of two potentially efficacious treatments for depression – sertraline, an established SSRI, and aerobic exercise – in cardiac patients with elevated symptoms of depression. Because depressive symptoms can be highly variable, and the placebo response rate can be high, the design of the UPBEAT study incorporates a placebo control arm with built in safeguards to ensure patient safety. In addition, several intermediate endpoints, heart rate variability, baroreflex control, inflammation and platelet aggregation, and vascular function, were selected because of their association with depression and potential prognostic significance and serve as the primary outcomes in the study. The UPBEAT study promises to provide important information about the value of exercise and medication in treating depression in cardiac patients, and will provide insights into the mechanisms by which these interventions may reduce the risk for future adverse events in this vulnerable patient population.
Acknowledgments
This research was supported by Grant No. HL 080664 from the National Institutes of Health, Bethesda, Maryland and the National Center for Research Resources, Clinical Research Centers Program, NIH MO1-RR-30. Dr Doraiswamy has received research grants and honoraria from several pharmaceutical companies and antidepressant manufacturers, including Pfizer, but does not own stock in these companies.
References
- 1.American Heart Association. Heart and stroke statistical update. American Heart Association; Dallas, TX: 2001. [Google Scholar]
- 2.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–72. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 3.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108:1772–78. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 4.Barefoot JC. Depression and coronary heart disease. Cardiologia. 1997;42:1245–50. [PubMed] [Google Scholar]
- 5.Carney RM, Freedland KE, Sheline YI, Weiss ES. Depression and coronary heart disease: a review for cardiologists. Clin Cardiol. 1997;20:196–200. doi: 10.1002/clc.4960200304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lett H, Blumenthal J, Babyak M, Sherwood A, Strauman T, Robins C. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305–15. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 7.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 8.Barth J, Schumacher M, Hermann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802–13. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 9.van Melle JP, de Jong P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta analysis. Psychosom Med. 2004;66:814–22. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 10.Davidson KW, Rieckman N, Lesperance F. Psychological theories of depression: potential application for the prevention of acute coronary syndrome recurrence. Psychosom Med. 2004;66:165–73. doi: 10.1097/01.psy.0000116716.19848.65. [DOI] [PubMed] [Google Scholar]
- 11.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction: impact on 6-month survival. JAMA. 1993;270:1819–25. [PubMed] [Google Scholar]
- 12.Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 13.Frasure-Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom Med. 1999;61:26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–53. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 15.Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88:337–41. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 16.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 17.Thakore JH, Richards PJ, Reznek RH, Martin A, Dinan TG. Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biological Psychiatry. 1997;41:1140–42. doi: 10.1016/S0006-3223(97)85394-2. [DOI] [PubMed] [Google Scholar]
- 18.Rich MW, Saini JS, Kleiger RE, Carney RM, Tevelde A, Freedland KE. Correlation of heart rate variability with clinical and angiographic variables and late mortality after coronary angiography. Am J Cardiol. 1988;62:1–7. doi: 10.1016/0002-9149(88)91208-8. [DOI] [PubMed] [Google Scholar]
- 19.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–62. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 20.Nolan J, Batin PD, Andrews R, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998;98:1510–16. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- 21.Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol. 1995;76:562–64. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- 22.Carney RM, Blumenthal JA, Stein PK, et al. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–28. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- 23.Casolo G, Balli E, Taddei T, Amuhasi J, Gori C. Decreased spontaneous heart rate variability in congestive heart failure. Am J Cardiol. 1989;64:1162–67. doi: 10.1016/0002-9149(89)90871-0. [DOI] [PubMed] [Google Scholar]
- 24.Watkins LL, Grossman P. Association of depressive symptoms with reduced baroreflex cardiac control in coronary artery disease. Am Heart J. 1999;137:453–57. doi: 10.1016/s0002-8703(99)70491-6. [DOI] [PubMed] [Google Scholar]
