Abstract
Umbilical cord blood (CB) transplantation is a promising therapeutic approach but continues to be associated with delayed engraftment and infections. Here, we explored in our macaque CB transplant model expansion and engraftment kinetics of cells expanded with the combination of HOXB4 and Delta-1. CB cells were divided into two equal fractions; one fraction was transduced with HOXB4 yellow fluorescent protein (YFP) and expanded on control OP9 cells, and the other was transduced with HOXB4 green fluorescent protein (GFP) and expanded on Delta-expressing OP9 cells (OP9-DL1). Both fractions were transplanted into myeloablated subjects. Neutrophil and platelet recovery occurred within 7 and 19 days respectively, which was significantly earlier than in our previous study using cells expanded with HOXB4 alone, which resulted in neutrophil recovery within 12 days (P = 0.05) and platelet recovery within 37 days (P = 0.02). Furthermore, two of three animals in the current study remained fully transfusion-independent after transplantation. By day 30, reconstitution of lymphocytes was significantly greater with the HOXB4/OP9-DL1 expanded cells in all animals (P = 0.05). In conclusion, our data show that the combination of OP9-DL1 and HOXB4 can result in increased numbers of repopulating cells, thus leading to rapid engraftment and transfusion independence in macaques transplanted with autologous, expanded CB cells.
Introduction
The number of patients undergoing umbilical cord blood transplant (CBT) for treatment of both malignant and non-malignant diseases is increasing steadily; in fact, the number of CBT performed annually has surpassed the number of bone marrow transplants for the last 3 years (National Marrow Donor Program website: http://www.marrow.org, accessed 19 November 2012). Currently, over 20,000 patients have received umbilical CBTs worldwide.1 As a source of donor hematopoietic stem cells (HSC), CB offers distinct advantages over other sources of stem cells such as bone marrow and mobilized adult peripheral blood. For example, allogeneic stem cell transplantation patients have only a 25% likelihood of human leukocyte antigen matching with a sibling and <1% chance of matching with another relative.1 In fact, nearly 40% of patients in need of a stem cell transplant (~16,000 people per year) are unable to find a match; CB grafts, on the contrary, can be found for nearly all patients in need of transplant. CB offers many benefits including rapid accessibility, reduced risk of transmitted infections, and (perhaps most importantly) lower risk of chronic graft-versus-host disease due to a higher tolerance for human leukocyte antigen mismatches between donor and host. Even so, the field continues to be limited by the low number of cells in a single CB unit which often translates to a suboptimal cell dose, especially in adolescent and adult patients. Cell dose is widely known to be a major predictor of engraftment kinetics and transplantation success;2 thus, this limitation must be overcome in order for CBT to reach its full potential. Existing methods that have been implemented to overcome cell dose barriers include double CB unit transplant (dCBT) and pre-transplant ex vivo expansion of CB-derived hematopoietic stem and progenitor cells.
Although dCBT is now routinely used in adults to overcome cell dose limitations, it is not without flaws. Typically in this transplant setting, a single unit emerges over time as the dominant source of long-term hematopoiesis.3 This method continues to be associated with delayed engraftment, infectious complications resulting from slow immune reconstitution, and graft-versus-host disease. In addition to these limitations, cost is a major consideration in dCBT: the cost of a single unit ranges between $25,000 and 40,000, thus causing dCBT to be an expensive therapeutic option. This has led many researchers to pursue alternate techniques, including ex vivo expansion of stem cells to augment cell doses without the need for cotransplantation of cells from additional donors.
Initial attempts to expand stem cells ex vivo have implemented the use of cytokine cocktails. However, although cytokines are necessary for stem cell proliferation and survival in ex vivo culture, they have not ultimately led to significant expansion of repopulating cells. Furthermore, cytokine-mediated approaches often lead to enhanced differentiation in lieu of proliferation/maintenance of true “stem” cells. Consequently, clinical trials have not successfully been able to show improved engraftment using cytokine-only expanded cells.4,5,6 Alternative methods, including the use of stem cell self-renewal genes (for example: HOXB4)7 and manipulation of cell fate pathways such as the Notch/Delta signaling pathway8 have shown more promise.
