Abstract
Background
Irregular, sporadic episodes of ischemic brain injury are known to occur in sickle cell anemia (SCA), resulting in overt stroke and silent cerebral infarction. Ongoing ischemia in other organs is common in SCA but has never been documented in the brain.
Objective
To test the hypothesis that acute silent cerebral ischemic events (ASCIEs) are frequent and potentially transient.
Design
Cross-sectional and cohort study of children with SCA screened by magnetic resonance imaging (MRI) of the brain for a randomized clinical trial.
Setting
Clinical trial setting in tertiary care centers.
Patients
Asymptomatic children with SCA without known stroke, neurologic injury, or epilepsy not receiving treatment with transfusions or hydroxyurea.
Main Outcome Measure
Incidence of ASCIEs calculated using single diffusion-weighted MRI scans (acute ischemic events that occurred within 10 days of the MRI).
Results
Acute silent cerebral ischemic events were detected on 1.3% of MRIs (10 of 771) in 652 children (mean age, 10.0 years), with an incidence of 47.3 events per 100 patient-years (95% CI, 22.7–87.2). Two of 10 children with ASCIEs had follow-up MRIs of the brain; only 1 had silent cerebral infarction in the same location as the previously detected ASCIE.
Conclusions
Children with SCA experience ongoing (chronic, intermittent) cerebral ischemia, sometimes reversible, far more frequently than previously recognized. The brain in SCA is at constant threat of ischemia.
Sickle cell anemia (SCA) is a chronic, debilitating disease characterized by recurring episodes of ischemiareperfusion injury and is the most common cause of overt stroke in children. Primary prevention of strokes, using transcranial Doppler ultrasonography screening to direct the initiation of chronic transfusion therapy, has greatly decreased the frequency of overt stroke,1,2 but silent cerebral infarction (SCI) still occurs, often despite transfusion, and is more common than overt stroke.1–3 By definition, SCI produces no motor or sensory deficits, but it is associated with neuro-cognitive impairment, poor school performance, neurologic soft signs, and increased risk for subsequent overt stroke.4–7
The results of critical cerebral ischemia—stroke and SCI—can readily be detected and quantified by MRI of the brain. However, the combined frequency of these discrete, permanent brain lesions might underestimate the frequency of all possible ischemic insults to the brain in SCA. Some ischemic events could be transient, reversible, and leave no permanent tissue damage or produce a lesion that is smaller than the limits of detection by magnetic resonance imaging (MRI). Frequent, recurrent, and potentially reversible ischemia occurs in many organ systems in SCA, and we postulated that the brain was not an exception. Evidence to support this postulate was provided by 2 recent reports of acute silent cerebral ischemic events (ASCIEs) that were detected during the ischemic phase by diffusion-weighted MRI in children who were acutely ill with exacerbation of chronic anemia.1,8 Some ASCIEs were not associated with detectable lesions on follow-up imaging.8
We postulated that ASCIEs would occur more frequently than SCI, and that ASCIEs could be detected in the asymptomatic baseline state, consistent with the contention that the brain is at continual risk for ischemia. The incidence of SCI has only been reported from the Cooperative Study of Sickle Cell Disease. The incidence (the number of new SCI events in a designated period) of first and progressive SCI was 1.01 per 100 patient-years (95% CI, 0.4–2.7) and 7.06 per 100 patient-years (95% CI, 4.2–11.8), respectively.9 The incidence rate of ASCIEs is unknown, and whether all ASCIEs evolve into SCI lesions that are detectable on MRI performed beyond the acute or subacute phase of injury is also unknown.
To explore the hypothesis that ASCIEs were frequent (and potentially transient), we measured and compared the incidence of ASCIEs and progressive SCI in the group of children screened for SCI in the Silent Cerebral Infarct Transfusion (SIT) Trial. We also investigated the clinical circumstances antecedent to ASCIEs to help understand their causes.
METHODS
PARTICIPANTS AND SITES
The primary aim of the SIT Trial (clinicaltrials.gov Identifier: NCT00072761) is to determine whether chronic red blood cell transfusions prevent new or enlarging SCI. The trial design has been reported elsewhere.10,11 Briefly, individuals with sickle cell anemia (SCA; homozygous SCA) or sickle-β0-thalassemia (both genotypes herein referred to as SCA) between 5 and 14 years of age without a history of overt cerebral infarction were enrolled beginning in 2004. Subjects were screened for SCI by MRI of the brain and neurologic examination. Those who had SCI were eligible to be randomly allocated to observation or chronic transfusions for 3 years. A second MRI was obtained within 6 months of the screening MRI and immediately before randomization. The presence of new or enlarging SCI lesions was a disqualification for randomization. Study sites comprised tertiary care centers specializing in the treatment of children with SCA in the United States, England, and France. Participants were recruited for participation at the site of their medical care. Informed consent and assent were obtained in accordance with the requirements and guidelines of the human subjects committees at the participating sites.
