Abstract
Purpose
During cell cycle progression, D-cyclins activate cyclin-dependent kinases (CDKs) 4/6 to inactivate Rb, permitting E2F1-mediated S-phase gene transcription. This critical pathway is typically deregulated in cancer, and novel inhibitory strategies would be effective in a variety of tumors. The protein synthesis inhibitor silvestrol has potent activity in B-cell leukemias via the mitochondrial pathway of apoptosis, and also reduces cyclin D1 expression in breast cancer and lymphoma cell lines. We hypothesized that this dual activity of silvestrol would make it especially effective in malignancies driven by aberrant cyclin D1 expression.
Experimental Design
Mantle Cell Lymphoma (MCL), characterized by elevated cyclin D1, was used as a model to test this approach. The cyclin D/Rb/E2F1 pathway was investigated in vitro using MCL cell lines and primary tumor cells. Silvestrol was also evaluated in vivo using an aggressive model of MCL.
Results
Silvestrol showed low nanomolar potency both in MCL cell lines and primary MCL tumor cells. D-cyclins were depleted with just 10 nM silvestrol at 16 hr, with subsequent reductions of phosphorylated Rb, E2F1 protein, and E2F1 target transcription. As demonstrated in other leukemias, silvestrol caused Mcl-1 depletion followed by mitochondrial depolarization and caspase-dependent apoptosis, effects not related to inhibition of CDK4/6. Silvestrol significantly (P<0.0001) prolonged survival in a MCL xenograft model without detectable toxicity.
Conclusions
These data indicate that silvestrol effectively targets the cyclin/CDK/Rb pathway, and additionally induces cytotoxicity via intrinsic apoptosis. This dual activity may be an effective therapeutic strategy in MCL and other malignancies.
Keywords: translation, cyclin, Rb, lymphoma, cell cycle
INTRODUCTION
In normal cells, the progression from G1 to S phase of the cell cycle is tightly controlled by a conserved mechanism involving cyclins D1, D2 and/or D3, cyclin-dependent kinases (CDK) 4 and/or 6, CDK inhibitory proteins of the INK4 family, the tumor suppressor Rb, and transcription factors of the E2F family. In non-dividing cells, hypophosphorylated Rb binds E2F proteins to suppress their activity. Upon appropriate signaling, D-cyclins bind and activate CDK4/6 to phosphorylate and inactivate Rb, freeing E2F to form a complex with other factors to drive the transcription of genes required for cell cycle progression and DNA synthesis (reviewed in (1)). Nearly all tumors are defective in some aspect of this pathway, e.g. through cyclin overproduction, INK4 mutations, or Rb inactivation, providing tumor cells a strong growth advantage and escape from normal mitotic control. Components of this pathway are proposed to constitute valuable therapeutic targets (2, 3), and considerable efforts are underway to develop specific pharmacologic inhibitors. As an example, the CDK4/6-specific inhibitor PD-0332991 (4) has efficacy in a variety of tumor models (5–9), and is currently undergoing clinical testing (10, 11). However, as a single agent PD-0332991 was reported to be cytostatic rather than cytotoxic, although it sensitizes cells to cytotoxic agents (6). Owing to the near universal dysfunction of the cyclin/Rb pathway across cancer types, a dual strategy to block the cyclin D/CDK4,6/Rb pathway while concurrently activating apoptosis has the potential to provide broad therapeutic benefit.
A prime example of a tumor with a disrupted cyclin D/Rb axis is the B-cell malignancy Mantle Cell Lymphoma (MCL), in which the t(11;14)(q13;q32) translocation places CCND1, the gene for cyclin D1, under the control of an immunoglobulin promoter. This results in elevated and sustained cyclin D1 expression in tumor cells and concomitant Rb inactivation, S phase entry and cell division (12). Furthermore, in more aggressive cases mutations/deletions in the genes for DNA damage response factors such as ataxia telangiectasia mutated (ATM) and p16ARF are likely to contribute to aberrant mitotic progression by impeding the activities of CHK1/2 and p53 (13). MCL is a relatively uncommon subset of Non-Hodgkin Lymphoma, but accounts for a disproportionate number of deaths. Treatment options are limited and relapses are nearly universal, highlighting the need for new therapeutic approaches. Beyond the obvious clinical need, however, MCL provides an excellent model to investigate therapeutic targeting of the D-cyclin CDK4,6/Rb pathway.
Silvestrol is a structurally unique, plant-derived cyclopenta[b]benzofuran (14) with potent in vitro and in vivo anti-tumor activity in B-cell malignancies including acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL) (15). Silvestrol is reported to block the initiation step of translation by promoting an aberrant interaction of the RNA helicase eIF4A with capped mRNA, thus preventing assembly into the eIF4F complex (16, 17). This effect leads to selective depletion of short half-life proteins, including Mcl-1 (15) and cyclin D1 (17, 18). The therapeutic benefit of protein synthesis inhibition in MCL and other B-cell malignancies is well-substantiated by the vast amount of data with mTOR inhibitors, and both Mcl-1 and cyclin D1 are commonly shown to be affected by these agents (19). Although multiple studies show that inhibiting of either cyclin D1 alone (20) or CDK4/6 alone (5) is not cytotoxic, the resulting interference with tumor cell growth in vivo may be sufficient to provide therapeutic benefit. More importantly, however, recent work indicates that inhibition of the D-cyclin/CDK4,6 pathway can sensitize tumor cells to targeted agents including bortezomib (21) and imatinib (22). Thus, we hypothesized that silvestrol, through its dual activities of D-cyclin inhibition and direct induction of apoptosis, would be especially effective in rapidly proliferating B-cell malignancies.
Here, we demonstrate that silvestrol shows potent cytostatic as well as cytotoxic activity in MCL primary cells and cell lines. Low doses of silvestrol cause the loss of D-cyclins followed by Rb dephosphorylation and abrogation of E2F1-mediated transcription. Additionally, as we previously reported in chronic and acute lymphocytic leukemias, silvestrol induces depletion of Mcl-1 with subsequent mitochondrial depolarization and apoptosis via the intrinsic pathway, thus providing a dual anti-tumor effect. Importantly, silvestrol provides a significant survival advantage in an aggressive mouse model of MCL. Together, these data support further pre-clinical investigation of this novel agent in MCL as well as other malignancies with a hyperactivated D-cyclin/CDK4,6 axis.
