Summary
Microenvironmental factors play a critical role in B-cell lymphomas. Most studies emphasize the role of lymphoma-infiltrating T-cells and macrophages, with few studies on natural killer cells. Natural killer cells include a less mature (CD56bright/CD16−) subset that is more common in lymph nodes and a more mature (CD56dim/CD16+) subset that is more numerous in peripheral blood. Therefore, the proportions of natural killer cells, natural killer subsets, and natural killer–like T-cells (CD3+, CD56+, and/or CD16/57+) were determined by flow cytometry in 150 cases of tissue-based B-cell non-Hodgkin lymphoma and 89 nonneoplastic tissue biopsies. Results were correlated with the clinicopathologic findings. A higher percentage of natural killer cells was found in nonneoplastic spleen versus other nonneoplastic tissue (P <.001), in splenic-based B-cell non-Hodgkin lymphoma versus other B-cell non-Hodgkin lymphoma (P < .01), and in stage II to IV diffuse large B-cell lymphoma versus stage I diffuse large B-cell lymphoma (n = 19, P = .02). The more mature natural killer subset was increased in benign spleen (P < .001) and nonsplenic B-cell non-Hodgkin lymphoma (P < .01) versus nonsplenic, nonneoplastic tissue; in diffuse large B-cell lymphoma versus other B-cell non-Hodgkin lymphoma (P < .001); and in follicular lymphoma with an intermediate follicular lymphoma international prognostic index score (n = 17, P = .04). A higher proportion of natural killer–like T-cells was seen in diffuse large B-cell lymphoma versus other B-cell non-Hodgkin lymphoma (P = .001), whereas chronic lymphocytic leukemia/small lymphocytic lymphoma contained fewer natural killer–like T-cells (P < .001). The proportions of natural killer cells, natural killer subsets, and natural killer–like T-cells vary with tissue site, type of B-cell non-Hodgkin lymphoma, and clinical stage in diffuse large B-cell lymphoma and follicular lymphoma. A higher proportion of CD56dim/CD16/57+ natural killer cells is found in spleen, in more aggressive B-cell non-Hodgkin lymphoma, and in follicular lymphoma with an intermediate follicular lymphoma international prognostic index score. This may be of importance with increasing therapeutic use of immunomodulatory agents.
Keywords: NK cells, NKT-cells, B-cell non-Hodgkin lymphoma, Flow cytometry
1. Introduction
The microenvironment of B-cell non-Hodgkin lymphoma (B-NHL) has garnered increasing attention in recent years, particularly with the introduction of new drugs that specifically target the tumor microenvironment [1, 2]. The T-cell, macrophage, and stromal microenvironments in B-NHL have been extensively investigated, with many studies indicating that patients with enhanced host immune responses may have better clinical outcomes [1, 3–6]. However, the investigation of natural killer (NK) cells in B-NHL has been more limited [7–10].
Mature NK cells are functionally and phenotypically heterogeneous and include 2 main subsets: less mature CD3−, CD56bright NK cells and more mature CD3−, CD56dim NK cells [11, 12]. CD56bright NK cells, which are mainly present in lymph node, tonsil, and mucosa-associated lymphoid tissue, demonstrate dim or negative expression of CD16 and produce abundant cytokines including tumor necrosis factor–α and interferon-γ [11, 12]. In contrast, CD56dim NK cells are more commonly found in peripheral blood and spleen, demonstrate bright expression of CD16, and mediate natural cytotoxicity and antibody-dependent cellular cytotoxicity [11, 12]. NKT-cells (or so-called NK-like T-cells) are CD3+ T-cells that coexpress CD56, CD16, and/or CD57. They are usually derived from lymphokine (especially interleukin-2) activated T-cells and appear to also be involved in the innate immune system through cytoxic killing that is not restricted by major histocompatibility complex recognition [13, 14].
Previous studies of NK cells in B-NHL have suggested that higher proportions of peripheral blood NK cells may be a favorable prognostic factor in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and diffuse large B-cell lymphoma (DLBCL) [7, 8]. In addition, some studies have suggested that enhancement of peripheral blood NK cells may improve the cytotoxic effect of rituximab in patients with B-NHL [15, 16]. However, these investigations have focused primarily on peripheral blood NK cells; and to our knowledge, there are no previously published studies that analyze intratumoral NK cell subsets in B-NHL by flow cytometry [7–9].
For these reasons, flow cytometric immunophenotypic studies performed on 150 cases of tissue-based B-NHL and 89 nonneoplastic tissue biopsies were retrospectively reviewed to determine the relative proportions of NK cells, NK subsets, and CD56- and/or CD16/57-positive NKT-cells in neoplastic and nonneoplastic B-cell proliferations. The relationship between the NK and NKT-cell populations and clinicopathologic features including type of B-NHL, tissue site, and clinical stage at diagnosis were evaluated.
2. Materials and methods
2.1. Case selection
The study was approved by the University of Pittsburgh Institutional Review Board. One hundred fifty cases of tissue-based B-NHL and 89 nonneoplastic tissue biopsies with NK subset analysis were identified among specimens analyzed in the clinical flow cytometry laboratory of the University of Pittsburgh Medical Center, Presbyterian Hospital, between October 2006 and September 2008. B-NHL cases, as defined in the 2008 World Health Organization classification of tumors of hematopoietic and lymphoid tissues [17], included 38 follicular lymphomas (FLs), 35 DLBCLs, 29 CLL/SLLs, 25 marginal zone lymphomas, 17 mantle cell lymphomas, 1 Burkitt lymphoma, 1 lymphoplasmacytic lymphoma, and 4 B-cell lymphomas, not otherwise specified. Deidentified pathology reports and flow cytometry histograms were reviewed. Patient clinical characteristics (sex, age, stage at diagnosis, and laboratory values including peripheral blood white blood cell count, hemoglobin, and lactate dehydrogenase) were obtained from available deidentified clinical records. Patients with newly diagnosed B-NHL were staged when sufficient clinical data were available using Ann Arbor staging, international prognostic index for DLBCL, follicular lymphoma international prognostic index (FLIPI) for FL, and Rai staging for CLL/SLL [18–21].
