Abstract
Stress activates the hypothalamus- pituitary-adrenal (HPA) axis leading to the release of glucocorticoids (GC). Increased activity of the HPA axis and GC exposure has been suggested to facilitate the development of obesity and metabolic syndrome. Nonetheless, different stressors can produce distinct effects on food intake and may support different directions of food learning e.g. avoidance or acceptance. This study examined whether interoceptive (LiCl and exendin-4) and restraint stress support similar or distinct food learning. Female rats were exposed to different stressors after their consumption of a palatable food (butter icing). After 4 palatable food-stress pairings, distinct intakes of the butter icing were observed in rats treated with different stressors. Rats that received butter icing followed by intraperitoneal injections of LiCl (42.3 mg/Kg) and exendin-4 (10 μg/Kg) completely avoided the palatable food with subsequent presentations. In contrast, rats experiencing restraint stress paired with the palatable food increased their consumption of butter icing across trials and did so to a greater degree than rats receiving saline injections. These data indicate that interoceptive and psychosocial stressors support conditioned food avoidance and acceptance, respectively. Examination of c-Fos immunoreactivity revealed distinct neural activation by interoceptive and psychosocial stressors that could provide the neural basis underlying opposite direction of food acceptance learning.
Keywords: interoceptive stress, restraint stress, food preference learning, HPA axis, visceral pathway, binge eating
Introduction
Multiple stressors have been shown to affect food intake. Although most stressors produce similar hypothalamic-pituitary-adrenal (HPA) axis activation and corticotropin-releasing hormone (CRH) is known to be a potent anorexigenic agent (Krahn et al., 1990, Glowa and Gold, 1991, Benoit et al., 2000), the effects of stress on food intake are not always the same. Physical challenge by exercise (Looy and Eikelboom, 1989, Kawaguchi et al., 2005) and drugs e.g. LiCl (Bernstein and Goehler, 1983, Curtis et al., 1994, Rinaman and Dzmura, 2007) and glucagon like peptide 1 (GLP-1) analogs reduce food intake (Kinzig et al., 2002, Baraboi et al., 2011, Liang et al., 2013). While some 20% of people do not change food intake during stressful period, the remaining 80% evenly divide into hypophagic and hyperphagic responders to stress (Gibson, 2006, Dallman, 2010). That is, stress can produce either increase or decrease in food intake in both humans and rodents (Rybkin et al., 1997, Pecoraro et al., 2004, Tamashiro et al., 2007a, Tamashiro et al., 2007b, Torres and Nowson, 2007, Foster et al., 2009). These data indicate that multiple neural systems/factors activated by different stressors are involved to influence food intake. However, the distinction of these neural activations and their influence on stress related food intake has not been elucidated. While many studies have examined the effects of stress on food intake, less research has focused on stress related food avoidance or acceptance learning. This study aims to determine how different types of stressors affect food acceptance learning.
Conditioned food avoidance (CFAV) or acceptance (CFAC) results from learned associations between a novel food and a postingestive event. An aversive postingestive event e.g. malaise, will result in avoidance of the novel food and conversely, a positive postingestive event e.g. rewarding effects will result in future preference or overconsumption of the novel food. In these cases the novel food is a conditioned stimulus (CS) and the treatment that produces a postingestive event is an unconditioned stimulus (US). In this study, different stressors were applied after the presentation of a novel highly palatable food. The stressors that served as the US included intraperitoneal injection (IP) of LiCl or exendin-4 (Ex-4) or 30 min of restraint stress (RS). Lithium chloride is known to support long lasting CFAV (or conditioned taste aversion) with novel food but suppression of a familiar diet depends on dosage (Bernstein and Goehler, 1983, McCann et al., 1989, Benoit et al., 2003, Rinaman and Dzmura, 2007). Exendin-4 is a long acting analog of GLP-1. It produces consistent suppression on food intake and has been suggested to support CFAV (Kanoski et al., 2012, Liang et al., 2013). Both LiCl and Ex-4 can be considered as interoceptive stressors (Rinaman, 1999). Despite having a physical component, RS is mostly considered as a psychosocial stressor. Previous studies have demonstrated that unconditioned RS can decrease regular chow intake, but results for its effects on palatable food intake are inconsistent (Rybkin et al., 1997, Pecoraro et al., 2004, Kinzig et al., 2008). Using the conditioned food learning paradigm, we sought to determine whether different types of stressors support different learning to novel food i.e. avoidance or acceptance. After the acquisition of CFAV/CFAC, central neural activations by these stressors were examined by detecting a marker of neural activation c-Fos protein with immunohistochemistry.
Experimental Procedures
Animals
The subjects were 30 female Sprague-Dawley rats (Harlan, Frederick, MD) weighing 200–225 g at the beginning of the study. Female rats were used because based on hormone results they tend to be more responsive to stress (Bangasser and Valentino, 2012) and to palatable foods (Eckel and Moore, 2004). They were single housed in stainless steel wire mesh hanging cages in a colony room with temperature, humidity, and light cycle (12:12-h light-dark, lights on at 0100 h) automatically controlled. Body weight was measured daily. Food (Harlan Teklad diet 2018, 3.1 Kcal/g) and water were available ad libitum during acclimation while overnight food deprivation was applied during conditioning training. The rats were divided into groups of receiving IP saline, LiCl or exendin-4 (Ex-4) or restraint stress (RS) by matching average body weights. The first saline and LiCl groups had n=7 while the Ex-4 and restraint stress groups had n=8. All animal protocols were approved by the Institutional Animal Care and Use committee of the Johns Hopkins University.
Procedures
Palatable food
The palatable food (butter icing) served as a CS to examine how different stressful US affects palatable food acceptance learning. The butter icing was made by blending 4:3 portions of unsalted butter (Land O' Lakes) and confectioners sugar (Domino sugar 10-X powdered pure cane sugar) together. This resulted in a butter icing paste that contained 5.8 Kcal per g metabolizable energy. The butter icing was kept at 4 °C and served at room temperature during each conditioning trial.