MATERIALS AND METHODS
Reagents
Isolation and characterization of silvestrol has been described (14). The caspase inhibitor Q-VD-OPH (Enzyme Systems Products, Aurora, OH) was used at 20 micromolar (µM). JC-1 (5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolylcarbocyanine iodide) and dihydroethidium (DHE) were obtained from Invitrogen (Carlsbad, CA). The CDK4/6 inhibitor PD-0332991(4) was purchased from Active Biochem (Maplewood, NJ). TRAIL was purchased from R&D Systems (Minneapolis, MN).
Cells and cell lines
MCL cell lines were kindly provided by Dr. Raymond Lai, University of Alberta (Edmonton, Alberta, Canada) and have been previously described (23). Primary MCL cells were obtained from the blood or marrow of patients diagnosed with MCL by World Health Organization criteria (24), following written informed consent according to the Declaration of Helsinki. Tumor cells were enriched to at least 85% by CD45 expression (StemCell Technologies, Vancouver, British Columbia, Canada). All cells were incubated in RPMI 1640 supplemented with heat-inactivated fetal bovine serum (10%), l-glutamine (2 mM), and penicillin (100 U/mL)/streptomycin (100 g/mL) (all from Sigma, St. Louis MO) at 37°C in a humidified atmosphere of 5% CO2.
Growth inhibition
To assess growth inhibition, CellTiter96® (MTS) assays were performed according to the manufacturer’s instructions (Promega, Madison, WI). IC50 values with 90% confidence intervals were calculated using Prism (GraphPad Software, San Diego, CA).
Flow cytometry studies
Cell viability was measured by flow cytometry using annexin-V-FITC/Propidium Iodide (annexin/PI) according to the manufacturer’s instructions (BD Pharmingen). Mitochondrial membrane depolarization was assessed using JC-1 and reactive oxygen species generation was assessed using DHE as previously reported (25, 26). Cell cycle studies were performed according to standard procedures (27). All studies were conducted using a Beckman Coulter FC500 instrument (Brea, CA).
Immunoblot analyses
SDS-PAGE and immunoblotting were performed according to standard procedures. Antibodies to cyclin D1 and Mcl-1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); phosphorylated and total Rb from Cell Signaling Technology (Beverly, MA); E2F1 from Sigma; and GAPDH from Chemicon (Temecula, CA). Species-appropriate secondary antibodies conjugated with horseradish peroxidase were obtained from Santa Cruz Biotechnology. Protein bands were quantified by integration of the chemiluminescence signals on an AlphaImager system (Proteinsimple, Santa Clara, CA).
Real-time reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA). Real-time RT-PCR was performed using TaqMan Universal Master Mix (Applied Biosystems, Foster City CA), using TATA binding protein (TBP) as an endogenous control. Primers and labeled probes were obtained from Applied Biosystems. Mean threshold cycle (Ct) values were calculated by ABI PRISM software (Applied Biosystems) and used to determine fold differences according to manufacturer’s instructions.
In vivo studies
The JeKo-1 xenograft mouse model of MCL was used as previously described by our group (28–30). In this model, mice develop an aggressive, widely disseminated lymphoma with circulating and organ-infiltrating tumor cells and a median survival of four weeks post-tumor injection. CB17 SCID mice (Taconic, Hudson NY) were depleted of murine natural killer (NK) cells with weekly intraperitoneal injections of 0.2 mg anti-mouse interleukin-2 receptor β monoclonal antibody (TMβ1), starting the day prior to engraftment. Mice were injected via the tail vein with 4×107 JeKo-1 cells, then randomized to two groups. Fifteen days post-injection, treatment was initiated either with vehicle (30% hydroxypropyl-β-cyclodextrin in water) or silvestrol at 1.5 mg/kg in vehicle, intraperitoneally every 48 hr. Animals were monitored daily for signs of tumor burden (weight loss, hind limb paralysis, respiratory distress, ruffled coat, and distended abdomen). Animals were euthanized if they exhibited either hind limb paralysis, 30% reduction in body mass, or 10% reduction in body mass together with respiratory distress and/or ruffled coat or lethargic behavior. The primary endpoint was survival as defined by the lack of euthanasia criteria. All animal work was reviewed and approved by the OSU University Laboratory Animal Resources (ULAR)-Institutional Animal Care and Use Committee. For the pharmacodynamics experiment, a subset of mice at 3 weeks post-engraftment were injected with either silvestrol or vehicle. Spleen cells were obtained from euthanized mice at 24 hr and lysates were immediately prepared. Immunoblots for cyclin D1 were performed as described above, using an identical aliquot of JeKo-1 lysate as a normalizing control across blots.
Statistics
Linear mixed effects models were used to assess the average interaction effect between TRAIL and all five silvestrol doses for Mino and Jeko-1 cells separately, using an adjusted α=0.025 level of significance for each comparison to control the overall Type I error rate at α=0.05. For the mouse experiments, Kaplan-Meier estimates of the survival function for vehicle and silvestrol conditions were generated, and median survival times with 95% confidence intervals were calculated. Overall survival between the two groups was compared using the log-rank test using an α=0.05 level of significance. For the pharmacodynamics experiment, a mixed effects model was fit to account for experimental variability. SAS/STAT software (Version 9.2; SAS Institute Inc.) was used for all statistical analyses.
