Assembly of bacterial 30S ribosomal subunits requires structural rearrangements to both its 16S rRNA and ribosomal protein components. Ribosomal protein S4 nucleates 30S assembly and associates rapidly with the 5′ domain of the 16S rRNA. Using autogenesis, chemical probing, and gel shift analyses, the authors show that early S4–RNA interactions guide rRNA folding and impact late steps of 30S assembly.
Keywords: ribosome assembly, ribosomal protein S4, SHAPE, α-operon, pseudoknot, RNA–protein interaction
Abstract
Assembly of bacterial 30S ribosomal subunits requires structural rearrangements to both its 16S rRNA and ribosomal protein components. Ribosomal protein S4 nucleates 30S assembly and associates rapidly with the 5′ domain of the 16S rRNA. In vitro, transformation of initial S4–rRNA complexes to long-lived, mature complexes involves refolding of 16S helix 18, which forms part of the decoding center. Here we use targeted mutagenesis of Geobacillus stearothermophilus S4 to show that remodeling of S4–rRNA complexes is perturbed by ram alleles associated with reduced translational accuracy. Gel mobility shift assays, SHAPE chemical probing, and in vivo complementation show that the S4 N-terminal extension is required for RNA binding and viability. Alanine substitutions in Y47 and L51 that interact with 16S helix 18 decrease S4 affinity and destabilize the helix 18 pseudoknot. These changes to the protein–RNA interface correlate with no growth (L51A) or cold-sensitive growth, 30S assembly defects, and accumulation of 17S pre-rRNA (Y47A). A third mutation, R200A, over-stabilizes the helix 18 pseudoknot yet results in temperature-sensitive growth, indicating that complex stability is finely tuned by natural selection. Our results show that early S4–RNA interactions guide rRNA folding and impact late steps of 30S assembly.
INTRODUCTION
Bacterial ribosome assembly is necessary for cellular growth and requires the precise formation of many protein–rRNA contacts. The hierarchical addition of ribosomal proteins to the 16S rRNA (Held et al. 1974) arises from conformational changes to the rRNA induced by early binding proteins. In some examples, primary assembly proteins capture the folded structure of the rRNA (Weeks and Cech 1996; Menichelli et al. 2007), in turn stabilizing rRNA tertiary interactions and the binding sites of secondary assembly proteins (Agalarov and Williamson 2000; Ramaswamy and Woodson 2009a). Many ribosomal proteins also change structure when they join the complex, however, and the resulting mutually induced fit is expected to increase the specificity of assembly (Williamson 2000; Bokinsky et al. 2006). How specific ribosomal proteins have evolved to restructure the rRNA is not understood.
Protein S4 nucleates assembly of the 16S 5′ and central domains (Nowotny and Nierhaus 1988; Williamson 2000) and is one of the first proteins to associate with pre-rRNA transcripts (Talkington et al. 2005; Adilakshmi et al. 2008; Bunner et al. 2010; Mayerle et al. 2011). S4 forms extensive contacts with a five-helix junction in the 5′ domain (5′ dom) of the 16S rRNA (Powers and Noller 1995a; Brodersen et al. 2002), and S4 binding induces widespread conformational rearrangements in the 16S RNA (Stern et al. 1989). Previous studies using a minimal RNA containing just the five-way-junction (5WJ) (Bellur and Woodson 2009) showed that S4 initially forms a labile complex, stabilizing only a subset of native 16S interactions within the junction region, including a conserved pseudoknot in helix (H) 18 that orients G530 for its proper interactions with A-site tRNAs in the decoding center of the ribosome (Powers and Noller 1991). During the slower transition to a stable, mature complex, H3 and H4 stack at right angles, and loops in H16 and H18 take on their native state (Mayerle et al. 2011). Similar structural differences were observed between S4–16S complexes formed at 0°C and 42°C (Powers and Noller 1995b), and in both cases, co-incubation of S4 and the rRNA is required. How S4 directs these structural rearrangements in the 16S rRNA after initial binding is not known.
In addition to its roles in 30S assembly, Escherichia coli S4 maintains the balance between rRNA and ribosomal proteins by repressing translation of the α-operon (Nomura et al. 1980; Yates et al. 1980; Deckman and Draper 1987), which encodes S4 together with ribosomal proteins S13, S11, and L17 and the α-subunit of RNA polymerase (Olsson and Isaksson 1979b). When rRNA levels are limiting, S4 also up-regulates rRNA expression by acting as a general transcription anti-terminator (Torres et al. 2001) and increasing the transcription of ribosomal rRNA rrn operons (Takebe et al. 1985).
Free S4 consists of two conserved globular domains (Davies et al. 1998; Markus et al. 1998) and a 40-residue N-terminal extension, which appears disordered in solution NMR experiments (Sayers et al. 2000). The N-terminal peptide makes extensive and sequence-specific interactions with 16S helix 16 in the ribosome (Brodersen et al. 2002). As this extension is missing in RPS9 eukaryotic and archaeal homologs of S4, it may have evolved in concert with H16 in the bacterial ribosome (Chen et al. 2009). The globular domains of S4 contact H3, H4, H17, and H18 in the 5WJ.
E. coli S4 was first genetically identified in screens for translation factors. The ram or ribosomal ambiguity mutants characterized by increased error rates (Rosset and Gorini 1969; Olsson et al. 1974) mapped to amino acid substitutions and frequent C-terminal truncations of S4 and occasionally to S5 (Olsson and Isaksson 1979b). In vitro experiments using S4 obtained from ram strains showed that many of these mutant proteins were able to incorporate into ribosomes (Changchien and Craven 1978). However, the ribosomes were dysfunctional, and the mutants bound the rRNA less tightly than wild-type (WT) S4 (Olsson and Isaksson 1979a; Allen and Noller 1989). Chemical probing studies suggested at least some ram mutations lower fidelity by changing the structure of the protein–rRNA interface rather than the S4–S5 protein interface (Allen and Noller 1989; Vallabhaneni and Farabaugh 2009).
To ask if conserved S4 residues act directly in remodeling the 16S structure, we introduced S4 mutations designed to disrupt the formation of stable S4:rRNA complexes. S4 binding assays, selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE) structure probing, and in vivo complementation of an rpsD chromosomal deletion showed these mutations change S4 affinity and shift the relative populations of stable and labile S4 complexes in vitro. These structural perturbations to the S4–rRNA complex correlated with cold- and temperature-sensitive bacterial growth, incomplete 16S maturation, and strong defects in 30S biogenesis. In vitro binding assays showed the point mutations have little effect on S4 binding to a regulatory pseudoknot in the α-operon mRNA, indicating these in vivo effects are independent of S4’s regulation of ribosomal protein synthesis.
RESULTS
The flexible N terminus of S4 is required for stable binding
To understand how S4 specifically induces the conformation of the 16S rRNA observed in the ribosome, we first deleted the disordered N-terminal peptide (S4:Δ41) (Fig. 1A, red), which is conserved among bacteria but lacking in eukaryotes and archaea. The truncated S4 protein and other mutant S4 proteins were correctly folded at 25°C, as judged by far-UV circular dichroism spectroscopy (data not shown).
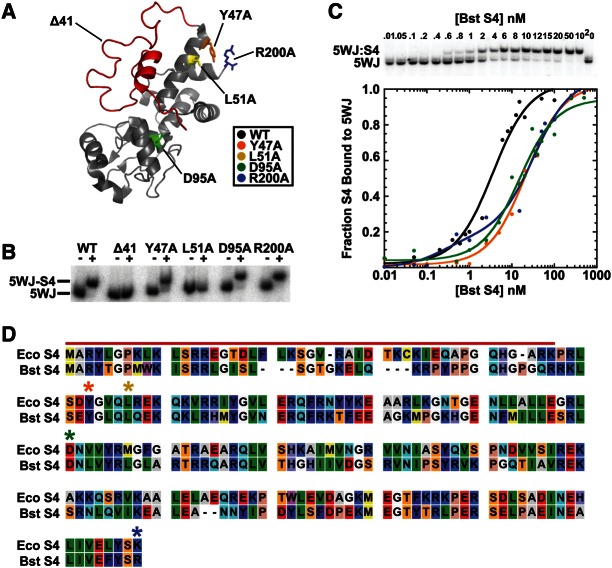
FIGURE 1.