We have recently published our studies in which we developed a nonhuman primate CBT model.9 Using this model, we subsequently compared engraftment kinetics of HOXB4 green fluorescent protein (GFP)-transduced, expanded cells and control yellow fluorescent protein (YFP)-transduced, non-expanded cells. Neutrophil recovery occurred within 19 days in each of three animals studied, and both neutrophil and platelet recovery were accelerated compared with human single unit CBT. Furthermore, we demonstrated that HOXB4-transduced and ex vivo expanded cells resulted in superior engraftment of all hematopoietic lineages in all animals over non-expanded controls. These studies were based upon previous research in our lab involving competitive repopulation of HOXB4-transduced and expanded bone marrow CD34+ cells and control YFP-transduced, non-expanded bone marrow CD34+ cells.10 In these earlier studies, we showed that HOXB4 appeared to have a more substantial effect on short-term repopulating cells than long-term repopulating cells and resulted in a 56-fold higher short-term engraftment compared with control-transduced, non-expanded cells. Subsequent studies by our group demonstrated that HOXB4-transduced and expanded CB cells could promote enhanced short-term engraftment, thereby providing hematopoietic rescue until engraftment of long-term repopulating cells could occur.9
The discovery that all four Notch receptors were detectable in human HSCs11 laid the foundation for the hypothesis that Notch genes and the Notch signaling pathway play a role in hematopoiesis. In the last decade, these studies have evolved into research involving ligands that activate the Notch pathway as a means of extrinsically modulating stem cell development. One of these ligands, Delta-1, has been studied extensively for its ability to induce Notch signaling in HSCs. Culture of human CB-derived CD34+ cells with Delta-1 ligand and cytokines led to a 100- to 200-fold increase in CD34+ cells over control cultures, as well as generation of nonobese diabetic/severe combined immunodeficient mouse repopulating cells.12,13 Methods to expand CB hematopoietic stem and progenitor cells in the presence of the Notch ligand Delta-1 are now being used in clinical trials.14
In order to determine whether the combination of Delta-1 ligand and HOXB4 exhibits a synergistic effect on HSC expansion, we studied this technique on human CD34+ cells and macaque CD34+ cells in vitro.15 In addition, we investigated engraftment kinetics of HOXB4 + Delta expanded human CD34+ cells in vivo in NSG mice.15 We found that cells cultured in the presence of Delta ligand and HOXB4 yielded higher percentages of CD34+ cells and CD7+ cells than non-Delta cultures. Furthermore, human CB CD34+ cells expanded in the combination of Delta ligand and HOXB4 yielded enhanced generation of NSG repopulating cells with higher levels of engraftment of human CD45, CD34, CD3, CD20, and CD41 cells compared with either factor individually. The results of this study prompted us to try a similar ex vivo expansion technique in a clinically relevant large animal model, which was our rationale for the work presented here using our nonhuman primate CBT model.
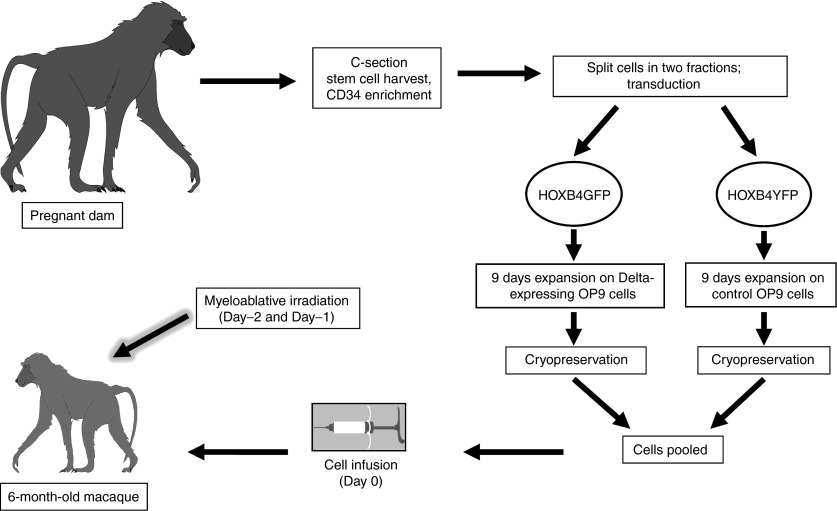
Our goal in the current study was to establish a technique to overcome the cell dose limitation associated with CBT. Our approach was based upon our earlier findings that the combination of HOXB4 and Delta-1 ligand leads to increased in vitro expansion of multilineage progenitors;15 here, we explored whether this combination can also improve overall engraftment kinetics and multilineage recovery in a clinically relevant large animal model (Figure 1).