RESULTS
Silvestrol has potent cytotoxic activity in MCL cells
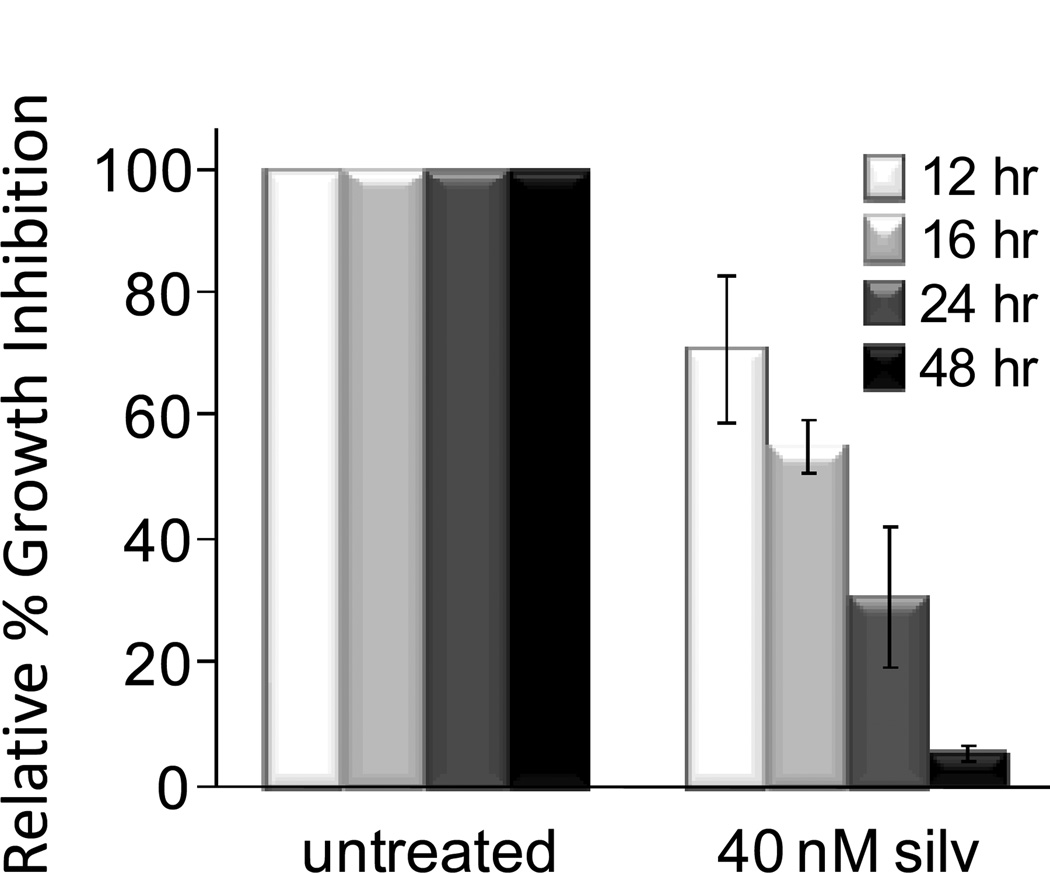
The activity of silvestrol was examined using a panel of MCL cell lines that included Mino, JeKo-1, and SP-53. As shown in Fig 1A, silvestrol showed low nanomolar IC50 values (concentration required for 50% growth inhibition) in the Mino and JeKo-1 cell lines at 48 hr by MTS assay. We previously reported that silvestrol is a substrate of P-glycoprotein (31). As SP-53 cells were notably more resistant to silvestrol, we hypothesized that P-gp expression was responsible for this difference. In support of this, SP-53 cells readily effluxed the fluorescent P-gp substrate rhodamine 123 (flow cytometry; data not shown), and the 48-hr IC50 was reduced approximately 4-fold in the presence of the P-gp inhibitor verapamil (Fig 1A). As the MTS assay does not distinguish cytotoxicity from cytostasis, cell death was confirmed in each case by annexin/propidium iodide (PI) staining and flow cytometric evaluation (Fig 1B and additional data not shown). We next evaluated the effects of silvestrol in primary tumor cells from six MCL patients (clinical characteristics of patients summarized in Table). Cells were isolated by CD45 selection and incubated without or with 10 nM silvestrol for 48 hr, and cell viability was examined by annexin/PI flow cytometry (Fig 1C). Data were plotted as percent live (annexin-negative and PI-negative) cells relative to each sample’s time-matched untreated control. The results indicated variability in silvestrol sensitivity between patient samples, but a median sensitivity comparable to the JeKo-1 and Mino cell lines. As we previously reported that silvestrol shows a relatively short half-life in vivo (32), we tested the effects with various exposure times. A 16 hr exposure of JeKo-1 cells to silvestrol produced approximately 50% of the effect achieved with continuous 48 hr exposure. Removing silvestrol after 24 hr reduced its efficacy only minimally (Fig 2; growth inhibition assessed at 48 hr in each case). Interestingly, at 16 and 24 hr the cells remained largely viable (>90% and >80% annexin/PI negative, respectively; data not shown). These results are similar to our findings in CLL primary cells (15), and together indicate that although the cytotoxic effects of silvestrol require at least 8–12 hours to initiate, they are largely irreversible by 24 hr even though cell death as measured by PI is minimal at that time. Our prior pharmacokinetics study (32) indicated that with a single-dosing strategy, concentrations achievable in vivo for similar time periods remain in the low nanomolar range. Thus, to reflect these data, further experiments to assess the mechanism utilized 10 and 40 nM silvestrol for 16 and 24 hr.
Figure 1. Efficacy of silvestrol in MCL cells.
(A) The indicated cell lines were incubated with various concentrations of silvestrol, and growth inhibition was assessed at 48 hr by MTS assay (N ≥ 3 each). Additionally, the SP-53 cell line was re-tested under identical conditions in the presence of verapamil (10 µM). IC50 and 95% confidence intervals are shown. (B) JeKo-1 cells were incubated 24 hr as indicated, then analyzed by annexin (x-axis) and PI (y-axis) flow cytometry. Mino results were similar. Data are representative of three individual experiments. (C) MCL patient samples (N=6; see Table) were incubated with silvestrol for 24 hr and evaluated by annexin/PI flow cytometry. Percent live (annexin- and PI-) are plotted relative to each sample’s untreated control. The horizontal black bar shows the median value.
TABLE.
| Pt # | Age/ Sex |
MCL variant |
Stage | Source of tumor cells |
Previous therapy |
Symbol on chart |
|---|---|---|---|---|---|---|
| 1 | 55/F | Classic | IVB | lymph node | No | ◇ |
| 2 | 59/M | Classic | IVA | blood | No | □ |
| 3 | 65/M | Classic | IVB | blood | No | Δ |
| 4 | 52/M | Blastoid | IVB | blood | No | × |
| 5 | 60/M | Blastoid | IVB | blood | Yes | + |
| 6 | 63/F | Classic | IVB | blood | Yes | ○ |
Figure 2. Effects of exposure time.
JeKo-1 cells were incubated with or without 40 nM silvestrol. At each timepoint shown, cells were re-plated in fresh media without silvestrol. All incubations were continued to a total of 48 hr. Growth inhibition was then assessed by MTS assay. Data are the averages of three experiments and bars indicate −/+ standard deviation.
Silvestrol depletes oncogenic proteins in MCL cells
Cyclins D1 and D3 were dramatically reduced in MCL cell lines with just 10 nM silvestrol at 16 hr (Fig 3A). Cyclin D2 could be detected in Mino, but not JeKo-1, cells and appeared to be reduced with silvestrol treatment, although levels were insufficient to allow quantification (data not shown). Silvestrol also caused the loss of both Mcl-1 and c-myc, as was previously reported along with cyclin D1 in the Granta-519 MCL cell line and other tumor types (15, 17, 18). Dramatic loss of cyclin D1 was also evident in primary MCL tumor cells with just 10 nM silvestrol (Fig 3B). These changes were not a consequence of an apoptotic process; as mentioned above, viability of cells at this time point (16 hr) were not different than untreated as determined by annexin/PI flow cytometry (data not shown). Furthermore, depletion of each of these proteins was not the result of transcriptional effects, as cyclin D1, Mcl-1 and c-myc mRNA levels were unchanged or moderately increased in these same samples (Fig 3C). As cyclin D1 is known to be degraded by the ubiquitin-proteasome pathway, we repeated this experiment in the presence of the proteasome inhibitor bortezomib to determine if the loss of this protein was due to increased proteasomal degradation. As shown in Fig 3D, cyclin D1 depletion following silvestrol treatment was unaffected by the addition of bortezomib. Together, these data are consistent with the hypothesis that silvestrol inhibits cyclin D1 production at the translation stage.
