Abstract
S-nitrosothiols (RSNO) have been used widely as experimental NO-donors but their clinical use remains limited. Recent data support a role for endogenous RSNO as mediators of nitric oxide (NO) signaling via post-translational modification of proteins. This new understanding together with emerging insights into NO-donor dependent and independent mechanisms of RSNO actions are discussed in this review in the context of emerging and potential therapeutics that target endogenous RSNOs, or utilize synthetic RSNOs to stimulate NO-signaling with a focus on treatment of diabetes and metabolic disease, pathologies in which dysfunction in NO-signaling is clearly implicated.
Keywords: Nitric oxide, thiol, signaling, redox, vascular
1. Introduction
Emerging evidence underscores the important role for S-nitrosothiols (thionitrite esters) as effectors of nitric oxide (NO) function both in vivo and therapeutically [1, 2]. S-nitrosothiols are adducts between the nitrosonium cation (NO+) and a thiolate anion (RS−) and conform to the general structure RSNO, where R represents the side chains of a peptide containing cysteine. Importantly, not all RSNO’s are equal with the ‘R’ group playing an important role in mechanisms by which the RSNO may be formed but also its chemical properties [3, 4]. As a result RSNO’s can be low (e.g. S-nitrosocysteine or S-nitrosoglutathione) or high molecular weight compounds (with more recent synthetic RSNO’s being polymer bound) with varied stability and reactivity. Moreover, the spectrum of biological responses associated with different RSNO’s mirror that of NO itself and provide the foundation for therapeutic development and use of RSNO to treat diseases in which NO-dysfunction is implicated. This includes complications associated with diabetes and metabolic syndrome, disease states that are of increasing prevalence and now considered to be epidemic. The goal of this review is to discuss the potential for RSNOs as both targets for therapeutic modulation and as direct therapeutics to affect NO-dependent processes. With this in mind, the biochemical properties and mechanisms that control RSNO metabolism which dictate their ability to control biological processes by NO-dependent or independent processes will be discussed, followed by discussion of the current generations of RSNO donors that are being tested as NO-based therapeutics for treating complications associated with metabolic syndrome.
2. Diabetes and NO dysfunction
Metabolic disease epitomized by diabetes mellitus (insulin independent or dependent) is increasing in frequency and contributes to significant morbidity and mortality worldwide. Over the last decade a role for dysfunctional NO-signaling in the pathogenesis of this disease has been recognized and can occur by too little or too much NO formation depending on the tissue and functional end point. For example, decreased NO-bioavailability in the vasculature in diabetics (via compromised NO production from endothelial NOS or increased production of reactive oxygen species) contributes to vascular inflammation, diminished wound healing and increased smooth muscle proliferation which collectively contribute to increased ischemia-reperfusion injury and an enhanced restenosis response after ballon angioplasty in diabetics [5–14]. On the other hand, too much NO formation (secondary to induction of inducible NOS) in islets has been associated with the development of insulin resistance [15, 16]. These select examples highlight the potential for therapeutic targeting of NO in these diseases [17]. The focus of this review is RSNO as targets for therapy as well as their use as therapeutics to replete NO-signaling. We note that this area of research and clinical development is in its infancy and will focus therefore on recent evidence implicating S-nitrosylation (the terms the terms S-nitrosation and S-nitrosylation are both used in the literature to denote the same modification. The former is correct with respect to chemical nomenclature, with the latter used more frequently to denote the potential signaling role for this modification. For the sakes of consistency we will use S-nitrosylation in this review) in metabolic disease, discuss key questions in the development of RSNO based therapies and highlight recent RSNO donors that are being tested as NO-repleting agents
3. NO donor vs. direct protein modifying functions of RSNO’s
Initially, RSNO’s were used widely as experimental tools to deliver NO and activation of soluble guanylate cyclase (sGC) and formation of the second messenger cyclic guanosine monophosphate (cGMP). However, this approach is now recognized to be problematic as understanding of NO-release mechanisms from RSNO has improved together with the appreciation that not all RSNO-dependent effects are mediated by NO release [1, 3, 18, 19]. In fact, the ability of RSNO to affect specific protein function by thiol modifications is now emerging as the primary mechanism of action of endogenous S-nitrosylation [1, 20]. It is not the purview of this article to list all reported effects of RSNO that are NO-sGC dependent and those that are not, but it is important to highlight that these dual mechanisms are critical in the context of RSNO based therapeutics. Each of these will be discussed with a focus on current therapeutics utilizing each function of RSNOs pertaining to diabetes and associated vascular complications. First however, a brief overview of the the chemical properties of RSNO’s pertaining to NO-release vs protein modification is provided
3.1. NO liberation from RSNO’s
Nitric oxide formation from RSNO’s can occur by homolytic and heterolytic mechanisms. Light dependent decomposition of occurs by a homolytic process yielding NO and the corresponding thiyl radical [21–23]. Nitric oxide release from RSNO’s can also occur by a reductive mechanism involving heterolytic cleavage of the S-N bond. Biologically relevant reductants include transition metal ions such as copper, ascorbate, superoxide and thiols [2, 3]. With copper, NO-formation occurs with cuprous ion (Cu+) reducing RSNO and forming cupric ion (Cu2+) [23, 24]. Unlike photolysis this process does not involve thiyl radical formation and importantly, since it is the reduced copper that reacts with the RSNO, reducing agents such as ascorbate or cysteine can accelerate RSNO decomposition by regenerating Cu2+ from Cu+. From a methodological standpoint the sensitivity of RSNO’s to light and metal ions is important since different lighting conditions and different buffer preparations containing different levels of contaminating metals or metal chelators, will result in variable RSNO stabilities in solution (Feelisch & Stamler, 1996). Also these properties have been harnessed to detect RSNO in biological matrices by selective release and detection of NO from RSNO by either light or metals[25–27]. It is important to appreciate however, the ‘R’ group of the RSNO will affect the photo-lability and reactivity with metals. For example if the ‘R’ group can absorb light or chelate metals, RSNO decomposition will be slower[28]. Finally, it should be stressed that the current generation of RSNO which are integrated into different matrices (see below) can release NO via reactions with light or metals [29] and opens the possibility for site-directed NO-generation [21, 22].