Figure 1.
Schematic of experimental design for nonhuman primate cord blood transplant model. This model was used to perform a competitive repopulation analysis of HOXB4-only expanded cells and HOXB4 + Delta ligand expanded cells.
Results
Efficient transduction and in vitro expansion of macaque CB cells
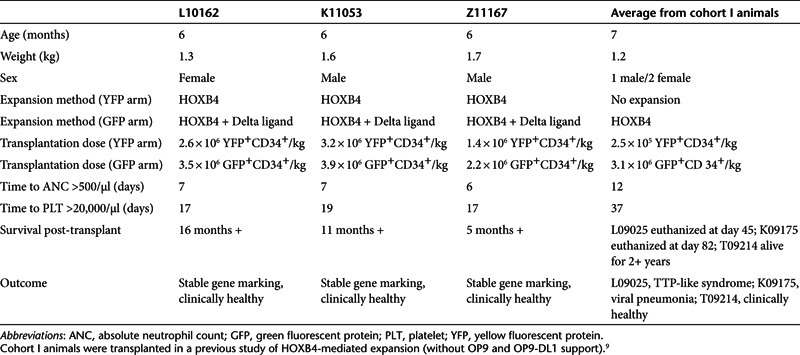
We have previously demonstrated that macaque CB CD34+ cells can be efficiently transduced with HOXB4.9 Here, we wished to determine the in vitro expansion potential of HOXB4-transduced CB cells grown on OP9-DL1 feeder layers. Transduction efficiencies ranged from 32 to 44% for the YFP-HOXB4 arm and 26 to 41% for the GFP-HOXB4 arm (n = 3). During the 9-day expansion protocol, YFP cells (cultured under the influence of HOXB4 alone) expanded on average 54-fold, whereas GFP cells (cultured under the influence of HOXB4 and Delta-1) expanded on average 64-fold. Overall CD34 percentages at the conclusion of the expansion period were, on average, 57% for the YFP arm and 66% for the GFP arm. Cell doses infused (summarized in Table 1) in the YFP arm ranged between 1.4 × 106 and 3.2 × 106 YFP+CD34+/kg, with an average of 2.4 × 106 YFP+CD34+/kg. Cell doses in the GFP arm ranged from 2.2 × 106 to 3.9 × 106 GFP+CD34+/kg, with an average of 3.2 × 106 GFP+CD34+/kg. Pre-freeze and post-thaw plating of colony-forming units showed that there was no significant loss of repopulating cell viability during the cryopreservation process. In summary, here we have shown that inclusion of Delta ligand during in vitro expansion yields ~20% higher total nucleated cell expansion and ~33% higher CD34+ cell dose than expansion in the absence of Delta-1.
Table 1. Pre-transplantation data and post-transplantation data from macaques involved in study.
Accelerated hematopoietic recovery in recipients of HOXB4 + Delta-1–expanded grafts