2.2. Flow cytometric immunophenotyping
Four-color flow cytometric analysis was performed using, at a minimum, the following antibody-fluorochrome combinations: κ-fluorescein isothiocyanate (FITC)/λ-phycoerythrin (PE)/CD20-peridinin chlorophyll-cyanine 5.5 (PerCP-Cy5.5)/CD10-allophycocyanin (APC) or κ-FITC/λ-PE/CD19-PerCP-Cy5.5/CD5-APC, and CD16/57-FITC/ CD7-PE/CD3-PerCP-Cy5.5/CD56-APC (Becton Dickinson, San Jose, CA, and Beckman Coulter, Miami, FL). Briefly, cells were incubated with antibody-fluorochrome cocktails on ice for 15 minutes and then incubated with ammonium chloride lysing solution at room temperature for 8 minutes. After washing with phosphate-buffered saline azide solution, the cell pellets were resuspended and fixed with 2% formaldehyde.
Data were acquired using Becton Dickinson FACSCalibur flow cytometers and analyzed with CellQuest software on specimens obtained between October 2006 and December 2007 and acquired using FACSCanto flow cytometers and analyzed with FACSDiva software on specimens obtained between December 2007 and September 2008 (Becton Dickinson). Flow cytometric analysis of CD56 and CD16/ 57 expression on T-cells and NK cells was performed for routine clinical purposes on all cases.Aplot of forward versus side scatter was used to identify cells falling in the “lymphoid region.” The lymphoid cells were displayed on a plot of CD3 versus CD7, and the T-cells (CD3 positive, CD7 positive) and NK cells (CD3 negative, CD7 positive) were then visualized on a plot of CD16/57 versus CD56. Light chain class-restricted B-cell populations were determined by analysis of the plots of CD19 or CD20 versus surface immunoglobulin light chain. For cases without clearly demonstrable surface light chain expression, the abnormal B-cell populations were determined by examination of the plots of either CD20 versus CD10 or CD19 versus CD5. The proportions of NK cells, NK cell subsets, T-cells with or without expression of CD56 and/ or CD16/57, and monoclonal B-cells were determined in each case. Only CD3 negative, CD7 positive cells with at least dim expression of CD56 were defined as NK cells.
2.3. Statistical analysis
For statistical analyses, t tests were performed using GraphPad Prism 5 software package (GraphPad Software, Inc, La Jolla, CA).
3. Results
3.1. NK cells, NK cell subsets, and NKT-cells in nonneoplastic tissue
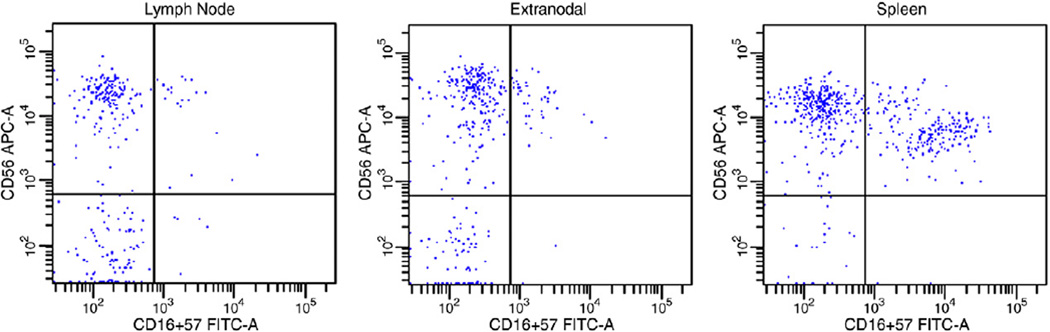
The relative proportions of T-cells, NKT-cells, NK cells, and NK subsets in nonneoplastic tissues are summarized in Table 1. The proportion of NK cells varied based on tissue site, with relatively more numerous NK cells present in nonneoplastic spleens (mean, 8.1% of total events; range, 0.2%–23.1%) versus other nonneoplastic tissues (mean, 1.4% of total events; range, 0.2%–8.3%) (P < .001). The percentage of less mature CD56bright, CD16/57− and more mature CD56dim, CD16/57+ NK cells also varied based on tissue site, with relatively more numerous CD56bright NK cells in lymph nodes and extranodal tissues (mean, 80.9% of total NK cells; range, 22.9%–100%) and more numerous CD56dim NK cells in spleens (mean, 36.9% of total NK cells; range, 2.5%–95.9%) compared with other tissue sites (P < .001) (Fig. 1). The proportion of NKT-cells also varied based on tissue site, with fewer NKT-cells seen in nonneoplastic lymph nodes (mean, 2.0% of total events; range, 0.4%–6.7%) compared with other tissue sites (mean, 5.2% of total events; range, 0.01%–27.4%) (P < .001).
Table 1.
Lymphocyte subsets in nonneoplastic tissue (mean ± SD)
| n | % Total T-cells |
% Total NKT-cells |
% Total NK cells |
% CD56bright, CD16/57− of total NK cells |
% CD56dim, CD16/57+ of total NK cells |
|
|---|---|---|---|---|---|---|
| All nonneoplastic | 89 | 52.1 ± 20.5 | 4.0 ± 4.1 | 3.5 ± 4.8 | 75.0 ± 19.4 | 25.0 ± 19.4 |
| Nonneoplastic lymph node | 32 | 63.8 ± 13.9 | 2.0 ± 1.3 a | 0.9 ± 0.5 | 80.2 ± 10.4 | 19.8 ± 10.4 |
| Nonneoplastic spleen | 29 | 35.5 ± 18.3 | 5.7 ± 4.3 | 8.1 ± 5.8 b | 63.1 ± 22.4 | 36.9 ± 22.4 c |
| Nonneoplastic extranodal | 28 | 55.5 ± 17.6 | 4.7 ± 5.0 | 1.8 ± 2.3 | 81.7 ± 18.3 | 18.3 ± 18.3 |
A relatively lower proportion of NKT-cells was present in nonneoplastic lymph nodes compared with other nonneoplastic tissues (P < .001).