3 RSNO-dependent modulation of protein function via thiol modification
The molecular diversity of thiols embodied by a multiple modifications that include S-nitrosylation, different oxidation states (reduced thiol (SH), disulfide (RSSR) mixed disulfides (RSSR’), sulfenic acid (RSOH) and sulfinic acids (RSO2H), and adducts with an array of electrophilic compounds, are now recognized to play important roles in redox cell signaling [30–35]. The list of proteins whose function is potentially regulated by one or more of the above mentioned thiol modifications are increasing rapidly. However, it should be stated that in most part these insights emanate from either purified protein, organelle or cell culture studies in which exposure to range of concentrations (often supra-physiological) of the thiol modifying agent of interest (electrophile, oxidant etc) is performed. In principle any protein with a critical thiol can be modified and will likely lead to a change in activity / function. Therefore it is imperative, that identification of a given protein thiol as target for S-nitrosylation be verified in vivo where multiple thiol targets including glutathione (which is typically present at significantly higher concentrations that a protein thiol) are present. Despite this caution, it is clear that many proteins are S-nitros(yl)ated in vivo with this post-translational modification subject to dynamic regulation consistent with key roles in signaling [36].
Specific mechanisms for RSNO formation in vivo remains an active, but still not well understood area of investigation [1, 3, 37]. Note RSNO are not formed from the direct reaction with NO and a RSH. RSNO formation requires NO or thiol oxidation in this reaction and not surprisingly then roles for metalloproteins in RSNO formation is emerging (e.g. ceruloplasmin [38], cytochrome c [39, 40]and iron-sulfur centers [40]). Irrespective of formation mechanisms, RSNO’s are clearly present under basal physiological conditions and in general their concentrations increase during inflammatory diseases characterized by increased formation of NO from iNOS, and increased reactive oxygen and nitrogen species collectively termed oxidative / nitrosative stress [41–46]. This paradigm has been demonstrated in insulin resistance and type 2 diabetes also with several proteins in the insulin signaling cascade now being identified as having increased levels of S-nitrosylation during disease pathogenesis (see below). With respect to protein S-nitrosylation,a key reaction is transnitrosation from one RSNO to another thiol (equation 1).
| Equation 1 |
This reversible reaction can occur between any RSNO and RSH with the rate of transnitrosation depending on the relative concentrations, thiol pKa’s and local environment of donor and acceptor thiols (since the reactions occur with the thiolate anion, rates of transnitrosation will be faster with more acidic acceptor thiols [18, 47–49]), and on the relative stabilities of the respective RSNO’s. This latter point has emerged to be critical in the context of protein denitrosylation with two principal pathways identified that involve transnitrosation reactions between the SNO-protein and either glutathione (GSH) to form S-nitrosoglutathione (GSNO) or with thioredoxin (Trx) to form SNO-Trx [36, 50, 51], note other mechanisms may also be present (e.g. carbonyl reductase 1 [52]). In the case of the former, GSNO-reductase (GSNOR, also GSH dependent formaldehyde dehydrogenase) is an enzyme that consumes GSNO driving the equilibrium away from protein S-nitrosylation. This mechanism and its emerging importance in RSNO biology has been supported primarily from gene knockout studies, and more recently from studies testing inhibitors of denitrosylation pathways [36, 50, 53] with both beneficial and detrimental role for GSNO implicated depending on the organ system and disease under study [36, 45, 46, 51]. For example GSNOR depletion protects against reactive airways in asthma and myocardial ischemia reperfusion injury [54–56] but potentiates sepsis induced mortality and promotes hepatocarcinogenesis [46, 57]. With the more recently identified Trx-dependent denitrosylation system, reduced Trx is regenerated by Thioredoxin reductase and this cycle has been shown to modulate levels of caspase-3 S-nitrosylation and hence control programmed cell death [50].
The discussion above is limited effects of S-nitrosylation itself on protein function, but it should be stressed that by S-nitrosylating a thiol, subsequent modification of by oxidation, alkylation etc will be preventing and therefore RSNO formation may regulate thiol based signaling via indirect effects.
4.1 Diabetes and S-nitrosylation
Although many studies have developed the paradigm that dysfunction and decreased NO bioavailability contribute to the pathogenesis of metabolic disease and diabetes, relatively little is known on whether S-nitrosylation is altered, and if so how, in these diseases. Emerging data suggest that RSNO levels are increased in both db/db and ob/ob murine models of type 2 diabetes secondary to induction of iNOS [58]. Based on insights in denitrosylationmechanisms, increased RSNO levels may also be due to decreased activities of the aforementioned pathway and it is interesting to speculate that since reducing equivalents from NADH/NADPH are required for catalytic denitrosylation by GSNOR and TRx dependent pathways, increased oxidative stress during diabetes may impair this process.