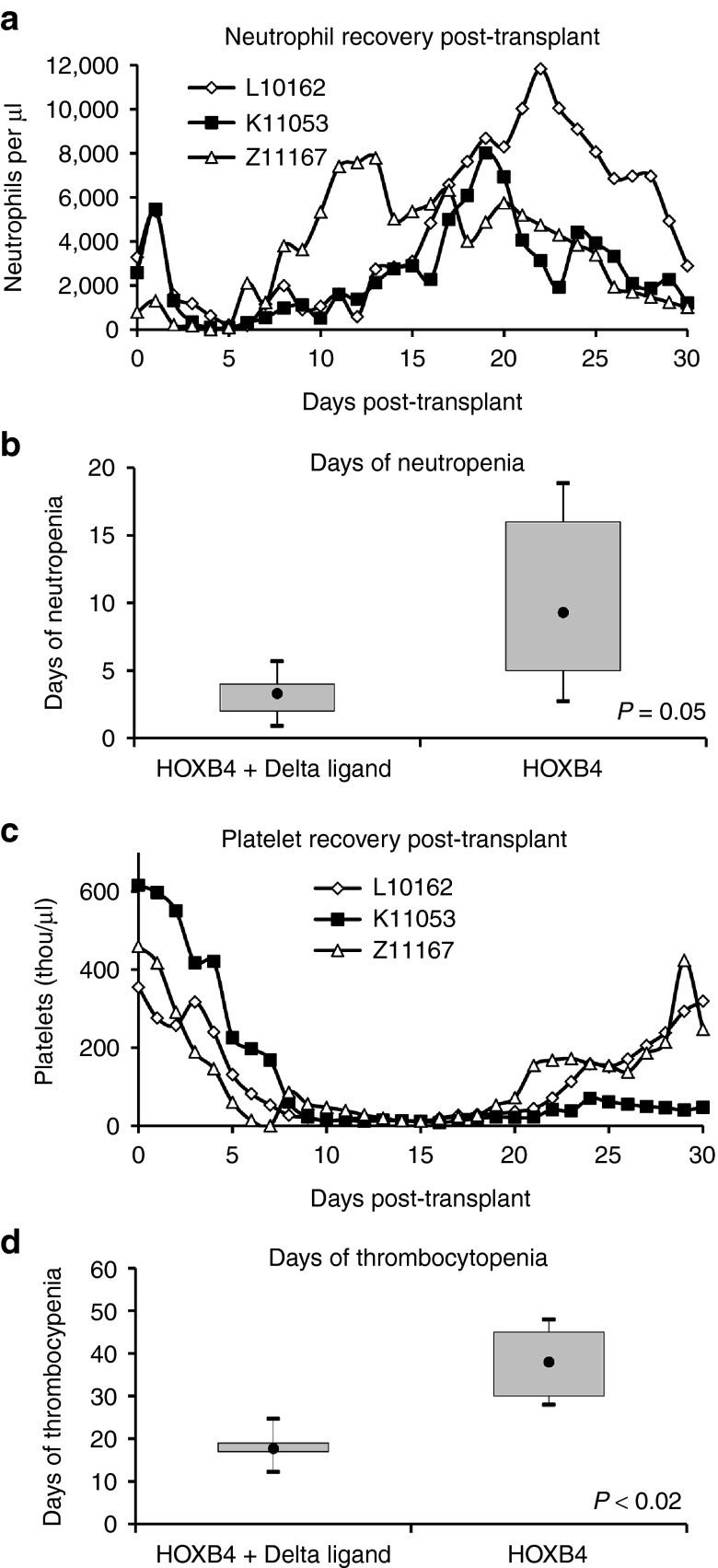
Having demonstrated increased in vitro expansion of HOXB4-transduced macaque CB cells in the presence of OP9-DL1 versus OP9 alone, we proceeded to carry out competitive repopulation transplantation studies in three macaques to determine the engraftment kinetics of expanded cells in vivo. All three macaques reached absolute neutrophil count (ANC) >500/μl within 7 days (Table 1 and Figure 2a). Granulocyte colony-stimulating factor (G-CSF) was discontinued after it was confirmed that neutrophil engraftment was sustained (ANC >1,000/μl for 3 days). This occurred on day 17 for L10162, day 13 for K11053, and day 8 for Z11167. Furthermore, we observed that these animals were neutropenic (ANC <500/μl) for a abbreviated period of time, averaging only 3.3 days (Figure 2b). This is substantially more rapid than the period of 9.3 days (P value = 0.08) of neutropenia that we observed in our previous cohort of animals9 receiving cells expanded with HOXB4 and no Delta ligand receiving the same post-transplant administration of G-CSF.
Figure 2.
Kinetics of hematopoietic recovery. (a) Neutrophil recovery after autologous cord blood transplant using cells expanded with the combination of HOXB4 + Delta ligand. (b) Box and whisker plots illustrate the duration of neutropenia (ANC <500) for animals receiving cells expanded with HOXB4 + Delta ligand versus HOXB4 alone (data from ref. 9). The closed circles represent the mean; the box contains the minimum and maximum values; and the whiskers indicate ±1.96 × SE. (c) Platelet recovery after transplant. (d) Box and whisker plots illustrate the time to platelet recovery, defined as platelet count >20,000/μl and unsupported by transfusions for at least 7 days. ANC, absolute neutrophil count; GFP, green fluorescent protein; YFP, yellow fluorescent protein.
