Abstract
We have previously reported a subpopulation of bone marrow cells (BMC) that express Clara cell secretory protein (CCSP), generally felt to be specific to lung Clara cells. Ablation of lung Clara cells has been reported using a transgenic mouse that expresses thymidine kinase under control of the CCSP promoter. Treatment with ganciclovir results in permanent elimination of CCSP+ cells, failure of airway regeneration, and death. To determine if transtracheal delivery of wild-type bone marrow CCSP+ cells is beneficial after ablation of lung CCSP+ cells, transgenic mice were treated with ganciclovir followed by transtracheal administration of CCSP+ or CCSP− BMC. Compared with mice administered CCSP− cells, mice treated with CCSP+ cells had more donor cells lining the airway epithelium, where they expressed epithelial markers including CCSP. Although donor CCSP+ cells did not substantially repopulate the airway, their administration resulted in increased host ciliated cells, better preservation of airway epithelium, reduction of inflammatory cells, and an increase in animal survival time. Administration of CCSP+ BMC is beneficial after permanent ablation of lung Clara cells by increasing bronchial epithelial repair. Therefore, CCSP+ BMC could be important for treatment of lung diseases where airways re-epithelialization is compromised.
Introduction
Airway epithelial cells play a central role in the pathogenesis of chronic lung diseases, including chronic obstructive pulmonary disease, asthma, obliterative bronchiolitis, and cystic fibrosis.1,2,3 When the airway epithelium is injured a succession of cellular events take place, from the loss of surface epithelial integrity to partial shedding of the epithelium or even complete denudation of the basement membrane.4 In the classical view, airway epithelium is maintained in the steady state by the infrequent proliferation of Clara cells.1,5 Clara cells have the capacity to repair the airway epithelium producing both more Clara cells and ciliated cells; they also play a role in host defense and may control the extent of inflammation through secretion of Clara cell secretory protein (CCSP).6 Severe injury resulting in the depletion of Clara cells is repaired through the activation of local tissue stem cells residing at airway branch point associated neuroepithelial bodies and the bronchioalveolar duct junction; these stem cells also express CCSP.1,7 Chronic injury to the airway inhibits normal epithelial repair and differentiation and is characterized by a decreased abundance of Clara cells and reductions in lung and serum levels of CCSP.2,3,8
Permanent ablation of CCSP-expressing cells (CCSP+) in the lungs has been reported using a transgenic mouse (CCtk) which expresses the Herpes simplex thymidine kinase suicide gene under regulation of the mouse CCSP promoter.9 Treatment of these mice with ganciclovir results in elimination of Clara cells and CCSP+ stem cells, the initiation of a stress response by remaining lung cells,10 excessive extracellular matrix deposition without resolution,11 and a failure of airway regeneration that is associated with rapid mortality.9
Several studies in animal models and humans have suggested the involvement of bone marrow cells (BMC) in lung repair following injury.12,13,14,15 Our group has previously described that bone marrow has a population of CCSP+ cells which increase in peripheral blood and home to the lung in response to injury. These cells express CD45 and the surface markers CD73, CD90, and CD105. They express airway and alveolar proteins following culture at air-liquid interface.16
The aim of this study was to determine if transtracheal delivery of wild-type CCSP+ BMC could reduce disease following ablation of lung CCSP+ cells in CCtk mice. Compared with control mice administered with CCSP− cells, mice administered with CCSP+ BMC had more donor cells retained in the lung. These cells were mainly found lining the airways where they expressed epithelial cell markers, including CCSP, cytokeratin, and ion channel proteins. Administration of donor CCSP+ BMC resulted in increased numbers of host ciliated cells, better airway epithelium preservation, reduction of inflammatory cells in the bronchoalveolar lavage, and an increase in survival time. As in other studies, the increase in survival time seemed out of proportion to the numerical contribution of the donor cell repopulation. However, of significant interest, although donor BMC appeared to contribute to numerous cell lineages within the airway, there was no contribution to the ciliated cell lineage specifically.
Results
Characterization of the CCSP+ BMC population in FVB/n mice
We previously reported the existence of the CCSP+ BMC in C57BL/6 mice.16 In this study, we make use of FVB/n mice to determine the contribution of CCSP+ BMC following ablation of airway Clara cells. As in our initial observations in C57BL/6 mice, flow cytometry analysis of freshly isolated BMC showed a population of 1.74 ± 0.16% CCSP+ cells which expanded after 7 days in culture to 22.42 ± 1.66% (Supplementary Figure S1a,b). To rule out the possibility that the detection of CCSP protein on the cell surface was simply passive adsorption, gene expression for cubilin and megalin which can bind CCSP,17 was assessed on flow cytometry–sorted CCSP+ and CCSP− BMC. Only CCSP− BMC had any cubilin expression (Supplementary Figure S1c and Table S1), whereas no megalin expression was seen on either cell type (Supplementary Figure S1d and Table S1). This would seem to rule out passive adsorption. Further characterization of flow cytometry–sorted CCSP+ BMC showed the transcription of the CCSP gene by quantitative real-time PCR (RT-PCR), whereas the CCSP− BMC did not express this gene (Supplementary Figure S1e). CCSP+ BMC also expressed low levels of other epithelial markers, such as Claudin 10, paraoxanase 1, cytokeratins 5 and 14, CFTR, and ENaC (Supplementary Table S1). Immunocytochemistry using three different antibodies for CCSP corroborated that 98% of CCSP+ BMC had positive staining for these antibodies after sorting, and retained the CCSP expression 3 days after sorting. In contrast, the CCSP− BMC did not show expression of CCSP at any time (Supplementary Figure S1f–m).