A relatively higher proportion of NK cells was seen in nonneoplastic spleens compared with other nonneoplastic tissues (P < .001).
Relatively more numerous mature CD56dim, CD16/57+ NK cells were seen in nonneoplastic spleens compared with other nonneoplastic tissues (P < .001).
Fig. 1.
The CD3−, CD7+ events are displayed in these histograms. Note in these examples of nonneoplastic tissue that lymph node and extranodal tissue (tonsil) predominantly contain less mature CD56bright, CD16/57− NK cells (91.4% and 90.5% of total NK cells, respectively), whereas the spleen contains a distinct population of CD56dim, CD16/57+ NK cells (40.2% of total NK cells).
3.2. NK cells, NK cell subsets, and NKT-cells in B-NHL
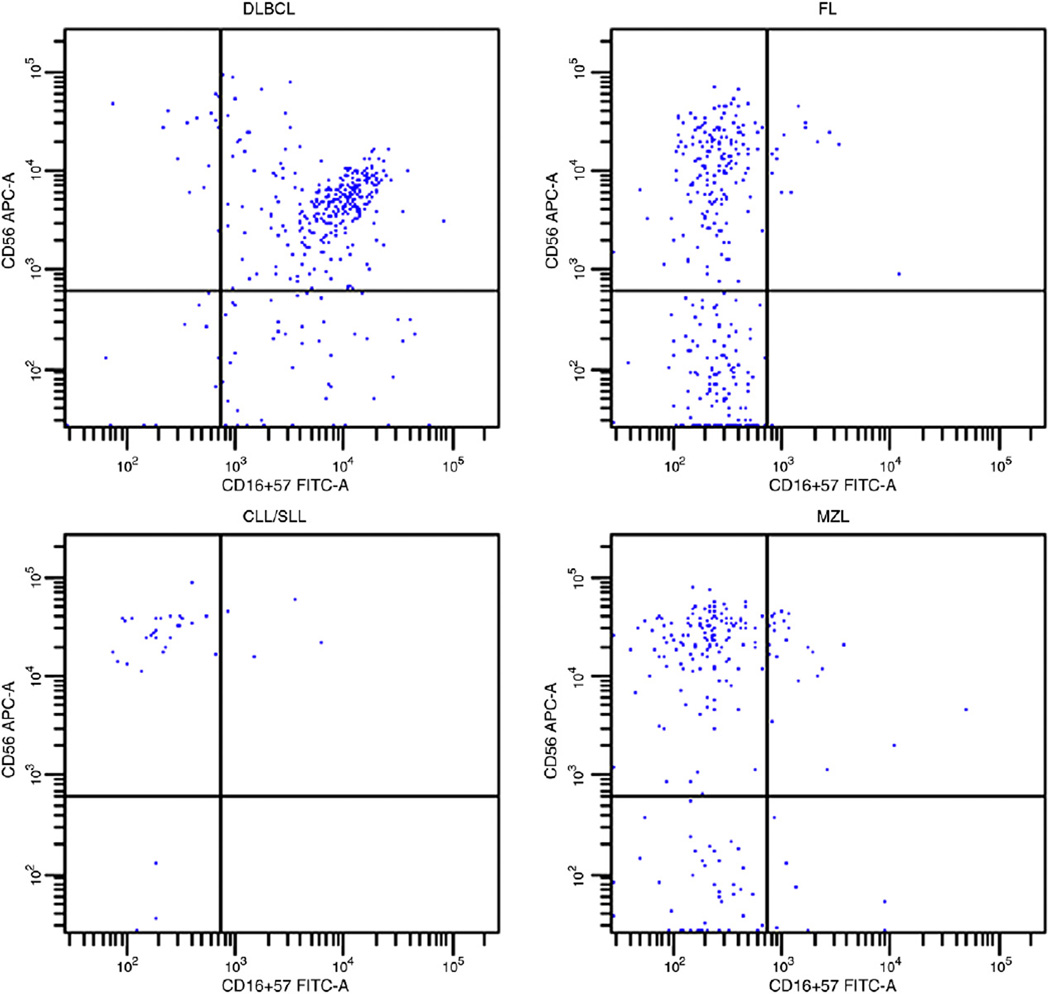
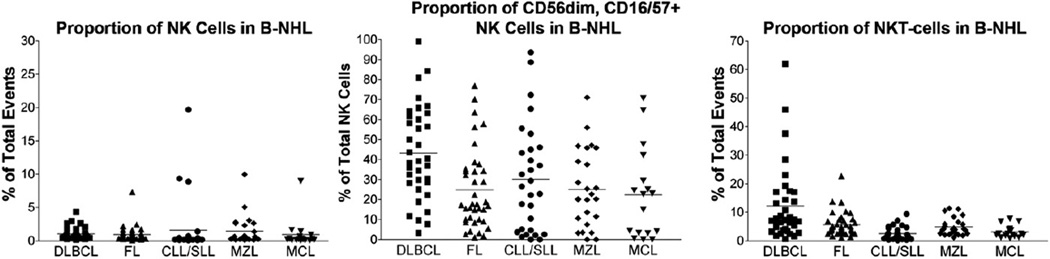
The relative proportions of T-cells, NKT-cells, NK cells, and NK subsets in B-NHL are summarized in Table 2. Similar to nonneoplastic tissue, the proportion of NK cells varied based on tissue site in B-NHL. Splenic-based B-NHL contained relatively more numerous NK cells (mean, 5.9% of total events; range, 0.6%–19.7%) than B-NHL at other sites (mean, 0.8% of total events; range, 0.03%–9.0%) (P < .01). In contrast to nonneoplastic tissue, the proportions of less mature CD56bright, CD16/57− and more mature CD56dim, CD16/57+ NK cells did not vary significantly based on site of B-NHL. Restricting the analyses to nonsplenic sites, more mature CD56dim, CD16/57+ NK cells were relatively more numerous in B-NHL (mean, 29.5% of total NK cells; range, 0%–99.1%) compared with nonneoplastic tissue (mean, 19.1% of total NK cells; range, 0%–77.1%) (P < .01). There was also variation in the proportion of NK cell subsets based on type of B-NHL, with DLBCL containing a higher percentage of more mature CD56dim NK cells (mean, 43.3% of total NK cells; range, 3.3%–99.1%) compared with other types of B-NHL (mean, 25.8% of total NK cells; range, 0%–97.2%) (P < .001) (Figs. 2 and 3).