Palatable food acceptance learning
Rats were acclimated to the animal room for 5 days, and the ad lib chow intake was measured daily. After acclimation, rats were placed on an overnight 19 h food deprivation schedule. Food was available daily according to the following schedule: 15 min beginning at 7:00 AM and then 1.5 h beginning at 11:00 AM. During the 1.5 h food access, the rats always receive regular chow. For the 15 min access, they receive chow on regular training days but butter icing on conditioning days. The baseline training days lasted 7 days and the first conditioning cycle began on day 8. The conditioning cycle consisted of one conditioning day followed by 2 regular chow days. On the conditioning day, all rats received 15 min access to a previously weighed food jar containing butter icing (the CS). At the end of butter icing access, intake was measured by weighing the food jar. No treatment was given if a rat did not consume any butter icing. For the rats that consumed the butter icing, a treatment was given 10 min after the end of the butter icing access. Depended on their group assignment, rats received an IP injection (1.33ml/100g) of 0.9% saline in the saline group, of 0.075M LiCl (42.3 mg/Kg, 213233, Sigma Aldrich) in the LiCl group and of 10 μg/Kg exendin-4 (H-8730, Bachem) in the Ex-4 group. Doses of LiCl and exendin-4 were chosen based on our previous results indicating that these resulted in comparable learning curves. Rats in the RS group were placed in Plexiglas restraint tubes with several bored holes for 30 min. The 30 min restraint period was chosen based on the demonstration that such treatment significantly increases plasma corticosterone (CORT) and decreases food intake (Girotti et al., 2006, Kinzig et al., 2008). The conditioning cycle continued until rats in each group received 4 conditioning parings e.g. CS-saline, CS-LiCl, CS-Ex-4 and CS-RS. If the rats failed to consume any butter icing after 3 conditioning days, no pairing was done for these rats. As a result, all rats in the saline, LiCl and Ex-4 group and 6 rats in the RS group completed the study. After the 4th conditioning pairing, rats experienced 2 additional regular chow days and on the following day they received their respective US treatment and 85–90 min afterward, they were sacrificed. The rats were deeply anesthetized with isoflurane (10019-360-60, Baxter) and then perfused with ~200 ml of heparinized 0.9% saline and followed by ~150 ml of ice cold 4% paraformaldehyde in PBS. Brains were removed, stored overnight in 4% paraformaldehyde in PBS and then in 25% (wt/vol) sucrose until processed for c-Fos immunostaining.
c-Fos immunohistochemistry
To assess the neural activation produced by the four different US's, brains were frozen, and sectioned at 40 μm on a cryostat from the brainstem (for 5 series) through the forebrain (for 4 series). One of these series was taken for c-Fos protein immunohistochemistry. Thus, each brainstem section was 200 μm apart and each forebrain section was 160 μm apart. Briefly for the immunostaining, sections were incubated for 20 h with c-Fos primary antibody (1:10,000 dilution; PC38, Calbiochem) and 2 h with secondary donkey anti-rabbit (1:500 dilution; 711-065-152, Jackson Immuno Research). Then, sections were processed according to standard immunoperoxidase methods (ABC reagent, PK-6100, Vector Laboratories) and colorized with a commercially available peroxidase kit (SK-4100, Vector Laboratories). To control for staining variability, each immunohistochemistry run contained matched sections from all experimental groups and controls. Quantitative analysis of c-Fos immunoreactivity was done using the IP Laboratory Imaging System (Scanalytics, Vienna, VA) image analysis software. Coronal bilateral sections from two rostrocaudal levels of the area postrema (AP), caudal nucleus of the solitary tract (cNTS), intermediate NTS (imNTS), lateral parabrachial nucleus (LPBN) and the paraventricular nucleus of the hypothalamus (PVN) and three rostrocaudal levels of the central nucleus of the amygdala (CeA) were analyzed per animal. Sections selected for each brain structure across rats were as close in coordinate as possible. The anterior-posterior levels were determined by coordinates from the bregma following Paxinos and Watson (Paxinos and Watson, 2005). The NTS areas included from caudal (cNTS; −14.28 mm), corresponding to the AP and through the intermediate regions (imNTS; −13.08 mm). The LPBN included regions located dorsal lateral to the brachium conjunctivum (BC) in sections between −8.8 mm and −9.36 mm. The CeA included the area surround and adjacent to the commissural stria terminalis (CST) from sections between −2.4 mm and −3.0 mm. The PVN included the area next to the upper third ventricle from sections between −1.2 mm and −1.56 mm. The c-Fos-positive cells were counted bilaterally for each structure by individuals blind to the group design. The statistics were done using the average total counts per structure across rats.
Statistical Analysis
Data were analyzed by Statistica 7.0 (Systat, Tulsa, OK) by ANOVA. Repeated-measures ANOVA were performed on the intake data during training. One way ANOVA was performed to compare the number of Fos-positive neurons in each brain region across the US treatment. For all of the variables measured, data from 2 rats in the RS group was excluded because they failed to consume any butter icing throughout the experiment. Based on the food intake and body weight measurement, one rat was not responsive to the Ex-4 treatment and thus data from it was also excluded. Subsequent comparisons between groups used Newman-Keuls procedures. Data are presented as the means ± SEM.
Results
Intakes and body weight
After acclimation, rats were divided into 4 groups of similar body weight (average between 216.4 and 223.5 g). Average daily chow intakes of the last 4 acclimation days were similar as well (between 56.1 and 60 Kcal/day). Initially, the food deprivation and scheduled feeding regimen significantly decreased total daily food intake and body weight. After 2 days of training on this regimen, intake and body weight quickly stabilized. Before the conditioning cycle, total daily energy intake stabilized at about 60% of ad lib intake. There was no group difference in the 15 min and total daily (sum of 15 min and 1.5 h intake) intake and body weight at the time when the conditioning cycles began.