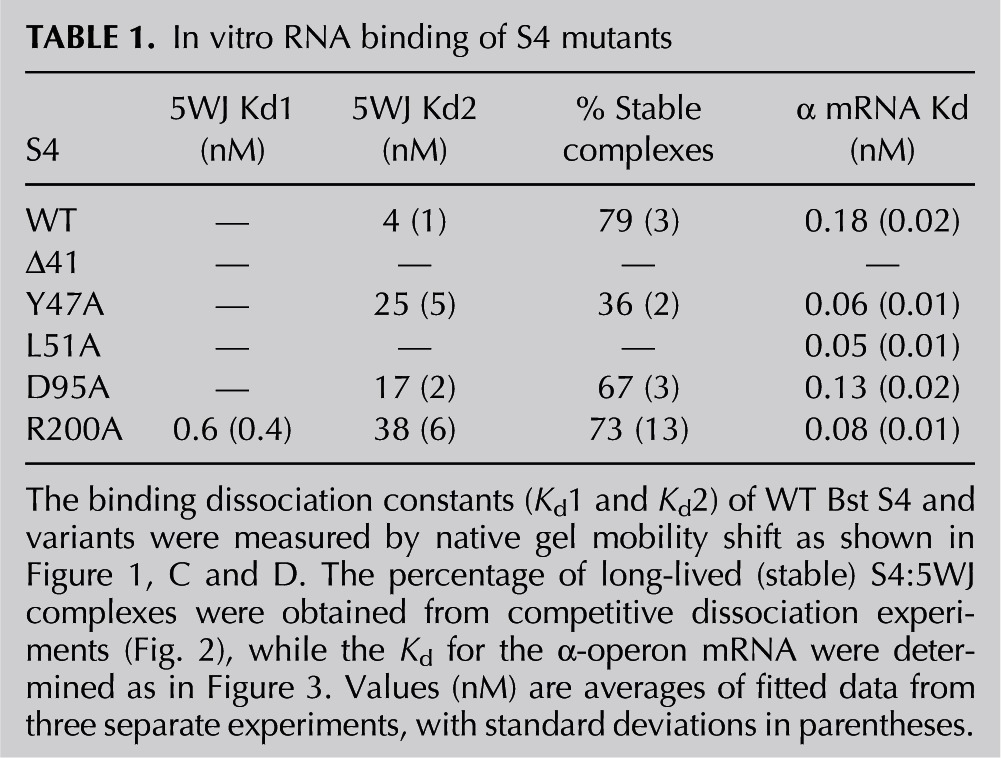
S4 mutations affect binding to 5WJ RNA. (A) Sites of mutated residues in Bst S4 are depicted on the crystal structure of Eco S4 (PDB: 2AVY). Red indicates N-terminal residues truncated in S4:Δ41. Other colors indicate individual alanine substitutions (Y47[Y50], orange; L51[L54], yellow; D95[D98], green; and R200[L205], blue). (B) Native gel mobility shift (EMSA) showing 32P-labeled 5WJ RNA alone (−) or with an excess of the indicated Bst S4 protein (+). (C) Representative EMSA experiment used to determine binding affinity for the 5WJ RNA. The proportion of 5WJ bound to S4 (fB) is plotted versus S4 concentration for all variants able to bind in the EMSA assay and fit to fB = ([S4]/Kd)/(1 + [S4]/Kd). Values for the Kd are listed in Table 1. (D) Alignment of Eco and Bst S4 protein sequences. Red bar indicates the S4:Δ41 deletion. Colored asterisks indicate sites of Bst S4 mutations. The alignment was made using CLC Free Workbench 4 (CLC Bio).
We chose to use Geobacillus stearothermophilus (Bst) S4 in this study, because Bst S4 is more stable than E. coli (Eco) S4 in solution, and binds the E. coli 16S rRNA with equal affinity and specificity (Gerstner et al. 2001). The secondary and tertiary structure of the S4 binding site is strongly conserved among different bacteria (Woese et al. 1975; Gutell et al. 1985), and the structure of S4 itself, particularly at positions that contact the rRNA, is also well conserved (Davies et al. 1998; Chen et al. 2009). As a result, Bst S4 forms specific native-like complexes with the E. coli 16S 5′ dom and a minimal 5WJ fragment (Mayerle et al. 2011). We used the minimal 5WJ RNA for our in vitro assays, because S4 binds tightly to the 5WJ in the absence of other proteins, nonspecific binding is minimized, and native-like complexes form in 4 mM Mg2+ compared with the less physiologically relevant 20 mM Mg2+ used in ribosome reconstitution experiments.
Early studies demonstrated that an S4 fragment missing the N-terminal flexible extension (S4:Δ41) (Fig. 1A, red) bound 16S rRNA with low micromolar affinity (Changchien and Craven 1976, 1978; Conrad and Craven 1987) and does not significantly rearrange its structure upon binding (Newberry et al. 1977). Ribosome reconstitution experiments by Changchien and Craven (1976) and, later, Conrad and Craven (1987) showed that the S4 fragment could incorporate into ribosomal particles, although these particles were missing a subset of secondary and tertiary binding proteins. Structural studies show that truncation of S4 does not change the structure of the globular domain (Markus et al. 1998).
To test if S4:Δ41 was capable of binding the 5WJ RNA with high (nanomolar) affinity, subnanomolar concentrations of 32P-labeled 5WJ were folded and then incubated with an excess of protein. Complexes were detected by native gel mobility shift. As shown in Figure 1B, S4:Δ41 was not able to stably bind the 5WJ, indicating that the N terminus of S4 is required for high-affinity 5WJ binding. We were also unable to detect binding by SHAPE modification (see below). The observation that the N terminus is needed for tight binding is supported by previous chemical probing experiments showing that 16S H16, which interacts with the S4 N terminus, rearranges during stable complex formation (Mayerle et al. 2011). Interactions between H16 and the N terminus of S4 were also important for binding in MD and Gö simulations (Chen et al. 2012).
S4 point mutants can both strengthen and decrease 5WJ affinity
To test the role of the C-terminal domains in 16S rRNA recognition, we introduced single alanine substitutions designed to disrupt specific S4:5WJ interactions previously suggested by genetic or biochemical experiments to play a role in ribosome assembly and translation. These sites were conserved in bacteria and, in most cases, also conserved in archaeal and eukaryotic homologs (RPS9) of S4 (Ben-Shem et al. 2010; Chen et al. 2010). D95 is located in the globular domain 2 of S4 (Fig. 1A, green), where it forms a salt bridge with R93, ordering the surrounding basic amino acids (especially the conserved R111). Proper ordering of these amino acids is hypothesized to be crucial for RNA binding and protein stability (Davies et al. 1998). Next, we mutated a conserved tyrosine (Bst Y47) (Fig. 1A, orange) and a leucine (L51) (Fig. 1A, yellow) that contact the 16S rRNA at nucleotides 509–510, which are adjacent to the H18 pseudoknot (nucleotides 505–507). A missense mutation at the tyrosine (E. coli Y51D) is cold-sensitive and suppresses a mutant RF1 temperature-sensitive phenotype, but at the cost of increased read-through of amber stop codons (Dahlgren and Ryden-Aulin 2000). Finally, we substituted the C-terminal R200 (Fig. 1A, blue), which lies between H18 and H3, and is predicted to be critical for pseudoknot formation (Davies et al. 1998). Modification of the N-terminal lysine of E. coli S4 decreased binding affinity for 16S rRNA (Daya-Grosjean et al. 1974). In addition, many ram mutants are C-terminal truncations, indicating the importance of the basic residue at the S4 C terminus in both ribosome assembly and translation (Olsson and Isaksson 1979b; Olsson 1979).
The S4:Y47A, S4:D95A, and S4:R200A variants formed stable complexes with the 5WJ RNA (Fig. 1B). In contrast, S4:L51A interacted weakly with the RNA and did not produce a clear shift in gel mobility (Fig. 1B). To determine more precisely how the mutations affect RNA binding, 32P-labeled 5WJ RNA was titrated with S4 in 4 mM MgCl2 at 42°C (Fig. 1C). Bst S4 binds the 5WJ with an apparent Kd of 4 nM (Fig. 1C; Table 1; Bellur and Woodson 2009). All of the mutations (S4:Y47A, S4:D95A, and S4:R200A) weakened S4 affinity for the 5WJ, raising the Kd to 25 nM, 17 nM, and 38 nM, respectively (Fig. 1C; Table 1). Interestingly, the S4:R200A mutation increased the apparent proportion of a high-affinity complex (Kd = 0.6 nM) (Table 1), suggesting this mutation stabilizes a subset of stable S4:5WJ complexes. Thus, mutations at residues in the C-terminal domains linked to defects in ribosome function perturb the S4–RNA interactions.
TABLE 1.
In vitro RNA binding of S4 mutants
Mutants alter how stable complexes form
We previously used kinetic competition assays to show that Bst S4 and the 5WJ first form a labile complex, which rearranges to a stable, long-lived complex after 1–3 min co-incubation (Mayerle et al. 2011). The same assay was used to determine if the S4 mutations alter its propensity to form a stable complex with the 5WJ RNA (see Materials and Methods). S4 complexes with 32P-labeled 5WJ were chased with an excess of unlabeled 5WJ competitor to trap any released S4. The rate of loss of the 32P-labeled S4 complex after addition of competitor 5WJ revealed two populations of S4:5WJ complexes with different half-lives, representing labile and stable complexes.
The S4:R200A variant produced a similar proportion of stable complexes (black squares and dashed line, 0.73 ± 0.13) as WT S4 (black circles and solid line, 0.79 ± 0.03). In contrast, S4:D95A modestly reduced this fraction (gray triangles and line, 0.67 ± 0.03), while S4:Y47A was substantially less likely to form a long-lived complex than WT S4 (open circles and dashed line, 0.36 ± 0.02) (Fig. 2; Table 1). The inability to convert to a long-lived complex likely contributes to the decreased equilibrium binding affinity of this mutant and indicates that the S4:Y47A mutation may impair remodeling of the 5WJ structure, such as the stabilization of the H18 pseudoknot that occurs in the transition between labile and stable complexes. A similar remodeling defect could explain why S4:L51A did not produce a stable shift in gel mobility (Fig. 1B).
FIGURE 2.