Table 2 .
Lymphocyte subsets in B-NHL (mean ± SD)
| n | % Total T-cells |
% Total NKT-cells |
% Total NK cells |
% CD56bright, CD16/57− of total NK cells |
% CD56dim, CD16/57+ of total NK cells |
|
|---|---|---|---|---|---|---|
| All B-NHL | 150 | 36.5 ± 21.5 | 6.1 ± 7.8 | 1.2 ± 2.3 | 70.1 ± 24.0 | 29.9 ± 24.0 |
| LN-based B-NHL | 96 | 35.9 ± 21.6 | 5.7 ± 8.9 | 0.8 ± 1.2 | 71.5 ± 24.4 | 28.5 ± 24.4 |
| Splenic-based B-NHL | 12 | 37.0 ± 14.6 | 8.5 ± 5.4 | 5.9 ± 5.5 a | 65.2 ± 26.1 | 34.8 ± 26.1 |
| Extranodal-based B-NHL | 42 | 37.5 ± 2.3 | 6.4 ± 5.4 | 0.7 ± 0.7 | 68.3 ± 22.9 | 31.7 ± 22.9 |
| DLBCL | 35 | 42.8 ± 26.9 | 12.2 ± 13.3 c | 1.0 ± 0.9 | 56.7 ± 23.3 | 43.3 ± 23.3 b |
| FL | 38 | 45.4 ± 18.1 | 5.6 ± 4.2 | 0.9 ± 1.2 | 75.2 ± 19.9 | 24.8 ± 19.9 |
| CLL/SLL | 29 | 24.8 ± 16.1 | 2.5 ± 2.3 c | 1.6 ± 4.2 | 69.8 ± 26.9 | 30.2 ± 26.9 |
| MZL | 25 | 36.3 ± 15.9 | 4.9 ± 3.0 | 1.5 ± 2.2 | 74.9 ± 19.2 | 25.0 ± 19.2 |
| MCL | 17 | 22.0 ± 13.3 | 3.2 ± 2.1 | 0.9 ± 2.1 | 77.6 ± 22.6 | 22.4 ± 22.6 |
Abbreviations: LN, lymph node; MZL, marginal zone lymphoma; MCL, mantle cell lymphoma.
A relatively higher proportion of NK cells was seen in splenic-based B-NHL compared with B-NHL at other sites (P < .01).
Relatively more numerous mature CD56dim, CD16/57+ NK cells were seen in DLBCL compared with other B-NHL (P < .001).
A relatively higher proportion of NKT-cells was seen in DLBCL compared with other B-NHL (P = .001), whereas relatively fewer NKT-cells were present in CLL/SLL compared with other B-NHL (P < .001).
Fig. 2.
The CD3−, CD7+ events are displayed in these histograms. Note that almost all of the NK cells in the illustrated DLBCL are of the more mature CD56dim, CD16/57+ type (84.3% of total NK cells), whereas, in the other illustrated B-NHL, the NK cells are almost all of the less mature CD56bright, CD16/57− type (FL, 96.8%; CLL/SLL, 96.3%; and marginal zone lymphoma, 96.7% of total NK cells). Abbreviation: MZL, marginal zone lymphoma.
Fig. 3.
The proportions of NK cells; CD56dim, CD16/57+ NK cells; and NKT-cells in B-NHL vary based on lymphoma type. The mean within each subgroup is represented by a line. Relatively more numerous CD56dim, CD16/57+ NK cells and NKT-cells are seen in DLBCL compared with other types of B-NHL (P < .001). Relatively fewer NKT-cells are seen in CLL/SLL compared with other B-NHL (P < .001).
Although the percentage of NKT-cells also varied by site in B-NHL, the differences were not statistically significant. However, there was significant variation in the proportion of NKT-cells based on type of B-NHL (Fig. 3). DLBCL contained relatively more numerous NKT-cells (mean, 12.2% of total events; range, 0.7%–61.9%) than other types of B-NHL (mean, 4.3% of total events; range, 0.1%–22.7%) (P = .001); and interestingly, fewer NKT-cells were present in CLL/SLL (mean, 2.5% of total events; range, 0.1%–9.3%) compared with other B-NHL (mean, 4.9% of total events; range, 0.9%–22.7%), even when cases of DLBCL were excluded (P < .001). The differences in the proportion of NKT-cells in DLBCL or CLL/SLL versus other B-NHL remained significant regardless of tissue site (DLBCL, P < .05; CLL/SLL, P < .001). In addition, when the proportions of NKT-cells were evaluated in FL based on histologic grade, more numerous NKT-cells were seen in grade 3 FL (mean, 7.5% of total events; range, 1.1%–22.7%) compared with grade 1 to 2 FL (mean, 4.6%; range, 1.4%–13.7%) (P = .04) (Fig. 4).