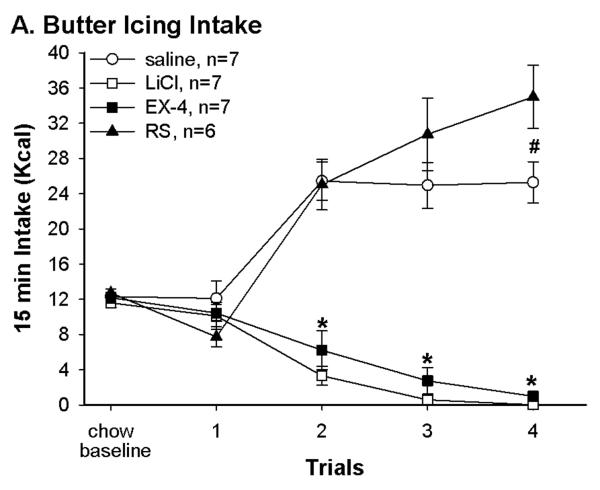
Different US treatment resulted in different responses to the palatable food (Fig. 1A). Average 15 min intakes of butter icing were not different between groups when the rats started to consume the butter icing. Repeated measures ANOVA of the butter icing during the 4 conditioning trials indicate significant effects of group, trial and group × trial interaction [F(3, 23)=55.95, F(3, 69)=9.4, and F(9, 69)=24.81 in the presenting order; P < 0.0001]. While no group difference was seen in the first pairing trial, two distinct patterns of butter icing intake appeared in the following pairing trials. Intakes for the LiCl and Ex-4 groups decreased while intakes increased for the saline and RS groups over trials. By the 4th pairing trial, rats in the LiCl and Ex-4 almost completely avoided the butter icing while in the RS continued to increase intake such that they were consuming significantly more than the saline group.
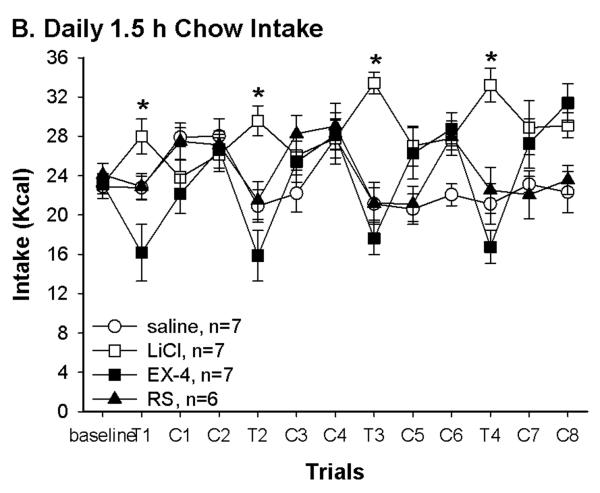
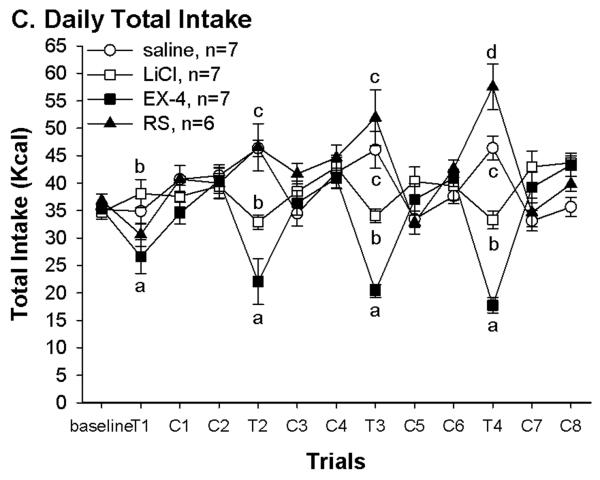
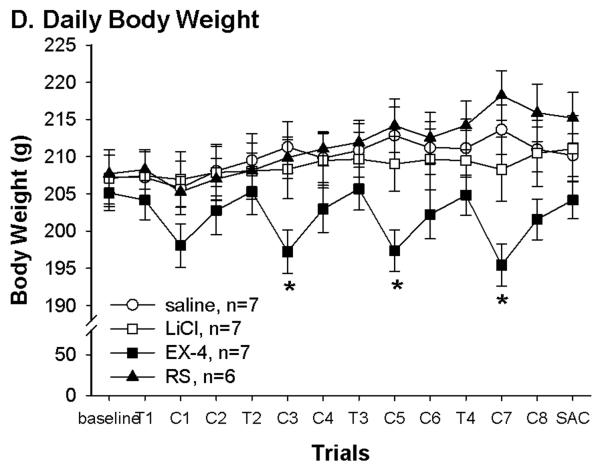
Fig. 1.
(A) Pairing with different US resulted in differences in butter icing intakes during conditioning trials. There was no difference in baseline chow and in first trial butter icing intake. At trial 2–4, rats in both LiCl and Ex-4 suppressed intakes while rats in both saline and RS groups increased butter icing intakes. *: LiCl and Ex-4 vs. saline and RS, P < 0.05; #: saline vs. RS, P < 0.05. (B) Daily 1.5 hr intake indicated that Ex-4 continued to suppress food intake 4 hrs after the injection. The saline and RS groups consumed less to compensate for their overeating during butter icing presentation. Thus, over the conditioning days, LiCl treated rats consumed significantly more chow than the other groups. *: LiCl vs. the other 3 groups P < 0.05. (C) Daily total energy intakes were in corresponding with the group treatment. Rats in the LiCl and Ex-4 groups had lower total energy intake on the conditioning day. Different letters represent significant differences P < 0.05. (D) Daily body weight was consistent with daily total energy intake. Rats in the Ex-4 group significantly decreased their body weight following the conditioning day. *: Ex-4 vs. the other 3 groups P < 0.05. T: conditioning day; C: regular chow training day; SAC: the day the rats were sacrificed
Significant group differences were also seen in total daily intake on conditioning days. One way ANOVA revealed significant group difference in total intakes on the first conditioning day [F(3, 23)=3.9, P=.02, T1 of Fig. 1C)]. This is due to decreased 1.5 h intake in the rats received Ex-4 injection relative to rats receiving the LiCl. This continued on the following conditioning days. Although rats in the LiCl and Ex-4 groups both avoided the butter icing, only the Ex-4 rats continued to decrease chow intake in the 1.5 hr access session (Fig. 1B) resulting in significant reduction on total energy intake on conditioning days. On the other hand, rats in the saline and RS groups had a trend toward increased total energy intake across conditioning days. Such increases are consistent with their increased 15 min butter icing intake across conditioning days. In fact, compared to the LiCl group, both saline and RS groups consumed significantly less chow during the 1.5 hr access time on conditioning days. Unlike the Ex-4 treated rats, this decrease in chow intake during 1.5 hr access is likely compensatory to their overconsumption of butter icing. One way ANOVA results confirmed these 1.5 hr intake differences from conditioning day 1 to 4 [F(3,23)=6.4, 8.6, 18.6, and 13.5, P < 0.003], and total intake group differences from conditioning day 2 to 4 [F(3, 23)=14.9, 21.5, and 48.7, P < 0.0001]. Furthermore, the body weights during this period were consistent with the intakes. That is, rats in the Ex-4 group decreased body weight following each conditioning day (Fig. 1D). Although the RS group weighed more than the LiCl and saline groups, body weights of these 3 groups did not differ significantly. One way ANOVA revealed significant group effects on days following each conditioning day [C1,F(3, 23)=1.5, P=0.2; C3, C5 and C7: F(3, 23)=3.5, 4.6 and 7.3, P < 0.04].