Moreover, specific targets that are S-nitros(yl)ated are also being identified with the overall effect being impaired insulin signaling. Specifically, S-nitrosylation of the early and key players in the insulin signaling pathway namely insulin receptor (IRβ), insulin receptor substrate (IRS1) and protein kinase B (Akt) have been demonstrated in vitro and in vivo during diabetes and result in their inactivation and an inhibition of insulin signaling [59, 60]. Moreover, S-nitrosylation is dependent upon iNOS activity and reversed by interventions (e.g. exercise, aspirin, thiazolidinedione therapy [59, 61, 62]) that improve insulin sensitivity. This novel paradigm of iNOS-derived NO leading to S-nitrosylation of key proteins that inhibit insulin signaling is likely to be supported further as new metabolic proteins (e.g. insulin degrading enzyme [63]) are identified as targets for S-nitrosylation. Lastly, it should be noted that GSNO itself may affect glucose metabolism with low micromolar concentrations of GSNO acutely augmenting glucose uptake in diabetic rat skeletal muscle; whereas, high millimolar GSNO decreases glucose uptake [64, 65].
It should also be stressed not all S-nitrosylation is detrimental. S-nitro(sy)lation of glucokinase under basal (i.e. non inflammatory conditions) activates this enzyme which is important in coupling increased glucose levels to insulin secretion [66]. Loss of S-nitrosylation of glucokinase can impair activation and potentially lead to hyperglycemia. In this model then, S-nitrosylation plays an important physiologic role in preventing insulin resistance. A final area of cross talk between glucose concentrations and S-nitrosylation is the potential for hyperglycemia to increase reactive species which can oxidize protein thiols precluding their ability to be S-nitros(yl)ated [67, 68]. The potential impact of this on signaling is exemplified by the example of high glucose preventing NO-dependent inhibition of smooth muscle cell proliferation via oxidation of cys-674 of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase [67]. This discussion serves to highlight the multiple roles for protein and low molecular weight thiol S-nitrosylation in metabolic disease and to underscore the complexity of therapeutically targeting this post-translational modification.
4.2 Therapeutic modulation of protein S-nitrosylation
In the examples discussed above, both protective and disease promoting effects of S-nitrosylation were illustrated depending on the protein and tissue of interest raising the question can S-nitrosylation of a specific protein be targeted therapeutically? This goal remains a challenge, but insights from recent studies suggest that targeted S-nitrosylation to specific tissue / organelles can be achieved. For example RSNO concentrations can be increased by several routes. Exogenous administration of an RSNO can undergo transnitros(yl)ation reactions with acceptor thiols (see above). More recently, reduction of endogenous or exogenously applied nitrite to nitroso species (including RSNO) in hypoxic tissues has been demonstrated [42, 44, 69–71]. Similarly, administration of nitrosating agents will result in increased SNO-formation. In principal RSNO can also be generated by nitrosating intermediates formed from the NO-autoxidation reaction [3]. Therapeutically this is only likely to occur upon addition of relatively high NO concentrations however. Finally, protein S-nitrosylation can be increased by inhibiting GSNOR and Trx dependent denitros(yl)ation, with development of inhibitors of these mechanisms under way [53]. Inhibitors / activators of denitrosylation processes are clearly of therapeutic interest but will likely require targeting to specific tissues for viability.
One key question remaining is how can these formation pathways be targeted? Recent studies have yielded promising results in this regard. For example nitrite-derived RSNO is focused in hypoxic areas which are important in ischemia-reperfusion injury [71]. Continuous nitrite therapy has also been reported to increase chronically ischemic tissue RSNO levels which could be important for diabetic complications such as peripheral vascular disease [72]. Moreover, inhalation of nitrosating species can limit RSNO formation to the pulmonary compartment (e.g. inhaled ethyl nitrite treatment of pulmonary hypertension[73]). With respect to RSNO’s as therapeutic S-nitros(yl)ating agents recent studies have shown that low molecular weight RSNO can be targeted to specific intracellular organelles by introducing targeting moieties to the parent structure. This is exemplified by mitochondrial targeting of the SNO-derivative of 2-mercaptopropionylglycine (a thiol that accumulates in mitochondria) or tagging S-nitroso-N-acetylpenicillamine (SNAP) with a triphenylphosphonium cation moiety (which imparts a positive charge and lipophilicity to the molecule, two properties that drive accumulation into the mitochondria). Both compounds preferentially increased S-nitrosylation of mitochondrial proteins and importantly, this resulted in protection against ischemia-reperfusion injury in vitro and in vivo, via S-nitrosylation of complex 1 of the respiratory chain which prevents formation of reactive species formation during reperfusion [74–77]. Finally, it is now appreciated that the L-type amino acid transport system mediates movement of extracellular SNOC into cells and that blocking this system blunts extracellular SNOC dependent S-nitrosylation of intracellular proteins and signaling [19, 78–80] and thereby provide a novel pathway to affect intracellular RSNO levels. These data suggest the potential for development of RSNO compounds that can be targeted to tissues and also to subcellular compartments to mediate specific functions.
5. RSNO as NO-donors
As mentioned earlier, the NO group in an RSNO is formally NO+, necessitating a redox reaction that results in 1 electron reduction of this group to produce NO. Several biological systems can provide this redox couple including transition metal ions (e.g. copper) [3]. Importantly, the rate of NO liberation will vary depending on the mechanism of reduction and nature of the RSNO, properties that have been used extensively to model NO release for varying durations and at different concentrations in experimental systems. Moreover, Early studies demonstrated that GSNO inhibits coagulation in patients undergoing percutaneous transluminal coronary angioplasty [81] and more recently synthesized RSNO’s are showing promise as anti-platelet effectors also [81, 82]. Despite these early studies and extensive use in cell culture and animal studies, RSNO have not developed significantly as NO-donor therapeutics. Many reasons may account for this discordance primary amongst them is the lack of ability to target NO-release to specific tissues and thereby avoid unwanted systemic effects (e.g. hypotension) together with rapid clearance of the administered RSNO.