Macaques reached platelet nadirs within the first 16 days following transplant (i.e., within 18 days following the first dose of irradiation). Platelet engraftment (defined as platelet count >20,000/μl and unsupported by transfusions for at least 7 days) was achieved at day 17 (L10162), day 19 (K11053), and day 17 (Z11167) (Table 1 and Figure 2c). L10162 and K11053 did not require a single transfusion over the course of the study; Z11167 required one transfusion on day 7. Platelets nadirs for each animal were 11,000/μl (L10162), 8,000/μl (K11053), and 3,000/μl (Z11167). The average period of thrombocytopenia (platelet count <20,000/μl) in these animals was 17.7 days, which is a 20-day decrease over HOXB4-only animals that were thrombocytopenic for an average of 38 days (Figure 2d). This difference is statistically significant (P < 0.02) and of particular importance given that these animals received a myeloablative dose of total body irradiation (1,100 cGy) before transplant. Thus, we have demonstrated a shorter period of neutropenia and only modest thrombocytopenia with a mean low of 7,300 platelets/μl, in addition to complete transfusion independence in two animals.
Gene-marking analysis indicates that Delta-1 improves lymphoid engraftment
By including the reporter genes GFP and YFP, we were able to track the in vivo contribution of cells expanded with either HOXB4 + Delta-1 or HOXB4 alone (Figure 3a). All animals showed a similar pattern in that gene marking peaked during the first 3 weeks and then declined and stabilized, remaining stable from ~2 months post-transplant. In K11053 and Z11167, GFP+ granulocytes outnumber YFP+ granulocytes at all time points post-transplant; in L10162, this trend is reversed, though not consistently over all time points. Among lymphocytes, however, the percentage of GFP+ cells is higher than the percentage of YFP+ cells in each animal; this trend is most clearly illustrated in K11053. In addition, statistical analysis of all three animals shows that at 30 days post-transplant, the percentage of GFP+ lymphocytes is significantly higher than the percentage of YFP+ lymphocytes (P = 0.05).
Figure 3.
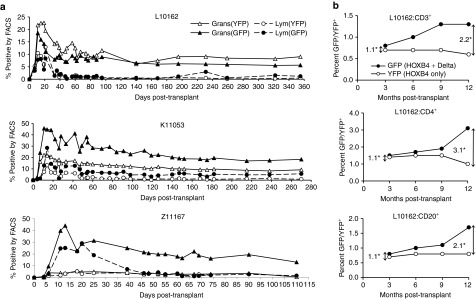
Percent gene-marked cells by fluorescence-activated cell sorting (FACS) analysis. (a) GFP+ and YFP+ granulocytes and lymphocytes in each animal over the course of the study. (b) GFP+ and YFP+ lymphoid cells in L10162 over the first year post-transplant. Vertical arrows and corresponding numbers (denoted by an asterisk) represent the fold difference between GFP+ cells and YFP+ cells at the 3-month and 12-month time points; these data demonstrate an increasing role of Delta ligand among lymphoid cells. GFP, green fluorescent protein; YFP, yellow fluorescent protein.
L10162 is the only of the three animals for which overall GFP marking is not higher than YFP marking. However, as mentioned above, this animal does consistently have higher GFP marking among lymphoid populations. Figure 3b depicts the percentage of GFP+ and YFP+ cells over time in three different lymphoid lineages; this figure illustrates the two- to threefold increase in GFP+ cells compared with YFP+ cells over time in these subsets. Thus, here we show that cells expanded with HOXB4 + Delta ligand make a greater contribution to lymphopoiesis than cells expanded with HOXB4 alone.
Combination of Delta-1 and HOXB4 leads to normal post-transplant hematopoiesis and multilineage recovery
Flow cytometry-based subset stains were performed early (1 and 3 months post-transplant) and later (9 months post-transplant) to determine both the percentages of, and gene marking within, various hematopoietic lineages. Here, peripheral blood cells were stained for CD3, CD13, CD20, and CD41; bone marrow cells were stained for CD34. We then determined the individual contribution of certain subsets to overall hematopoiesis in the macaque K11053 (Figure 4a) compared with a control (non-transplanted) animal, and an animal from our previous study transplanted with cells expanded with HOXB4 alone (T09214). Results show that, within the lymphoid lineages, CD3 and CD20, the macaque transplanted with cells expanded in the presence of Delta-1 and HOXB4 is quicker to regain normal hematopoiesis than the macaque receiving HOXB4-only expanded cells. Indeed, by 3 months post-transplant, experimental animals have regained normal hematopoiesis; furthermore, in comparison to T09214, K11053 (HOXB4 and Delta-1) has higher percentages of cells of each lineage except CD13. This indicates that pre-transplant Delta- and HOXB4-mediated expansion does not lead to any long-term skewing of hematopoiesis. To confirm this, we analyzed the hematopoietic composition of GFP+, YFP+, and unmarked populations (Figure 4b); the data confirms that there is no evidence of lineage skewing as a result of the expansion protocol.