Neuronal activation by the unconditioned stimuli
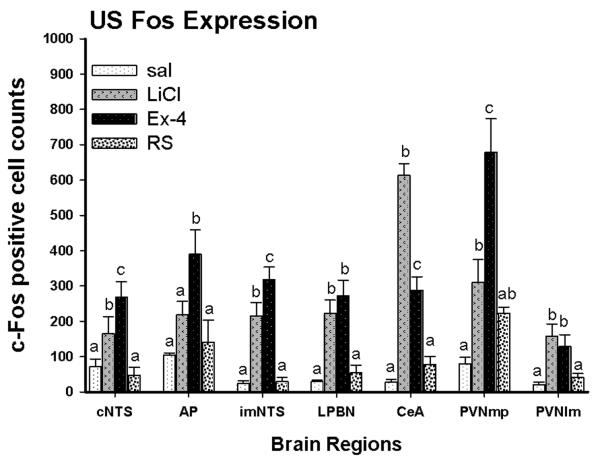
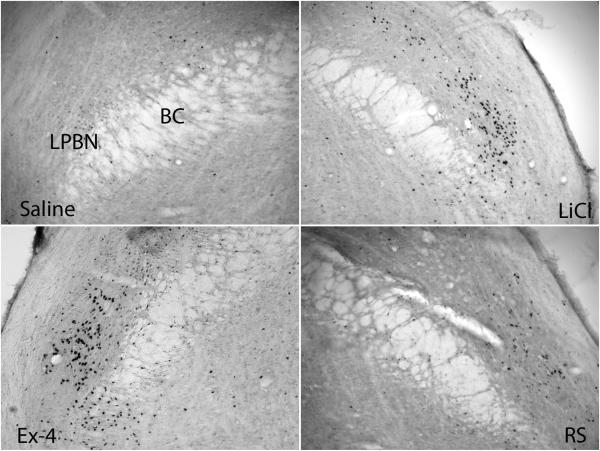
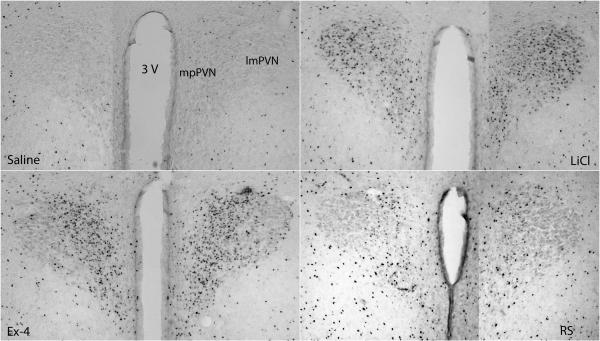
c-Fos immunohistochemistry was done to determine whether different neuronal activation might contribute to the different responses to stress. Fos-positive staining was examined in the visceral and HPA regions. Distinct positive Fos expression was seen between groups (Fig. 2). One way ANOVA revealed significant group effects at all regions examined including the cNTS, imNTS, AP, LPBN, CeA and PVN [ in the presenting order: F(3, 23)=15, F(3, 23)=27.5, F(3, 22)= 10.7, F(3, 23)=15.1, F(3, 23)=83.6 and F(3, 23)=13.8; P < 0.001]. Saline and restraint rats had fewer c-Fos positive cells in the caudal brainstem than did LiCl and Ex-4 rats. Compared to saline, brains from LiCl treated rats had significantly more Fos expression in the caudal and intermediate NTS and LPBN; Ex-4 treated rats had significantly more Fos expression in the NTS, AP and LPBN. At forebrain sites, saline treated rats had low numbers of Fos positive cells. Restraint rats only had a trend for increased Fos expression in the PVN (post hoc P=0.097; P < 0.05 if a t-test comparison is done between saline and RS groups). In contrast, treatment with LiCl and Ex-4 both resulted in increased Fos expression in the CeA and PVN. Furthermore, the expression patterns of Fos between LiCl and Ex-4 treatments differed. Exendin-4 activated more Fos than did LiCl in the PVN and conversely, LiCl activated more Fos than did Ex-4 in the CeA. Furthermore, while both LiCl and Ex-4 treatment induce significantly more Fos in the PVN, Ex-4 induced significantly more Fos in the medial parvicellular (mp) subdivision of the PVN than did LiCl. Images of Fos expression in each brain region examined are shown from Fig 3 to 7.
Fig. 2.
(A) Activation of c-Fos expression in different brain regions after different US. The c-Fos activation patterns were similar after LiCl and Ex-4 injection. Injection of Ex-4 activated significantly more Fos in the medial parvicellular (mp) subdivisions of the PVN than did injection of LiCl. Fos activation in the lateral magnocellular (lm) division did not differ between the LiCl and Ex-4 treatment. Different letters indicate significant group differences, P < 0.05.