Figure 3. Effects of silvestrol on key MCL survival and proliferation proteins.
(A) JeKo-1 or Mino cells were incubated 16 or 24 hr with silvestrol as indicated, and lysates were analyzed by immunoblot. Results are representative of at least three separate experiments. Vertical lines indicate deletion of an irrelevant lane in the same blot. (B) MCL primary tumor cells were incubated 16 hr −/+ silvestrol and immunoblotted for cyclin D1 (representative of six patient samples). (C) RNA was extracted from the cells in (A) at the 16 hr timepoint and analyzed by real-time RT-PCR. Data were normalized to TBP and are shown relative to the untreated time-matched sample. Results are averages of three separate experiments, and the bars show −/+ standard deviation. (D) JeKo-1 cells were incubated with or without silvestrol (10 nM) or bortezomib (BTZ; 10 nM) for 4 hr (left 4 lanes). Additionally, BTZ was added 2 hr prior to silvestrol treatment (right two lanes), and incubations continued for 4 hr. Mcl-1 was also included as a known proteasome target. Lower bands in the GAPDH blot are residual cyclin D1 (36 kDa). Similar results were observed in two additional experiments.
Downstream effects of cyclin D1 depletion
During cell cycle progression, cyclin D1 translocates into the nucleus and forms a holoenzyme with CDK4/6 to phosphorylate the Rb resulting in the release of E2F transcription factors and G1/S phase transition (1). We therefore assessed the effects of silvestrol-mediated cyclin D1 depletion in Mino and JeKo-1 MCL cell lines incubated for 16 or 24 hr in the presence of 10–40 nM silvestrol. As cyclin D1 depletion is expected to have the immediate effect of CDK4/6 inactivation, we also included the CDK4/6-specific inhibitor PD-0332991 (4) as a control. We performed preliminary experiments to determine the minimum effective dose of PD-0332991 for these cell lines. Concentrations between 100 and 1000 nM produced similar effects as measured by growth inhibition (MTS assay), and none were cytotoxic as determined by annexin/PI flow cytometry (data not shown). We therefore used 100 nM in the remaining experiments. As expected, silvestrol and PD-0332991 both resulted in the loss of the phosphorylated form of Rb, while total Rb levels were generally not affected (Fig 4A). Additionally, silvestrol caused a notable reduction in E2F1 protein (Fig 4A). This reduction was also observed with PD-0332991, although to a lesser extent, suggesting that silvestrol-mediated E2F1 loss might be partly attributable to loss of CDK4/6 activity following cyclin depletion. Thus, silvestrol treatment may produce E2F1 inactivation both through hypophosphorylation of Rb as well as total E2F1 protein reduction. As shown in Fig 4B, message levels of classical E2F1 targets including cyclin E, proliferating cell nuclear antigen (PCNA), thymidine kinase, CDK’s 2 and 4, MCM10, CDC45, and CDC6 were notably reduced in Mino and JeKo-1 cells with just 10 nM silvestrol. Results using PD-0332991 were similar in Mino cells, although in JeKo-1 cells PD-0332991 had little effect on these targets at the 100 nM concentration used. These experiments also showed silvestrol-mediated loss of E2F1 mRNA. As E2F1 is regulated by multiple factors including cyclin D1 and c-myc as well as itself, this result is not unexpected. Finally, low concentrations of silvestrol consistently produced an increase in the G1 population and a decrease in the intermediate (S-phase) population, suggestive of arrest at the G1 transition (Fig 4C).
Figure 4. Effects of silvestrol on cyclin D downstream events.
(A) JeKo-1 and Mino cells were incubated as indicated, and lysates were analyzed for the indicated proteins by immunoblot. The CDK4/6 inhibitor PD-0332991 (100 nM) was included as a control. Results shown are representative of at least three experiments. (B) Transcriptional effects of silvestrol on E2F1 targets in JeKo-1 (black bars) and Mino (grey bars) MCL cells as assessed by real-time RT-PCR. N=5 (except for cyclin E1, CDK2 and PCNA, N=4) and bars indicate −/+ standard deviation). (C) Cell cycle effects of silvestrol in JeKo-1 and Mino MCL cell lines (16 hr). All results are representative of at least three individual experiments.
Mechanism of cell death
As loss of the Bcl-2 family member protein Mcl-1 is consistently observed in silvestrol-treated cells and E2F1 has been reported to modulate the transcription of apoptosis-related genes (33), we analyzed silvestrol-treated JeKo-1 and Mino cells for several of these factors. We did not observe substantial changes in protein levels of Bcl-2, Bax, Bak, Bag-1, Bim, or XIAP (data not shown). Similar to what we previously reported in CLL and ALL cells, silvestrol treatment consistently induced mitochondrial depolarization in JeKo-1 and Mino cells as early as 16 hr that increased over time (24 hr data shown; Fig 5A). This effect was not related to the inhibition of CDK4/6, as 100 nM PD-0332991 produced little or no change. Addition of the caspase inhibitor Q-VD-OPH reduced but did not prevent silvestrol-mediated mitochondrial depolarization, suggesting the involvement of caspases in this process. Increases in reactive oxygen species (ROS) production are indicative of mitochondrial perturbation. Therefore, we next evaluated silvestrol-induced ROS generation using Q-VD-OPH and gating around the live population in the forward/side scatter to focus the analysis on non-apoptotic cells. By 24 hr, ROS production was notably increased by silvestrol in both JeKo-1 and Mino cells (Fig 5B), indicating that silvestrol induces ROS generation that is not simply a consequence of cell death.