The current generation of synthetic RSNO has evolved on the basis that the ‘R’ group of RSNO can be modified and readily incorporated into different polymeric matrices and scaffolds. Polymer chemistry is relatively advanced allowing the generation of different compounds that can have different water solubilities / hydrophobicity, can be applied as films and gels, be of different sizes which collectively can allow better drug targeting, increase stability, allow incorporation of multiple RSNO’s and allow a range of NO-release rates over long time periods. Specific examples include various polymer conjugates containing multiple S-nitrosothiol moieties (e.g. low Molecular weight RSNOs polymer conjugates [83, 84], polynitros(yl)ated polyesters (with the thiol of mercaptosuccinic acid being used as the thiol substrate) [85], incorporation of SNOC or GSNO into polymeric hydrogels [85–87], S-nitrosothiol modified hyperbranched dendrimers, S-nitrosothiol modified xerogels [29] and more recently S-nitrosylation of phytochelatins (poly thiols) and subsequent cross-linking of these to other polymers [11].
The therapeutic focus of RSNO polymers is wound healing and restensois after angioplasty, two processes that are regulated by endogenous NO-formation via multiple mechanisms including stimulating angiogenesis and inhibition of smooth muscle cell proliferation / platelet aggregation respectively. Furthermore, these pathologies are highly accentuated in the diabetic milieu, in part due to abherrant NO-signaling and contribute significantly to morbidity and mortality of metabolic disease [5–8, 17]. Therefore these processes represent important targets for NO-repletion therapy and recent studies support further development of these RSNO-based compounds. For example, GSNO containing hydrogels can be applied topically to cutaneous wound sites in rats and shown to accelerate wound closure and re-epithelialization [87] and a single topical application of polymer complexes of GSNO promote wound healing in a rat model of streptozotocin induced diabetes [11]. With respect to prevention of restenosis, the strategy has been to incorporate an RSNO-releasing polymer to stents that are inserted during balloon angioplasty. Again proof of concept studies in rodent models support further development with S-nitros(yl)ated polyetheyleneglycol hydrogels inhibiting smooth muscle proliferation during restenosis, consistent with local NO-generation [86]. Similarly polyS-nitros(yl)ated polyesters blended with poly(methyl methacrylate) inhibited platelet aggregation in whole blood [88]. An important consideration and control to all studies evaluating RSNOs as NO-donors is to assess the effects of the thiol containing product after NO-release. Not all studies have reported these controls and it is reasonable to expect that some effects may in fact be mediated by the parent thiol and not any released NO per se. This also highlights the need to evaluate any toxicities associated with polymeric S-nitrosothiols and derived products. Nevertheless, the potential for various polymers to be modified to contain RSNO and then used to replete NO in the area of application is clear and an exciting area of NO-therapeutics.
6. Summary
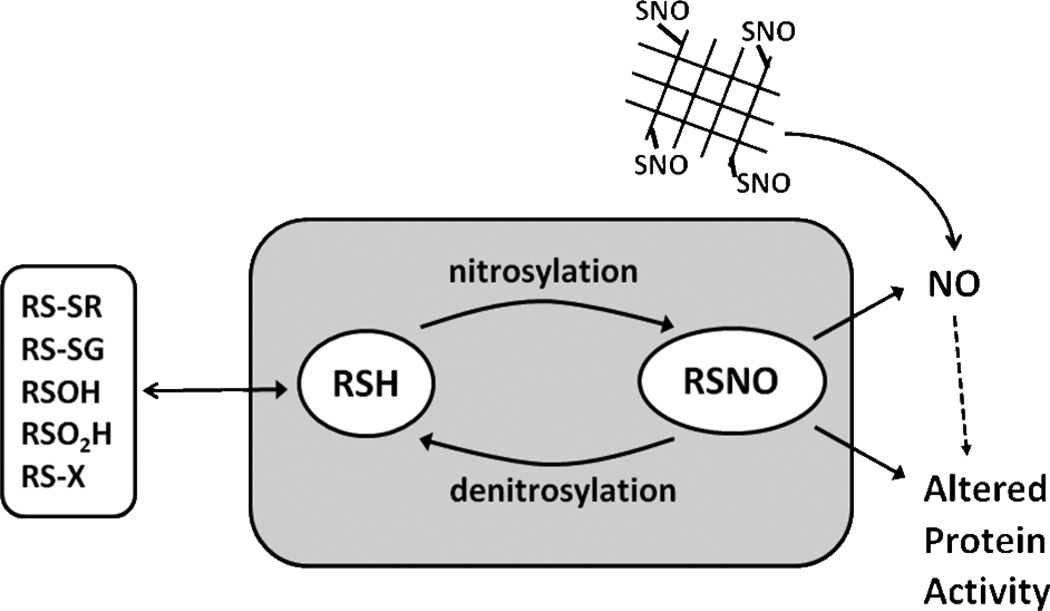
Figure 1 highlights key points discussed above. It is clear that changes in S-nitrosylation are observed throughout numerous disease states including diabetes. Moreover, recent discoveries suggest the intracellular S-nitrosothiol formation and metabolism clearly influence glucose metabolism in different cell types. Coupled with the clear defect in systemic NO bioavailability and enhanced oxidative stress during cardiometabolic syndrome, S-nitrosothiol based therapeutic approaches may be useful for rectifying metabolic dysfunction during diabetes. Building on current concepts and developing strategies that can compartmentalize RSNO-based therapies (including modifying endogenous S-nitrosylation or using RSNO donors to replete NO-signaling) will be critical for further therapeutic development.