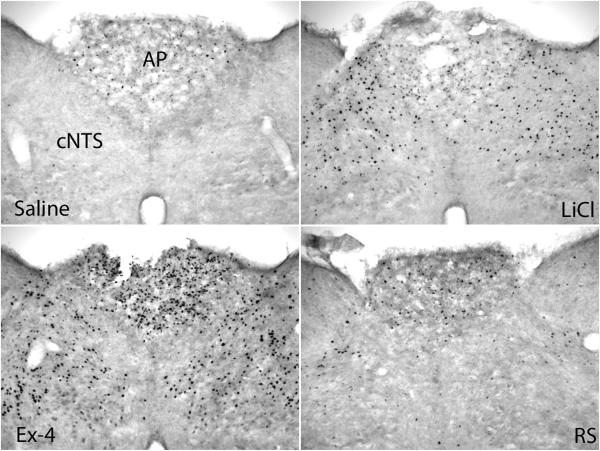
Fig. 3.
Fos expression in the cNTS and AP. Significantly more Fos expression was shown in the cNTS and AP in LiCl and Ex-4 treated brains. AP: area postrema; cNTS: caudal nucleus of the solitary tract; imNTS: intermediate nucleus of the solitary tract; 4V: the 4th ventricle
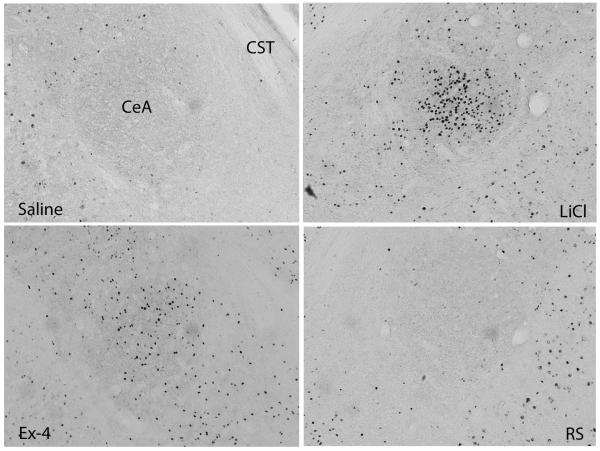
Fig. 7.
Fos expression in the CeA. Significantly more Fos expression was shown in the CeA in LiCl and Ex-4 treated brains. CST: commissural stria terminalis
Discussion
Acceptance and avoidance to food can be based on associated postingestive consequences. In this study, a classical conditioning paradigm is used to examine whether different types of stressors support different directions of food learning. The palatable food butter icing was served as the CS and the stressors were provided as the postingestive consequences or the US. Previous studies in rodents have demonstrated that both types of stressors can reduce intake of familiar food e.g. standard chow diet (Ervin et al., 1995, Rybkin et al., 1997, Rinaman and Dzmura, 2007). Fewer studies have determined and compared how these different stressors affect food acceptance learning. The results here indicate that the interoceptive stressors, LiCl and Ex-4, support food avoidance learning i.e. they decrease intake over time. In contrast, the psychosocial stressor, restraint stress, supports food acceptance learning i.e. increased intake of the CS over time more than did the controls. Results of c-Fos labeling in the brain indicate that the two interoceptive stressors activate similar brainstem and forebrain pathways while restraint stress induced some activation of the forebrain HPA region, these increases were not significant. In addition to HPA relays, LiCl and Ex-4 also activate the brainstem visceral pathway including the NTS and LPBN. These results are consistent with previous studies indicating that drugs such as LiCl and Ex-4 produce negative physiological effects and induce protective food avoidance learning (Rinaman, 1999, Baraboi et al., 2011, Liang et al., 2013). Intriguingly, although restraint stress can reduce food intake and body weight (Rybkin et al., 1997, Harris et al., 2006), it facilitates palatable food intake when serving as an US. Overall, the results of this study suggest that different types of stressors can support opposite food learning responses i.e. avoidance and acceptance.
Conditioned taste/food aversion is a paradigm commonly used to determine whether the anorexic/hypophagic effect of an agent is due to its aversive property. A standard comparison is LiCl because its aversive property has been well characterized. Animals not only avoid the CS associated with the LiCl but also show gaping orofacial expression after ingesting the CS e.g. negative taste reactivity (Grill and Norgren, 1978, Cantora et al., 2006). Exendin-4 is an FDA approved drug for type 2 diabetes. Patients taking Ex-4 have reported adverse effects such as nausea although this normally disappears after a period of usage (Buse et al., 2004, Blonde et al., 2006). In animal research there have been conflicting results as to whether Ex-4 produces significant negative physiological effects to support CFA. Such discrepancy could be due to differences in experimental methods and species tested [for detail discussion see (Liang et al., 2013)]. With the paradigm used in our laboratory, negative symptoms e.g. laying on the belly, are consistently observed in rats receiving IP injection of 10 μg/Kg Ex-4. Accordingly, it appears that LiCl and Ex-4 have similar aversive properties. While having similar aversive properties, doses used in this study demonstrated that their effects on food intake may differ. Our data demonstrate that LiCl (42.3 mg/Kg) treated rats learned to avoid the butter icing CS but did not decrease regular chow intake or body weight following the CS-US conditioning. In contrast, Ex-4 not only supported avoidance learning to the palatable CS but also decreased total energy intake and body weight (Fig. 1 B – D). The doses used here also resulted in different degrees of Fos activation at multiple brain regions. Ex-4 at 10 μg/Kg activates significantly more Fos in the NTS, AP and the medial parvicellular division of the PVN than does LiCl at 42.3 mg/Kg. Alternatively, LiCl activates significantly more Fos in the CeA than does Ex-4. Finally, although the tested doses of LiCl and Ex-4 showed similar food avoidance acquisition curve, it is not clear whether one of them resulted in stronger CFAV. Comparisons of trials required to extinguish the CFA by LiCl and Ex-4 would provide an indication of the strength of CFAV.