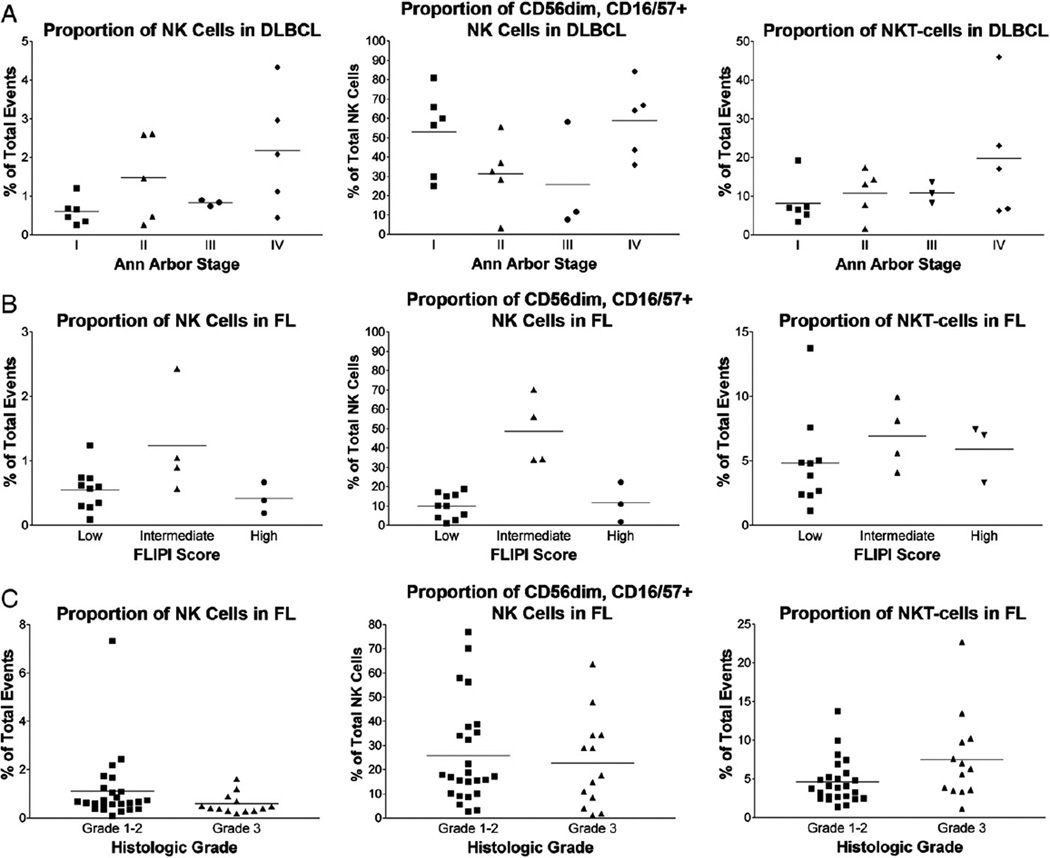
Fig. 4.
The proportions of NK cells; CD56dim, CD16/57+ NK cells; and NKT-cells in DLBCL and FL are illustrated. The mean within each subgroup is represented by a line. A, Relatively more numerous NK cells are present in stage II to IV DLBCL versus stage I (P = .02); and relatively more numerous NKT-cells are present in stage IV DLBCL versus stage I to III (P = .04). B, NK cells and CD56dim, CD16/57+ NK cells are relatively more numerous in FL with an intermediate FLIPI score versus cases with a low or high FLIPI score (P ≤ .04). C, Relatively more numerous NKT-cells are present in histologic grade 3 versus grade 1 to 2 FL (P = .04).
The relative proportions of NK cells, NK subsets, and NKT-cells were further analyzed in patients with sufficient clinical information (56 patients) to determine if differences in these lymphocyte populations correlated with clinical features and in particular clinical stage at diagnosis. Although there were differences in the proportion of total NK cells in patients with Ann Arbor stage I DLBCL (mean, 0.6% of total events; range, 0.3%–1.2%) compared with stage II to IV DLBCL (mean, 1.6% of total events; range, 0.3%–4.3%) (n = 19, P = .02), there were no significant differences in the percentages of CD56bright versus CD56dim NK cells (Fig. 4). NKT-cells also varied based on stage, with relatively more numerous NKT-cells present in Ann Arbor stage IV DLBCL (mean, 19.8% of total events; range, 6.2%–45.9%) versus stage I to III DLBCL (mean, 9.7% of total events; range, 1.6%–19.2%) (n = 19, P = .04) (Fig. 4). No significant variations were seen in the proportion of NK cells or NKT-cells in DLBCL based on international prognostic index.
In FL, the proportions of total NK cells, CD56bright versus CD56dim NK cells, and NKT-cells were similar based on Ann Arbor stage (n = 21). However, the proportions of NK cells varied based on FLIPI score, with more numerous NK cells present in FL with an intermediate FLIPI score (mean, 1.24% of total events; range, 0.9%–2.4%) versus FL with a low FLIPI score (mean, 0.55% of total events; range, 0.09%–1.2%) (n = 17, P = .04) (Fig. 4). Although the proportion of NK cells also appeared to be relatively higher in FL with an intermediate FLIPI score compared with cases with a high FLIPI score, this difference was not statistically significant (P = .2). The percentage of more mature CD56dim, CD16/57+ NK cells was also relatively higher in FL with an intermediate FLIPI score (mean, 48.7% of total NK cells; range, 34.0%–70.2%) compared with FL with a low FLIPI score (mean, 10.0% of total NK cells; range, 1.1%–18.8%) (P < .001) or FL with a high FLIPI score (mean, 11.7% of total NK cells; range, 1.8%–22.4%) (P = .02). No differences in the proportions of NK cells or NKT-cells were seen in CLL/SLL based on Rai stage (n = 16). Insufficient clinical information was available in other types of B-NHL for a meaningful evaluation.