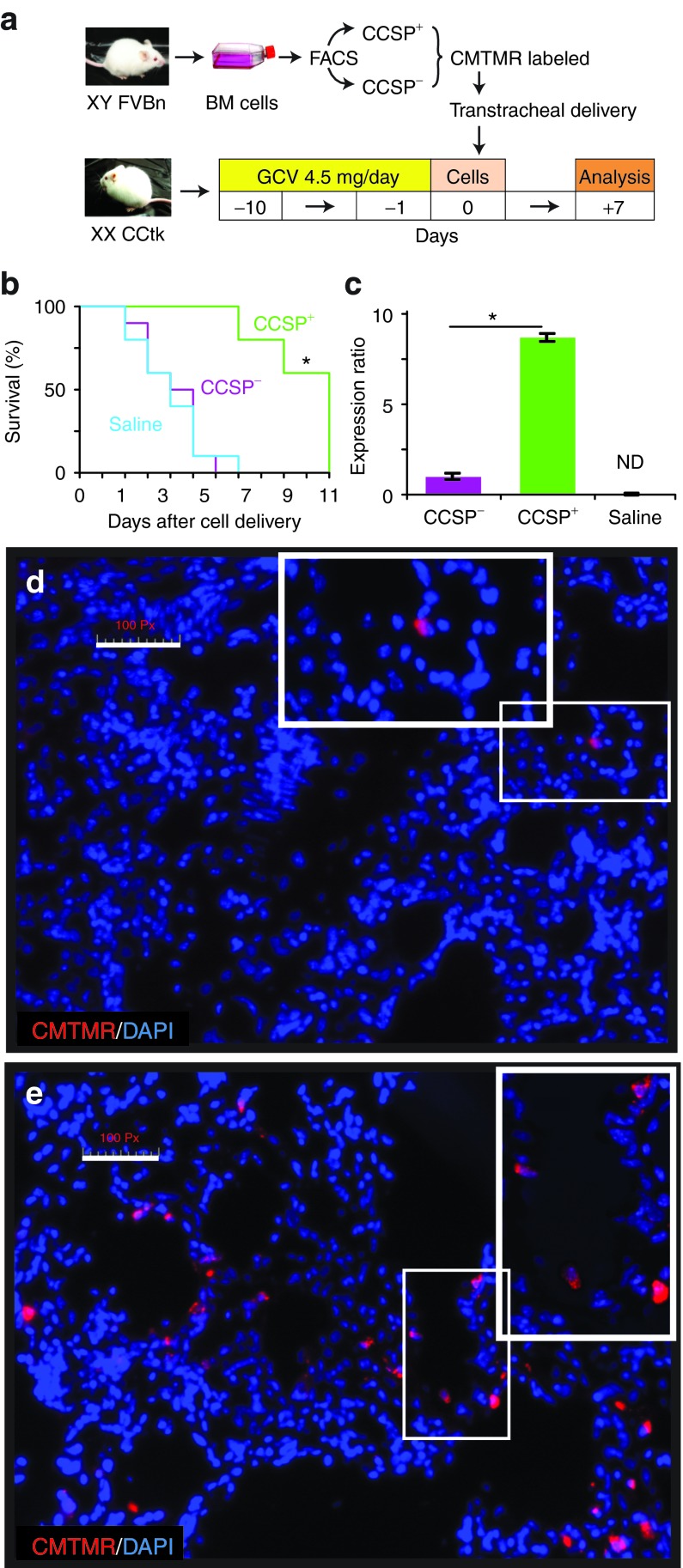
CCSP+ BMC are more efficiently retained in the lung and increase survival of CCtk mice
To confirm that Clara cell ablation in airways of CCtk transgenic mice was occurring in this model, immunohistochemical analysis of CCSP expression in lung tissue from CCtk mice treated with ganciclovir for 10 days was performed (Supplementary Figure S2). To determine the impact of CCSP+ BMC administration following ablation of lung Clara cells, female CCtk mice were treated with ganciclovir for 10 days followed by transtracheal administration of CellTracker Orange CMTMR (5-(and-6)-(((4 chloromethyl) benzoyl) amino) tetramethylrhodamine) (CMTMR)–labeled CCSP+ or CCSP− BMC from male wild-type mice. Analysis was performed 7 days after cell delivery (Figure 1a). Figure 1b demonstrates that 60% of CCtk mice administered CCSP+ BMC survived at least 10 days after cell administration, whereas none of the mice treated with CCSP− cells or saline survived >7 days (P = 0.001). Thus, transtracheal administration of CCSP+ BMC has a beneficial effect after ablation of lung CCSP+ cells increasing the survival time although ultimately there was no change in mortality.
Figure 1.
CCSP+ BMC extend survival after ablation of lung CCSP+ cells. (a) Female CCtk mice were treated with ganciclovir (GCV) 4.5 mg/day for 10 days, followed by transtracheal administration of sorted CCSP+ or CCSP− BMC from male wild-type mice. Lung analysis was performed 7 days after cell delivery. (b) Administration of CCSP+ BMC increased survival after ganciclovir treatment, whereas none of the mice treated with CCSP− BMC survived 10 days (*P = 0.001). (c) Quantitative RT-PCR for the Sry gene showed that mice treated with CCSP+ BMC had 8.9 times more male cells in the lung compared with mice treated with CCSP− BMC (*P < 0.001), whereas no amplification of male DNA was detected (ND) in lungs from mice treated with saline. (d) Lung sections from mice treated with CCSP− BMC showed areas with low density of donor cells identified by CMTMR+ signal (red cytoplasm), whereas (e) mice treated with CCSP+ BMC showed areas with high density of donor cells. Insets show areas with donor cells under high-power magnification. Scale bars represents 100 µm. Data shown are means ± SEM. n = 8 mice per group. BMC, bone marrow cells; CCSP, Clara cell secretory protein.
Levels of male donor-derived cells in the lungs of female recipients were measured by RT-PCR for Sry gene using genomic DNA showing an 8.9-fold increase in the group of mice administered with CCSP+ cells compared with mice administered with CCSP− cells (P < 0.001; Figure 1c). To confirm these findings, the number of donor-derived, CMTMR-labeled BMC was assessed by fluorescence microscopy. There were less CMTMR-labeled CCSP− donor cells (0.17 ± 0.04) per 100 nuclei (Figure 1d) in contrast to CCSP+ BMC (1.92 ± 0.33; P = 0.01; Figure 1e). Under high-power magnification 65.2 ± 1.12% of all CCSP+ BMC appeared to be in or on an airway in contrast to only 18.13 ± 3.15% of CCSP− BMC (P = 0.0002). These results are consistent with our RT-PCR data and confirm that CCSP+ BMC were more efficiently retained in the lung lining the airway epithelium. Similar results of preferential retention and improved survival were found when mice were treated with CCSP+ cells after only 5 days of ganciclovir treatment (Supplementary Figure S3).