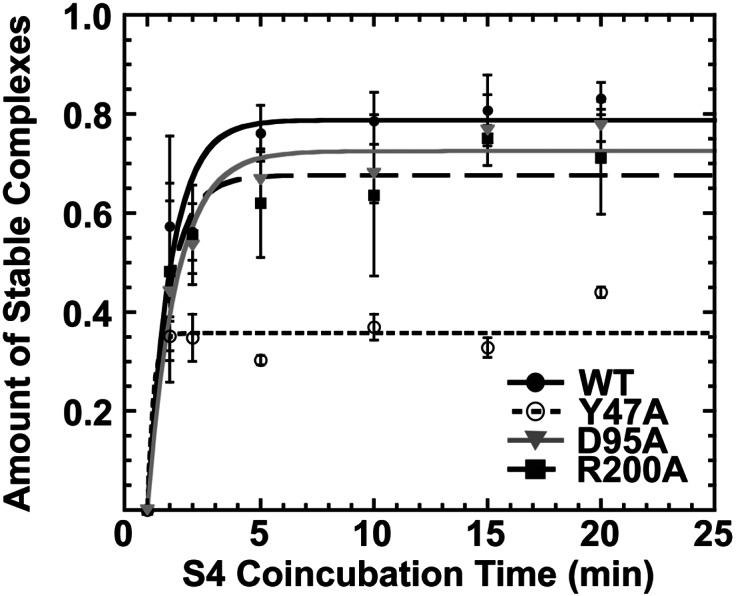
S4:Y47A forms fewer stable complexes. Kinetic competition assays (Mayerle et al. 2011) were used to determine the proportion of stable complexes after 1–20 min incubation with each S4 variant (see Materials and Methods). Symbols and error bars represent the average and standard deviation of at least three experiments. The results are summarized in Table 1.
Only Δ41 strongly affects α-operon mRNA binding in vitro
We used native EMSA to determine if the S4 mutants could bind the α-operon mRNA to assess whether changes in its regulation could account for any potential ribosome biogenesis defects. Subnanomolar prefolded 32P-labeled α-operon pseudoknot RNA (α-mRNA) was incubated with increasing concentrations of S4 protein at 42°C to allow pseudoknot:S4 complexes to come to equilibrium (Schlax et al. 2001). The pseudoknot adopts two conformers, the higher mobility of which preferentially binds S4 and shifts to the top of the gel (Fig. 3; Table 1). Depletion of free α-mRNA band was used to measure the extent of S4 binding, as a small fraction of the α-mRNA migrates at the top of the gel even in the absence of S4 (Fig. 3; Table 1). The shift in α-mRNA mobility due to S4 binding was reversed upon treatment of samples with Proteinase K, confirming it represents the S4–RNA complex and not an aggregate of α-mRNA (Fig. 3).
FIGURE 3.
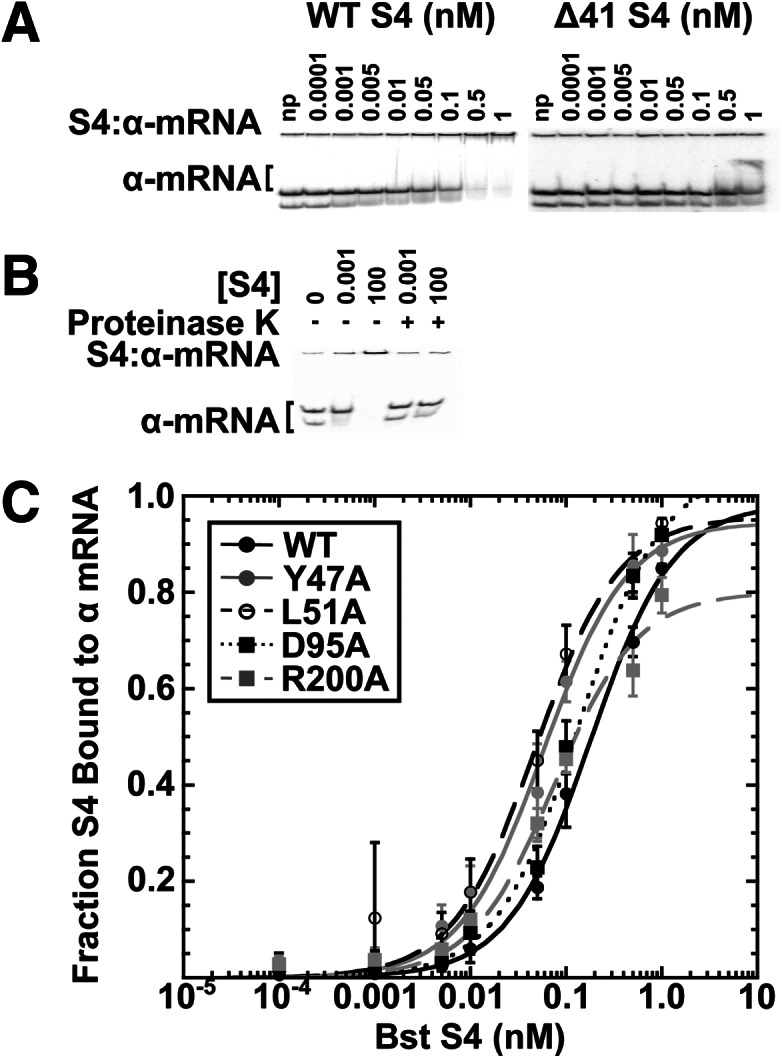
S4:Δ41 cannot stably bind the α-operon mRNA in vitro. (A) Representative EMSA experiment used to determine the Kd of Bst S4 and mutants for the pseudoknot in the α-operon mRNA leader. The α-operon mRNA leader (Schlax et al. 2001) migrates as doublet, and added WT S4 preferentially depletes the faster mobility band (left). The Δ41 S4 (right) was unable to shift the RNA mobility. Protein concentrations shown above the lanes; np indicates no protein. A constant fraction of α-mRNA migrates at the top of the gel in the absence of S4 and likely represents an inert aggregate. (B) The gel mobility shift caused by 0.001 or 100 nM S4 (− lanes) is reversed upon incubation of complexes with proteinase K (+ lanes). (C) The proportion of α-mRNA bound to S4 versus S4 concentration for WT S4 and mutants shown in the key; no shift was observed for S4Δ41. The values are fit to fB = ([S4]/Kd)/(1 + [S4]/Kd), and the fit parameters are summarized in Table 1.
WT Bst S4 and S4:D95A both bound the pseudoknot with approximately equal affinity (0.18 nM for WT, 0.13 nM for D95A). Three mutants, S4:Y47A (0.06 nM), S4:L51A (0.05 nM), and S4:R200A (0.08 nM) showed a modest two- to threefold increased affinity compared with WT Bst S4 (Table 1; Fig. 3). S4 mutant Δ41 showed greatly decreased binding affinity for the pseudoknot (Fig. 3, upper right gel), indicating that the N-terminal extension is also important for pseudoknot recognition. These data indicate that, aside from S4:Δ41, α-mRNA misregulation by our S4 mutants is unlikely.
SHAPE chemical probing correlates 5WJ affinity to alterations in 5WJ structure
We performed SHAPE to examine how S4 mutants affect the 5WJ RNA structure. SHAPE reports on the relative flexibility of the rRNA backbone (Wilkinson et al. 2006; Mortimer and Weeks 2007; Deigan et al. 2009; Low and Weeks 2010) and also reflects protein–rRNA contacts that alter the relative disorder of the rRNA (Gherghe et al. 2008). Folded 5WJ RNA (see Materials and Methods) was co-incubated with S4 for 10 min to produce stable 5WJ:S4 complexes before modification at 42°C. Primer extension (Fig. 4A) was used to quantify the extent of ribose modification at each position (Fig. 4B,C).
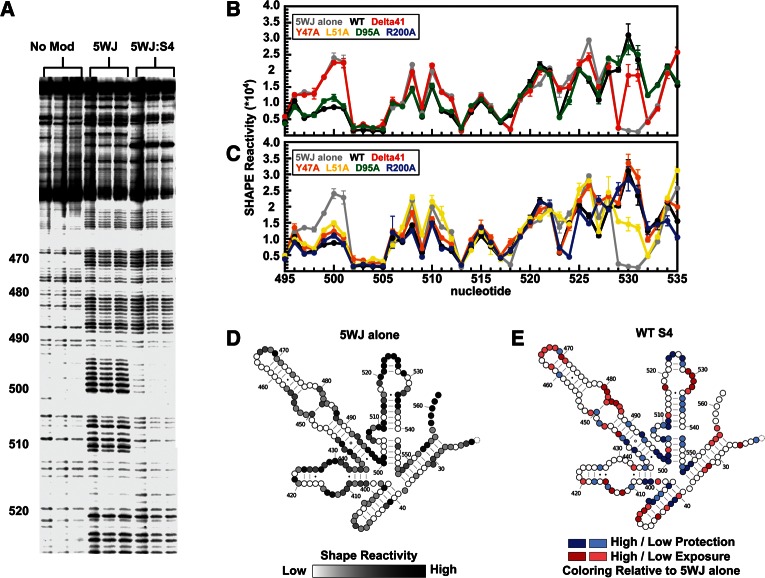
FIGURE 4.
Structural changes to the 5WJ RNA caused by S4 mutants. (A) Representative primer extension gel of NMIA modified 5WJ RNA in 4 mM MgCl2 at 42°C. No mod indicates three duplicate control reactions on unmodified 5WJ RNA; 5WJ, modified 5WJ RNA in the absence of S4; and 5WJ:S4, the effect of S4 on the SHAPE modification pattern. (B,C) Relative SHAPE reactivity, ρ, of nucleotides in H18. Nucleotide number is given on the x-axis, and the value of ρ (see Materials and Methods) is given by the y-axis. Error bars, SD from the mean of three trials. Each mutant is colored as in Figure 1, and the reactivity of the 5WJ alone is shown in gray. (D) Relative NMIA reactivity of the 5WJ RNA in the absence of S4. Darker coloring indicates heavier modification. (E) Effect of WT Bst S4 on 5WJ structure after 10 min co-incubation. Blue indicates moderately and highly protected residues relative to 5WJ alone; red, exposed residues. The stabilizing effect of S4 agrees well with previous 1M7 modification experiments (Mayerle et al. 2011).