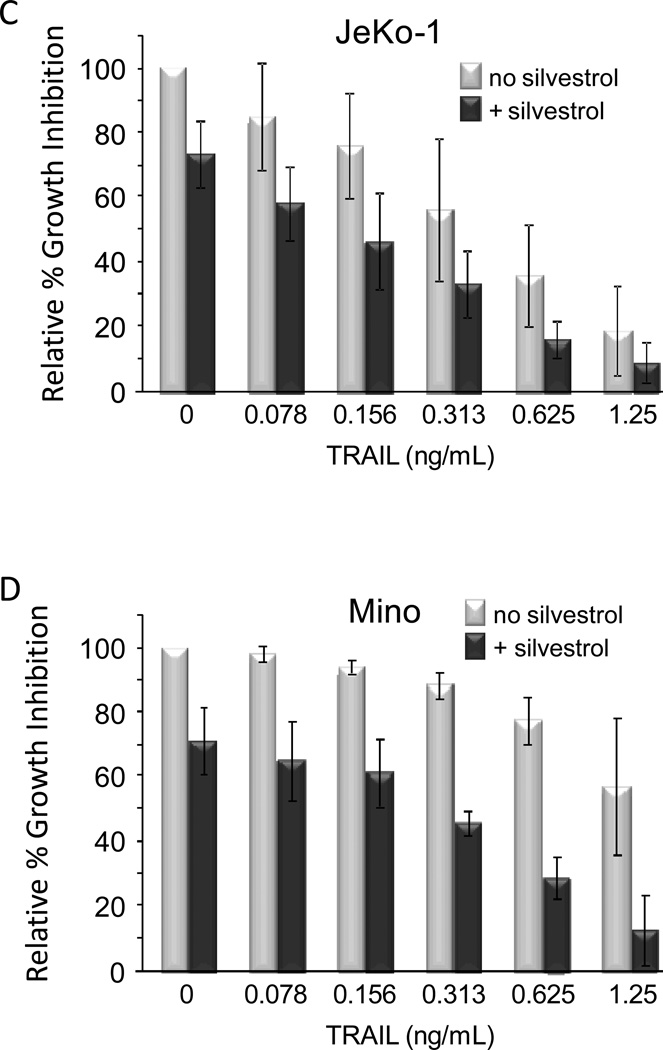
Figure 5. Silvestrol mechanism of cell death.
(A) JeKo-1 (top) and Mino (bottom) cells were incubated 24 hr as indicated and stained using the voltage-sensitive dye JC-1. Mitochondrial integrity was assessed as percentage of cells remaining in the FL2 (y-axis) high, FL1 (x-axis) low population. Data are representative of three separate experiments. (B) JeKo-1 and Mino cells were incubated with or without silvestrol in the presence of Q-VD-OPH (20 µM). ROS production was analyzed by DHE flow cytometry. Results representative of three separate experiments. (C) JeKo-1 and (D) Mino cells were incubated 24 hr without or with silvestrol (10 nM). TRAIL was then added at the indicated concentrations. After an additional 24 hr, cell growth inhibition was assessed by MTS assay. Data are shown relative to time-matched untreated cells. Data shown are the average of three separate experiments, and bars show −/+ standard deviation. A significant synergistic interaction between silvestrol and TRAIL was observed in Mino (p<0.0001) but not JeKo-1 (p=0.351) cells.
Loss of Mcl-1 is known to sensitize tumor cells to apoptosis mediated by TNF-receptor associated ligand (TRAIL) (34, 35). We therefore investigated whether silvestrol produced this effect. JeKo-1 and Mino cells were incubated 24 hr with or without 10 nM silvestrol before adding TRAIL at various concentrations and incubating an additional 24 hr. In JeKo-1 cells, responses were additive although a significant interaction effect (synergism) was not observed (p=0.351) (Fig 5C). However in Mino cells, the interaction effect across all doses was highly significant (p<0.0001), indicating a much greater effect of TRAIL in the presence of silvestrol (Fig 5D). As TRAIL sensitization can also be mediated by the loss of c-FLIP (36), we also investigated c-FLIP expression by immunoblot. No changes in c-FLIP expression were observed in either cell line (data not shown).
In vivo activity
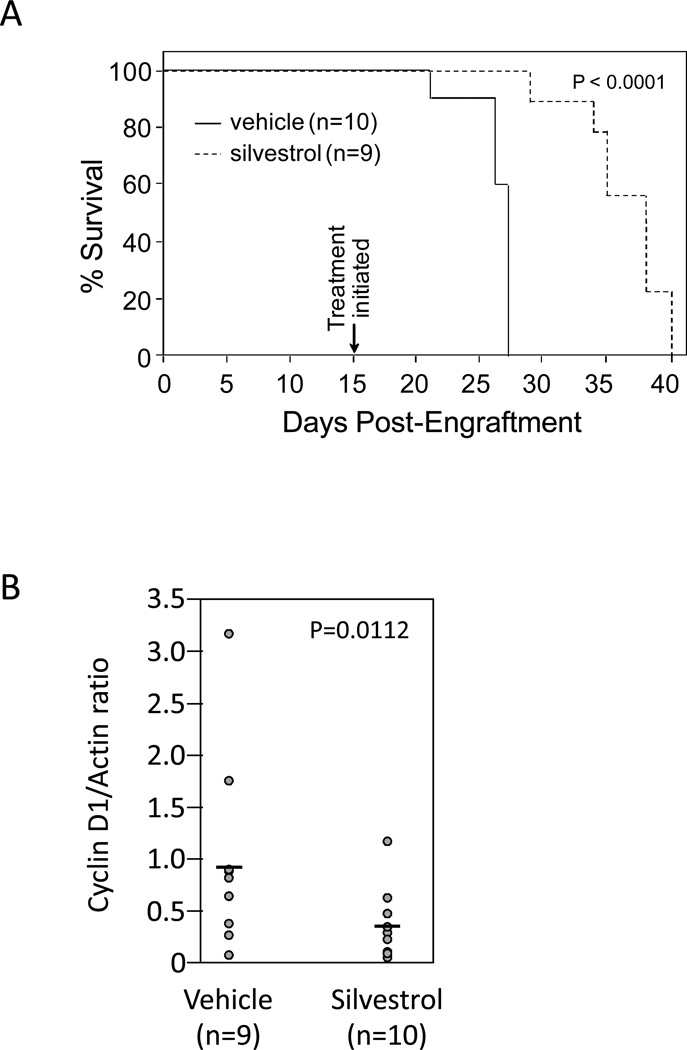
We and others previously showed that silvestrol produces significant in vivo benefit in diverse cancer models that include prostate and breast cancer (17) and chronic and acute B-cell leukemias/lymphomas (15, 18). To evaluate the single-agent activity of silvestrol in MCL, we employed the JeKo-1/SCID model as recently reported by our group (28). In this model, engraftment of JeKo-1 cells produces an aggressive disseminated leukemia that, in untreated animals, results in a median survival of just 28 days. For these studies, NK cell-depleted SCID mice were injected intravenously with 40 million JeKo-1 cells. Day 15 post-inoculation, treatments were initiated with either vehicle alone (hydroxypropyl beta-cyclodextrin, 30% in sterile water) or 1.5 mg/kg silvestrol in vehicle (n = 10 per group), administered intraperitoneally every 48 hr. This dose was selected for its known safety in studies using non-tumored animals (data not shown). The median survival of vehicle-treated vs. silvestrol-treated mice was 27 vs. 38 days (N = 10 and 9 respectively; log rank P<0.0001) (Fig 6a). These results were confirmed in a follow-up experiment using the identical regimen, in which the median survivals were 28.5 vs. 36 days for vehicle- and silvestrol-treated mice, respectively (N=10 each group; log rank P=0.0008). Importantly, there was no evidence of toxicity in silvestrol-treated animals as noted by weight loss; however, detailed toxicity studies remain to be conducted. Separately, a cohort of identically engrafted animals was treated 23 days post-engraftment with a single injection either of silvestrol (N=10) or vehicle (n=9). 24 hr later, spleen cells were collected for immunoblotting. As shown in Fig 6b, cyclin D1 levels were moderately but significantly lower in the group of mice receiving silvestrol (0.47-fold versus vehicle; 95% CI = 0.29, 0.75; P=0.0112).