- 25.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106:945–49. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]
- 26.Hohnloser SH, Klingenheben T, van de LA, Hablawetz E, Just H, Schwartz PJ. Reflex versus tonic vagal activity as a prognostic parameter in patients with sustained ventricular tachycardia or ventricular fibrillation. Circulation. 1994;89:1068–73. doi: 10.1161/01.cir.89.3.1068. [DOI] [PubMed] [Google Scholar]
- 27.De Ferrari GM, Landolina M, Mantica M, Manfredini R, Schwartz PJ, Lotto A. Baroreflex sensitivity, but not heart rate variability, is reduced in patients with life-threatening ventricular arrhythmias long after myocardial infarction. Am Heart J. 1995;130:473–80. doi: 10.1016/0002-8703(95)90354-2. [DOI] [PubMed] [Google Scholar]
- 28.Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation. 1982;66:874–80. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy GJ, Hofer MA, Cohen D, Shindledecker R, Fisher JD. Significance of depression and cognitive impairment in patients undergoing programed stimulation of cardiac arrhythmias. Psychosom Med. 1987;49:410–21. doi: 10.1097/00006842-198707000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093–98. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 31.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arteriosclerosis, Thrombosis & Vascular Biology. 2003;23:168–75. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 32.Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol. 2001;88:196–98. doi: 10.1016/s0002-9149(01)01623-x. [DOI] [PubMed] [Google Scholar]
- 33.Broadley AJ, Korszun A, Jones CJ, Frenneaux MP. Arterial endothelial function is impaired in treated depression. Heart (British Cardiac Society) 2002;88:521–23. doi: 10.1136/heart.88.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris KF, Matthews KA, Sutton-Tyrrell K, Kuller LH. Associations between psychological traits and endothelial function in postmenopausal women. Psychosom Med. 2003;65:402–09. doi: 10.1097/01.psy.0000035720.08842.9f. [DOI] [PubMed] [Google Scholar]
- 35.Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. JACC. 2005;46:656–59. doi: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 36.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. NEJM. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 38.Tall AR. C-reactive protein reassessed. NEJM. 2004;350:1450–52. doi: 10.1056/NEJMe048020. [DOI] [PubMed] [Google Scholar]
- 39.Ford DE, Erlinger TP. Depression and C-reative protein in US adults. Arch Intern Med. 2004;164:1010–14. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 40.Patrono C, Renda G. Platelet activation and inhibition in unstable coronary syndromes. Am J Cardiol. 1997;80:17E–20E. doi: 10.1016/s0002-9149(97)00484-0. [DOI] [PubMed] [Google Scholar]
- 41.Thaulow E, Erikssen J, Sandvik L, Stormorken H, Cohn PF. Blood platelet count and function are related to total and cardiovascular death in apparently healthy men. Circulation. 1991;84:613–17. doi: 10.1161/01.cir.84.2.613. [DOI] [PubMed] [Google Scholar]
- 42.Markovitz JH, Shuster JL, Chitwood WS, May RS, Tolbert LC. Platelet activation in depression and effects of sertraline treatment: an open-label study. Am J Psychiat. 2000;157:1006–08. doi: 10.1176/appi.ajp.157.6.1006. [DOI] [PubMed] [Google Scholar]
- 43.Musselman DL, Tomer A, Manatunga AK, et al. Exaggerated platelet reactivity in major depression. Am J Psychiat. 1996;153:1313–17. doi: 10.1176/ajp.153.10.1313. [DOI] [PubMed] [Google Scholar]
- 44.Kop WJ, Gottdiener JS, Tangen CM, et al. Inflammation and coagulation factors in persons >65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89:419–24. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 45.Laghrissi-Thode F, Wagner WR, Pollock BG, Johnson PC, Finkel MS. Elevated platelet factor 4 and beta-thromboglobulin plasma levels in depressed patients with ischemic heart disease. Biological Psychiatry. 1997;42:290–95. doi: 10.1016/S0006-3223(96)00345-9. [DOI] [PubMed] [Google Scholar]
- 46.Depression Guideline Panel. Treatment of Depression, Clinical Practice Guideline. Vol. 2. Vol. 5. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, HHCPR; 1993. Depression in Primary Care. [Google Scholar]
- 47.Lesperance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian cardiac randomized evaluation of antidepressant and psychotherapy efficacy (CREATE) Trial. JAMA. 2007;297:367–79. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- 48.Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–09. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 49.Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction. JAMA. 2003;289:3106–16. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 50.Taylor CB, Youngblood ME, Catellier D, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792–98. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- 51.Sherwood A, Blumenthal JA, Trivedi R, et al. Relationship of depression to death or hospitalization in patients with heart failure. Arch Int Med. 2007;167:367–73. doi: 10.1001/archinte.167.4.367. [DOI] [PubMed] [Google Scholar]