SHAPE results on the 5WJ RNA in the absence of S4 (Fig. 4D) agreed with previously published SHAPE results on the native 16S in the absence of proteins (Deigan et al. 2009), indicating that the minimal 5WJ forms the correct secondary structure. As previously observed (Mayerle et al. 2011), WT Bst S4 strongly protected the central 5WJ from modification (nucleotides 402, 437, 439, 440, 497–501, and 545–547) (Fig. 4E). S4 also stabilized the H18 pseudoknot (nucleotides 505, 524, 526) and induced a conformational rearrangement in the H18 530 loop that strongly enhances the reactivity of G530. H16 modifications were consistent with the expected contacts between the internal loop of H16 and the N-terminal extension of S4. Finally, protection of nucleotides 397–400 (H4) reflected the formation of a right angle between H3 and H4 (Fig. 4E).
Figure 5A compares the SHAPE modification patterns of the mutant and WT S4:5WJ complexes. The S4:Δ41 deletion mutant effected virtually no changes to the 5WJ RNA structure (Fig. 4B, cf. red and gray lines). Overall, the pattern and extent of modification with S4:Δ41 was very similar to that of the RNA alone (see opposite colors in Figs. 5A and 3E). These results were consistent with native EMSA results showing that the truncated protein lacking the N-terminal extension cannot bind the RNA in vitro (Fig. 1B).
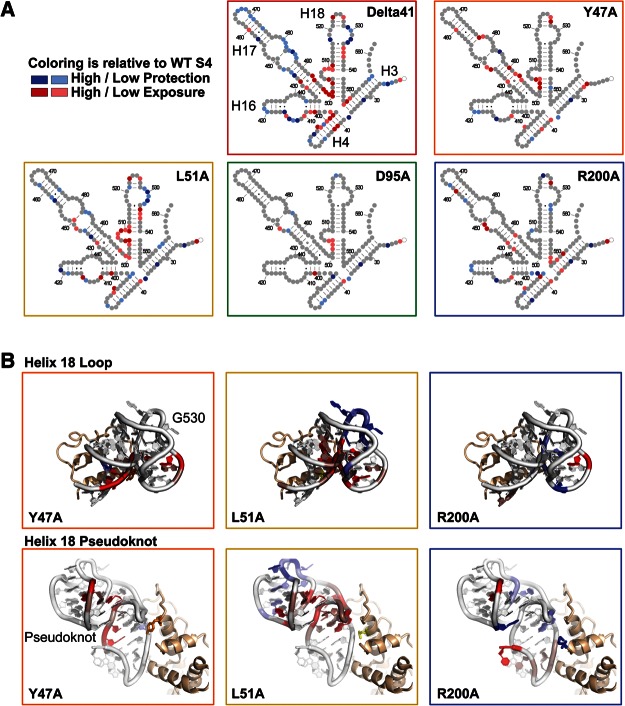
FIGURE 5.
S4 Mutations affect the structures of 5WJ complexes. (A) Secondary structure representations of the 16S 5WJ indicating the relative SHAPE reactivity (τ) of complexes with S4 mutants relative to WT S4 complexes, as indicated in each panel. Color scheme as in Figure 4E. Residues that do not show a significant difference in reactivity in mutant vs. WT complexes are light gray. (B) Relative reactivity (τ) depicted on the mature 16S structure in E. coli ribosomes (PDB 2AVY). Top panels show the “530 loop” of H18 loop; bottom panels show the H18 pseudoknot.
As expected from our binding assays, the single amino acid variants stabilized the 5WJ almost as well as the WT protein (Fig. 5A, gray schematics). However, both S4:Y47A (orange box) and S4:L51A mutations (yellow box) destabilized the H18 pseudoknot (Fig. 5B, red), while S4:L51A also perturbed the structure of the H18 G530 loop (Fig. 5B, blue). Since both of these mutants bind the RNA less tightly than WT S4, these data indicate that specific S4 interactions are needed to stabilize the pseudoknot and refold the 530 loop (Fig. 1C) and that these interactions lead to more stable S4 complexes. Consistent with its tighter binding (Fig. 1C; Table 1), S4:R200A stabilized the pseudoknot slightly better than WT S4 while also increasing the stacking of H3 and H4 and restructuring of H16 (Fig. 5A). The S4:D95A mutation in the core of the protein did not significantly change the structure of the complex, indicating that the slight destabilization of the complex caused by this mutation (Fig. 1C) is not due to the loss of specific protein–RNA interactions.
Bst S4 can complement an E. coli S4 deletion strain
To test the effects of our S4 mutants on ribosome assembly in vivo, we asked whether they complement a partial rpsD chromosomal deletion (Shoji et al. 2011). In strain DH10β, deletion of the initial 176 amino acids encoded by rpsD (S4) is covered by an arabinose-inducible plasmid expressing WT E. coli S4. This plasmid (pCDSSara-S4) also carries a sacB marker to facilitate exchange with plasmids expressing Bst S4 behind an IPTG-inducible promoter (Materials and Methods).
While it had previously been shown that Bst S4 functions in reconstituted 30S subunits in vitro (Higo et al. 1973), we found that Bst S4 can support 30S biogenesis and function in vivo. When WT Bst S4 expressed from pTRC-S4 was the sole source of S4 protein, the doubling time at 37°C (95 min) (Table 2) was similar to that of the same strain expressing Eco S4 (ΔrpsD/CDSSara-S4; 87 min). This doubling time was twice that of a S16 deletion on the same genetic background (43 min), however, indicating a deleterious effect of the chromosomal S4 deletion and plasmid over-expression of either Eco S4 or Bst S4. Interestingly, these strains readily accumulated mutations in the S4 coding sequence, which were usually C-terminal truncations. These S4 mutations may be favored because they compensate the streptomycin-resistant rpsL (S12) allele in the parental strain (Shoji et al. 2011). Because of this, we sequenced rpsD DNA from all bacterial cultures used in subsequent experiments, both before and after the experiment was completed.
TABLE 2.
In vivo characterization of S4 mutants
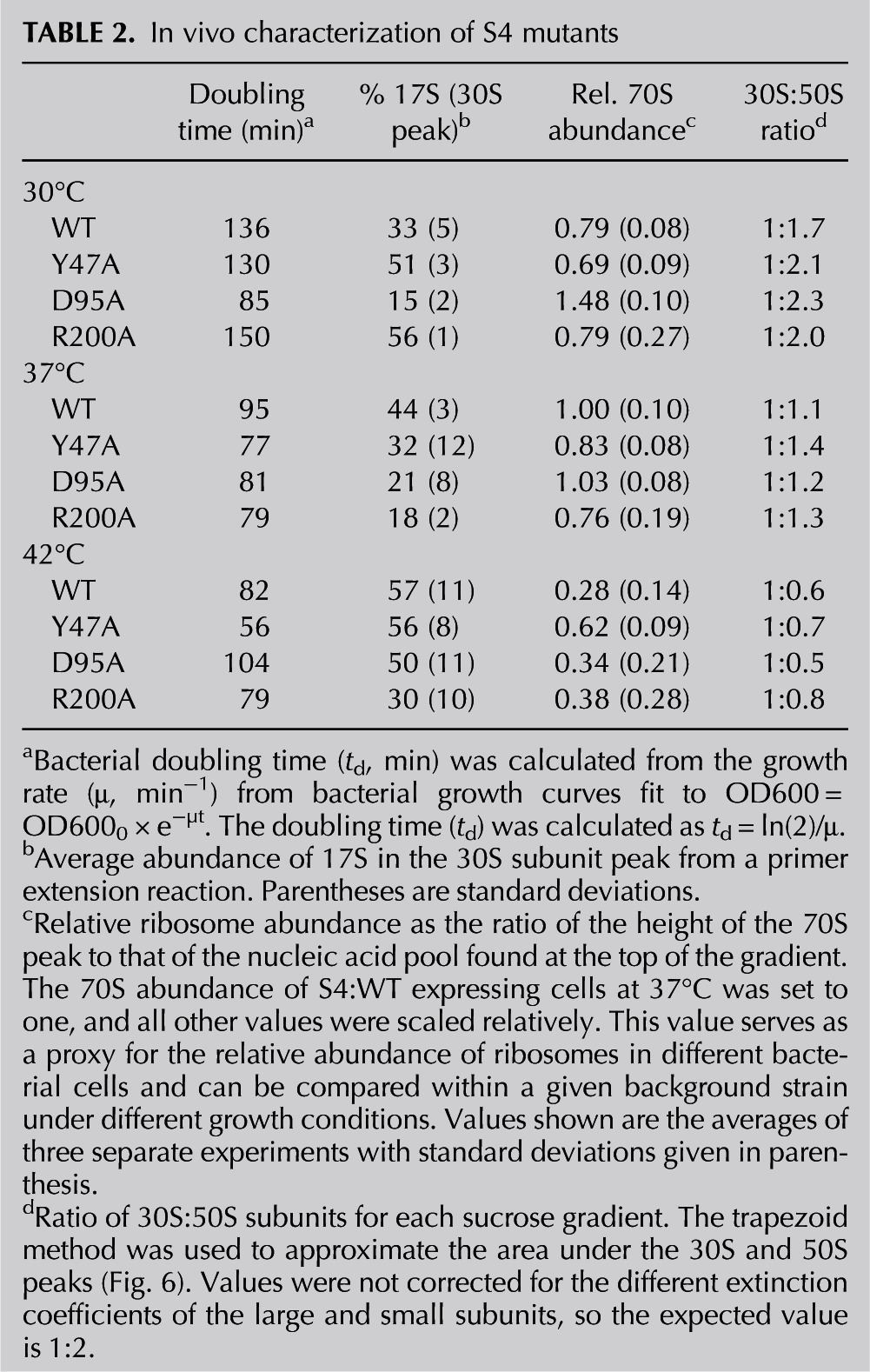
To determine how the Bst S4 variants affected ribosome assembly, we analyzed the ribosomal subunits from actively growing cultures on sucrose gradients. Profiles are shown in Figure 6, and the ratio of 30S:50S peaks are given in Table 2. We also estimated the relative abundance of ribosomes as a ratio of the height of the 70S peak to the height of the cellular RNA peak at the top of the sucrose gradient, scaled to the same ratio for WT Bst S4 at 37°C (Table 2). While this ratio is an approximation, it provides an internal reference for the total level of ribosomes in the bacteria relative to other RNAs.