Figure 1.
S-nitrosylation and denitrosylation of thiols plays than important role in the biological actions of NO. This modification can regulate protein function by directly changing activity or by affecting other thiol modifications including (disulfide (RSSR) formation, glutathionylation (RSSG), sulfenic acid formation (RSOH), sulfinic acid formation (RSO(OH), adduct formation with different electrophils (RSX). RSNO can be reduced to yield NO and thereby provide a vehicle for endocrine NO signaling. With respect to diabetes, emerging data suggest that both nitrosation and denitrosation of specific proteins are altered in the disease and therefore represent novel therapeutic targets. The current generations of RSNO therapeutics are polymer based and being developed to allow site specific generation of NO which could of benefit in preventing diabetic vascular injury
Acknowledgments
This work was supported by NIH grant HL80482 to C.G.K. and HL092624 to R.P.P.
References
- 1. Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15(9):391–404. doi: 10.1016/j.molmed.2009.06.007. •• A comprehensive review of the role of S-nitrosylation as a post translational modification to regulate protein function.
- 2.Hogg N. Biological chemistry and clinical potential of S-nitrosothiols. Free Radic Biol Med. 2000;28(10):1478–1486. doi: 10.1016/s0891-5849(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 3.Hogg N. The biochemistry and physiology of S-nitrosothiols. Annu Rev Pharmacol Toxicol. 2002;42:585–600. doi: 10.1146/annurev.pharmtox.42.092501.104328. [DOI] [PubMed] [Google Scholar]
- 4.Stamler JS, Toone EJ. The decomposition of thionitrites. Curr Opin Chem Biol. 2002;6(6):779–785. doi: 10.1016/s1367-5931(02)00383-6. [DOI] [PubMed] [Google Scholar]
- 5.Hink U, Tsilimingas N, Wendt M, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus: therapeutic implications. Treat Endocrinol. 2003;2(5):293–304. doi: 10.2165/00024677-200302050-00001. [DOI] [PubMed] [Google Scholar]
- 6.Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance. Biofactors. 2009;35(1):21–27. doi: 10.1002/biof.3. [DOI] [PubMed] [Google Scholar]
- 7.Shelton J, Wang D, Gupta H, Wyss JM, Oparil S, White CR. The neointimal response to endovascular injury is increased in obese Zucker rats. Diabetes Obes Metab. 2003;5(6):415–423. doi: 10.1046/j.1463-1326.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 8.Ding H, Triggle CR. Endothelial dysfunction in diabetes: multiple targets for treatment. Pflugers Arch. 2010;459(6):977–994. doi: 10.1007/s00424-010-0807-3. [DOI] [PubMed] [Google Scholar]
- 9.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30(4):653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med. 2006;23(6):594–608. doi: 10.1111/j.1464-5491.2006.01773.x. [DOI] [PubMed] [Google Scholar]
- 11. Li Y, Lee PI. Controlled nitric oxide delivery platform based on S-nitrosothiol conjugated interpolymer complexes for diabetic wound healing. Mol Pharm. 2010;7(1):254–266. doi: 10.1021/mp900237f. •• provides evidence that localized delivery of NO via RSNO can be achieved to replete NO-signalingin diabetic wound healing.
- 12.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57(3):696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 13.Elrod JW, Duranski MR, Langston W, Greer JJ, Tao L, Dugas TR, Kevil CG, Champion HC, Lefer DJ. eNOS gene therapy exacerbates hepatic ischemia-reperfusion injury in diabetes: a role for eNOS uncoupling. Circ Res. 2006;99(1):78–85. doi: 10.1161/01.RES.0000231306.03510.77. [DOI] [PubMed] [Google Scholar]
- 14.Pereira EC, Ferderbar S, Bertolami MC, Faludi AA, Monte O, Xavier HT, Pereira TV, Abdalla DS. Biomarkers of oxidative stress and endothelial dysfunction in glucose intolerance and diabetes mellitus. Clin Biochem. 2008;41(18):1454–1460. doi: 10.1016/j.clinbiochem.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 15.Corbett JA, Sweetland MA, Wang JL, Lancaster JR, Jr, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A. 1993;90(5):1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDaniel ML, Corbett JA, Kwon G, Hill JR. A role for nitric oxide and other inflammatory mediators in cytokine-induced pancreatic beta-cell dysfunction and destruction. Adv Exp Med Biol. 1997;426:313–319. doi: 10.1007/978-1-4899-1819-2_41. [DOI] [PubMed] [Google Scholar]
- 17.Ahanchi SS, Varu VN, Tsihlis ND, Martinez J, Pearce CG, Kapadia MR, Jiang Q, Saavedra JE, Keefer LK, Hrabie JA, Kibbe MR. Heightened efficacy of nitric oxide-based therapies in type II diabetes mellitus and metabolic syndrome. Am J Physiol Heart Circ Physiol. 2008;295(6):H2388–H2398. doi: 10.1152/ajpheart.00185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konorev EA, Kalyanaraman B, Hogg N. Modification of creatine kinase by S-nitrosothiols: Snitrosation vs. S-thiolation. Free Radic Biol Med. 2000;28(11):1671–1678. doi: 10.1016/s0891-5849(00)00281-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Hogg N. S-Nitrosothiols: cellular formation and transport. Free Radic Biol Med. 2005;38(7):831–838. doi: 10.1016/j.freeradbiomed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol Med. 2003;9(4):160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 21.Sexton DJ, Muruganandam A, McKenney DJ, Mutus B. Visible light photochemical release of nitric oxide from S-nitrosoglutathione: potential photochemotherapeutic applications. Photochem Photobiol. 1994;59(4):463–467. doi: 10.1111/j.1751-1097.1994.tb05065.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Photosensitized decomposition of S-nitrosothiols and 2-methyl-2-nitrosopropane. Possible use for site-directed nitric oxide production. FEBS Lett. 1995;360(1):47–51. doi: 10.1016/0014-5793(95)00065-h. [DOI] [PubMed] [Google Scholar]