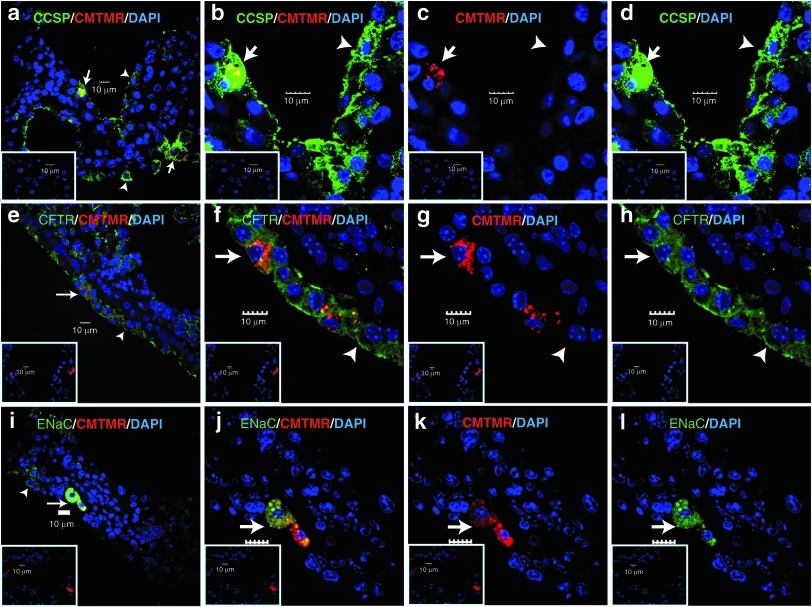
CCSP+ BMC were localized in the airway epithelium and expressed epithelial markers
Analysis of small and large airways by colocalization of CMTMR-labeled donor cells with the Clara cell marker CCSP (Figure 2a–d and Supplementary Figure S4) and the ion channel proteins CFTR (Figure 2e–h) and ENaC (Figure 2i–l) was assessed by immunofluorescence microscopy. CCSP+ BMC expressed all these epithelial markers and, interestingly there were areas in the airways where both donor and host cells expressed epithelial proteins forming a chimeric tissue, as shown for CCSP and CFTR staining. The percentage of donor CCSP+ BMC that expressed these epithelial proteins in the lungs was much higher compared with CCSP− BMC (Supplementary Table S2). Before transtracheal injection, CCSP+ and CCSP− cells in culture had either minimal or no expression of these markers (Supplementary Figure S5). These data show that the CCSP+ BMC are retained in the lung, appear to form part of the airway and express proteins generally considered to be specific to epithelium.
Figure 2.
CCSP+ BMC express epithelial and ion channel proteins in the host lung. Confocal microscopic analysis of Clara cell marker (a–d) CCSP, (e–h) CFTR, and (i–l) ENaC. Donor cells were identified by CMTMR positive fluorescence (red). The first and second columns show merged images with donor double-positive cells; the third column shows donor cells (red), whereas the fourth column shows single color imaging for each protein analyzed. Insets are representative isotype staining controls. Arrows point to donor double-positive cells and arrowheads indicate host single-positive cells. Scale bar represents 10 µm. n = 4 mice per group. Samples from mice administered with donor cells after 10 days of ganciclovir. BMC, bone marrow cells; CCSP, Clara cell secretory protein.
To evaluate whether the CMTMR (red fluorescence) label could be acquired by recipient phagocytes, we evaluated the donor origin of the CCSP+ BMC by analyzing the expression of major histocompatibility complex (MHC) class II, a marker of antigen presenting cells, such as dendritic cells, B lymphocytes, macrophages, and activated epithelial cells.18,19,20 Fluorescence microscopy analysis showed that only 1.2 ± 0.87% of the CMTMR+ cells were positive for MHC II for the CCSP+ BMC (Supplementary Figure S6a–c), whereas 10.67 ± 3.49% of the CMTMR+ cells were positive for MHC II for the CCSP− BMC (P = 0.0052). Before transtracheal delivery, <5% of the CCSP+ BMC were positive for MHC II, whereas none of the CCSP− BMC were stained for MHC II (Supplementary Figure S7). Thus, it is indeed possible that some of the CMTMR+ cells seen after injection of CCSP− BMC are phagocytes, this seems unlikely following injection of CCSP+ cells. As donors and recipients were sex-mismatched, it was also possible to track the donor cells by confocal microscopy analysis of lung sections using fluorescence in situ hybridization for Y-chromosome and then subjected to immunohistochemistry for pan-cytokeratin, an epithelial cell marker. This demonstrated that only CMTMR+ cells had the Y chromosome signal in epithelia whereas cytokeratin was expressed by both CCSP+ BMC and host cells (Supplementary Figure S6d–f). These findings diminish the possibility that the CMTMR+ cells were phagocytes and also showed that the majority of cells that remained lining the airway were of host origin. Conversely, the expression of the cell proliferation marker, Ki-67 (Supplementary Figure S6g–i) showed no significant differences in proliferative index of donor CCSP+ BMC (1.8 ± 0.69%) and CCSP− BMC (0%; P = 0.08). These data suggest that CCSP+ BMC did not contribute substantially by directly repopulating the airway although they may play a paracrine role in the survival of host epithelial cells.