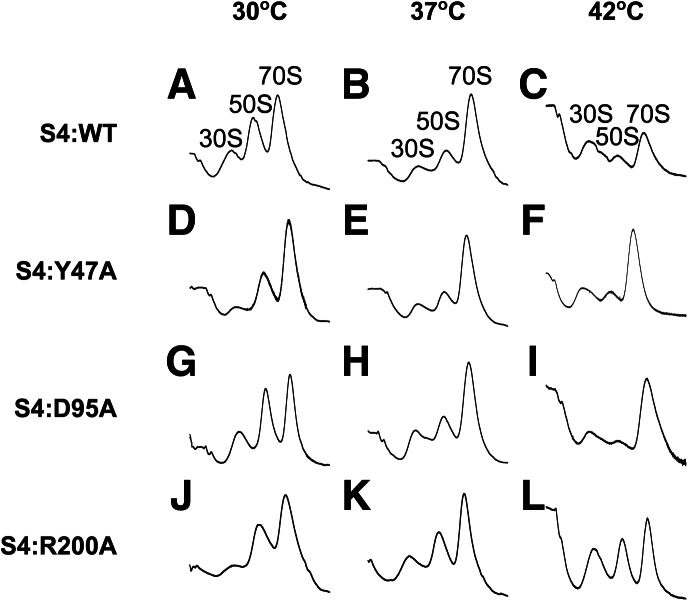
FIGURE 6.
S4 mutations perturb ribosome biogenesis. Ten percent to 40% sucrose gradient profiles from ΔrpsD E. coli expressing Bst S4 (WT and mutants) from pTrc-S4. Cells were grown at 30°C, 37°C, or 42°C. (A–C) S4:WT; (D–F) S4:Y47A; (G–I) S4:D95A; (J–L) S4:R200A. The ratio of the 30S:50S peaks was determined using the trapezoid method and is given in Table 2. The parental strain expressing Eco S4 had equal or greater amounts of free subunits as the strain expressing WT Bst S4.
E. coli expressing WT Bst S4 37°C showed slightly increased and broadened peaks for both the 30S and 50S fractions relative to the 70S. Because we observed similar profiles in the parental strain expressing Eco S4, we believe the numbers of free subunits and the amount of 17S pre-rRNA (see below) are elevated due to S4 overexpression and the genotype of the parental strain (DH10β is rpsL). Nevertheless, we cannot exclude that some effects arise from overexpression of Bst S4 instead of Eco S4. Therefore, we only mutant Bst S4 proteins with the same strain expressing WT Bst S4.
Bacteria with defects in ribosome assembly tend to show temperature-dependent growth defects, especially cold sensitivity (Rosset and Gorini 1969). Lowering the growth temperature to 30°C increased the levels of free subunits, while raising it to 42°C diminished the number of 70S ribosomes and increased the accumulation of 21-26S pre-30S complexes (Fig. 6, Table 2). Despite repeated attempts, we were unable to grow these strains at lower (20°C) or higher (50°C) temperatures.
The Δ41 mutation is lethal
The truncated S4:Δ41 lacking the N-terminal extension was unable to complement growth of the S4 deletion strain under any of the growth conditions tested. While lack of growth may be due to constitutive expression of the α-operon (Fig. 3), there is evidence this truncation leads to improper 30S ribosome assembly. Not only was S4:Δ41 unable to bind the 5WJ in vitro (Fig. 1B), previous reconstitution experiments (Changchien and Craven 1976; Conrad and Craven 1987) showed that complexes containing S4:Δ41 lack a subset of secondary and tertiary binding proteins and were therefore nonfunctional. Thus the intrinsically disordered N-terminal extension of bacterial S4 contributes essential contacts for RNA recognition and viability.
S4 stabilization of the H18 pseudoknot is essential for ribosome function
Our in vitro data show that S4:Y47A and S4:L51A do not fully stabilize the H18 pseudoknot and therefore are unable to properly structure the G530 loop (see Fig. 5, red and yellow boxes), leading to potential decoding defects. This effect is more pronounced in S4:L51A. In contrast, S4:R200A strengthens the interaction between S4 and the 5WJ portion of 16S rRNA (Figs. 1C, 5). Strikingly, bacteria expressing S4:L51A, S4:Y47A, or S4:R200A as their sole source of S4 exhibited different phenotypes that correlate to pseudoknot stability in vitro. S4:Y47A was cold-sensitive, growing more slowly than the WT Bst S4 strain at 30°C and more rapidly at 42°C (Table 2). This faster growth rate correlated with a greater proportion of 70S ribosomes at 42°C in the Y47A strain (Table 2). The S4:L51A mutation was lethal, suggesting that the destabilization of H18 for this mutant is too severe. In contrast, expression of S4:R200A, which stabilizes the H18 pseudoknot, grew similarly to WT Bst S4 at 37°C but much slower than the comparison strain at warmer temperatures (Table 2). Thus, despite the many steps in 30S synthesis, we observe a link between the pseudoknot stability and growth rate consistent with the important role of the H18 pseudoknot in translation (Powers and Noller 1995a).
Growth and rRNA processing in S4 D95A are cold-sensitive
The S4:D95A mutation was predicted to disrupt a network of salt-bridges and other stabilizing interactions in the globular domain of S4, thereby weakening interactions between the globular domain and the RNA. Although this destabilization had no effect on 5WJ binding in vitro, it resulted in poor growth at 42°C (Table 2), consistent with diminished stability of the protein. The D95A mutant grew better than the control strain expressing WT Bst S4 at 30°C and 37°C (Table 2). At 42°C, however, the cell density increased only linearly and leveled off after 350 min, although the fraction of 70S ribosomes remained slightly higher than in the WT control strain (Table 2).
S4 mutants alter 17S to 16S rRNA processing
We next asked whether slow growth and the broad 30S peaks in our sucrose gradients arise from improper assembly of 30S ribosomes. Following cleavage by RNase III to liberate the 17S pre-rRNA, further maturation of the 16S 5′ end by RNase E and RNase G is linked to recruitment of tertiary assembly proteins and proper assembly of the 30S subunit (Culver 2003). Thus incomplete assembly often results in accumulation of 17S pre-rRNA.
RNA from 30S and 70S peaks in sucrose gradients was isolated, and the degree of processing was measured by extending a primer bound near the 5′ end of the 16S rRNA. The 70S fractions contained little 17S pre-rRNA, while the lightest 30S fractions contained the most 17S RNA, as expected. In the WT Bst S4 strain, 16S processing became less efficient with temperature, based on the proportion of 17S rRNA remaining (Table 2). Decreased rRNA processing correlated with an increased heterogeneity of small subunits at 42°C (Fig. 6). Thus, while the strain expressing Bst S4 grew slightly faster at 42°C, this was offset by temperature-dependent defects in 30S biogenesis.
Individual S4 mutations affected rRNA processing differently. In the S4:D95A strain, 17S rRNA levels were correlated with growth, with less 17S at 30°C and 37°C, at which this strain grows better than the WT control, and much more 17S at 42°C, at which the D95A strain grew poorly (Table 2). Contrary to this, 17S rRNA processing was relatively unaffected by temperature in S4:Y47A cells (Table 2). Expression of S4:R200A resulted in similar rRNA processing as the S4:Y47A strain at 30°C but became more efficient at 37°C and 42°C, although the rate of cell growth diminished (Table 2). These data showed that S4 alleles that alter the protein–RNA interface also affect rRNA processing and maturation. As S4 alleles that stabilize and destabilize 16S H18 both perturbed cell growth, these data suggested that S4 acts at more than one stage of 30S biogenesis.
DISCUSSION
Ribosomal protein S4 plays a crucial role in the early stages of 30S assembly. S4 binding not only stabilizes the conformation of the 5WJ but induces widespread conformational rearrangements in the 16S RNA (Stern et al. 1989), explaining its role in nucleating assembly of the 16S 5′ and central domains. Co-incubation with S4 induces specific conformational changes in the RNA, including a kink in H16, docking of H3 with H18, and stabilization of the universal H18 pseudoknot that is essential for translation fidelity (Woese et al. 1975) and that orients G530 for participation in mRNA decoding in the mature ribosome (Schuwirth et al. 2005). Here we show that specific S4 interactions are needed to remodel the structure of the 5WJ and that incomplete remodeling by S4 mutants correlates with defects in 30S biogenesis and poor growth. As discussed below, our S4 binding assays suggest the physiological effects of the S4 mutations are best explained if S4 acts during multiple stages of 30S assembly, rather than by misregulation of the α-operon. Below we propose a model for how S4, which binds the 16S rRNA early in assembly, can guide rRNA folding at late stages of assembly and subunit maturation.