- 23.Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J Biol Chem. 1996;271(31):18596–18603. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- 24.Gorren AC, Schrammel A, Schmidt K, Mayer B. Decomposition of S-nitrosoglutathione in the presence of copper ions and glutathione. Arch Biochem Biophys. 1996;330(2):219–228. doi: 10.1006/abbi.1996.0247. [DOI] [PubMed] [Google Scholar]
- 25.Forrester MT, Foster MW, Stamler JS. Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J Biol Chem. 2007;282(19):13977–13983. doi: 10.1074/jbc.M609684200. [DOI] [PubMed] [Google Scholar]
- 26.Marley R, Feelisch M, Holt S, Moore K. A chemiluminescense-based assay for S-nitrosoalbumin and other plasma S-nitrosothiols. Free Radic Res. 2000;32(1):1–9. doi: 10.1080/10715760000300011. [DOI] [PubMed] [Google Scholar]
- 27.Cha W, Lee Y, Oh BK, Meyerhoff ME. Direct detection of S-nitrosothiols using planar amperometric nitric oxide sensor modified with polymeric films containing catalytic copper species. Anal Chem. 2005;77(11):3516–3524. doi: 10.1021/ac048192u. [DOI] [PubMed] [Google Scholar]
- 28.Singh RJ, Hogg N, Goss SP, Antholine WE, Kalyanaraman B. Mechanism of superoxide dismutase/H(2)O(2)-mediated nitric oxide release from S-nitrosoglutathione--role of glutamate. Arch Biochem Biophys. 1999;372(1):8–15. doi: 10.1006/abbi.1999.1447. [DOI] [PubMed] [Google Scholar]
- 29.Riccio DA, Dobmeier KP, Hetrick EM, Privett BJ, Paul HS, Schoenfisch MH. Nitric oxide-releasing S-nitrosothiol-modified xerogels. Biomaterials. 2009;30(27):4494–4502. doi: 10.1016/j.biomaterials.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bindoli A, Fukuto JM, Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal. 2008;10(9):1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper CE, Patel RP, Brookes PS, Darley-Usmar VM. Nanotransducers in cellular redox signaling: modification of thiols by reactive oxygen and nitrogen species. Trends Biochem Sci. 2002;27(10):489–492. doi: 10.1016/s0968-0004(02)02191-6. [DOI] [PubMed] [Google Scholar]
- 32.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45(1):1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landar A, Darley-Usmar VM. Nitric oxide and cell signaling: modulation of redox tone and protein modification. Amino Acids. 2003;25(3–4):313–321. doi: 10.1007/s00726-003-0019-7. [DOI] [PubMed] [Google Scholar]
- 34.Poole LB, Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol. 2008;12(1):18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38(12):1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 36. Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nature Reviews Molecular Cell Biology. 2009;10(10):721–732. doi: 10.1038/nrm2764. •• A comprehensive review summarising current concepts of denostrosylation as a means to regulate biological actions of S-nitrosothiols.
- 37.Akaike T. Mechanisms of biological S-nitrosation and its measurement. Free Radic Res. 2000;33(5):461–469. doi: 10.1080/10715760000301001. [DOI] [PubMed] [Google Scholar]
- 38.Inoue K, Akaike T, Miyamoto Y, Okamoto T, Sawa T, Otagiri M, Suzuki S, Yoshimura T, Maeda H. Nitrosothiol formation catalyzed by ceruloplasmin. Implication for cytoprotective mechanism in vivo. J Biol Chem. 1999;274(38):27069–27075. doi: 10.1074/jbc.274.38.27069. [DOI] [PubMed] [Google Scholar]
- 39.Basu S, Keszler A, Azarova NA, Nwanze N, Perlegas A, Shiva S, Broniowska KA, Hogg N, Kim-Shapiro DB. A novel role for cytochrome c: Efficient catalysis of S-nitrosothiol formation. Free Radic Biol Med. 2010;48(2):255–263. doi: 10.1016/j.freeradbiomed.2009.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosworth CA, Toledo JC, Jr, Zmijewski JW, Li Q, Lancaster JR., Jr Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc Natl Acad Sci U S A. 2009;106(12):4671–4676. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ckless K, van der Vliet A, Janssen-Heininger Y. Oxidative-nitrosative stress and post-translational protein modifications: implications to lung structure-function relations. Arginase modulates NF-kappaB activity via a nitric oxide-dependent mechanism. Am J Respir Cell Mol Biol. 2007;36(6):645–653. doi: 10.1165/rcmb.2006-0329SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crawford JH, Chacko BK, Pruitt HM, Piknova B, Hogg N, Patel RP. Transduction of NO-bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104(5):1375–1382. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]
- 43.Eiserich JP, Patel RP, O'Donnell VB. Pathophysiology of nitric oxide and related species: free radical reactions and modification of biomolecules. Mol Aspects Med. 1998;19(4–5):221–357. doi: 10.1016/s0098-2997(99)00002-3. [DOI] [PubMed] [Google Scholar]
- 44.Feelisch M, Rassaf T, Mnaimneh S, Singh N, Bryan NS, Jourd'Heuil D, Kelm M. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of NO in vivo. FASEB J. 2002;16(13):1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS, Steverding D. Nitrosative stress: protection by glutathione-dependent formaldehyde dehydrogenase. Redox Rep. 2001;6(4):209–210. doi: 10.1179/135100001101536337. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116(4):617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 47.Arnelle DR, Stamler JS. NO+, NO, NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch Biochem Biophys. 1995;318(2):279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 48.Liu Z, Rudd MA, Freedman JE, Loscalzo J. S-Transnitrosation reactions are involved in the metabolic fate and biological actions of nitric oxide. J Pharmacol Exp Ther. 1998;284(2):526–534. [PubMed] [Google Scholar]
- 49.Rossi R, Lusini L, Giannerini F, Giustarini D, Lungarella G, Di Simplicio P. A method to study kinetics of transnitrosation with nitrosoglutathione: reactions with hemoglobin and other thiols. Anal Biochem. 1997;254(2):215–220. doi: 10.1006/abio.1997.2424. [DOI] [PubMed] [Google Scholar]