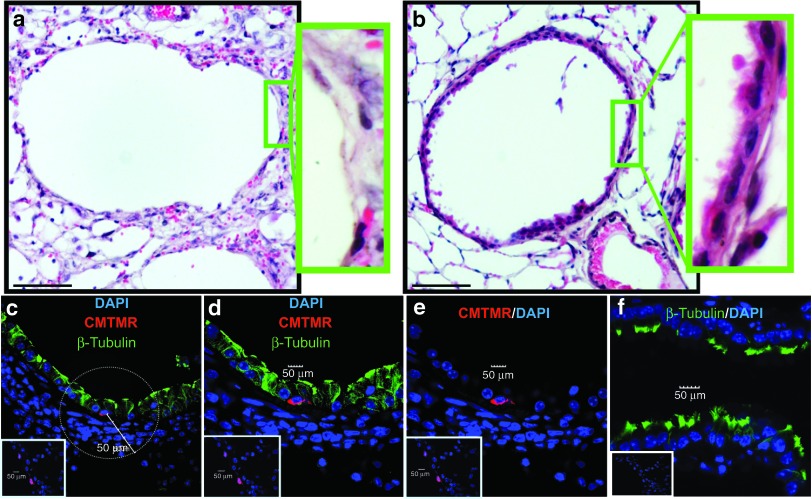
Treatment with CCSP+ BMC results in preservation of host airway epithelium
Subjective evaluation of the lungs of animals treated with CCSP+ BMC demonstrated apparent preservation of airway epithelial integrity, out of keeping with the magnitude of contribution of donor cells to airway chimerism. To quantitatively determine the impact of CCSP+ BMC on airway maintenance, an airway epithelium score was calculated based on the percentage of cells lining the large and small airways after ganciclovir treatment (Figure 3a,b and Supplementary Figure S4a,b). Hematoxylin and eosin staining of lung tissue sections from ganciclovir-exposed CCtk mice showed that mice treated with CCSP− BMC have a poorly preserved airway epithelium (score 1.3 ± 0.17) compared with mice treated with CCSP+ BMC where the epithelium is better preserved (3.25 ± 0.16; P < 0.001). These data suggest that administration of CCSP+ BMC reduced the extent of denudation of airways previously described using this model.10
Figure 3.
CCSP+ BMC preserve the epithelium in large airways. (a) Mice treated with CCSP− BMC have a poorly preserved epithelium compared with (b) mice treated with CCSP+ BMC. Green insets: higher magnification image of boxed region. (c–e) Confocal microscopic analysis of the ciliated cell marker β-tubulin IV (green fluorescence) showed that donor cells (CMTMR+; red fluorescence) do not express β-tubulin but are preferentially surrounded by host β-tubulin+ cells (c1 and c2) in the CCSP+ BMC group. (f) The host β-tubulin+ cells exhibited reduced numbers of organized cilia on the cell surface which make them fluorescent not only in the apical region but also in the cytoplasm in contrast to ciliated cells from normal lung that only showed β-tubulin expression in the apical cilia. Insets are representative isotype staining controls. Scale bar represents 10 µm. n = 4 mice per group. Samples from mice administered with donor cells after 10 days of ganciclovir. BMC, bone marrow cells; CCSP, Clara cell secretory protein.
To further characterize the phenotype of the cells that were lining the airways, we analyzed the expression of the ciliated cell protein β-tubulin (Figure 3c–f and Supplementary Figure S4c–f for large and small airways, respectively). Immunofluorescence staining showed that donor CCSP+ BMC were negative for the ciliated cell marker, β-tubulin IV, but were preferentially located in the airways surrounded by β-tubulin+ cells. We then counted β-tubulin+ host cells within a 50-µm radius of a donor cell (Figure 3c), finding that CCSP+ BMC were surrounded by many more ciliated cells compared with the CCSP− BMC group (4.9 ± 0.13 versus 0.24 ± 0.45 β-tubulin+ cells per donor cell; P < 0.01). These findings suggest that CCSP+ BMC were preferentially associated with host ciliated cells. These ciliated cells were the main population remaining to form the lining of both large and small airways 7 days after donor cell delivery. The distribution of β-tubulin observed is in agreement with previous publications that describe β-tubulin+ cells which exhibit reduced numbers of organized cilia on the cell surface as well as cytoplasmic fragments of internalized cilia. These cells spread beneath injured Clara cells after naphthalene injury, maintaining the integrity of the epithelium.21,22
Lungs from mice treated with CCSP+ BMC express higher levels of epithelial and secretory cell markers
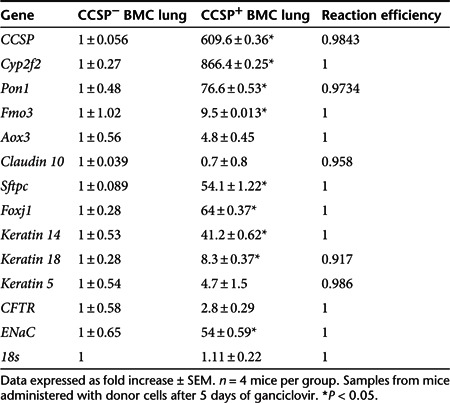
To further understand the mechanism of prolonged survival following administration of CCSP+ BMC, the expression of the number of epithelial genes was assessed. Quantitative RT-PCR showed higher expression of CCSP, which could reflect the contribution of CCSP+ BMC or an indirect mechanism whereby the CCSP+ BMC augmented survival of airway Clara cells, reducing the initial ganciclovir-induced ablation. Moreover, other Clara cell markers, such as Cyp2f2, Pon1, and Fmo3, were also increased. The ion channel protein ENaC, the type 2 pneumocyte marker Sftpc, the ciliated cell marker Foxj1, and the epithelial-specific cytokeratins Krt14 and Krt18 were all increased in lungs of mice treated with CCSP+ BMC compared with CCSP− BMC group (P < 0.05; Table 1). Taken together, these data show that transtracheal administration of CCSP+ BMC results in higher expression of epithelial genes after ganciclovir lung injury compared with mice administered CCSP− BMC. This is consistent with our earlier observation that lungs from CCtk mice treated with CCSP+ BMC had a preserved recipient airway epithelium.
Table 1. Lung epithelial gene expression.