4. Discussion
NK cells are functionally and phenotypically heterogeneous and include 2 main subsets: less mature CD56bright NK cells and more mature CD56dim NK cells [11, 12]. CD56bright NK cells, which are mainly present in lymph node, tonsil, and mucosa-associated lymphoid tissue, demonstrate dim or negative expression of CD16 and produce abundant cytokines including tumor necrosis factor–α and interferon-γ [11, 12]. Via cytokine production, this less mature NK cell subset influences the Th1 immune response, activates antigen-presenting cells, augments macrophage killing of intracellular pathogens, and represses proliferation of transformed cells [11].
In contrast, CD56dim NK cells are more commonly found in peripheral blood and spleen, demonstrate bright expression of CD16, and mediate natural cytotoxicity and antibody-dependent cellular cytotoxicity [11, 12]. Antibody-dependent cellular cytotoxicity is mediated by CD16, the low-affinity Fcγ receptor IIIA, which activates NK cell degranulation and perforin-dependent target cell lysis when bound to the constant region of immunoglobulin immobilized to a cell surface [11]. This NK cell–mediated antibody-dependent cellular cytotoxicity appears to play a major role in the antitumor activity of rituximab [2, 11, 15, 16, 22]. The majority of CD56dim, CD16+ NK cells also appear to express CD57 [23]. Previous studies have suggested that CD57 expression is acquired later in the differentiation pathway of CD56dim, CD16+ NK cells and is associated with increased cytotoxic activity [13, 14, 23–25].
Antigens typically associated with NK cells may also be expressed on T-cells, in particular CD8-positive T-cells that have a large granular lymphocyte cytology and cytotoxic function similar to NK cells [13]. Although NKT-cells constitute a small proportion of lymphocytes in normal individuals, this subset of T-cells may be expanded in the presence of chronic immune system activation, as can be seen with autoimmune disorders or viral infections, and may also be expanded during marrow regeneration after hematopoietic stem cell transplantation [13, 14]. Increased numbers of peripheral blood NKT-cells have also been described in patients with hematolymphoid neoplasms [26].
As new therapies have developed in recent years that specifically target tumor microenvironments, it has become increasingly important to understand the specific components of the B-NHL microenvironment [1]. Previous studies of the B-NHL microenvironment have primarily focused on the characterization of T-cells, macrophages, and stromal cells [1, 3–6, 27–30]. Intratumoral T-cells, and in particular T-helper cells and regulatory T-cells, may be prominent in B-NHL [3, 6]. T-helper cells have been shown to be in close association with neoplastic B-cells in the proliferation centers of CLL/SLL and in the neoplastic follicles of FL [3, 6, 27]. This T-cell subset is thought to play a role in the proliferation of neoplastic B-cells in CLL/SLL and may be associated with transformation to more aggressive disease in FL [3, 6]. Peripheral blood and intratumoral regulatory T-cells are increased in B-NHL and may contribute to the systemic immunosuppression commonly seen in B-cell lymphomas. However, high numbers of tumor-infiltrating regulatory T-cells have also been associated with improved survival in FL, although this association may be dependent on the distribution of regulatory T-cells within the lymphoma [28]. In addition, lymphoma-associated macrophage content appears to be an independent predictor of survival in FL, although this association appears to be influenced by type of chemotherapeutic regimen [4, 5].
The investigation of NK cells in B-NHL has been more limited and has primarily focused on the analysis of peripheral blood NK cells [7–10, 15, 16]. In contrast to previous studies, we focused on the investigation of intratumoral NK cells, NK cell subsets, and NKT-cells. We found that the relative proportions of NK cells, NK cell subsets, and NKT-cells varied both in nonneoplastic tissue and in B-NHL based on different parameters. The proportion of total NK cells was higher in nonneoplastic spleen compared with other nonneoplastic tissue and in splenic-based B-NHL versus B-NHL at other sites. The spleen is recognized to be a major tissue site where NK cells are found, with NK cells comprising up to 17% of lymphocytes in the spleen compared with usually less than 1% in lymph nodes [31, 32]. The increased proportion of NK cells in splenic B-NHL compared with nonsplenic B-NHL most likely reflects a normal variation in NK cell distribution rather than differences intrinsic to splenic B-NHL.
Similar to what has been reported in the literature, nonneoplastic lymph nodes and extranodal tissues contained relatively larger proportions of less mature CD56bright, CD16/57− NK cells compared with nonneoplastic spleen [11, 12]. This finding likely reflects normal NK cell maturation, which is thought to occur primarily in secondary lymphoid tissue and possibly in mucosa-associated lymphoid tissue, as well as the normal mucosal concentration of noncytotoxic CD56bright, CD16/57− NK cells that are thought to play a role in mucosal immune surveillance [11, 33, 34]. Interestingly, the relative proportion of more mature CD56dim, CD16/57+ NK cells was higher in nonsplenic B-NHL than in nonsplenic, nonneoplastic tissue (P < .01), but was not significantly different between neoplastic and nonneoplastic spleen. The mechanism underlying the increased proportion of CD56dim, CD16/57+ NK cells in nonsplenic B-NHL is unclear based on our study, but may suggest that more matureNKcells, which have a primary cytotoxic function and are normally more numerous in peripheral blood and spleen, are recruited from the peripheral blood or stimulated to differentiate from less mature CD56bright, CD16/57− NK cells within the B-NHL microenvironment at nodal and extranodal sites. Additional functional studies would be of interest to elucidate the mechanism underlying this phenomenon.