- 50.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320(5879):1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410(6827):490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 52.Bateman RL, Rauh D, Tavshanjian B, Shokat KM. Human carbonyl reductase 1 is an S-nitrosoglutathione reductase. J Biol Chem. 2008;283(51):35756–35762. doi: 10.1074/jbc.M807125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanghani PC, Davis WI, Fears SL, Green SL, Zhai L, Tang Y, Martin E, Bryan NS, Sanghani SP. Kinetic and cellular characterization of novel inhibitors of S-nitrosoglutathione reductase. J Biol Chem. 2009;284(36):24354–24362. doi: 10.1074/jbc.M109.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choudhry S, Que LG, Yang Z, Liu L, Eng C, Kim SO, Kumar G, Thyne S, Chapela R, Rodriguez-Santana JR, Rodriguez-Cintron W, et al. GSNO reductase and beta2-adrenergic receptor gene-gene interaction: bronchodilator responsiveness to albuterol. Pharmacogenet Genomics. 2010;20(6):351–358. doi: 10.1097/FPC.0b013e328337f992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308(5728):1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, et al. Endogenous S-nitrosothiols protect against myocardial injury. Proc Natl Acad Sci U S A. 2009;106(15):6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei W, Li B, Hanes MA, Kakar S, Chen X, Liu L. S-nitrosylation from GSNOR deficiency impairs DNA repair and promotes hepatocarcinogenesis. Sci Transl Med. 2010;2(19):19ra13. doi: 10.1126/scitranslmed.3000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaneki M, Shimizu N, Yamada D, Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid Redox Signal. 2007;9(3):319–329. doi: 10.1089/ars.2006.1464. [DOI] [PubMed] [Google Scholar]
- 59.Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JB, de Oliveira MG, Velloso LA, Curi R, Saad MJ. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes. 2005;54(4):959–967. doi: 10.2337/diabetes.54.4.959. [DOI] [PubMed] [Google Scholar]
- 60.Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;280(9):7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 61.Carvalho-Filho MA, Ropelle ER, Pauli RJ, Cintra DE, Tsukumo DM, Silveira LR, Curi R, Carvalheira JB, Velloso LA, Saad MJ. Aspirin attenuates insulin resistance in muscle of diet-induced obese rats by inhibiting inducible nitric oxide synthase production and S-nitrosylation of IRbeta/IRS-1 and Akt. Diabetologia. 2009;52(11):2425–2434. doi: 10.1007/s00125-009-1498-1. [DOI] [PubMed] [Google Scholar]
- 62.Pauli JR, Ropelle ER, Cintra DE, Carvalho-Filho MA, Moraes JC, De Souza CT, Velloso LA, Carvalheira JB, Saad MJ. Acute physical exercise reverses S-nitrosation of the insulin receptor, insulin receptor substrate 1 and protein kinase B/Akt in diet-induced obese Wistar rats. J Physiol. 2008;586(2):659–671. doi: 10.1113/jphysiol.2007.142414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cordes CM, Bennett RG, Siford GL, Hamel FG. Nitric oxide inhibits insulin-degrading enzyme activity and function through S-nitrosylation. Biochem Pharmacol. 2009;77(6):1064–1073. doi: 10.1016/j.bcp.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 64.McGrowder D, Ragoobirsingh D, Brown P. Modulation of glucose uptake in adipose tissue by nitric oxide-generating compounds. J Biosci. 2006;31(3):347–354. doi: 10.1007/BF02704107. [DOI] [PubMed] [Google Scholar]
- 65.McGrowder D, Ragoobirsingh D, Brown P. Acute effects of exogenous nitric oxide on glucose uptake in skeletal muscle of normoglycaemic and diabetic rats. Med Sci Monit. 2006;12(1):BR28–BR35. [PubMed] [Google Scholar]
- 66.Ding SY, Tribble ND, Kraft CA, Markwardt M, Gloyn AL, Rizzo MA. Naturally occurring glucokinase mutations are associated with defects in posttranslational S-nitrosylation. Mol Endocrinol. 2010;24(1):171–177. doi: 10.1210/me.2009-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tong X, Ying J, Pimentel DR, Trucillo M, Adachi T, Cohen RA. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J Mol Cell Cardiol. 2008;44(2):361–369. doi: 10.1016/j.yjmcc.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wadham C, Parker A, Wang L, Xia P. High glucose attenuates protein S-nitrosylation in endothelial cells: role of oxidative stress. Diabetes. 2007;56(11):2715–2721. doi: 10.2337/db06-1294. [DOI] [PubMed] [Google Scholar]
- 69.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci U S A. 2006;103(22):8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1(5):290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 71.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115(5):1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH, Jr, Langston W, Teng X, et al. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci U S A. 2008;105(21):7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moya MP, Gow AJ, Califf RM, Goldberg RN, Stamler JS. Inhaled ethyl nitrite gas for persistent pulmonary hypertension of the newborn. Lancet. 2002;360(9327):141–143. doi: 10.1016/S0140-6736(02)09385-6. [DOI] [PubMed] [Google Scholar]
- 74.Chouchani E, Hurd T, Nadtochiy SM, Brookes PS, Fearnley IM, Lilley KS, Smith RA, Murphy MP. Identification of S-nitrosated mitochondrial proteins by S-nitrosothiol Difference In Gel Electrophoresis (SNO-DIGE): implications for the regulation of mitochondrial function by reversible S-nitrosation. Biochem J. 2010 doi: 10.1042/BJ20100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol. 2007;42(4):812–825. doi: 10.1016/j.yjmcc.2007.01.010. •• Provides data supporting that S-nitrosothiols can be targetted to mitochondria and transnitrosate specific protein thiols.