DEFINITIONS AND CALCULATIONS
The definition of SCI has 2 components: (1) an infarct-like lesion on MRI and (2) a normal neurologic examination or an abnormality on neurologic examination that cannot be explained by the location of the infarct-like lesion.10,11 An infarct-like lesion was defined as an MRI signal abnormality (increased signal), at least 3 mm in 1 dimension, that was visible on at least 2 views of T2–fluid-attenuated inversion recovery images of the brain. The presence of infarct-like lesions was adjudicated by 3 study neuroradiologists. The infarct-like lesion was independently determined to be clinically silent by the neurology committee, based on a review of each patient's standardized neurologic examination performed at the clinical site by a pediatric neurologist. Similarly, we defined an ASCIE to be an area of restricted diffusion on diffusion-weighted imaging (DWI) sequences with a corresponding decrease in signal intensity on the apparent diffusivity coefficient map in the absence of focal neurologic findings that could be explained by the location of the DWI-positive lesion.
The incidence of ASCIEs was calculated on the subset of SIT Trial participants who had screening or prerandomization MRIs that included optional DWI sequences. Individual study sites made a practical, not patient-specific, decision whether to perform DWI sequences for all site participants. All DWI sequences were reviewed for quality; nondiagnostic scans were excluded. Because DWI signal abnormalities occur within 24 hours of the onset of cerebral ischemia and persist for approximately 10 days,12–14 all DWI-positive lesions were considered new (incident) and each MRI was considered to provide 10 patient-days of observation. As a secondary analysis, we also considered the effects of using 7 or 14 patient-days of observation for each MRI scan with DWI sequences.
The incidence of progressive SCI was calculated in the subset of SIT Trial participants who were adjudicated to have SCI on a screening MRI at study entry and also had a second, prerandomization MRI as required by the study protocol. Progressive (incident) SCI was defined as new or enlarging (increased in size) SCI that did not show evidence of acute ischemia on DWI on the prerandomization MRI compared with the screening MRI. The interval between the screening and prerandomization MRI was the period for the calculation of the incidence rate. No case of ASCIE was also designated as progressive SCI.
The clinical management of cases of ASCIEs, including the plan for follow-up imaging, was not specified in the study protocol and left to the discretion of the site clinical team because these incidental events were not within the scope of the study. Therefore, we used a formal medical record review tool to ascertain the clinical circumstances surrounding each case of ASCIEs. Based on a prior study,15 the elements of this retrospective tool included documentation of fever, acute chest syndrome, acute anemia, or transfusion therapy in the 2 weeks preceding the DWI-positive MRI. The tool also recorded the highest documented blood pressure in the 3 days preceding the MRI because acute stroke can be associated with an abrupt change in blood pressure. The site neurologist examined each case and recorded the presence or absence of aphasia, visual field deficits, motor or coordination deficits, sensory deficits, or other neurologic findings. We also obtained any available, clinically indicated follow-up MRI scans for patients with ASCIEs.
We generated summary statistics for variables of interest and calculated 95% confidence intervals for incidence rates. All incidence rates were expressed as the number of events per 100 patient-years of observation. This was a secondary analysis of SIT Trial data. We did not perform sample size calculations for this analysis because the SIT Trial was designed for a different primary aim, and we simply used all available data for this analysis. We considered α<0.05 to be statistically significant and did not correct for multiple comparisons. Data were analyzed using PASW Statistics version 18 for Mac (SPSS Inc) and MedCalc for Windows version 11.3.3.0 (MedCalc Software).