The S4 N terminus is required for stable S4:5WJ complexes
The S4 N terminus accounts for a relatively small proportion of the total contacts between the 5WJ and S4 in crystal structures of 30S subunits but is nonetheless essential for stable binding, giving it a disproportionate significance in rRNA recognition. S4 amino acids 31–46, which bridge the interface between H16 and H18, are required for the RI to RI* transition (Changchien and Craven 1978). These observations support a role for the S4 N terminus during the transition to the stable complex. Recent MD and Gö simulations show that rearrangements to the N terminus of S4 are required to restructure H16 and form the native 5WJ:S4 complex (Chen et al. 2012). The simulation results agree with SHAPE probing data showing that changes to H16 occur slowly and correlate with increased stability of the 5WJ:S4 complex (Mayerle et al. 2011).
H18 is remodeled in an S4 dependent manner during 30S subunit maturation
Three of the S4 amino acids tested here, Y47, L51 and R200, directly contact H18, and mutations in these residues alter the stability of the H18 pseudoknot. These mutations also impair 30S assembly in vivo, leading to accumulation of assembly intermediates and immature 17S pre-rRNA. The conformational changes in the 5WJ rRNA specifically induced by S4 are also observed during assembly of complete 30S subunits. Chemical probing and base-modification assays of low-temperature and heat-activated reconstitution intermediates (RI and RI*) showed that nucleotides G505, G506, A509, A510, and A531 in H18 become buried in the transition from RI to RI*, while nucleotides G529 and G530 become more accessible (Holmes and Culver 2005). G529 and G530 become even more reactive as RI* complexes convert to 30S complexes (Holmes and Culver 2005).
In mature E. coli ribosomes, S4 residues Y47 and L51 interact with U508, A509, and A510, stabilizing the H18 pseudoknot between 16S residues 505–507 and 524–526 (Fig. 7C). The ring of Y47 is bent backward forming a pi-stack interaction with the phosphate of U508, likely stabilizing a sharp turn in the RNA backbone at this position (Schuwirth et al. 2005). The ε-amino group of S4:L205 (Bst R200) contacts A8 at the 5′ end of 16S H1 and is also only ∼7 Å from C507 of the H18 pseudoknot and ∼6 Å from A26 at the H1/H3 junction (Fig. 7D). A26 is highly conserved in bacterial 16S sequences (Woese et al. 1975; Masquida et al. 1997) and forms a sheared base pair with G558 in the mature ribosome (Fig. 7C). The position of S4:R200 between the H18 pseudoknot and the minor groove of H1 and H3 suggests that the C terminus of S4 (R200) stabilizes the final orientation of H1 and H3, together with proteins S5 and S12 (Fig. 7B,D; Schuwirth et al. 2005).
FIGURE 7.
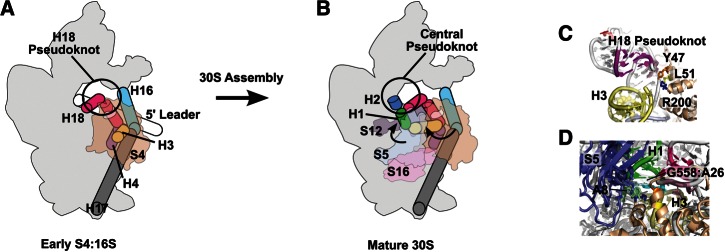
Model for S4 function in ribosome assembly. Schematic of early S4:16S RNP (A) and mature 30S ribosome (B). The 5′ leader sequence is indicated as a white cylinder. H3 (yellow), H4 (purple), H16 (cyan), H17 (gray), and H18 (magenta) are indicated as well. S4 (tan), S5 (blue), S16 (pink), and S12 (brown) are shown as transparent. H1 and the 5′ mRNA leader sequence can form an alternative structure (Dammel and Noller 1993) in assembling RNPs. As 30S RNPs mature, H3 changes its orientation and is stabilized in its native form by S16 binding. H1 (green) and H2 (blue) form after cleavage of the 5′ leader sequence, linking all domains of the 30S subunit in their final orientations. (C) S4 amino acids Y47 (orange), L51 (yellow), and R200 (blue) are in close proximity to the H18 pseudoknot (magenta) and H3 (yellow) in mature ribosomes. (D) A26 (cyan) binds G558 (pale cyan) in mature ribosomes, helping to orient H1 (green) relative to H3 (yellow). S5 binds at A8 (pale green), which also interacts with R200 of S4, locking the orientation H1 in mature 30S subunits (PDB 2AVY).
S4 stabilizes alternative rRNA conformations during assembly
The 5′ end of the pre-rRNA is known to form alternative structures that are eventually remodeled to the native conformation (Fig. 7). We propose that S4 amino acids Y47, L51, and R200 form alternative interactions with the rRNA during assembly that promote these structural rearrangements. Different contacts between these residues and the rRNA at different stages of assembly could explain the varied phenotypes of our mutations, and the resultant defects in 5′ processing of the 16S rRNA.
First, H3 samples at least two different conformations during assembly of the 5′ dom (Fig. 7; Ramaswamy and Woodson 2009b), and our SHAPE results showed that H3 docks with H18 during the transition to long-lived complexes of S4 and the 16S 5WJ (Mayerle et al. 2011). Second, chemical probing of reconstitution intermediates showed that the H1/H2 central pseudoknot forms late in assembly (Powers et al. 1993; Holmes and Culver 2005) and is therefore absent when S4 binds initially. Third, H1 competes with an alternative helix in the unprocessed 17S pre-rRNA that involves base-pairing with nucleotides in the 5′ leader (Dammel and Noller 1993), and thus H1 may also be absent when S4 first binds. Mutations that favor this alternative helix cause cold-sensitive growth and 30S biogenesis, demonstrating the importance of these intermediate structures to accurate 30S assembly (Dammel and Noller 1993).
Individual S4 amino acids could form different interactions in these assembly intermediates than they do in the mature ribosome (Holmes and Culver 2005). For example, before interacting with the turning phosphate (nucleotides 507–508) of the H18 pseudoknot, Y47 may stack with a nearby nucleobase. R200 is positioned to flexibly interact with nucleotides in H3 or alternative structures at the 5′ end of the pre-rRNA; loss of this positive charge hyperstabilizes the H18 pseudoknot, suggesting a loss of rRNA flexibility.
In summary, our results show that specific S4 mutations impair 30S biogenesis and 5′ processing of the pre-rRNA even though the protein can still bind the 16S rRNA. These alleles also perturb the stability of the H18 pseudoknot and the 530 loop and the transition to stable S4 complexes, indicating that they do not restructure the 16S rRNA precisely as the WT protein does. From these results, we conclude that the S4–RNA interface is likely remodeled at several stages of 30S subunit assembly and that early assembly proteins can therefore influence assembly and maturation of the ribosome long after they join the complex.
MATERIALS AND METHODS
S4 mutagenesis
S4:Y47A, S4:L51A, S4:D95A, and S4:R200A S4 amino acid substitutions were introduced into pET11WT Bst S4 (Quikchange, Stratagene) (Gerstner et al. 2001). The N-terminal deletion S4:Δ41 was created using standard cloning methods. WT and mutant S4 proteins were overexpressed and purified according to the method described previously (Gerstner et al. 2001; Bellur and Woodson 2009; Mayerle et al. 2011). Protein concentration was calculated both by BioRad protein assay and by absorbance at 280 nm (Bst S4 extinction coefficient 18,490 M−1cm−1) (Gerstner et al. 2001). Protein folding was verified by far-UV circular dichroism spectroscopy.
RNA preparation
DNA templates for the 5WJ (16S nucleotides 21–46, 395–562) (Bellur and Woodson 2009), 5WJ-1199 (3′ extension of 5WJ as described by Mayerle et al. 2011), and p127 RNAs (Schlax et al. 2001) were transcribed in vitro, purified, and labeled with 32P according to the method previously described (Bellur and Woodson 2009; Mayerle et al. 2011).
Native EMSA
Uniformly 32P-labeled 5WJ RNA (0.5 nM) was prefolded at 65°C for 20 min in HKM4 (80 mM K-Hepes at pH 7.5, 330 mM KCl, 4 mM MgCl2) in the presence of 20 ng/µL poly dI-dC (Invitrogen). Varying concentrations of S4 protein were then added to each tube, and the tubes were incubated for 20 min at 42°C. Loading buffer (10% glycerol, 0.05% XC dye final concentration) was added to each tube, and the samples were immediately loaded onto a native 6% polyacrylamide gel in TKM2 (30 mM Tris-HCl at pH 7.5, 100 mM K-acetate, 2 mM MgCl2) and run for 5–6 h at 10°C at 15 W.
The fraction of bound RNA was calculated from the ratio of the counts in the shifted RNA band over the total counts in the lane and fit to fB = ([S4]/Kd)/(1 + [S4]/Kd) using Kaleidagraph (Synergy Software). Kinetic competition assays to measure the rate of S4 dissociation were performed according to the method previously described (Mayerle et al. 2011). Briefly, S4 complexes were formed as above with 25 nM Bst S4, 1 µM unlabeled 5WJ competitor was added at 42°C, and reaction aliquots were loaded directly on a running native gel at various times after the addition of competitor. The proportion of stable complexes was determined from the difference between the initial rRNA fraction bound in a control reaction with no competitor (time “zero”) and the fraction bound after 15 sec in the presence of competitor RNA. The maximum extent of binding was ∼80%, presumably due to dissociation of residual nonspecific complexes during electrophoresis.