- 52.Carney RM, Blumenthal JA, Freedland KE, et al. Depression and late mortality after myocardial infarction in the enhancing recovery in coronary heart disease (ENRICHD) Study. Psychosom Med. 2004;66:466–74. doi: 10.1097/01.psy.0000133362.75075.a6. [DOI] [PubMed] [Google Scholar]
- 53.Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Medicine. 2002;32:741–60. doi: 10.2165/00007256-200232120-00001. [DOI] [PubMed] [Google Scholar]
- 54.Folkins CH, Sime WE. Physical fitness training and mental health. Am Psychologist. 1981;36:373–89. doi: 10.1037//0003-066x.36.4.373. [DOI] [PubMed] [Google Scholar]
- 55.Hughes JR. Psychological effects of habitual aerobic exercise: a critical review. Prev Med. 1984;13:66–78. doi: 10.1016/0091-7435(84)90041-0. [DOI] [PubMed] [Google Scholar]
- 56.Greist JH, Klein M. Running as a treatment for depression. Comp Psychiat. 1979;20:41–54. doi: 10.1016/0010-440x(79)90058-0. [DOI] [PubMed] [Google Scholar]
- 57.Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. Br Med J. 2001;322:763–67. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Int Med. 1999;159:2349–56. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 59.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: Efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD trial. Medicine and Science in Sports and Exercise. 2004;36:746–55. doi: 10.1249/01.mss.0000125997.63493.13. [DOI] [PubMed] [Google Scholar]
- 61.Bigger JT, Jr, Albrecht P, Steinman RC, Rolnitzky LM, Fleiss JL, Cohen RJ. Comparison of time- and frequency domain-based measures of cardiac parasympathetic activity in Holter recordings after myocardial infarction. Am J Cardiol. 1989;64:536–38. doi: 10.1016/0002-9149(89)90436-0. [DOI] [PubMed] [Google Scholar]
- 62.Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–71. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 63.Welch PD. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short modified periodograms. IEEE Trans Audio Electroacoust. 1967;15:70–73. [Google Scholar]
- 64.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–15. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 65.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task. JACC. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 66.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. JACC. 1994;24:1468–74. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 67.Kuipers PM, Hamauluak K. β Thromboglobulin and platelet factor 4 levels in post-MI patients with major depression. Psychiat Res. 2002;109:207–10. doi: 10.1016/s0165-1781(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 68.Martinez-Sales V, Vila V, Reganon E, Goberna MA, et al. Elevated thrombotic activity after myocardial infarction: a 2-year follow-up study. Haemostasis. 1998;28:301–6. doi: 10.1159/000022446. [DOI] [PubMed] [Google Scholar]
- 69.Serebruany VL, Glassman AH, Malinin AI, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline Anti-Depressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation. 2003;108:939–44. doi: 10.1161/01.CIR.0000085163.21752.0A. [DOI] [PubMed] [Google Scholar]
- 70.Muthen B. A general structural equation model with dichotomous, ordered categorical, and continuous latent variable indicators. Psychometrika. 1984;49:115–32. [Google Scholar]
- 71.Blumenthal JA, Sherwood A, Babyak MA, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease; a randomized controlled trial. JAMA. 2005;293(13):1626–34. doi: 10.1001/jama.293.13.1626. [DOI] [PubMed] [Google Scholar]
- 72.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc, Series B. 1995;57:289–300. [Google Scholar]
- 73.Levine R. The use of placebos in randomized clinical trials. IRB. 1985;7:1–4. [PubMed] [Google Scholar]
- 74.Rothman KJ, Michels KB. The continuing unethical use of placebo controls. NEJM. 1994;331:394–8. doi: 10.1056/NEJM199408113310611. [DOI] [PubMed] [Google Scholar]
- 75.Addington D, Williams R, Lapierre Y, el Guebaly N. Placebos in clinical trials of psychotropic medication. Canadian Journal of Psychiatry - Revue Canadienne de Psychiatrie. 1997;42:1–6. doi: 10.1177/070674379704200312. [DOI] [PubMed] [Google Scholar]
- 76.Bialik RJ, Ravindran AV, Bakish D, Lapierre YD. A comparison of placebo responders and nonresponders in subgroups of depressive disorder. J Psychiat & Neurosci. 1995;20:265–70. [PMC free article] [PubMed] [Google Scholar]
- 77.Klein DF. Preventing hung juries about therapy studies. J Consult & Clin Psychol. 1996;64:81–87. doi: 10.1037//0022-006x.64.1.81. [DOI] [PubMed] [Google Scholar]
- 78.Klerman GL. Scientific and ethical considerations in the use of placebo controls in clinical trials in psychopharmacology. Psychopharmacol Bull. 1986;22:25–29. [PubMed] [Google Scholar]
- 79.Prien RF. Method and models for placebo use in pharmacotherapeutic trials. Psychopharmacol Bull. 1988;24:4–8. [PubMed] [Google Scholar]
- 80.Temple R. Difficulties in evaluating positive control trials. Clinic Eval. 1993;21:141–149. [Google Scholar]
- 81.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice. JACC. 2005;45:637–51. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]