RESULTS
PATIENTS AND SCANS
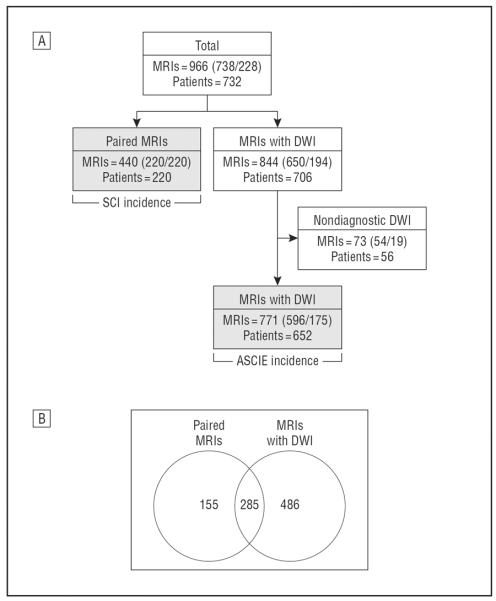
In total, we studied 966 MRIs from 732 children with a mean (SD) age of 9.5 (2.4) years, who were 51% male and 93% with homozygous SCA (Figure 1). The incidence of ASCIEs was determined using 771 MRIs from 652 children. The incidence of SCI was determined in 220 children with a mean interval between scans of 124 days (range, 14–645 days), providing a total of 74.9 patient-years of observation. The clinical characteristics of participants are shown in Table 1.
Figure 1.
Diagrams of magnetic resonance images (MRIs) and patients. A, Diagram shows how the final 2 MRI groups, the acute silent cerebral ischemic events (ASCIEs) incidence population and the silent cerebral infarction (SCI) incidence population (filled gray rectangles), were assembled to calculate the incidence rates. The 440 paired MRIs (220 patients) are the subjects who agreed to further screening after the initial MRI and possible randomization (not the entire screening population). A few patients may have had more than 1 screening or prerandomization MRI to qualify. The numbers in each box indicate the total number of MRIs considered and how many were screening or prerandomization MRIs (total [screening/prerandomization]). B, Diagram shows the degree of overlap between these 2 MRI groups, the ASCIE incidence population and the SCI incidence population, because some patients’ MRIs were used for both determinations. The number of MRIs is shown in the Venn diagram (some patients had more than 1 MRI). DWI indicates diffusion-weighted imaging.
Table 1.
Clinical Characteristics of the Study Populations
| Characteristic | ASCIE Incidence Population | SCI Incidence Population |
|---|---|---|
| Age, mean (SD), y | 10.0 (2.5) | 9.5 (2.4) |
| Sex, % | ||
| Male | 51 | 53 |
| Female | 49 | 47 |
| Genotype: HbSS (HbSβ0), % | 92 (8) | 94 (6) |
| Hemoglobin, mean (SD), g/dLa | 8.1 (1.1) | 8.1 (1.1) |
| Pain events count, median (IQR)b | 1 (0–3) | 1 (0–3) |
| ACS events count, median (IQR)c | 0 (0–1) | 0 (0–1) |
| SCI present, % | 44.3 | 100 |
| TCD, % | ||
| Normal | 84 | 89 |
| Conditional | 13 | 11 |
| Abnormal | 4 | 0 |
Abbreviations: ACS, acute chest syndrome; ASCIE, acute silent cerebral ischemic event; HbSS, homozygous sickle cell anemia; HbSβ0, sickle-β0-thalassemia; IQR, interquartile range; SCI, silent cerebral infarction; TCD, transcranial Doppler ultrasonography.
Well or steady state hemoglobin concentration. To convert hemoglobin to grams per liter, multiply by 10.0.
Hospitalizations for pain events in the preceding 3 years.
Hospitalizations for ACS in the preceding 3 years.
INCIDENCE OF ASCIEs
Eleven patients had evidence of acute ischemia (DWI-positive lesions) during scheduled, study-related MRIs not obtained for clinical indications. One patient was ascertained to have a clinically overt stroke and was excluded from this analysis. The remaining 10 events were ASCIEs (8 on screening MRIs and 2 on prerandomization MRIs). The 771 MRIs with DWI sequences provided 21.1 patient-years of observation. The estimated incidence of ASCIEs was 47.3 events per 100 patient-years (95% CI, 22.7–87.2), based on all MRIs with DWI sequences (Table 2). The incidence of ASCIEs was not different between the screening and prerandomization MRI groups if considered separately (P = .82; Table 2). Finally, if we assume that each scan with DWI sequences provided 7 or 14 patient-days of observation (instead of 10), then the estimated incidence of ASCIEs would be 67.6 events per 100 patient-years (95% CI, 32.4–124.3) or 33.8 events per 100 patient-years (95% CI, 16.2–62.1) of observation, respectively.
Table 2.