In contrast to the results discussed above, when a palatable food serves as a CS, restraint stress increases the intake of the CS. To date, this is the first study to demonstrate in a learning paradigm that restraint stress facilitates overconsumption of an energy dense palatable food. In general, acute unconditioned RS reduces regular chow intake and the longer the restraint (30 min to 3 hr) the more the suppression of intake (Rybkin et al., 1997, Kinzig et al., 2008, Calvez et al., 2011). Chronic RS also results in decreased chow intake, but the effects can decline overtime, probably due to adaptation to the stress. In contrast, the effects of unconditioned RS on palatable food intake are inconsistent. For rats that have been consuming fat and sugar mixture, acute 30min RS or daily 3 hr RS has been shown to significantly decrease the intake of the mixture (Pecoraro et al., 2004, Kinzig et al., 2008). Another group, however, has demonstrated that acute RS did not change palatable “Froot Loop” intake (Ely et al., 1997). Furthermore, they showed that chronic daily RS reduces intake of chow but increases palatable “Froot Loop” intake (Ely et al., 1997, Silveira et al., 2000). While the results of the effects of unconditioned RS on palatable food intake are inconsistent, effects on food preference do seem to be consistent. Restraint stressed rats maintained their preference to the palatable energy dense food (fat and sugar) although total energy intake was reduced (Pecoraro et al., 2004, Kinzig et al., 2008). This is consistent with the study that demonstrated unconditioned RS reduces intake but not preference for saccharin solution (Howell et al., 1999).
In this study, the intake of the palatable CS reached a plateau in the saline treated rats while it continued to increase in the restraint stress treated rats such that by the fourth exposure their intakes were significantly elevated relative to that of the saline treated rats. Such a difference between the saline and RS group suggests that indeed a psychosocial stressor like restraint stress can facilitate overconsumption and likely promote the development of a learned preference for palatable energy dense food. Because butter icing is innately palatable, it is unclear whether restraint stress will support a CFAC when the CS is a neutral food. A demonstration that restraint stress as an US also increases intake of a flavored chow or control diet would further confirm that psychosocial stress can support food acceptance learning in general. Furthermore, one difference between previous studies with restraint stress and this one is the sex of the rats. Sex differences in stress responses and palatable food intake have been reported (Eckel and Moore, 2004, Bangasser and Valentino, 2012). Future studies are required to investigate whether restraint stress in male rats would also facilitate overconsumption of palatable foods.
In addition to affecting the HPA axis, different stressors activate different pathways to adapt and maintain physiological homeostasis during stress. The results of Fos labeling indicate that LiCl and Ex-4 activate visceral relays including the caudal and intermediate NTS, the LPBN, and the CeA. These results are consistent with previous studies (Yamamoto et al., 1992, Baraboi et al., 2011, Spencer et al., 2012). On the other hand, 30 min restrain stress only induced a trend of more Fos in the PVN compared to the saline treatment. The lack of significant c-Fos activation by the RS is not likely due to our long interval (90 min) between the end of restraint and sacrifice. It has been demonstrated that sacrificing rats 30 min or 2 hrs after the end of acute restraint stress resulted in similar amount of Fos activation in the PVN (Viau and Sawchenko, 2002). Fos activation in the PVN and other forebrain regions (Sterrenburg et al., 2012), however, are greatly reduced after repeated daily restraint (Chen and Herbert, 1995, Stamp and Herbert, 1999, Viau and Sawchenko, 2002) even when significant increases of plasma CORT after restraint was detected (Viau and Sawchenko, 2002). In our study, the restraint had been repeated 5 times by the time of sacrificing. Although we did detect significant increase of CORT (pre vs. post restraint stress, 363 ± 87.8 vs. 667.3 ± 65 ng/ml, P < 0.05) after the restraint on the day of sacrifice, our Fos activation could still be greatly reduced. Furthermore, while we did not detect Fos activation in the brainstem regions examined, others have demonstrated increased Fos in the NTS (Chen and Herbert, 1995, Stamp and Herbert, 1999, Banihashemi et al., 2011) and locus coeruleus (Chen and Herbert, 1995, Stamp and Herbert, 1999) after acute restraint stress. These activations are also likely to be reduced after repeated restraint exposure (Stamp and Herbert, 1999).
The Fos results have implications related to the differences in food acceptance learning by the interoceptive and restraint stress. The fact that the restraint stress induced less Fos in both the brainstem and forebrain compared to those induced by LiCl and Ex-4 raises several issues. First, the intensity of the RS might not be strong enough to produce sufficient negative effects to support a CFAV. Given longer restraint (e.g. 3 hrs) has been shown to produce long lasting reduction in food intake and body weight (Harris et al., 1998), it is likely that longer restraint stress would result in food avoidance learning. Second, if adaptation occurs to all stressors used here, the Fos results would suggest that rats habituated to the effects of restraint to a larger degree than they did to the LiCl or Ex-4 treatment. The differences in stress adaptation could be due to the type of stressor as well as their previous experience with consuming butter icing. While it has been demonstrated that consuming palatable energy dense food can dampen the effects of restraint stress (Pecoraro et al., 2004), it is likely that a more intense and less adaptive form of psychogenic stress such as predator stress e.g. cat exposure (Figueiredo et al., 2003) would support a different pattern of food acceptance learning. Along similar issues, as demonstrated in the literature, the same taxionomical stressors do not always support the same patterns of food acceptance learning. While we demonstrated that the psychogenic (or exteroceptive) restraint stress can lead to increased intake of the CS, another group demonstrated that the same restraint stress did not change the intake of the CS (saccharin) (Lockwood et al., 2003). Using another exteroceptive stress, footshock, as the US has produced inconsistent results relative to conditioned food avoidance (Garcia et al., 1968, Pelchat et al., 1983). These discrepancies within the same type of stressor could be due to specifics of research methodology, and warrant further study.