Figure 6. Efficacy of silvestrol in vivo.
SCID mice were NK cell-depleted with weekly administration of anti-IL2R antibody, then engrafted with 40×106 JeKo-1 cells via the tail vein. (A) Mice were then randomized, and 15 days post-engraftment treatment was initiated with vehicle (N=10) or silvestrol at 1.5 mg/kg ip every 48 hr (N=9). Median survival was significantly prolonged (P<0.0001). A repeat experiment showed similar results (N=10 per group; p=0.0008). (B) In a separate cohort, 3 weeks post-engraftment mice were randomized to receive a single injection of either vehicle (N=9) or silvestrol (N=10). 24 hr after injections, mice were euthanized and lysates prepared from spleen cells. Cyclin D1 levels were then assessed by immunoblot. Results show the cyclin D1 to actin ratios of each sample. Results were normalized across blots using an identical aliquot of JeKo-1 lysate.
DISCUSSION
MCL is an aggressive, incurable B-cell malignancy for which novel therapeutic strategies are desperately needed. Additionally, it represents a valuable model of disrupted cell cycle control, a nearly universal characteristic of cancer. Thus, the use of MCL as a system to investigate mechanisms of tumor cell killing related to cell cycle may identify factors with relevance to a variety of malignancies. Here, we show that silvestrol potently induces cell growth inhibition and cell death in MCL cell lines and primary cells, and significantly prolongs survival in an aggressive in vivo model of MCL. These effects appear to be due to dual inhibition of cell cycle progression via depletion of D-cyclins and E2F1, as well as induction of mitochondrial depolarization and intrinsic apoptosis via depletion of Mcl-1 as previously reported by our group (15). Although either cyclin D depletion (20) or CDK4/6 inhibition (6, 7) alone are not necessarily sufficient to induce cell death, the data presented here suggest that the dual effect of cell cycle inhibition and mitochondrial depolarization readily induces cell death, potentially representing an effective therapeutic strategy in MCL. Furthermore, this strategy sensitizes cells to additional agents such as TRAIL, as demonstrated here, or to a variety of chemotherapies (16, 18, 37).
Silvestrol appears to exert its effects via inhibition of translation by promoting an abnormal association of capped mRNA with the RNA helicase eIF4A (16, 17), thus sequestering mRNA from the eIF4F initiation complex. This translation inhibition mechanism likely explains the depletion of both cyclin D1 and Mcl-1 reported here, as mRNA for both genes is unaffected or moderately increased and proteasome inhibition does not fully prevent protein loss. Agents with similar effects, but different mechanisms of translation inhibition, include sorafenib (38), homoharringtonine and its derivatives (39), and mTOR inhibitors (40). These agents have obvious therapeutic benefit in multiple cancer types, and together with the data shown here, strongly support translation inhibition as a valuable therapeutic strategy. However it will be important to ascertain the relative benefits of direct translation inhibition with silvestrol, versus indirect inhibition with various kinase inhibitors. Hypothetically, direct inhibition of translation avoids the potential feedback activation of survival pathways (41, 42), although we have not yet shown this to provide an advantage in vivo. Furthermore, as both rapamycin (41, 42) and silvestrol (31) have been shown to activate resistance mechanisms, their optimal benefit is likely to be observed in combination strategies.
Silvestrol is a unique member of an intriguing class of natural products, rocaglates, that are being investigated for activity in hematologic malignancies alone or in combination with other agents (37, 43, 44). Efficacy of this class of agents in various solid tumors is also under evaluation, and our results showing inhibition of the D-cyclin/CDK4,6/Rb axis suggest that it will have activity in a subset of these as well. Interestingly, our results reveal subtle differences between the effects of silvestrol in B-leukemias versus prostate or breast cancer cells, which might signal variations in cytotoxic mechanisms despite the obvious potency of silvestrol in each of these tumor types. For example, as reported by Cencic et al. (17), silvestrol causes loss of Bcl-2 protein expression in MDA-MB-231 breast and PC-3 prostate cancer cells, whereas our results show very little effect of silvestrol on Bcl-2 protein levels prior to cell death, either in MCL as reported here, or in CLL or ALL ((15) and data not shown). Bcl-2 is a critical anti-apoptotic protein and a validated therapeutic target, and its reduction in solid tumor cell lines suggests combination strategies of silvestrol with agents that show reduced efficacy in the presence of elevated Bcl-2. However, the relative lack of Bcl-2 reduction in B-leukemias and lymphomas treated with silvestrol, coupled with their exquisite sensitivity to this agent, indicates that Bcl-2 loss is not essential for silvestrol-mediated cytotoxicity.
Previous reports suggest that rocaglates may act in part as inhibitors of the NF-kB pathway (45). As NF-kB is frequently activated in leukemias and serves a pro-survival function, it would be valuable to find that silvestrol blocked this important pathway. However, under the conditions reported here, silvestrol treatment did not reduce mRNA levels of classical NF-kB targets including XIAP, BCL2L1, NFKB1, CD74, and BCL3 (data not shown), and in fact moderate transcriptional increases were noted in putative NF-kB targets cyclin D1, c-myc and Mcl-1. This suggests that NF-kB inhibition is unlikely to be a component of silvestrol-mediated cytostasis or cytotoxicity in MCL cells. Additionally, although we observed the loss of the potential therapeutic target protein c-myc, classical c-myc-modulated genes were not found to be affected in these experiments. However, these observations do not rule out the potential importance of silvestrol-mediated c-myc depletion in other tumor types.