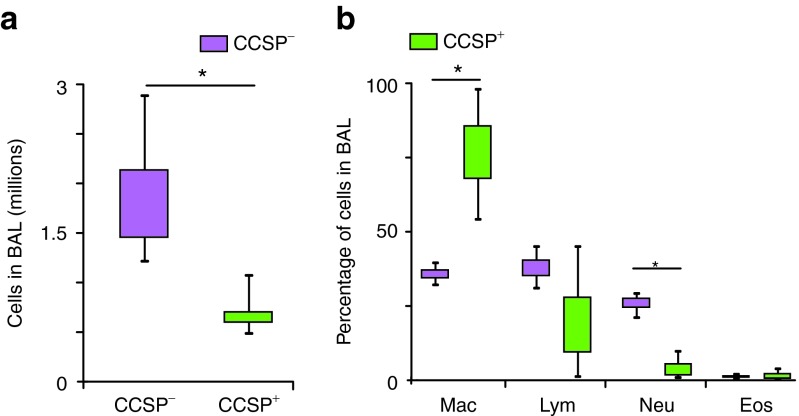
Lungs from mice treated with CCSP+ BMC have reduced inflammatory infiltrate
To determine whether administration of CCSP+ BMC impacted the number and type of inflammatory cells in the bronchoalveolar lavage, total and differential cells counts were assessed (Figure 4a,b). Mice treated with CCSP− BMC had more inflammatory cells compared with the CCSP+ BMC group (1.79 ± 0.34 versus 0.59 ± 0.10 million cells; P = 0.015). The differential cell count analysis of bronchoalveolar lavage cells indicate that mice treated with CCSP+ BMC shown fewer neutrophils (3.53 ± 0.0002%) with relatively more macrophages (76.6 ± 0.01%) compared with the CCSP− BMC group (neutrophils, 25.8 ± 1.6% and macrophages, 35.6 ± 1.4%; P < 0.01). These data demonstrate a decrease in inflammatory cells when the CCtk mice were treated with CCSP+ BMC suggesting that these cells play an immunomodulatory role.
Figure 4.
Mice treated with CCSP+ BMC have less inflammatory cells in bronchoalveolar lavage fluid. To avoid analyzing samples of moribund mice from the CCSP− BMC group, lungs from mice treated with cells after only 5 days of ganciclovir treatment were analyzed. (a) Bronchoalveolar lavage (BAL) from mice treated with CCSP− BMC had 1.79 ± 0.34 million cells in the total cell count, whereas the CCSP+ BMC group had 0.59 ± 0.10 million (*P = 0.015). (b) The differential cell count analysis of BAL cells shown significantly less neutrophils (3.53 ± 0.0002%) and more macrophages (76.6 ± 0.01%) in the CCSP+ BMC group compared with the CCSP− BMC group (neutrophils, 25.8 ± 1.6% and macrophages, 35.6 ± 1.4%; *P < 0.01). Data shown are means ± SEM. n = 8 mice per group. BMC, bone marrow cells; CCSP, Clara cell secretory protein; Eos, eosin; Mac, macrophages; Lym, lymphocytes; Neu, neutrophils.
CCSP protein increases epithelial cell proliferation while protecting against cell death by oxidative stress
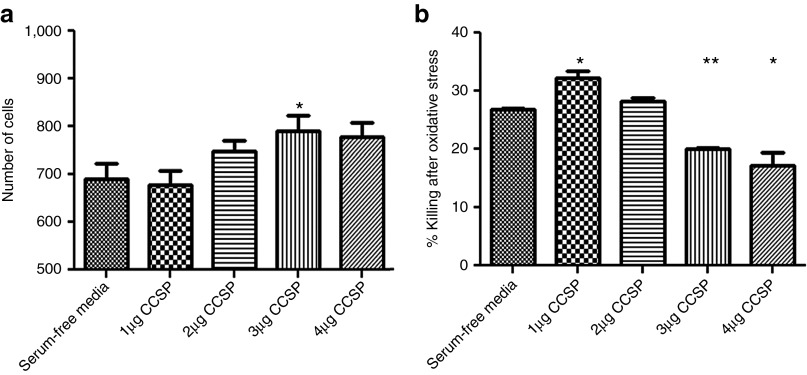
As the in vivo effects of BMC could be either to decrease the immune response or to have direct effects on the epithelial cell, affecting either cell proliferation or resistance to injury. To determine whether CCSP had direct effects on lung epithelium, CCSP was added to cultures of epithelial cells in vitro. In the cell line BEAS-2b, CCSP produced a dose-dependent increase in the rate of epithelial proliferation (Figure 5a). Addition of CCSP was also found to significantly decrease the level of cell death after the induction of cytotoxic stress using hydrogen peroxide (Figure 5b).
Figure 5.
CCSP protein causes an increase in the rate of epithelial proliferation while protecting against cell death. (a) The addition of CCSP to BEAS-2b cell cultures resulted in a significant increase in cell proliferation in a dose-dependent manner (*P < 0.002, n = 5). (b) CCSP also reduced the percentage killing after the addition of 10 mmol/l hydrogen peroxide (*P < 0.002, **P < 0.001, n = 4). Data are shown as mean ± SEM. CCSP, Clara cell secretory protein.
Discussion
The role of BMC in lung injury has been the subject of much study and indeed controversy. Initial reports of significant long-term engraftment have been revisited with more recent studies using more sophisticated techniques. Nonetheless, there remains general consensus that there is some therapeutic potential for cell therapy although the mechanisms remain uncertain. Given this uncertainty, it is difficult to optimize the treatment protocols. One area that has received relatively little attention is the concept that not all BMC are the same. Our observation of a somewhat unique CCSP+ population has been recently reviewed in full.23 The present study demonstrates that donor CCSP+ BMC are preferentially retained in the lung and are more likely to be found lining the airways than other types of BMC. In the lung, they express several epithelial proteins but do not contribute substantially by direct repopulation of the airway epithelium. They contribute in this acute and rapidly fatal model of airway epithelial injury by preserving host epithelial cells, reducing pulmonary inflammation, and increasing survival time.