It is still unclear why and how restraint stress would support the overconsumption of palatable CS. Since previous studies have demonstrated that palatable food can dampen the effects of restraint stress (Ulrich-Lai et al., 2007, Foster et al., 2009), it is possible that the positive effects after consuming the butter icing could overcome the distress by restraint stress. If this is the case, overconsumption of the CS after pairing with restraint stress would not be observed when the CS is not a highly palatable food. Moreover, a previous study indicated that the frequency of consuming sucrose is a determinant of its ability to dampen the effect of HPA activation. In that study, at least two weeks of sucrose exposure is required for dampening the effects of restraint stress (Ulrich-Lai et al., 2011). If the food acceptance learning in response to restraint stress is due to the rewarding effects of the high fat high sugar, the results of this study would suggest that high fat and high sugar is more potent than sucrose to dampen the effects of restraint stress. That is, only one pairing of high fat high sugar consumption with restraint stress may be sufficient to facilitate overeating of highly palatable energy dense food when experiencing a psychosocial stress is predicted. Finally, all rats that received restraint stress in this paradigm showed overconsumption of butter icing. This result seems to be inconsistent with the bimodal feeding response to stress in human subjects. Nevertheless, it has been demonstrated in humans that even the subjects that decreased overall food intake would increase intake of highly palatable food e.g. sweet and chocolate (Oliver and Wardle, 1999), which is consistent with the palatable butter icing used in this study.
In summary, this study demonstrates that different stressors can support different patterns of palatable food learning. In line with previous studies, experience with interoceptive stressors results in food avoidance learning. In contrast, the psychosocial stressor, restraint stress, which has been repeatedly shown to reduce daily energy intake can serve as a US to facilitate overeating of an energy-dense palatable CS. Such results suggest a role of psychosocial stress induced overeating of highly palatable energy-dense food in the development of obesity or binge eating disorders.
Research Highlights
Restraint stress as an unconditioned stimulus facilitates overconsumption of a palatable food.
Interoceptive stressors such as LiCl and exendin-4 support conditioned food avoidance learning.
Rats are less adaptive to stress induced by LiCl or exendin-4 than by restraint.
A psychogenic restraint stress may play a role in the development of binge eating disorders.
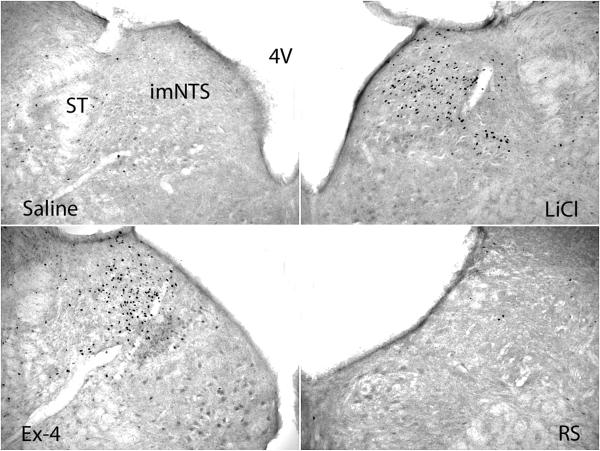
Fig. 4.
Fos expression in the imNTS. Significantly more Fos expression was shown in the imNTS in LiCl and Ex-4 treated brains. 4V: the 4th ventricle
Fig. 5.
Fos expression in the LPBN. Significantly more Fos expression was shown in the LPBN in LiCl and Ex-4 treated brains. BC: brachium conjunctivum
Fig. 6.
Fos expression in the PVN. Significantly more Fos expression was shown in the PVN in LiCl and Ex-4 treated brains. LiCl activates more even Fos expression in the medial parvicellular (mp) and lateral magnocellular (lm) subdivisions of the PVN while Ex-4 has most activation of Fos in the mp subdivision of the PVN. 3V: the 3rd ventricle
Acknowledgement
The authors thank Mr. Ryan Purcell for assisting restraint stress in rats and Dr. Kellie Tamashiro for valuable feedback on an earlier manuscript. This research is supported by NIH DK019302.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32:709–723. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi L, O'Neill EJ, Rinaman L. Central neural responses to restraint stress are altered in rats with an early life history of repeated brief maternal separation. Neuroscience. 2011;192:413–428. doi: 10.1016/j.neuroscience.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraboi ED, St-Pierre DH, Shooner J, Timofeeva E, Richard D. Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1011–1024. doi: 10.1152/ajpregu.00424.2010. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Air EL, Wilmer K, Messerschmidt P, Hodge KM, Jones MB, Eckstein DM, McOsker CC, Seeley RJ, Woods SC, Sheldon RJ. Two novel paradigms for the simultaneous assessment of conditioned taste aversion and food intake effects of anorexic agents. Physiol Behav. 2003;79:761–766. doi: 10.1016/s0031-9384(03)00189-6. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Thiele TE, Heinrichs SC, Rushing PA, Blake KA, Steeley RJ. Comparison of central administration of corticotropin-releasing hormone and urocortin on food intake, conditioned taste aversion, and c-Fos expression. Peptides. 2000;21:345–351. doi: 10.1016/s0196-9781(00)00153-4. [DOI] [PubMed] [Google Scholar]
- Bernstein IL, Goehler LE. Chronic lithium chloride infusions: conditioned suppression of food intake and preference. Behav Neurosci. 1983;97:290–298. doi: 10.1037//0735-7044.97.2.290. [DOI] [PubMed] [Google Scholar]
- Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- Calvez J, Fromentin G, Nadkarni N, Darcel N, Even P, Tome D, Ballet N, Chaumontet C. Inhibition of food intake induced by acute stress in rats is due to satiation effects. Physiol Behav. 2011;104:675–683. doi: 10.1016/j.physbeh.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Cantora R, Lopez M, Aguado L, Rana S, Parker LA. Extinction of a saccharin-lithium association: assessment by consumption and taste reactivity. Learn Behav. 2006;34:37–43. doi: 10.3758/bf03192869. [DOI] [PubMed] [Google Scholar]
- Chen X, Herbert J. Regional changes in c-Fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience. 1995;64:675–685. doi: 10.1016/0306-4522(94)00532-a. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Sved AF, Verbalis JG, Stricker EM. Lithium chloride-induced anorexia, but not conditioned taste aversions, in rats with area postrema lesions. Brain Res. 1994;663:30–37. doi: 10.1016/0006-8993(94)90459-6. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel LA, Moore SR. Diet-induced hyperphagia in the rat is influenced by sex and exercise. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1080–1085. doi: 10.1152/ajpregu.00424.2004. [DOI] [PubMed] [Google Scholar]