- 76.Nadtochiy SM, Burwell LS, Ingraham CA, Spencer CM, Friedman AE, Pinkert CA, Brookes PS. In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol. 2009;46(6):960–968. doi: 10.1016/j.yjmcc.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Prime TA, Blaikie FH, Evans C, Nadtochiy SM, James AM, Dahm CC, Vitturi DA, Patel RP, Hiley CR, Abakumova I, Requejo R, et al. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2009;106(26):10764–10769. doi: 10.1073/pnas.0903250106. •• Provides data supporting that S-nitrosothiols can be targetted to mitochondria and transnitrosate specific protein thiols.
- 78.Broniowska KA, Zhang Y, Hogg N. Requirement of transmembrane transport for S-nitrosocysteine-dependent modification of intracellular thiols. J Biol Chem. 2006;281(45):33835–33841. doi: 10.1074/jbc.M603248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hogg N, Broniowska KA, Novalija J, Kettenhofen NJ, Novalija E. Role of S-nitrosothiol transport in the cardioprotective effects of S-nitrosocysteine in rat hearts. Free Radic Biol Med. 2007;43(7):1086–1094. doi: 10.1016/j.freeradbiomed.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci U S A. 2004;101(21):7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Langford EJ, Brown AS, Wainwright RJ, de Belder AJ, Thomas MR, Smith RE, Radomski MW, Martin JF, Moncada S. Inhibition of platelet activity by S-nitrosoglutathione during coronary angioplasty. Lancet. 1994;344(8935):1458–1460. doi: 10.1016/s0140-6736(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 82.Zafar MU, Vilahur G, Choi BG, Ibanez B, Viles-Gonzalez JF, Salas E, Badimon JJ. A novel anti-ischemic nitric oxide donor (LA419) reduces thrombogenesis in healthy human subjects. J Thromb Haemost. 2007;5(6):1195–1200. doi: 10.1111/j.1538-7836.2007.02543.x. [DOI] [PubMed] [Google Scholar]
- 83. Bohl KS, West JL. Nitric oxide-generating polymers reduce platelet adhesion and smooth muscle cell proliferation. Biomaterials. 2000;21(22):2273–2278. doi: 10.1016/s0142-9612(00)00153-8. • provides evidence that localized delivery of NO via RSNO can be achieved to replete NO-signaling.
- 84. Masters KS, Lipke EA, Rice EE, Liel MS, Myler HA, Zygourakis C, Tulis DA, West JL. Nitric oxide-generating hydrogels inhibit neointima formation. J Biomater Sci Polym Ed. 2005;16(5):659–672. doi: 10.1163/1568562053783722. • provides evidence that localized delivery of NO via RSNO can be achieved to replete NO-signaling.
- 85. Seabra AB, da Silva R, de Oliveira MG. Polynitrosated polyesters: preparation, characterization, and potential use for topical nitric oxide release. Biomacromolecules. 2005;6(5):2512–2520. doi: 10.1021/bm050216z. • provides evidence that localized delivery of NO via RSNO can be achieved to replete NO-signaling.
- 86.Lipke EA, West JL. Localized delivery of nitric oxide from hydrogels inhibits neointima formation in a rat carotid balloon injury model. Acta Biomater. 2005;1(6):597–606. doi: 10.1016/j.actbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 87.Amadeu TP, Seabra AB, de Oliveira MG, Costa AM. S-nitrosoglutathione-containing hydrogel accelerates rat cutaneous wound repair. J Eur Acad Dermatol Venereol. 2007;21(5):629–637. doi: 10.1111/j.1468-3083.2006.02032.x. [DOI] [PubMed] [Google Scholar]
- 88.Seabra AB, da Silva R, de Souza GF, de Oliveira MG. Antithrombogenic polynitrosated polyester/poly(methyl methacrylate) blend for the coating of blood-contacting surfaces. Artif Organs. 2008;32(4):262–267. doi: 10.1111/j.1525-1594.2008.00540.x. [DOI] [PubMed] [Google Scholar]