Incidence Rates of SCI and ASCIE
| Event | MRI Type | Patients, No. | Observation Time, Patient-Years |
Incident Events, No. | Incidence, Events/100 Patient-Years |
p Valuea |
|---|---|---|---|---|---|---|
| SCI | Screening and prerandomization |
220 | 74.9 | 8 | 10.7 | |
| ASCIE | Screening and prerandomizationb |
652 | 21.1 | 10 | 47.3 | <.001 |
| Screening | 594 | 16.3 | 8 | 49.0 | <.001 | |
| Prerandomization | 170 | 4.8 | 2 | 41.7 | .06 |
Abbreviations: ASCIE, acute silent cerebral ischemic event; MRI, magnetic resonance imaging; SCI, silent cerebral infarction.
P value for comparison of incidence rates of ASCIE to SCI.
Patients may have had screening MRIs only or both screening and prerandomization MRIs, so the total number of patients (screening and prerandomization) is smaller than the sum of the screening and prerandomization groups.
INCIDENCE OF PROGRESSIVE SCI
Incident (new or enlarged) SCI occurred in 8 participants on the prerandomization MRI. Incident SCI was a new lesion in 5 patients, an enlarged lesion in 2, and both new and enlarged lesions in 1. The incidence of new or enlarging SCI was 10.7 events per 100 patient-years (95% CI, 4.6–21.0) (Table 2). The incidence of ASCIEs was statistically significantly higher than the incidence of progressive SCI (P = .001; Table 2).
MEDICAL EVENTS RELATED TO ASCIEs
Table 3 lists the known antecedent and concurrent medical events in the preceding 2 weeks collected using the medical history tool for each case of ASCIEs, which were all incidentally discovered. Nine of 10 cases of ASCIEs had no apparent medical illnesses in the 2 weeks before MRI. The remaining case occurred in a participant recovering from a recent episode of acute chest syndrome (onset 5 days before MRI) complicated by severe anemia and hypertension and treated with a simple transfusion. Cerebral ischemia or stroke was not suspected clinically in this case, and the study-related MRI was obtained on the day of discharge from the hospital. Two cases reported occasional headaches that were not clearly associated with the ASCIEs. Standard neurologic examination was normal for all cases, indicating that the acute cerebral ischemia was clinically covert or silent.
Table 3.
Medical Events Associated With ASCIE
| Case No./Sex/Age, y | MRI Type | Highest BP, mm Hg | ACSa | Acute Anemiaa | Transfusiona | Fevera | Standard Neurologic Examination |
Comments |
|---|---|---|---|---|---|---|---|---|
| 1/F/6.6 | Screening | 115/61 | − | − | − | − | Normal | |
| 2/F/10 | Screening | 104/77 | − | − | − | − | Normal | |
| 3/M/7.9 | Screening | 94/53 | − | − | − | − | Normal | |
| 4/F/6.4 | Screening | NA | − | − | − | − | Normal | |
| 5/F/7.9 | Screening | 144/77 | + | + | + | + | Normal | Hospitalized for ACS |
| 6/F/6.3 | Screening | 105/57 | − | − | − | − | Normal | |
| 7/M/11.7 | Screening | 116/61 | − | − | − | − | Normal | Headache in preceding week |
| 8/M/10.8 | Screening | 96/58 | − | − | − | − | Normal | |
| 9/M/10.2 | Prerandomization | NA | − | − | − | − | Normal | − |
| 10/F/12 | Prerandomization | NA | − | − | − | − | Normal | Occasional headaches |
Abbreviations: ACS, acute chest syndrome; ASCIE, acute silent cerebral ischemic event; BP, blood pressure (highest recorded in 3 days preceding MRI); F, female; M, male; MRI, magnetic resonance imaging; NA, not available.
+ indicates present or performed; −, not present or not performed.
FOLLOW-UP IMAGING
At the discretion of the site investigators, 2 of the 10 participants with ASCIEs had follow-up MRIs of the brain. Case 6 had a follow-up MRI 4 months after the ASCIE that showed SCI in the same location in the deep white matter as the previously detected acute ischemia (Figure 2). Case 7 had a follow-up MRI 10 months later that showed no residual lesion in the same location in the basal ganglia, although the lesion had been detected on T2–fluid-attenuated inversion recovery images at the time of the initial DWI (Figure 3). Thus, not all ASCIEs produce detectable SCI on follow-up imaging. During study monitoring visits to all sites, we also attempted to obtain follow-up MRIs for the other cases of ASCIEs, but no additional images were available for analysis (such MRIs were not mandated as part of the SIT Trial study protocol).