Figure 4.
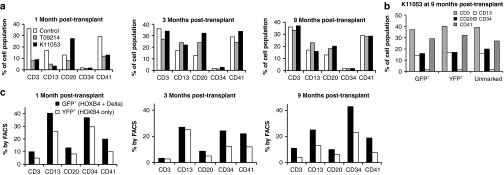
Example subset stain analysis from a macaque (K11053) receiving cells expanded with HOXB4 and Delta ligand. (a) Hematopoietic composition of K11053 at various time points post-transplantation, compared with a macaque receiving cells expanded with only HOXB4 (T09214) and a non-transplanted control macaque. (b) Hematopoietic composition of GFP+, YFP+, and unmarked cells in K11053 at 9 months post-transplant. (c) Percent GFP+ and YFP+ cells among hematopoietic lineages at various time points post-transplantation. FACS, fluorescence-activated cell sorting; GFP, green fluorescent protein; YFP, yellow fluorescent protein.
In addition, subset stain data was used to investigate the individual contributions of the differentially expanded cells to a particular lineage (Figure 4c). It is clear that GFP+ cells (those expanded with the combination of HOXB4 and Delta-1) contributed to a greater extent than cells expanded with HOXB4 alone in each lineage studied and at each follow-up time point. In fact, by the 9-month time point when hematopoiesis has stabilized, the percentage of GFP+ cells is between 1.7- and 2.8-fold higher than the percentage of YFP+ cells.
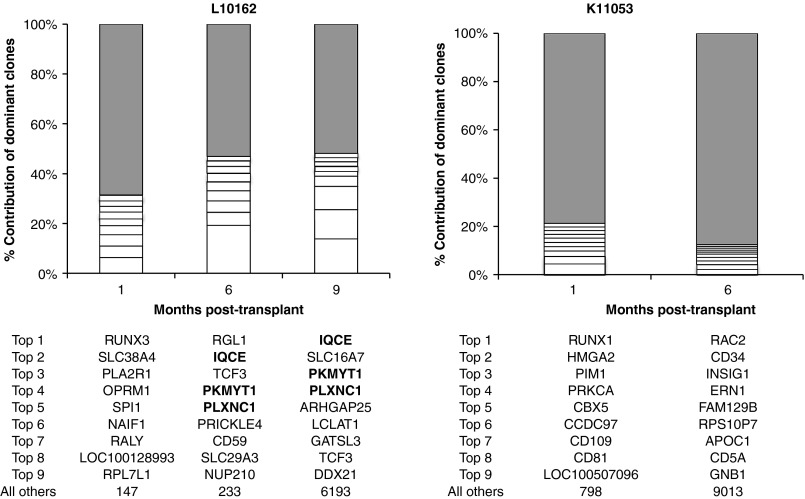
Retroviral integration site analysis indicates polyclonal expansion
We performed retroviral integration site analysis from whole peripheral blood at 1, 6, and 9 months post-transplant in L10162, and at 1 and 6 months post-transplant in K11053 (Figure 5). Here, it was our objective to determine the clonality of repopulating cells at time points early and later after transplant. The pyrosequencing technique we used here allows us to capture between several hundred and several thousand valid integration sites at each time point. Results showed a relatively uniform distribution of the top nine most frequently captured sites, with no single site showing particular dominance at any time point. The only possible exception is the RGL1 site identified in L10162 at 6 months post-transplant, present at a 19.3% frequency. However, it should be noted that this site does not show up again at the 9-month time point, thus there is no evidence of increasing dominance. Sites that increased in dominance over time are in bold font in Figure 5; these sites were only identified in one of the animals (L10162), at the 6- and 9-month time points. There were three such sites: the IQCE site, which increased from 5.2 to 13.9% frequency between 6 and 9 months post-transplant; the PKMYT1 site, which increased from 4.1 to 9.3%; and the PLXNC1 site, which increased from 3.6 to 4.1%. These sites will continue to be monitored, although all three are considered reasonably harmless in terms of oncogenic potential, based on established oncogene database searches. Furthermore, as established by the Food and Drug Administration, a clone does not become “dominant” in a clinical setting unless it comprises >20% of all gene-modified cells; in our studies, no clone fits this criteria.