After the donor BMC were administered to the lung, they were identified by their labeling with CMTMR (red fluorescence). There was better retention of the CCSP+ BMC compared with CCSP− BMC. These data were also corroborated by quantitative RT-PCR analysis. Furthermore, most of the CCSP+ BMC were found lining the airways which is precisely the site of Clara cell depletion after ganciclovir administration.10 This cell retention pattern is consistent with our previous publications using naphthalene-injured mice where transtracheal delivery shown a substantial improvement in retention compared with intravenous delivery.16,24
Once the CCSP+ BMC were retained in the lung, they maintained expression of the Clara cell marker CCSP, but also expressed the ion channel proteins CFTR, EnaC, and epithelial cytokeratins. The expression pattern of these epithelial proteins in the airways was similar in both donor and host cells resulting in a chimeric epithelium. These findings are consistent with results from our lab and others that suggest that bone marrow-derived populations can express CCSP, cytokeratins, and CFTR in the lung13,16,25 and ENaC in vitro.16 On the contrary, most of the CCSP+ BMC didn't express MHC II, which decreases the possibility that the CMTMR label was acquired by phagocytes. For CCSP− BMC, however, the level of dual staining with MHC II was 10.7%. The apparent retention of those cells may simply mean that they were engulfed by phagocytes and the retention numbers should be reduced by 10%. This further highlights the distinction between CCSP+ and CCSP− BMC.
The protective effect of donor CCSP+ BMC administration on maintenance of host ciliated epithelium in both large and small airways was shown by a reduction of the extent of denudation of the basal membrane with areas covered by β-tubulin+ cells. These findings are in accordance of previous publications that have shown β-tubulin+ cells derived from ciliated cells covering the airway surface after Clara cell depletion.21,22 Previous publications using CCtk mice have shown that depletion of CCSP+ cells was accompanied by a significant depletion of ciliated cells in the terminal bronchioles resulting in apparent denudation of the basal membrane, whereas the ciliated cells were not affected in the proximal airway.2,10,26 Our finding of ciliated cells lining the terminal bronchioles in association with donor CCSP+ BMC suggests that CCSP+ donor cells may protect the ciliated cells in injured airways by producing factors that increase their survival.
The quantitative expression of lung epithelial genes confirmed that administration of CCSP+ BMC preserved the airway epithelium showing increased expression of genes associated with ciliated cells, such as Foxj1, and epithelial genes generally (ENaC and cytokeratins). Our data also suggest that donor CCSP+ BMC help to protect other airway epithelial cell types as well. The upregulation of the basal cell marker Krt1427 in mice administered CCSP+ BMC is in agreement with a previous publication that has shown that a subset of basal cells upregulates the expression of Krt14, showing hyperplasia and increased proliferation in response to Clara cell injury.9 Another cell type that may be benefitted by CCSP+ BMC is the type 2 pneumocyte as shown by an increased expression of Sftpc.
Of particular interest is the possibility that donor CCSP+ BMC play a role increasing the survival of a population of CCSP+ host cells. In support of this view, our data showed an increased expression of the Clara cell markers CCSP, Cyp2f2, Pon1, and Fmo3. Overexpression of CCSP itself might be expected from the donor cell contribution but Cyp2f2, and Fmo3 are not found in the CCSP+ BMC after sorting (Supplementary Table S1). Conversely, Aox3 and Cldn10 that have also been described as Clara cell markers11 did not show differential expression between mice treated with CCSP+ or CCSP− BMC. It remains possible that donor CCSP+ BMC play a role increasing the survival of a population of CCSP+ host cells that differ from the classical Clara cells based on previously defined Clara cell markers.11 Consistent with this idea, previous publications have suggested the existence of different subpopulations of Clara cells.9,28,29 Conversely, the presence of CCSP protein, whether derived from the donor CCSP+ BMC or surviving host CCSP+ cells, may play a role in increasing survival given previous publications that suggest protective roles of CCSP in oxidative stress and inflammation.2,10,30,31
Lung inflammation was significantly attenuated in mice administered CCSP+ BMC showing a decrease in the total cell count of inflammatory cells with an important decrease in neutrophils while mice administered with CCSP− BMC show an inflammatory cell profile dominated by neutrophils and lymphocytes, in accordance with the inflammatory cell profile described for this model.9 Conversely, treatment with CCSP+ BMC showed a predominance of macrophages. Future studies will be needed to examine whether these are of the M2 phenotype and directly contributing to a reduction in inflammation and injury. Unfortunately, the available data does not allow determination of cause and effect. That is, whether donor CCSP+ BMC play an immunomodulatory role by reducing the production of inflammatory mediators and proinflammatory cytokines leading to better preservation of epithelial integrity or whether direct prosurvival effects on epithelial cells reduces proinflammatory signals.
Nonetheless, we have shown that transtracheal delivery of CCSP+ BMC increased the survival time of CCtk mice after ganciclovir injury. This observation suggests that transtracheal administration of CCSP+ BMC have a beneficial effect after ablation of lung CCSP+ cells. The significant change in survival time after the sudden and permanent loss of airway epithelium including their progenitors clearly indicates an important advance, highly relevant to end-stage lung disease where the loss of airway progenitor cells is much more gradual. Although this beneficial effect was not sufficient to permanently rescue the CCtk mice from their lethal phenotype, additional changes in delivery techniques such as repeated cell treatments, increased cell numbers, and/or selection of more proliferative subsets of BMC may allow even greater benefits. Taken together, these observations suggest that CCSP+ BMC will be of significant therapeutic relevance for the treatment of chronic lung diseases where re-epithelialization of the airways is compromised.