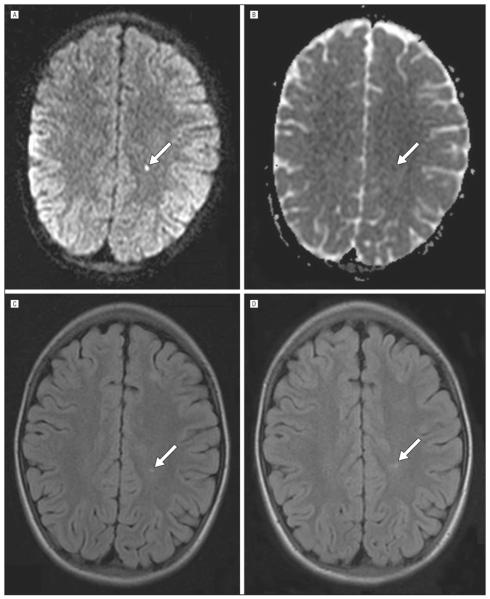
Figure 2.
Progression of an acute silent cerebral ischemic event to silent cerebral infarction. Initial screening magnetic resonance imaging (MRI) (A–C). A, A focus of restricted diffusion on diffusion-weighted imaging, with a corresponding region of decreased signal on the apparent diffusivity coefficient maps shown in panel B. These findings indicate that the cerebral ischemia occurred within the 10 days preceding the magnetic resonance imaging (MRI). The same lesion is shown on the T2–fluid-attenuated inversion recovery sequence in panel C. D, The lesion on a second MRI 4 months later on T2–fluid-attenuated inversion recovery sequence meets the study definition of silent cerebral infarction. The lesion is indicated by an arrow in all panels.
Figure 3.
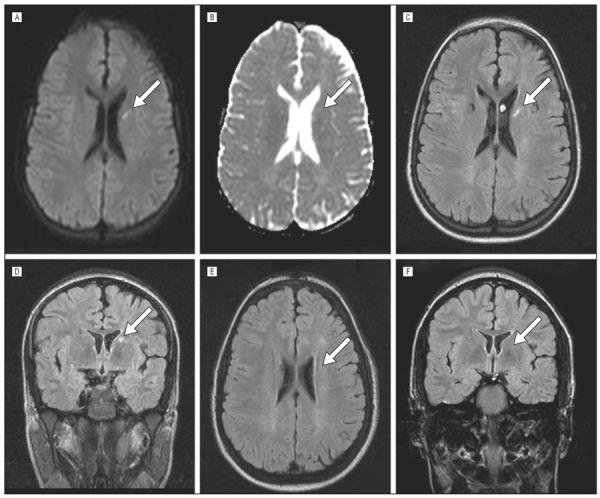
Radiographic resolution of an acute silent cerebral ischemic event. Initial screening magnetic resonance images (A–D). A, A focus of restricted diffusion on diffusion-weighted imaging, with a corresponding region of decreased signal on the apparent diffusivity coefficient maps shown in panel B. These findings indicate that the cerebral ischemia occurred within the 10 days preceding the magnetic resonance image. The same lesion is shown on the T2–fluid-attenuated inversion recovery sequences in the axial (C) and coronal (D) planes. The lack of any detectable lesion on a second magnetic resonance image 10 months later on T2–fluid-attenuated inversion recovery sequences in either the axial or coronal planes is shown (E and F). The region of interest is indicated by an arrow in all panels.
COMMENT
In this largest prospective study to date in SCA, to our knowledge, we have provided evidence that clinically silent cerebral ischemia can be detected acutely, and that ASCIEs occur in asymptomatic children without concurrent or antecedent illness. Only 1 case of an ASCIE was documented in association with an acute illness (following acute chest syndrome). Moreover, ASCIEs appear to be 4 times more frequent than progressive SCI. Along with limited follow-up imaging data, this suggests, but does not confirm, that a fraction of ASCIEs may be transient lesions. We provide evidence that some ASCIEs are either radiographically reversible, as has been reported in transient ischemic attacks in the general population,16 or produce permanent infarction that is smaller than the limits of detection by MRI of the brain.
Two previous single-center studies provided the proof of principle that acute ischemic lesions occur in children with SCA who have nonfocal neurologic examinations. Dowling et al8 published a case series of children with SCA who had clinically silent acute cerebral ischemia detected by MRI with DWI sequences. These events were termed acute SCI because 4 of 7 cases had SCI on follow-up MRI that corresponded to the location of the previously detected DWI signal abnormality. Enninful-Eghan et al1 also reported 5 cases of acute SCI in children with SCA. In both studies, acute SCI was found mainly in the context of acute anemic or febrile illnesses. We prefer the designation ASCIE to acute SCI because it appears, based on our data and that of Dowling et al,8 that not all ASCIEs progress to radiographically detectable, permanent infarction that would later be classified as SCI. To our knowledge, we are the first to calculate the incidence of ASCIEs. Moreover, we show that ASCIEs occur in asymptomatic children without antecedent or concurrent acute medical illnesses.