- Ely DR, Dapper V, Marasca J, Correa JB, Gamaro GD, Xavier MH, Michalowski MB, Catelli D, Rosat R, Ferreira MB, Dalmaz C. Effect of restraint stress on feeding behavior of rats. Physiol Behav. 1997;61:395–398. doi: 10.1016/s0031-9384(96)00450-7. [DOI] [PubMed] [Google Scholar]
- Ervin GN, Birkemo LS, Johnson MF, Conger LK, Mosher JT, Menius JA., Jr The effects of anorectic and aversive agents on deprivation-induced feeding and taste aversion conditioning in rats. J Pharmacol Exp Ther. 1995;273:1203–1210. [PubMed] [Google Scholar]
- Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150:2325–2333. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, McGowan BK, Ervin FR, Koelling RA. Cues: their relative effectiveness as a function of the reinforcer. Science. 1968;160:794–795. doi: 10.1126/science.160.3829.794. [DOI] [PubMed] [Google Scholar]
- Gibson EL. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiol Behav. 2006;89:53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-Fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Gold PW. Corticotropin releasing hormone produces profound anorexigenic effects in the rhesus monkey. Neuropeptides. 1991;18:55–61. doi: 10.1016/0143-4179(91)90164-e. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978;201:267–269. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- Harris RB, Palmondon J, Leshin S, Flatt WP, Richard D. Chronic disruption of body weight but not of stress peptides or receptors in rats exposed to repeated restraint stress. Horm Behav. 2006;49:615–625. doi: 10.1016/j.yhbeh.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Harris RB, Zhou J, Youngblood BD, Rybkin II, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol. 1998;275:R1928–1938. doi: 10.1152/ajpregu.1998.275.6.R1928. [DOI] [PubMed] [Google Scholar]
- Howell LA, Harris RB, Clarke C, Youngblood BD, Ryan DH, Gilbertson TA. The effects of restraint stress on intake of preferred and nonpreferred solutions in rodents. Physiol Behav. 1999;65:697–704. doi: 10.1016/s0031-9384(98)00223-6. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology. 2012;62:1916–1927. doi: 10.1016/j.neuropharm.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M, Scott KA, Moran TH, Bi S. Dorsomedial hypothalamic corticotropin-releasing factor mediation of exercise-induced anorexia. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1800–1805. doi: 10.1152/ajpregu.00805.2004. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzig KP, Hargrave SL, Honors MA. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiol Behav. 2008;95:108–113. doi: 10.1016/j.physbeh.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry. 1990;27:1094–1102. doi: 10.1016/0006-3223(90)90046-5. [DOI] [PubMed] [Google Scholar]
- Liang NC, Bello NT, Moran TH. Additive feeding inhibitory and aversive effects of naltrexone and exendin-4 combinations. Int J Obes (Lond) 2013;37(2):272–8. doi: 10.1038/ijo.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood DR, Kwon B, Smith JC, Houpt TA. Behavioral effects of static high magnetic fields on unrestrained and restrained mice. Physiol Behav. 2003;78:635–640. doi: 10.1016/s0031-9384(03)00040-4. [DOI] [PubMed] [Google Scholar]
- Looy H, Eikelboom R. Wheel running, food intake, and body weight in male rats. Physiol Behav. 1989;45:403–405. doi: 10.1016/0031-9384(89)90147-9. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Verbalis JG, Stricker EM. LiCl and CCK inhibit gastric emptying and feeding and stimulate OT secretion in rats. Am J Physiol. 1989;256:R463–468. doi: 10.1152/ajpregu.1989.256.2.R463. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol Behav. 1999;66:511–515. doi: 10.1016/s0031-9384(98)00322-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press; 2005. [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Grill HJ, Rozin P, Jacobs J. Quality of acquired responses to tastes by Rattus norvegicus depends on type of associated discomfort. J Comp Psychol. 1983;97:140–153. [PubMed] [Google Scholar]
- Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277:R582–590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1495–1503. doi: 10.1152/ajpregu.00393.2007. [DOI] [PubMed] [Google Scholar]
- Rybkin II, Zhou Y, Volaufova J, Smagin GN, Ryan DH, Harris RB. Effect of restraint stress on food intake and body weight is determined by time of day. Am J Physiol. 1997;273:R1612–1622. doi: 10.1152/ajpregu.1997.273.5.R1612. [DOI] [PubMed] [Google Scholar]
- Silveira PP, Xavier MH, Souza FH, Manoli LP, Rosat RM, Ferreira MB, Dalmaz C. Interaction between repeated restraint stress and concomitant midazolam administration on sweet food ingestion in rats. Braz J Med Biol Res. 2000;33:1343–1350. doi: 10.1590/s0100-879x2000001100013. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Eckel LA, Nardos R, Houpt TA. Area postrema lesions attenuate LiCl-induced c-Fos expression correlated with conditioned taste aversion learning. Physiol Behav. 2012;105:151–160. doi: 10.1016/j.physbeh.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Roubos EW, Peeters BW, Kozicz T. Sex-dependent and differential responses to acute restraint stress of corticotropin-releasing factor-producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis. J Neurosci Res. 2012;90:179–192. doi: 10.1002/jnr.22737. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Nguyen MM, Melhorn SJ, Ma LY, Woods SC, Sakai RR. Dynamic body weight and body composition changes in response to subordination stress. Physiol Behav. 2007a;91:440–448. doi: 10.1016/j.physbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Ostrander MM, Gardner SR, Ma LY, Woods SC, Sakai RR. Social stress and recovery: implications for body weight and body composition. Am J Physiol Regul Integr Comp Physiol. 2007b;293:R1864–1874. doi: 10.1152/ajpregu.00371.2007. [DOI] [PubMed] [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Herman JP. HPA axis dampening by limited sucrose intake: reward frequency vs. caloric consumption. Physiol Behav. 2011;103:104–110. doi: 10.1016/j.physbeh.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. J Comp Neurol. 2002;445:293–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Azuma S, Bai WZ, Wakisaka S. C-Fos expression in the rat brain after intraperitoneal injection of lithium chloride. Neuroreport. 1992;3:1049–1052. doi: 10.1097/00001756-199212000-00004. [DOI] [PubMed] [Google Scholar]