For binding to the α-operon mRNA pseudoknot, 0.5 nM uniformly 32P-labeled in vitro transcribed p127 mRNA pseudoknot (Schlax et al. 2001) was folded in TKM8 buffer (30 mM Tris at pH 7.5, 350 mM KCl, 8 mM MgCl2, 8 mM βME, 20 ng/μL poly dI-dC, 0.01% Nikkol) for 20 min at 42°C. S4 protein was added and samples analyzed by native PAGE as above. For protease controls, Proteinase K (20 μg; NEB) was added after S4 binding and incubated for 5 min at 42°C before native PAGE.
SHAPE chemical probing
5WJ-1199 RNA or 5WJ RNA (2 pmol) was prefolded for 10 min at 42°C in HKM4 buffer. Ten picamoles Bst S4 was added and allowed to bind for 10 min at 42°C. After S4 incubation, N-methylisatoic anhydride (NMIA) was added to a final concentration of 6.5 mM and allowed to react for 26 min at 42°C (five NMIA half-lives at 42°C) (Wilkinson et al. 2006). Total reaction volume was 10 μL. Modified RNA was extracted with phenol and chloroform, precipitated with ethanol, and then resuspended in TE buffer (10 mM Tris-HCl at pH 7.5, 1 mM EDTA) for primer extension with Superscript III Reverse Transcriptase according to the manufacturer’s directions (Invitrogen). Primers used for extension reactions annealed 3′ of 16S nt 546 (H18) and 433 (H16) and in a 3′ extension (Moazed et al. 1986; Bellur and Woodson 2009; Mayerle et al. 2011).
Analysis of SHAPE data
The SHAPE modification intensities (ρ) were normalized using an invariant band, and the effects of S4 co-incubation on 5WJ SHAPE reactivity at each nucleotide, α = log10(ρ10 min/ρ0 min), were evaluated according to the method described by Mayerle et al. (2011). The relative reactivity (τ) of each nucleotide in S4 mutant complexes and WT complexes was calculated as τ = log(ρmutant/ρWT). Colors indicate 5WJ nucleotides that are highly protected (τ < −0.25, 3% of total nucleotides), moderately protected (−0.10 > τ > −0.25, 6%), exposed (0.10 < τ < 0.25, 6%), or highly exposed (τ > 0.25, 4%) relative to the WT.
S4 complementation
Standard cloning methods were used to create plasmids pTrc-WT, pTrc-Δ41, pTrc-Y47A, pTrc-L51A, pTrc-D95A, and pTrc-R200A from plasmid pTrc-S16 (Shoji et al. 2011). pTrc plasmids confer ampicillin resistance (100 μg/mL) and are IPTG-inducible (0.75 mM) and were transformed into chemically competent ΔS4 DH10β cells carrying a helper plasmid, pCDDSara-S4, to allow for strain maintenance (Shoji et al. 2011). Strains carrying pCDDSara are streptomycin (50 μg/mL)-resistant, arabinose (0.2% weight/volume)-inducible, and sucrose-sensitive (5% weight/volume).
Transformed ΔS4 DH10β cells containing both pCDDSara-S4 and pTrc-S4WT (or mutants) were spread onto LB-Agarose plates supplemented with 50 μg/mL streptomycin, 100 μg/mL ampicillin, and 0.2% arabinose to select for the pTrc plasmid while allowing S4 expression from pCDDSara-S4. Colonies were grown overnight in LB supplemented with 100 μg/mL ampicillin and 0.75 mM IPTG to switch expression to the pTrc plasmid and, in the morning, were diluted 1:100 into LB supplemented with 100 μg/mL ampicillin, 0.75 mM IPTG, and 5% sucrose to induce the loss of the pCDSSara plasmid. Complemented ΔS4 DH10Β cells were grown shaking at 212 rpm at 30°C, 37°C, or 42°C until they reached an OD600 of ∼0.5. The bacteria were cooled on ice and pelleted in 3 mL aliquots. Cell pellets were rinsed with cold 10 mM Tris (pH 7.5), 20 mM MgCl2, 200 mM NH4Cl, 3 mM βME before dry storage at −80°C.
Sucrose gradient ultracentrifugation
Cell pellets were analyzed according to the method described by Kitahara and Suzuki (2009). Briefly, thawed pellets were resuspended in 10 mM Tris-HCl (pH 7.5), 15 mM MgCl2, 1 mg/mL lysozyme and then frozen in an ethanol dry ice bath and slowly thawed at 4°C. Cold deoxycholate was added a final concentration of 0.75%, vortexed, and incubated on ice for 3 min. The lysates were cleared (15,000g for 20 min), and supernatants were loaded onto a 10%–40% sucrose gradient (20 mM Tris-HCl at pH 7.8, 15 mM MgCl2, 100 mM NH4Cl, 2 mM dithiolthreitol) and centrifuged at 17,000 rpm for 15.5 h at 4°C (Beckman SW41). Gradients were fractionated at 4°C using a Piston Gradient Fractionator (BioComp) with monitoring at 256 nm using an Econo UV Monitor (BioRad).
Analysis of 16S 5′ end
Sucrose gradient fractions were precipitated with ethanol, extracted four times with phenol and twice with chloroform, and precipitated in 70% ethanol again. The total purified cellular RNA from each gradient fraction and 1 μL of 5′ 32P end-labeled 161 primer (Moazed et al. 1986) were annealed by heating for 5 min to 65°C followed by rapid cooling. Subsequent primer extension analysis with Superscript III was performed according to the manufacturer’s directions (Invitrogen).
ACKNOWLEDGMENTS
We thank David Draper for the p127 α-operon and S4 expression plasmids, Corey Dambacher and Shinichiro Shoji for S4 deletion strains and plasmids, Mollie Rappé and Sanjaya Abeysirigunawardena for assistance with experiments, Jonathon Merz for help with figures and scripts used in data analysis, and Reza Behrouzi and J. Duncan Kilburn for help with statistics and error analysis. This work was supported by the NIH (R01 GM60819).
REFERENCES
- Adilakshmi T, Bellur DL, Woodson SA 2008. Concurrent nucleation of 16S folding and induced fit in 30S ribosome assembly. Nature 455: 1268–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agalarov SC, Williamson JR 2000. A hierarchy of RNA subdomains in assembly of the central domain of the 30 S ribosomal subunit. RNA 6: 402–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PN, Noller HF 1989. Mutations in ribosomal proteins S4 and S12 influence the higher order structure of 16 S ribosomal RNA. J Mol Biol 208: 457–468 [DOI] [PubMed] [Google Scholar]
- Bellur DL, Woodson SA 2009. A minimized rRNA-binding site for ribosomal protein S4 and its implication for 30S assembly. Nucleic Acids Res 37: 1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shem A, Jenner L, Yusupova G, Yusupov M 2010. Crystal structure of the eukaryotic ribosome. Science 330: 1203–1209 [DOI] [PubMed] [Google Scholar]
- Bokinsky G, Nivon LG, Liu S, Chai G, Hong M, Weeks KM, Zhuang X 2006. Two distinct binding modes of a protein cofactor with its target RNA. J Mol Biol 361: 771–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen DE, Clemons WM Jr, Carter AP, Wimberly BT, Ramakrishnan V 2002. Crystal structure of the 30 S ribosomal subunit from Thermus thermophilus: Structure of the proteins and their interactions with 16 S RNA. J Mol Biol 316: 725–768 [DOI] [PubMed] [Google Scholar]
- Bunner AE, Beck AH, Williamson JR 2010. Kinetic cooperativity in Escherichia coli 30S ribosomal subunit reconstitution reveals additional complexity in the assembly landscape. Proc Natl Acad Sci 107: 5417–5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changchien LM, Craven GR 1976. The function of the N-terminal region of ribosomal protein S4. J Mol Biol 108: 381–401 [DOI] [PubMed] [Google Scholar]
- Changchien LM, Craven GR 1978. Studies on the role of amino acid residues 31 through 46 of ribosomal protein S4 in the mechanism of 30 S ribosome assembly. J Mol Biol 125: 43–56 [DOI] [PubMed] [Google Scholar]
- Chen K, Roberts E, Luthey-Schulten Z 2009. Horizontal gene transfer of zinc and non-zinc forms of bacterial ribosomal protein S4. BMC Evol Biol 9: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Eargle J, Sarkar K, Gruebele M, Luthey-Schulten Z 2010. Functional role of ribosomal signatures. Biophys J 99: 3930–3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Eargle J, Lai J, Kim H, Ha T, Abeysirigunawardena S, Mayerle M, Woodson S, Luthey-Schulten Z 2012. Assembly of the fiveway junction in the ribosomal small subunit using hybrid MD/Go simulation. J Phys Chem B 116: 6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad RC, Craven GR 1987. A cyanogen bromide fragment of S4 that specifically rebinds 16S RNA. Nucleic Acids Res 15: 10331–10343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver GM 2003. Assembly of the 30S ribosomal subunit. Biopolymers 68: 234–249 [DOI] [PubMed] [Google Scholar]
- Dahlgren A, Ryden-Aulin M 2000. A novel mutation in ribosomal protein S4 that affects the function of a mutated RF1. Biochimie 82: 683–691 [DOI] [PubMed] [Google Scholar]
- Dammel CS, Noller HF 1993. A cold-sensitive mutation in 16S rRNA provides evidence for helical switching in ribosome assembly. Genes Dev 7: 660–670 [DOI] [PubMed] [Google Scholar]
- Davies C, Gerstner RB, Draper DE, Ramakrishnan V, White SW 1998. The crystal structure of ribosomal protein S4 reveals a two-domain molecule with an extensive RNA-binding surface: One domain shows structural homology to the ETS DNA-binding motif. EMBO J 17: 4545–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya-Grosjean L, Reinbolt J, Pongs O, Garrett RA 1974. A study of the regions of ribosomal proteins S4, S8, S15 and S20 that interact with 16 S RNA of Escherichia coli. FEBS Lett 44: 253–256 [DOI] [PubMed] [Google Scholar]