We also find that SCI is a frequently progressive lesion in children with SCA. The incidence of progressive SCI, both new and enlarging lesions, is 10.7 events per 100 patient-years in a population of children with a mean age of 9.5 years (range, 5–15 years). The incidence of progressive SCI in 229 children aged 6 to 19 years in the Cooperative Study of Sickle Cell Disease was 7.1 per 100 patient-years (95% CI, 4.2 –11.8).9 Our similar findings (10.7 per 100 patient-years; 95% CI, 4.6–21.0, vs 7.1 per 100 patient-years; 95% CI, 4.2–11.8; P = .35) provide strong evidence that the true incidence of progressive SCI is near these estimates. To our knowledge, there are no other reports of the incidence of SCI in SCA for comparison.
In summary, we found evidence to support our hypothesis that ongoing (chronic, intermittent) ischemia occurs in the brain, just as it occurs in other organ systems in SCA. The clinical implications of our findings are that children who have SCA with no history of overt stroke experience spontaneous cerebral ischemic events far more frequently than previously recognized, and that most of this cerebral ischemia is clinically silent. We postulate that small foci of acute cerebral ischemia (ASCIEs), potentially leading to infarction of brain tissue, may occur repeatedly in asymptomatic children with SCA, but the affected tissue is potentially salvageable. As such, the ASCIE may represent a lesion analogous to the concept of the ischemic penumbra in other forms of acute ischemic stroke,17,18 except that the entire ASCIE lesion can be considered the penumbra. Like other ischemic processes, reperfusion of ASCIE lesions may bring oxidative and inflammatory injury.19 We propose that ASCIEs are a paradigm of ischemia-reperfusion injury, occurring in the brain just as it is thought to occur in other tissues and organs in SCA.20–22 Additionally, supporting this concept is the preliminary observation that the plasma concentration of glial fibrillary acidic protein, a known plasma biomarker of acute stroke and brain trauma, is increased in patients with SCA compared with individuals without SCA.23 The optimal management of patients with ASCIEs has not been established. Children with SCA and ASCIEs will need to be studied systematically to determine what treatment, if any, is warranted.
This study has several limitations. First, we did not study a random sample of children with SCA. The prevalence of SCI in the ASCIE incidence population was more than 40% compared with 20% to 37% in prior studies of SCI.2,9,24,25 Therefore, we may have studied a population enriched with children at risk for SCI and ASCIEs; however, our estimate of prevalence approximates that of Bernaudin et al (37% by 14 years of age).2 Nevertheless, this was a generally healthy population of children with SCA, without known stroke, neurologic injury, or epilepsy, and not receiving treatment with transfusion or hydroxyurea for severe SCA. Also, our estimate of the incidence of progressive SCI agrees with Pegelow et al.9 There are no reports of the incidence of ASCIEs for comparison, but the ratio of ASCIEs to SCI indicates that ASCIEs occur more frequently (4-fold) than SCI. Second, because of the SIT Trial design, we did not have follow-up MRIs on most cases of ASCIEs, thus we cannot confirm how many cases of ASCIEs progressed to SCI. Nevertheless, the disparate incidence rates (ASCIEs much higher than SCI), our limited follow-up imaging data, and the follow-up imaging data from Dowling et al8 together suggest that ASCIEs occur more frequently than SCI. Third, antecedent and concurrent medical events associated with ASCIEs were captured using a retrospective tool, thus mis-classification of silent status was possible. Nevertheless, all ASCIEs were discovered incidentally and only 1 case had new issues prompting medical attention. Fourth, because DWI and apparent diffusivity coefficient signal abnormalities can persist for 7 to 14 days after the onset of acute cerebral ischemia, choosing different time intervals will affect the calculated incidence rate of ASCIEs. For example, if we consider that diffusion-weighted scans provide only 7 patient-days of observation, the calculated incidence of ASCIEs would be approximately 7 times higher than SCI. We chose to be conservative in this analysis by using 10 days, which available evidence suggests is the reasonable upper limit.12–14 Fifth, we calculated the incidence of ASCIEs using a novel method that uses the temporal information contained within the DWI signal of a single MRI, rather than the classic method of using paired MRIs. Each DWI scan provides 10 continuous days of observation for acute ischemia; thus, it is a valid method for construction of an incidence. Finally, the different MRI methods used to calculate the incidence of ASCIEs and SCI may have different sensitivities for the lesion in question, thus this might limit precise comparisons of rates.