The primary factors in the D-cyclin/CDK/E2F pathway have been reported to participate in cellular processes as diverse as DNA damage response and repair, differentiation and apoptosis, in addition to cell cycle control (2, 3, 46). We have not yet evaluated the effect of silvestrol on each of these many pathways. Clearly, more research is needed to sort out the consequences of inhibiting D-cyclins and their downstream effector molecules. Regardless, our data demonstrate the strong therapeutic potential of concurrently interfering with this plus other pro-survival pathways (i.e. mitochondrial stabilization) through direct inhibition of translation.
STATEMENT OF TRANSLATIONAL RELEVANCE.
Tumor cells rely heavily on continued production of proteins involved in growth, proliferation, and protection from apoptosis. However, translation inhibition represents an underexplored approach in cancer therapy. Silvestrol is a unique agent that blocks translation directly at the eIF4F complex, avoiding the compensatory activation of the Akt pathway as is seen with mTOR inhibitors. Here we show that silvestrol exhibits potent growth inhibitory activity in mantle cell lymphoma cells and causes early depletion of cyclin D, with concomitant E2F1 deactivation and cell cycle arrest followed by apoptosis. These results potentially expand the impact of this work into diverse tumor types, most of which rely on sustained proliferation through defects in the cyclin D pathway. Thus, silvestrol may represent a proof-of-concept for an effective new therapeutic strategy in cancer.
ACKNOWLEDGMENTS
We are grateful to Yicheng Mao for excellent assistance with in vivo experiments.
GRANT SUPPORT
This work was funded by the National Institutes of Health (NCI P50 CA140158). Silvestrol for all experiments was provided by A. D. Kinghorn (NCI P01 CA125066).
Footnotes
Conflicts of Interest: No authors have conflicts of interest with this work
REFERENCES
- 1.Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- 2.Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9:785–797. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 4.Toogood PL, Harvey PJ, Repine JT, Sheehan DJ, VanderWel SN, Zhou H, et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem. 2005;48:2388–2406. doi: 10.1021/jm049354h. [DOI] [PubMed] [Google Scholar]
- 5.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–1438. [PubMed] [Google Scholar]
- 6.Baughn LB, Di Liberto M, Wu K, Toogood PL, Louie T, Gottschalk R, et al. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 2006;66:7661–7667. doi: 10.1158/0008-5472.CAN-06-1098. [DOI] [PubMed] [Google Scholar]
- 7.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Wang J, Blaser BW, Duchemin AM, Kusewitt DF, Liu T, et al. Pharmacologic inhibition of CDK4/6: mechanistic evidence for selective activity or acquired resistance in acute myeloid leukemia. Blood. 2007;110:2075–2083. doi: 10.1182/blood-2007-02-071266. [DOI] [PubMed] [Google Scholar]
- 9.Wiedemeyer WR, Dunn IF, Quayle SN, Zhang J, Chheda MG, Dunn GP, et al. Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc Natl Acad Sci U S A. 2010;107:11501–11506. doi: 10.1073/pnas.1001613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. [cited October 2009]; www.clinicaltrials.gov. Available from:
- 11.Leonard JP, Lacasce AS, Smith MR, Noy A, Chirieac LR, Rodig SJ, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood. 2012 doi: 10.1182/blood-2011-10-388298. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2010;117:26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez V, Hartmann E, Ott G, Campo E, Rosenwald A. Pathogenesis of mantle-cell lymphoma: all oncogenic roads lead to dysregulation of cell cycle and DNA damage response pathways. J Clin Oncol. 2005;23:6364–6369. doi: 10.1200/JCO.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Hwang BY, Su BN, Chai H, Mi Q, Kardono LB, Afriastini JJ, et al. Silvestrol and episilvestrol, potential anticancer rocaglate derivatives from Aglaia silvestris. J Org Chem. 2004;69:3350–3358. doi: 10.1021/jo040120f. ibid. 6156. [DOI] [PubMed] [Google Scholar]
- 15.Lucas DM, Edwards RB, Lozanski G, West DA, Shin JD, Vargo MA, et al. The novel plant-derived agent silvestrol has B-cell selective activity in chronic lymphocytic leukemia and acute lymphoblastic leukemia in vitro and in vivo. Blood. 2009;113:4656–4666. doi: 10.1182/blood-2008-09-175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bordeleau ME, Robert F, Gerard B, Lindqvist L, Chen SM, Wendel HG, et al. Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. J Clin Invest. 2008;118:1–11. doi: 10.1172/JCI34753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cencic R, Carrier M, Galicia-Vazquez G, Bordeleau ME, Sukarieh R, Bourdeau A, et al. Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, silvestrol. PLoS One. 2009;4:e5223. doi: 10.1371/journal.pone.0005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schatz JH, Oricchio E, Wolfe AL, Jiang M, Linkov I, Maragulia J, et al. Targeting cap-dependent translation blocks converging survival signals by AKT and PIM kinases in lymphoma. J Exp Med. 2011;208:1799–1807. doi: 10.1084/jem.20110846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coiffier B, Ribrag V. Exploring mammalian target of rapamycin (mTOR) inhibition for treatment of mantle cell lymphoma and other hematologic malignancies. Leuk Lymphoma. 2009;50:1916–1930. doi: 10.3109/10428190903207548. [DOI] [PubMed] [Google Scholar]
- 20.Klier M, Anastasov N, Hermann A, Meindl T, Angermeier D, Raffeld M, et al. Specific lentiviral shRNA-mediated knockdown of cyclin D1 in mantle cell lymphoma has minimal effects on cell survival and reveals a regulatory circuit with cyclin D2. Leukemia. 2008;22:2097–2105. doi: 10.1038/leu.2008.213. [DOI] [PubMed] [Google Scholar]
- 21.Menu E, Garcia J, Huang X, Di Liberto M, Toogood PL, Chen I, et al. A novel therapeutic combination using PD 0332991 and bortezomib: study in the 5T33MM myeloma model. Cancer Res. 2008;68:5519–5523. doi: 10.1158/0008-5472.CAN-07-6404. [DOI] [PubMed] [Google Scholar]