Although the proportion of total NK cells did not vary significantly based on type of B-NHL, there were differences in the relative proportions of NK subsets and NKT-cells. There appeared to be an increased relative proportion of mature CD56dim, CD16/57+ NK cells as well as an increased proportion of NKT-cells in DLBCL compared with other types of B-NHL and in histologic grade 3 FL compared with grade 1 to 2 FL. These differences remained significant regardless of the site of lymphoma and suggest that increased proportions of cytotoxic CD56dim NK cells and NKT-cells are associated with more aggressive forms of B-NHL. This is supported by previous studies that demonstrated a relatively higher proportion of CD8-positive T-cells or TIA-1–positive lymphocytes in higher-grade lymphomas compared with more indolent types of B-NHL [27, 29]. Furthermore, this finding may have clinical significance given that a higher proportion of NKT-cells was found in Ann Arbor stage IV DLBCL and CD56dim NK cells were relatively more numerous in cases of FL with a higher FLIPI score in our study. Previous studies have also suggested that an increased proportion of intratumoral cytotoxic T-cells may be a negative prognostic factor in DLBCL [30].
How increased intratumoral cytotoxic CD56dim, CD16/ 57+ NK cells and NKT-cells interact with neoplastic B-cells in the microenvironment of more aggressive B-NHL remains to be determined. A previous study of NK cells in B-NHL has shown that the proportion of peripheral blood NK cells, which normally are predominantly CD56dim, CD16/57+ NK cells, was increased in aggressive B-cell lymphomas compared with indolent B-cell lymphomas regardless of the extent of disease [9]. However, a more recent study of peripheral blood NK cells in CLL/SLL found that higher NK cell to monoclonal B-cell ratios were associated with early-stage disease [7]. Although there were only a limited number of CLL/SLL cases with adequate staging information in our study (n = 16), we found no stage-related differences in the intratumoral proportion of total NK cells or NK subsets in CLL/SLL, even when NK cell to monoclonal B-cell ratios were analyzed (data not shown). However, it is difficult to know how the intratumoral distribution of NK cells compares with the peripheral blood component given that, even under normal physiologic conditions, the distribution of lymphocyte subsets in the body varies based on tissue site, with higher proportions of NK cells present in liver, lung, and spleen and lower proportions present in peripheral blood, bone marrow, and lymph nodes [31]. We were not able to evaluate peripheral blood lymphocyte subsets in our cases because of the lack of a concurrent peripheral blood sample obtained at the time of tissue diagnosis. Additional studies comparing peripheral blood and intratumoral NK cell and T-cell subsets would be important to determine how these lymphocyte compartments are related in patients with B-NHL.
Thus, the proportions of NK cells, NK subsets, and NKT-cells vary based on tissue site in nonneoplastic and neoplastic settings, based on the type of B-NHL, and based on the clinical stage in DLBCL and FL. Specifically, relatively more numerous NK cells are present in DLBCL of higher clinical stage; and a higher relative proportion of more mature, cytotoxic CD56dim NK cells is present in DLBCL compared with other lymphomas studied and in FL with a less favorable prognosis. However, the question remains what roles NK cells and the CD56dim NK cell population play in the microenvironment of these more aggressive B-NHLs, and whether their manipulation can enhance patient outcome. Currently, there is growing interest in microenvironment-targeted therapies and regimens to improve rituximab-mediated cytotoxicity [1, 2, 15, 16, 35]. Recent studies have also focused on the addition of immunomodulatory drugs, such as lenalidomide, to rituximab-containing regimens as well as the development of new anti-CD20 monoclonal antibodies with improved binding to the various isoforms of the Fcγ receptor IIIA [2, 22, 35]. Lenalidomide has been shown to stimulate NK cell activity by increasing levels of cytokines such as interferon-γ as well as to enhance recruitment of NK cells to tumor sites in murine models of lymphoma [2, 16]. In addition, lenalidomide has been shown to enhance NK cell–mediated antibody-dependent cellular cytotoxicity, which appears to play a major role in the antitumor activity of rituximab [2, 16, 35]. Further phenotypic and functional studies are warranted to investigate the role of NK cell subsets within the B-NHL microenvironment and to determine if this lymphocyte subset can be manipulated to improve the treatment of B-NHL.