Materials and Methods
Animal procedures. FVB/n and CCtk mice on an FVB/n background were kindly provided by Barry Stripp (Duke University, NC). Mice were genotyped as described10 and received care in compliance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Experimental Animals formulated by the Canadian Council on Animal Care. Bone marrow from wild-type male mice was harvested and cultured for 7 days as described.24 For sorting or flow cytometry, plastic-adherent BMC were blocked with 10% normal goat serum (Vector Laboratories, Burlingame, CA), stained with rabbit antimouse CCSP (1:200; Upstate, Tremecula, CA) followed by AlexaFluor 488 secondary IgG (1:200; Invitrogen, Eugene, OR). Sorted CCSP+ or CCSP− cells were cultured for 3 days, in 50% conditioned media obtained from unsorted BMC culture and 50% of fresh media, and labeled with CellTracker Orange CMTMR (Invitrogen). Cells (1.3 × 106) were delivered in 40 µl via the trachea to CCtk female mice after 5 or 10 days of ganciclovir treatment. Four-month-old (24.13 ± 0.28 g), female CCtk mice received 4.5 mg/day of ganciclovir (Hoffmann-La Roche, Mississauga, ON) by subcutaneous osmotic pump (ALZET, Cupertino, CA). Lung analysis was performed 7 days after cell delivery.
Lung assessment. Animals were anesthetized with 5% isoflurane and killed by cervical dislocation. Lungs were removed en bloc together with the heart. After ligation of the left bronchus, bronchoalveolar lavage was performed twice on the right side using 0.5 ml saline through an endotracheal tube. Total and differential cell count in the bronchoalveolar lavage was perform as described.32 Genomic DNA and total RNA were prepared from the right lung using the DNeasy and RNeasy kits, respectively (Qiagen, Valencia, CA), and quality assessed by spectrophotometry. Left lungs were fixed by intratracheal instillation of 10% formalin (EMD Chemicals, Gibbstown, NJ) for 10 minutes and immersed for further fixation, before paraffin embedding. An airway epithelium score was calculated based on percentage of epithelial cells lining the airway (score 1 = 0–25%; 2 = 25–50%; 3 = 50–75%, and 4 = 75–100%) in sections stained with hematoxylin-eosin (Sigma-Aldrich, St Louis, MO). For each group, 16 fields were examined in each sample using a 20X objective. Two observers blinded as to group evaluated the images for four mice in each group.
Quantitative RT-PCR analysis. Differential gene expression (SYBR green detection method; Applied Biosystems, Carlsbad, CA) was determined for CCSP, Cyp2f2, Pon1, Fmo3, Aox3, Cldn10, Sftpc, Aqp-5, Foxj1, Krt14, Krt18, Krt5, Cubilin, Megalin, CFTR, and ENaC. Quantification of donor cells retained in the lung was performed using Sex determining region Y (Sry) primers (for details see Supplementary Table S3). Normalized mRNA or gDNA levels are expressed as relative to the control samples. 18S was used to normalize gene expression levels using the REST-384 program.33
Immunohistochemistry, immunocytochemistry, and fluorescent in situ hybridization. For immunohistochemistry, lung sections underwent deparaffinization and heat-antigen retrieval, whereas for immunocytochemistry, cells in culture were fixed in 4% paraformaldehyde and washed in phosphate-buffered saline, all the samples were blocked with 5% normal serum for 1 hour. Slides were then incubated at 4 °C overnight with primary antibodies against CCSP (Upstate), Ki-67 (Dako, Mississauga, Canada), β-tubulin IV (Sigma), CFTR (R&D, Minneapolis, MN), ENaC (Chemicon, Temecula, CA), pan-cytokeratin (Abcam, Cambridge, MA), or MHC II (eBioscience, San Diego, CA). After washing, samples were incubated for 1 hour with the appropriate secondary AlexaFluor-conjugated antibody and nuclei were counterstained with Vectashield mounting media with DAPI (Vector Laboratories). Dual Y-chromosome fluorescent in situ hybridization and immunohistochemical detection was performed as described.34 For negative controls, primary antibodies were replaced with isotype-specific IgG (see details in Supplementary Table S4).
Proliferation and cell death assay. The lung epithelial cell line BEAS-2b was cultured in 96-well plates at a concentration of 500 cells per well in DMEM without serum. After 24 hours, recombinant CCSP (R&D systems, Burlington, Canada) was added to the wells in triplicate for a further 24 hours. Cell proliferation was assessed using the XTT assay (Roche). For cell death assays, BEAS-2b cells were grown in 12-well plates for 72 hours (1.5 × 105 cells per well). Monolayers were washed and treated with 10 mmol/l hydrogen peroxide in serum-free media in the presence or absence of CCSP protein. After 2.5 hours, the cells were washed with DMEM and counted after trypan blue (Invitrogen) staining to determine the percentage of cell killing.
Statistical analysis. Data are presented as mean ± SE. Statistical analysis was performed using GraphPad Prism Software (GraphPad Software, La Jolla, CA). Survival curves were generated by the Kaplan–Meier method and groups compared with the log-rank test. Continuous variables were compared among different groups using the Student's t-test or one-way analysis of variance followed by Tukey's test. Significance was defined as P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Characterization of the CCSP+ BMC population in FVB/n mice. Figure S2. Ablation of CCSP+ cells in CCtk mice treated with ganciclovir. Figure S3. Transtracheal administration of CCSP+ BMC at 5 days of ganciclovir treatment has a beneficial effect. Figure S4. CCtk mice treated with CCSP+ BMC have preserved epithelium in small airways. Figure S5. CCSP+ BMC express CFTR and ENaC, while CCSP− cells express only CFTR. Figure S6. Corroboration of donor origin for CMTMR+ cells. Figure S7. Expression of MHC II. Table S1. CCSP+ BMC gene expression in culture. Table S2. Expression of epithelial proteins by donor cells in lung. Table S3. Primers for real-time PCR. Table S4. Immunohistochemistry, immunocytochemistry, and fluorescence in situ hybridization.
Acknowledgments
The authors thank Golnaz Karoubi for critical reading of the manuscript, Mingyao Liu, Masashi Gotoh and Dirk Wagnetz for experimental advice; and the staff of the TGH Animal Care facilities for animal manipulation advice. Funded by Canadian Institutes of Health Research (MOP 86760), Consejo Nacional de Ciencia y Tecnologia CONACyT and the Kinnear Foundation. The authors declared no conflict of interest.