We conclude that asymptomatic children with SCA experience cerebral ischemia far more frequently than previously recognized. By considering only discrete, easily visualized, and permanent brain lesions (stroke and SCI), one underestimates the true frequency of all ischemic insults to the brain in SCA. We propose that the brain in SCA is at constant threat of ischemic injury. Further research is needed to better understand the causes, consequences, and treatment options of ASCIEs in SCA.
Acknowledgments
Funding/Support: This study was supported by grant U01-NS42804 from the National Institutes of Health.
Footnotes
Author Contributions: Study concept and design: Quinn, McKinstry, Casella, Roach, Hirtz, and DeBaun. Acquisition of data: Quinn, McKinstry, Dowling, Ball, Casella, Dlamini, Ichord, Jordan, Kirkham, Noetzel, Roach, Strouse, Kwiatkowski, and DeBaun. Analysis and interpretation of data: Quinn, McKinstry, Dowling, Ball, Kraut, Casella, Jordan, Kirkham, Strouse, Kwiatkowski, Hirtz, and DeBaun. Drafting of the manuscript: Quinn, Ball, Casella, and DeBaun. Critical revision of the manuscript for important intellectual content: Quinn, McKinstry, Dowling, Ball, Kraut, Casella, Dlamini, Ichord, Jordan, Kirkham, Noetzel, Roach, Strouse, Kwiatkowski, Hirtz, and De-Baun. Statistical analysis: Quinn. Obtained funding: McKinstry, Casella, Noetzel, and DeBaun. Administrative, technical, and material support: McKinstry, Dowling, Ball, Casella, Roach, Kwiatkowski, Hirtz, and De-Baun. Study supervision: McKinstry, Kirkham, Noetzel, Kwiatkowski, and DeBaun.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank the following collaborators: Michele Afif, MD, Central Middlesex Hospital, London, England; Gladstone Airewele, MD, Baylor College of Medicine, Houston, Texas; Brian Berman, MD, Case Western Reserve University, Cleveland, Ohio; Fran-çoise Bernaudin, Hôpital Intercommunal de Créteil, Créteil, France; Ana Burgos, MD, Children's National Medical Center, Washington, DC; Charles Daeschner, MD, The Brody School of Medicine, Greenville, North Carolina; Mark Heiny, MD, Indiana University–Purdue University Indianapolis; Thomas Howard, MD, University of Alabama at Birmingham, Birmingham; Baba Inusa, MD, Guy's and St. Thomas' Hospital, London, England; Rathi V. Iyer, MD, University of Mississippi Medical Center, Jackson; Karen Kalinyak, MD, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio; Allison King, MD, MPH, Washington University School of Medicine, St. Louis, Missouri; Melanie Kirby-Allen, MD, The Hospital for Sick Children, Toronto, Ontario; Fenella Kirkham, MD, University College London Institute of Child Health, London, England; Julie Panepinto, MD, MPH, Medical College of Wisconsin, Milwaukee; Mark Ranalli, MD, The Ohio State University, Columbus; Rupa Redding-Lallinger, MD, University of North Carolina at Chapel Hill; Hernan Sabio, MD, Wake Forest School of Medicine, Winston-Salem, North Carolina; Suzanne Saccente, MD, University of Arkansas, Little Rock; Ingrid Sarnaik, MD, Wayne State University, Detroit, Michigan; Paul Telfer, MD, The Royal London Hospital, London, England; Alexis Thompson, MD, Northwestern University, Chicago, Illinois; and Gerald Woods, MD, University of Missouri–Kansas City.