References
- 1.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–3375. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140:36–45. doi: 10.1111/j.1365-2141.2007.06841.x. [DOI] [PubMed] [Google Scholar]
- 3.Glas AM, Knoops L, Delahaye L, et al. Gene-expression and immunohistochemical study of specific T-cell subsets and accessory cell types in the transformation and prognosis of follicular lymphoma. J Clin Oncol. 2007;25:390–398. doi: 10.1200/JCO.2006.06.1648. [DOI] [PubMed] [Google Scholar]
- 4.Farinha P, Masoudi H, Skinnider BF, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106:2169–2174. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 5.Taskinen M, Karjalainen-Lindsberg ML, Nyman H. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamidedoxorubicin-vincristine-prednisone. Clin Cancer Res. 2007;13:5784–5789. doi: 10.1158/1078-0432.CCR-07-0778. [DOI] [PubMed] [Google Scholar]
- 6.Caligaris-Cappio F. Role of the microenvironment in chronic lymphocytic leukemia. Br J Haematol. 2003;123:380–388. doi: 10.1046/j.1365-2141.2003.04679.x. [DOI] [PubMed] [Google Scholar]
- 7.Palmer S, Hanson CA, Zent CS, et al. Prognostic importance of T and NK-cells in a consecutive series of newly diagnosed patients with chronic lymphocytic leukaemia. Br J Haematol. 2008;141:607–614. doi: 10.1111/j.1365-2141.2008.07070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plonquet A, Haioun C, Jais JP, et al. Peripheral blood natural killer cell count is associated with clinical outcome in patients with aaIPI 2–3 diffuse large B-cell lymphoma. Ann Oncol. 2007;18:1209–1215. doi: 10.1093/annonc/mdm110. [DOI] [PubMed] [Google Scholar]
- 9.Kuriyama Y, Nakano M, Kawanishi Y, et al. Cytotoxic lymphocytes in the peripheral blood of patients with B cell lymphomas. Leukemia. 1995;9:2123–2126. [PubMed] [Google Scholar]
- 10.Tursz T, Dokhelar MC, Lipinski M, Amiel JL. Low natural killer cell activity in patients with malignant lymphoma. Cancer. 1982;50:2333–2335. doi: 10.1002/1097-0142(19821201)50:11<2333::aid-cncr2820501119>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oshimi K. Progress in understanding and managing natural killer-cell malignancies. Br J Haematol. 2007;139:532–544. doi: 10.1111/j.1365-2141.2007.06835.x. [DOI] [PubMed] [Google Scholar]
- 13.Tarazona R, DelaRosa O, Alonso C, et al. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121:77–88. doi: 10.1016/s0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 14.Olteanu H, Schur BC, Bredeson C, Atallah E, Kroft SH. Expression of natural killer receptors in T- and NK-cells: comparison of healthy individuals, patients with prior stem cell transplant, and patients undergoing chemotherapy. Leuk Lymphoma. 2010;51:481–487. doi: 10.3109/10428190903552120. [DOI] [PubMed] [Google Scholar]
- 15.Gluck WL, Hurst D, Yuen A, et al. Phase I studies of interleukin (IL)-2 and rituximab in B-cell non-Hodgkin's lymphoma: IL-2 mediated natural killer cell expansion correlations with clinical response. Clin Cancer Res. 2004;10:2253–2264. doi: 10.1158/1078-0432.ccr-1087-3. [DOI] [PubMed] [Google Scholar]
- 16.Wu L, Adams M, Carter T, et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–4656. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 17.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. [Google Scholar]
- 18.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin's disease staging classification. Cancer Res. 1971;31:1860–1861. [PubMed] [Google Scholar]
- 19.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 20.Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 21.Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. [Google Scholar]
- 22.de Romeuf C, Dutertre CA, Garff-Tavernier M, et al. Chronic lymphocytic leukaemia cells are efficiently killed by an anti-CD20 monoclonal antibody selected for improved engagement of FcgammaRIIIA/CD16. Br J Haematol. 2008;140:635–643. doi: 10.1111/j.1365-2141.2007.06974.x. [DOI] [PubMed] [Google Scholar]
- 23.Lanier LL, Le AM, Phillips JH, Warner NL, Babcock GF. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983;131:1789–1796. [PubMed] [Google Scholar]
- 24.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–3191. [PubMed] [Google Scholar]
- 25.Phillips JH, Lanier LLA. Model for the differentiation of human natural killer cells. Studies on the in vitro activation of Leu-11+ granular lymphocytes with a natural killer–sensitive tumor cell, K562. J Exp Med. 1985;161:1464–1482. doi: 10.1084/jem.161.6.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van den Hove LE, Van Gool SW, Vandenberghe P, Boogaerts MA, Ceuppens JL. CD57+/CD28- T cells in untreated hemato-oncological patients are expanded and display a Th1-type cytokine secretion profile, ex vivo cytolytic activity and enhanced tendendy to apoptosis. Leukemia. 1998;12:1573–1582. doi: 10.1038/sj.leu.2401146. [DOI] [PubMed] [Google Scholar]
- 27.Harris NL, Bhan AK. Distribution of T-cell subsets in follicular and diffuse lymphomas of B-cell type. Am J Pathol. 1983;113:172–180. [PMC free article] [PubMed] [Google Scholar]
- 28.Farinha P, Al-Tourah A, Gill K. The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood. 2010;115:289–295. doi: 10.1182/blood-2009-07-235598. [DOI] [PubMed] [Google Scholar]
- 29.Diaz J, Tubbs R, Stoler M, Grogan T. Cytolytic (TIA-1+) tumor infiltrating lymphocytes in B cell non-Hodgkin's lymphomas. Leuk Lymphoma. 1993;9:91–94. doi: 10.3109/10428199309148509. [DOI] [PubMed] [Google Scholar]
- 30.Hasselblom S, Sigurdadottir M, Hansson U, et al. The number of tumour-infiltrating TIA-1+ cytotoxic T cells but not FOXP3+ regulatory T cells predicts outcome in diffuse large B-cell lymphoma. Br J Haematol. 2007;137:364–373. doi: 10.1111/j.1365-2141.2007.06593.x. [DOI] [PubMed] [Google Scholar]
- 31.Westermann J, Pabst R. Distribution of lymphocyte subsets and natural killer cells in the human body. Clin Investig. 1992;70:539–544. doi: 10.1007/BF00184787. [DOI] [PubMed] [Google Scholar]
- 32.Witte T, Wordelmann K, Schmidt RE. Heterogeneity of human natural killer cells in the spleen. Immunology. 1990;69:166–170. [PMC free article] [PubMed] [Google Scholar]
- 33.Chinen H, Matsuoka K, Sato T, et al. Lamina propria c-kit+ immune precursors reside in human adult intestine and differentiate into natural killer cells. Gastroenterology. 2007;133:559–573. doi: 10.1053/j.gastro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Münz C. Non-cytotoxic protection by human NK cells in mucosal secondary lymphoid tissues. Eur J Immunol. 2008;38:2946–2948. doi: 10.1002/eji.200838849. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Qian Z, Cai Z, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol. 2009;84:553–559. doi: 10.1002/ajh.21468. [DOI] [PubMed] [Google Scholar]