Supplementary Material
References
- Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Reynolds PR, Snyder JC, Whyte F, Paavola KJ, Stripp BR. CCSP regulates cross talk between secretory cells and both ciliated cells and macrophages of the conducting airway. Am J Physiol Lung Cell Mol Physiol. 2007;293:L114–L123. doi: 10.1152/ajplung.00014.2007. [DOI] [PubMed] [Google Scholar]
- Snyder JC, Teisanu RM, Stripp BR. Endogenous lung stem cells and contribution to disease. J Pathol. 2009;217:254–264. doi: 10.1002/path.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:726–733. doi: 10.1513/pats.200605-126SF. [DOI] [PubMed] [Google Scholar]
- Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc. 2008;5:328–333. doi: 10.1513/pats.200711-167DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Malkinson AM. Clara cell: progenitor for the bronchiolar epithelium. Int J Biochem Cell Biol. 2010;42:1–4. doi: 10.1016/j.biocel.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci USA. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans C, Bernard A. Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med. 1999;159:646–678. doi: 10.1164/ajrccm.159.2.9806064. [DOI] [PubMed] [Google Scholar]
- Reynolds SD, Giangreco A, Hong KU, McGrath KE, Ortiz LA, Stripp BR. Airway injury in lung disease pathophysiology: selective depletion of airway stem and progenitor cell pools potentiates lung inflammation and alveolar dysfunction. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1256–L1265. doi: 10.1152/ajplung.00203.2004. [DOI] [PubMed] [Google Scholar]
- Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, et al. Conditional clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1256–L1263. doi: 10.1152/ajplung.2000.278.6.L1256. [DOI] [PubMed] [Google Scholar]
- Snyder JC, Zemke AC, Stripp BR. Reparative capacity of airway epithelium impacts deposition and remodeling of extracellular matrix. Am J Respir Cell Mol Biol. 2009;40:633–642. doi: 10.1165/rcmb.2008-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, et al. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest. 2009;119:336–348. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister R, Boe IM, Nykjaer A, Jacobsen C, Moestrup SK, Verroust P, et al. A two-receptor pathway for catabolism of Clara cell secretory protein in the kidney. J Biol Chem. 2001;276:13295–13301. doi: 10.1074/jbc.M010679200. [DOI] [PubMed] [Google Scholar]
- Domínguez PM, Ardavín C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev. 2010;234:90–104. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- Matsuyama T, Kawai T, Izumi Y, Taubman MA. Expression of major histocompatibility complex class II and CD80 by gingival epithelial cells induces activation of CD4+ T cells in response to bacterial challenge. Infect Immun. 2005;73:1044–1051. doi: 10.1128/IAI.73.2.1044-1051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal D, Bal V, Mayor S, George A, Rath S. A role for the Hsp90 molecular chaperone family in antigen presentation to T lymphocytes via major histocompatibility complex class II molecules. Eur J Immunol. 2006;36:828–841. doi: 10.1002/eji.200535326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkle LS, Buckpitt AR, Nishio SJ, Isaac JM, Plopper CG. Cellular response in naphthalene-induced Clara cell injury and bronchiolar epithelial repair in mice. Am J Physiol. 1995;269 6 Pt 1:L800–L818. doi: 10.1152/ajplung.1995.269.6.L800. [DOI] [PubMed] [Google Scholar]
- Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, et al. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol. 2006;34:151–157. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AP, Keating A, Waddell TK. Airway regeneration: the role of the Clara cell secretory protein and the cells that express it. Cytotherapy. 2009;11:676–687. doi: 10.3109/14653240903313974. [DOI] [PubMed] [Google Scholar]
- Wong AP, Dutly AE, Sacher A, Lee H, Hwang DM, Liu M, et al. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;293:L740–L752. doi: 10.1152/ajplung.00050.2007. [DOI] [PubMed] [Google Scholar]
- Bruscia EM, Ziegler EC, Price JE, Weiner S, Egan ME, Krause DS. Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem Cells. 2006;24:2299–2308. doi: 10.1634/stemcells.2006-0166. [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Schlage WK, Bülles H, Friedrichs D, Kuhn M, Teredesai A. Cytokeratin expression patterns in the rat respiratory tract as markers of epithelial differentiation in inhalation toxicology. I. Determination of normal cytokeratin expression patterns in nose, larynx, trachea, and lung. Toxicol Pathol. 1998;26:324–343. doi: 10.1177/019262339802600307. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Van Winkle LS, Fanucchi MV, Malburg SR, Nishio SJ, Chang A, et al. Early events in naphthalene-induced acute Clara cell toxicity. II. Comparison of glutathione depletion and histopathology by airway location. Am J Respir Cell Mol Biol. 2001;24:272–281. doi: 10.1165/ajrcmb.24.3.4247. [DOI] [PubMed] [Google Scholar]
- West JA, Williams KJ, Toskala E, Nishio SJ, Fleschner CA, Forman HJ, et al. Induction of tolerance to naphthalene in Clara cells is dependent on a stable phenotypic adaptation favoring maintenance of the glutathione pool. Am J Pathol. 2002;160:1115–1127. doi: 10.1016/S0002-9440(10)64932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TM, Reynolds SD, Mango GW, Boe IM, Lund J, Stripp BR. Altered lung gene expression in CCSP-null mice suggests immunoregulatory roles for Clara cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1523–L1530. doi: 10.1152/ajplung.2001.281.6.L1523. [DOI] [PubMed] [Google Scholar]
- Harrod KS, Mounday AD, Stripp BR, Whitsett JA. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol. 1998;275 5 Pt 1:L924–L930. doi: 10.1152/ajplung.1998.275.5.L924. [DOI] [PubMed] [Google Scholar]
- Mura M, Andrade CF, Han B, Seth R, Zhang Y, Bai XH, et al. Intestinal ischemia-reperfusion-induced acute lung injury and oncotic cell death in multiple organs. Shock. 2007;28:227–238. doi: 10.1097/01.shk.0000278497.47041.e3. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejman J, Colombo C, Conese M. Engraftment of bone marrow-derived stem cells to the lung in a model of acute respiratory infection by Pseudomonas aeruginosa. Mol Ther. 2009;17:1257–1265. doi: 10.1038/mt.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.