REFERENCES
- 1.Enninful-Eghan H, Moore RH, Ichord R, Smith-Whitley K, Kwiatkowski JL. Transcranial Doppler ultrasonography and prophylactic transfusion program is effective in preventing overt stroke in children with sickle cell disease. J Pediatr. 2010;157(3):479–484. doi: 10.1016/j.jpeds.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernaudin F, Verlhac S, Arnaud C, et al. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood. 2011;117(4):1130–1140. doi: 10.1182/blood-2010-06-293514. quiz 1436. [DOI] [PubMed] [Google Scholar]
- 3.Hulbert ML, McKinstry RC, Lacey JL, et al. Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood. 2011;117(3):772–779. doi: 10.1182/blood-2010-01-261123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong FD, Thompson RJ, Jr, Wang W, et al. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Pediatrics. 1996;97(6, pt 1):864–870. [PubMed] [Google Scholar]
- 5.Schatz J, Brown RT, Pascual JM, Hsu L, DeBaun MR. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56(8):1109–1111. doi: 10.1212/wnl.56.8.1109. [DOI] [PubMed] [Google Scholar]
- 6.Mercuri E, Faundez JC, Roberts I, et al. Neurological ‘soft’ signs may identify children with sickle cell disease who are at risk for stroke. Eur J Pediatr. 1995;154(2):150–156. [PubMed] [Google Scholar]
- 7.Miller ST, Macklin EA, Pegelow CH, et al. Cooperative Study of Sickle Cell Disease. Silent infarction as a risk factor for overt stroke in children with sickle cell anemia: a report from the Cooperative Study of Sickle Cell Disease. J Pediatr. 2001;139(3):385–390. doi: 10.1067/mpd.2001.117580. [DOI] [PubMed] [Google Scholar]
- 8.Dowling MM, Quinn CT, Rogers ZR, Buchanan GR. Acute silent cerebral infarction in children with sickle cell anemia. Pediatr Blood Cancer. 2010;54(3):461–464. doi: 10.1002/pbc.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pegelow CH, Macklin EA, Moser FG, et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood. 2002;99(8):3014–3018. doi: 10.1182/blood.v99.8.3014. [DOI] [PubMed] [Google Scholar]
- 10.Casella JF, King AA, Barton B, et al. Design of the Silent Cerebral Infarct Transfusion (SIT) trial. Pediatr Hematol Oncol. 2010;27(2):69–89. doi: 10.3109/08880010903360367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vendt BA, McKinstry RC, Ball WS, et al. Silent Cerebral Infarct Transfusion (SIT) trial imaging core: application of novel imaging information technology for rapid and central review of MRI of the brain. J Digit Imaging. 2009;22(3):326–343. doi: 10.1007/s10278-008-9114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Provenzale JM, Sorensen AG. Diffusion-weighted MR imaging in acute stroke: theoretic considerations and clinical applications. AJR Am J Roentgenol. 1999;173(6):1459–1467. doi: 10.2214/ajr.173.6.10584783. [DOI] [PubMed] [Google Scholar]
- 13.Muir KW, Buchan A, von Kummer R, Rother J, Baron JC. Imaging of acute stroke. Lancet Neurol. 2006;5(9):755–768. doi: 10.1016/S1474-4422(06)70545-2. [DOI] [PubMed] [Google Scholar]
- 14.Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology. 1997;49(1):113–119. doi: 10.1212/wnl.49.1.113. [DOI] [PubMed] [Google Scholar]
- 15.Scothorn DJ, Price C, Schwartz D, et al. Risk of recurrent stroke in children with sickle cell disease receiving blood transfusion therapy for at least five years after initial stroke. J Pediatr. 2002;140(3):348–354. doi: 10.1067/mpd.2002.122498. [DOI] [PubMed] [Google Scholar]
- 16.Lecouvet FE, Duprez TP, Raymackers JM, Peeters A, Cosnard G. Resolution of early diffusion-weighted and FLAIR MRI abnormalities in a patient with TIA. Neurology. 1999;52(5):1085–1087. doi: 10.1212/wnl.52.5.1085. [DOI] [PubMed] [Google Scholar]
- 17.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia: the ischemic penumbra. Stroke. 1981;12(6):723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 18.Paciaroni M, Caso V, Agnelli G. The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur Neurol. 2009;61(6):321–330. doi: 10.1159/000210544. [DOI] [PubMed] [Google Scholar]
- 19.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190(3):255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Conran N, Franco-Penteado CF, Costa FF. Newer aspects of the pathophysiology of sickle cell disease vaso-occlusion. Hemoglobin. 2009;33(1):1–16. doi: 10.1080/03630260802625709. [DOI] [PubMed] [Google Scholar]
- 21.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest. 2000;106(3):411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96(1):314–320. [PubMed] [Google Scholar]
- 23.Savage WJ, Barron-Casella E, Fu Z, et al. Plasma glial fibrillary acidic protein levels in children with sickle cell disease. Am J Hematol. 2011;86(5):427–429. doi: 10.1002/ajh.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwiatkowski JL, Zimmerman RA, Pollock AN, et al. Silent infarcts in young children with sickle cell disease. Br J Haematol. 2009;146(3):300–305. doi: 10.1111/j.1365-2141.2009.07753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steen RG, Emudianughe T, Hankins GM, et al. Brain imaging findings in pediatric patients with sickle cell disease. Radiology. 2003;228(1):216–225. doi: 10.1148/radiol.2281020943. [DOI] [PubMed] [Google Scholar]