- 22.Kuo TC, Chavarria-Smith JE, Huang D, Schlissel MS. Forced expression of cyclin-dependent kinase 6 confers resistance of pro-B acute lymphocytic leukemia to Gleevec treatment. Mol Cell Biol. 2011;31:2566–2576. doi: 10.1128/MCB.01349-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amin HM, McDonnell TJ, Medeiros LJ, Rassidakis GZ, Leventaki V, O'Connor SL, et al. Characterization of 4 mantle cell lymphoma cell lines. Arch Pathol Lab Med. 2003;127:424–431. doi: 10.5858/2003-127-0424-COMCLC. [DOI] [PubMed] [Google Scholar]
- 24.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of hematological malignancies report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Mod Pathol. 2000;13:193–207. doi: 10.1038/modpathol.3880035. [DOI] [PubMed] [Google Scholar]
- 25.Hussain SR, Lucas DM, Johnson AJ, Lin TS, Bakaletz AP, Dang VX, et al. Flavopiridol causes early mitochondrial damage in chronic lymphocytic leukemia cells with impaired oxygen consumption and mobilization of intracellular calcium. Blood. 2008;111:3190–3199. doi: 10.1182/blood-2007-10-115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucas DM, Davis ME, Parthun MR, Mone AP, Kitada S, Cunningham KD, et al. The histone deacetylase inhibitor MS-275 induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia cells. Leukemia. 2004;18:1207–1214. doi: 10.1038/sj.leu.2403388. [DOI] [PubMed] [Google Scholar]
- 27.Darzynkiewicz Z, Bedner E, Smolewski P. Flow cytometry in analysis of cell cycle and apoptosis. Semin Hematol. 2001;38:179–193. doi: 10.1016/s0037-1963(01)90051-4. [DOI] [PubMed] [Google Scholar]
- 28.Alinari L, Mahoney E, Patton J, Zhang X, Huynh L, Earl CT, et al. FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death. Blood. 2011;118:6893–6903. doi: 10.1182/blood-2011-06-363879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alinari L, Yu B, Christian BA, Yan F, Shin J, Lapalombella R, et al. Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma. Blood. 2011;117:4530–4541. doi: 10.1182/blood-2010-08-303354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Alinari L, Chen CS, Yan F, Dalton JT, Lapalombella R, et al. FTY720 shows promising in vitro and in vivo preclinical activity by downmodulating Cyclin D1 and phospho-Akt in mantle cell lymphoma. Clin Cancer Res. 2010;16:3182–3192. doi: 10.1158/1078-0432.CCR-09-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta SV, Sass EJ, Davis ME, Edwards RB, Lozanski G, Heerema NA, et al. Resistance to the translation initiation inhibitor silvestrol is mediated by ABCB1/P-glycoprotein overexpression in acute lymphoblastic leukemia cells. AAPS J. 2011;13:357–364. doi: 10.1208/s12248-011-9276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saradhi UV, Gupta SV, Chiu M, Wang J, Ling Y, Liu Z, et al. Characterization of silvestrol pharmacokinetics in mice using liquid chromatography-tandem mass spectrometry. AAPS J. 2011;13:347–356. doi: 10.1208/s12248-011-9273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanelle J, Putzer BM. E2F1-induced apoptosis: turning killers into therapeutics. Trends Mol Med. 2006;12:177–185. doi: 10.1016/j.molmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Henson ES, Gibson EM, Villanueva J, Bristow NA, Haney N, Gibson SB. Increased expression of Mcl-1 is responsible for the blockage of TRAIL-induced apoptosis mediated by EGF/ErbB1 signaling pathway. J Cell Biochem. 2003;89:1177–1192. doi: 10.1002/jcb.10597. [DOI] [PubMed] [Google Scholar]
- 35.Kim SH, Ricci MS, El-Deiry WS. Mcl-1: a gateway to TRAIL sensitization. Cancer Res. 2008;68:2062–2064. doi: 10.1158/0008-5472.CAN-07-6278. [DOI] [PubMed] [Google Scholar]
- 36.Roue G, Perez-Galan P, Lopez-Guerra M, Villamor N, Campo E, Colomer D. Selective inhibition of IkappaB kinase sensitizes mantle cell lymphoma B cells to TRAIL by decreasing cellular FLIP level. J Immunol. 2007;178:1923–1930. doi: 10.4049/jimmunol.178.3.1923. [DOI] [PubMed] [Google Scholar]
- 37.Cencic R, Carrier M, Trnkus A, Porco JA, Jr, Minden M, Pelletier J. Synergistic effect of inhibiting translation initiation in combination with cytotoxic agents in acute myelogenous leukemia cells. Leuk Res. 2010;34:535–541. doi: 10.1016/j.leukres.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005;280:35217–35227. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- 39.Tang R, Faussat AM, Majdak P, Marzac C, Dubrulle S, Marjanovic Z, et al. Semisynthetic homoharringtonine induces apoptosis via inhibition of protein synthesis and triggers rapid myeloid cell leukemia-1 down-regulation in myeloid leukemia cells. Mol Cancer Ther. 2006;5:723–731. doi: 10.1158/1535-7163.MCT-05-0164. [DOI] [PubMed] [Google Scholar]
- 40.Panwalkar A, Verstovsek S, Giles FJ. Mammalian target of rapamycin inhibition as therapy for hematologic malignancies. Cancer. 2004;100:657–666. doi: 10.1002/cncr.20026. [DOI] [PubMed] [Google Scholar]
- 41.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 43.Giaisi M, Kohler R, Fulda S, Krammer PH, Li-Weber M. Rocaglamide and a XIAP inhibitor cooperatively sensitize TRAIL-mediated apoptosis in Hodgkin's lymphomas. Int J Cancer. 2011;81:713–722. doi: 10.1002/ijc.26458. [DOI] [PubMed] [Google Scholar]
- 44.Kim S, Salim AA, Swanson SM, Kinghorn AD. Potential of cyclopenta[b]benzofurans from Aglaia species in cancer chemotherapy. Anticancer Agents Med Chem. 2006;6:319–345. doi: 10.2174/187152006777698123. [DOI] [PubMed] [Google Scholar]
- 45.Baumann B, Bohnenstengel F, Siegmund D, Wajant H, Weber C, Herr I, et al. Rocaglamide derivatives are potent inhibitors of NF-kappa B activation in T-cells. J Biol Chem. 2002;277:44791–44800. doi: 10.1074/jbc.M208003200. [DOI] [PubMed] [Google Scholar]
- 46.McClellan KA, Slack RS. Specific in vivo roles for E2Fs in differentiation and development. Cell Cycle. 2007;6:2917–2927. doi: 10.4161/cc.6.23.4997. [DOI] [PubMed] [Google Scholar]