- Deckman IC, Draper DE 1987. S4–α mRNA translation regulation complex. II. Secondary structures of the RNA regulatory site in the presence and absence of S4. J Mol Biol 196: 323–332 [DOI] [PubMed] [Google Scholar]
- Deigan KE, Li TW, Mathews DH, Weeks KM 2009. Accurate SHAPE-directed RNA structure determination. Proc Natl Acad Sci 106: 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner RB, Pak Y, Draper DE 2001. Recognition of 16S rRNA by ribosomal protein S4 from Bacillus stearothermophilus. Biochemistry 40: 7165–7173 [DOI] [PubMed] [Google Scholar]
- Gherghe CM, Shajani Z, Wilkinson KA, Varani G, Weeks KM 2008. Strong correlation between SHAPE chemistry and the generalized NMR order parameter (S2) in RNA. J Am Chem Soc 130: 12244–12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell RR, Weiser B, Woese CR, Noller HF 1985. Comparative anatomy of 16-S-like ribosomal RNA. Prog Nucleic Acid Res Mol Biol 32: 155–216 [DOI] [PubMed] [Google Scholar]
- Held WA, Ballou B, Mizushima S, Nomura M 1974. Assembly mapping of 30 S ribosomal proteins from Escherichia coli: Further studies. J Biol Chem 249: 3103–3111 [PubMed] [Google Scholar]
- Higo K, Held W, Kahan L, Nomura M 1973. Functional correspondence between 30S ribosomal proteins of Escherichia coli and Bacillus stearothermophilus. Proc Natl Acad Sci 70: 944–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KL, Culver GM 2005. Analysis of conformational changes in 16 S rRNA during the course of 30 S subunit assembly. J Mol Biol 354: 340–357 [DOI] [PubMed] [Google Scholar]
- Kitahara K, Suzuki T 2009. The ordered transcription of RNA domains is not essential for ribosome biogenesis in Escherichia coli. Mol Cell 34: 760–766 [DOI] [PubMed] [Google Scholar]
- Low JT, Weeks KM 2010. SHAPE-directed RNA secondary structure prediction. Methods 52: 150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus MA, Gerstner RB, Draper DE, Torchia DA 1998. The solution structure of ribosomal protein S4 Δ41 reveals two subdomains and a positively charged surface that may interact with RNA. EMBO J 17: 4559–4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masquida B, Felden B, Westhof E 1997. Context dependent RNA-RNA recognition in a three-dimensional model of the 16S rRNA core. Bioorg Med Chem 5: 1021–1035 [DOI] [PubMed] [Google Scholar]
- Mayerle M, Bellur DL, Woodson SA 2011. Slow formation of stable complexes during coincubation of minimal rRNA and ribosomal protein S4. J Mol Biol 412: 453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichelli E, Isel C, Oubridge C, Nagai K 2007. Protein-induced conformational changes of RNA during the assembly of human signal recognition particle. J Mol Biol 367: 187–203 [DOI] [PubMed] [Google Scholar]
- Moazed D, Stern S, Noller HF 1986. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol 187: 399–416 [DOI] [PubMed] [Google Scholar]
- Mortimer SA, Weeks KM 2007. A fast-acting reagent for accurate analysis of RNA secondary and tertiary structure by SHAPE chemistry. J Am Chem Soc 129: 4144–4145 [DOI] [PubMed] [Google Scholar]
- Newberry V, Yaguchi M, Garrett RA 1977. A trypsin-resistant fragment from complexes of ribosomal protein S4 with 16-S RNA of Escherichia coli and from the uncomplexed protein. Eur J Biochem 76: 51–61 [DOI] [PubMed] [Google Scholar]
- Nomura M, Yates JL, Dean D, Post LE 1980. Feedback regulation of ribosomal protein gene expression in Escherichia coli: Structural homology of ribosomal RNA and ribosomal protein mRNA. Proc Natl Acad Sci 77: 7084–7088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny V, Nierhaus KH 1988. Assembly of the 30S subunit from Escherichia coli ribosomes occurs via two assembly domains which are initiated by S4 and S7. Biochemistry 27: 7051–7055 [DOI] [PubMed] [Google Scholar]
- Olsson MO 1979. Analysis of rpsD mutations in Escherichia coli. II. Physiology of some representative mutants. Mol Gen Genet 169: 259–269 [DOI] [PubMed] [Google Scholar]
- Olsson MO, Isaksson LA 1979a. Analysis of rpsD mutations in Escherichia coli. I. Comparison of mutants with various alterations in ribosomal protein S4. Mol Gen Genet 169: 251–257 [DOI] [PubMed] [Google Scholar]
- Olsson MO, Isaksson LA 1979b. Analysis of rpsD mutations in Escherichia coli. III. Effects of rpsD mutations on expression of some ribosomal protein genes. Mol Gen Genet 169: 271–278 [DOI] [PubMed] [Google Scholar]
- Olsson M, Isaksson L, Kurland CG 1974. Pleiotropic effects of ribosomal protein s4 studied in Escherichia coli mutants. Mol Gen Genet 135: 191–202 [DOI] [PubMed] [Google Scholar]
- Powers T, Noller HF 1991. A functional pseudoknot in 16S ribosomal RNA. EMBO J 10: 2203–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T, Noller HF 1995a. Hydroxyl radical footprinting of ribosomal proteins on 16S rRNA. RNA 1: 194–209 [PMC free article] [PubMed] [Google Scholar]
- Powers T, Noller HF 1995b. A temperature-dependent conformational rearrangement in the ribosomal protein S4·16 S rRNA complex. J Biol Chem 270: 1238–1242 [DOI] [PubMed] [Google Scholar]
- Powers T, Daubresse G, Noller HF 1993. Dynamics of in vitro assembly of 16S rRNA into 30S ribosomal subunits. J Mol Biol 232: 362–374 [DOI] [PubMed] [Google Scholar]
- Ramaswamy P, Woodson SA 2009a. Global stabilization of rRNA structure by ribosomal proteins S4, S17, and S20. J Mol Biol 392: 666–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy P, Woodson SA 2009b. S16 throws a conformational switch during assembly of 30S 5′ domain. Nat Struct Mol Biol 16: 438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosset R, Gorini L 1969. A ribosomal ambiguity mutation. J Mol Biol 39: 95–112 [DOI] [PubMed] [Google Scholar]
- Sayers EW, Gerstner RB, Draper DE, Torchia DA 2000. Structural preordering in the N-terminal region of ribosomal protein S4 revealed by heteronuclear NMR spectroscopy. Biochemistry 39: 13602–13613 [DOI] [PubMed] [Google Scholar]
- Schlax PJ, Xavier KA, Gluick TC, Draper DE 2001. Translational repression of the Escherichia coli α operon mRNA: Importance of an mRNA conformational switch and a ternary entrapment complex. J Biol Chem 276: 38494–38501 [DOI] [PubMed] [Google Scholar]
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH 2005. Structures of the bacterial ribosome at 3.5 Å resolution. Science 310: 827–834 [DOI] [PubMed] [Google Scholar]
- Shoji S, Dambacher CM, Shajani Z, Williamson JR, Schultz PG 2011. Systematic chromosomal deletion of bacterial ribosomal protein genes. J Mol Biol 413: 751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S, Powers T, Changchien LM, Noller HF 1989. RNA–protein interactions in 30S ribosomal subunits: Folding and function of 16S rRNA. Science 244: 783–790 [DOI] [PubMed] [Google Scholar]
- Takebe Y, Miura A, Bedwell DM, Tam M, Nomura M 1985. Increased expression of ribosomal genes during inhibition of ribosome assembly in Escherichia coli. J Mol Biol 184: 23–30 [DOI] [PubMed] [Google Scholar]
- Talkington MW, Siuzdak G, Williamson JR 2005. An assembly landscape for the 30S ribosomal subunit. Nature 438: 628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Condon C, Balada JM, Squires C, Squires CL 2001. Ribosomal protein S4 is a transcription factor with properties remarkably similar to NusA, a protein involved in both non-ribosomal and ribosomal RNA antitermination. EMBO J 20: 3811–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhaneni H, Farabaugh PJ 2009. Accuracy modulating mutations of the ribosomal protein S4–S5 interface do not necessarily destabilize the rps4–rps5 protein–protein interaction. RNA 15: 1100–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks KM, Cech TR 1996. Assembly of a ribonucleoprotein catalyst by tertiary structure capture. Science 271: 345–348 [DOI] [PubMed] [Google Scholar]
- Wilkinson KA, Merino EJ, Weeks KM 2006. Selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE): Quantitative RNA structure analysis at single nucleotide resolution. Nat Protoc 1: 1610–1616 [DOI] [PubMed] [Google Scholar]
- Williamson JR 2000. Induced fit in RNA–protein recognition. Nat Struct Biol 7: 834–837 [DOI] [PubMed] [Google Scholar]
- Woese CR, Fox GE, Zablen L, Uchida T, Bonen L, Pechman K, Lewis BJ, Stahl D 1975. Conservation of primary structure in 16S ribosomal RNA. Nature 254: 83–86 [DOI] [PubMed] [Google Scholar]
- Yates JL, Arfsten AE, Nomura M 1980. In vitro expression of Escherichia coli ribosomal protein genes: Autogenous inhibition of translation. Proc Natl Acad Sci 77: 1837–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]