Figure 5.
Longitudinal analysis of integration sites among gene-modified cells. Unique sites are plotted on the basis of capture frequency. The top nine most frequently identified sites are depicted, along with the sum of “all others” (gray portions of columns). Integration sites in bold font are those that increased in frequency over time.
Discussion
In this study, we have utilized our nonhuman primate transplant model to study engraftment kinetics of macaque CB cells expanded with the combination of HOXB4 and Delta-1. Using this strategy, we have demonstrated for the first time that these two factors synergize to promote in vivo repopulation of HSCs in a clinically relevant model; this technique led to a substantially improved time to neutrophil recovery and significantly improved platelet recovery compared with transplantation of cells expanded with HOXB4 alone. Most importantly, two of three animals in this study needed no transfusion support. Current literature involving statistics on the number of platelet transfusions after CBT are limited; however, reports indicate an average of 12–25 transfusions following transplant.16,17 Herein, we have demonstrated the ability to abrogate post-transplant neutropenia and thrombocytopenia by infusion of expanded macaque CB progenitor cells. By abbreviating the period of transplant-associated neutropenia and thrombocytopenia which result in increased risk of morbidity and mortality, overall survival is likely to increase.
Previously, we have shown that (i) HOXB4-transduced and expanded bone marrow CD34+ cells demonstrate more rapid engraftment than either HOXB4-transduced, non-expanded cells or control-transduced, expanded cells,10 (ii) HOXB4 and Delta-1 synergize to promote expansion of CB CD34+ cells in vitro and in an NSG mouse model,15 and (iii) HOXB4-expanded CB cells facilitate multilineage engraftment in our macaque CBT model.9 Thus, our goal here was to determine whether the beneficial effect of combining these two expansion factors results in significantly enhanced recovery and engraftment in a paradigm that is of high clinical relevance.
In this study, we have demonstrated neutrophil recovery within 7 days of transplant, in comparison with our previous HOXB4-only study, which yielded an average neutrophil recovery period of 12 days (Table 1). To put this into perspective, in human CBT using non-manipulated cells, neutrophil recovery takes on average 3–4 weeks, even in the dCBT setting and results in an increased risk of early transplant-related mortality.18 Thus, reducing the time to neutrophil recovery to just 1 week, as demonstrated here, is a significant improvement. Furthermore, the period of neutropenia in our current animals has been shortened from that of our previous HOXB4-only animals by 6 days, from an average of 9.3 to 3.3 days (Figure 2a,b). This has significant implications in clinical CBT, as patients undergoing CBT are highly susceptible to infection during the sustained period of neutropenia that tends to follow transplantation. In addition, our data shows that the range of days of neutropenia for animals in the current study, which received HOXB4 + Delta-1 expanded cells, is much more narrow than that of animals in the previous study, which received HOXB4-only expanded cells. Though one could argue that this effect is due to the inclusion of OP9 co-culture and not Delta-1, our competitive repopulation results (illustrated in Figures 3a,b and 4b) suggest that it is the inclusion of Delta ligand that yields these results because the only difference between GFP+ and YFP+ arms is the Delta-1. We would like to point out, however, that by no means do we suggest that the effects seen here are due solely to the effect of Delta-1; and there is certainly an appreciable contribution of the HOXB4 as well (as seen in Figures 3a and 4b).
Our observation of improved engraftment kinetics is extended to platelet recovery as well. Animals in this study achieved platelet engraftment within 19 days, compared with animals on the previous HOXB4 study, which took an average of 37 days to achieve platelet recovery (Table 1). Platelet recovery following human single unit, non-expanded CBT takes an average of 60 days;19 once again, the improved kinetics using our expansion methodology prove to be remarkable. We show that the duration of thrombocytopenia was abbreviated from 38 days (in our previous HOXB4 cohort) to only 17.7 days in the current study (Figure 2c,d). Similar to above with neutrophil recovery, the range of days of thrombocytopenia is much narrower than the range for animals receiving HOXB4-only expanded cells, once again indicating the potential for a more predictable, reproducible post-transplant prognosis. Perhaps, the most noteworthy finding in our current macaque study is the fact that two of three animals were transfusion-independent for the entire duration of study and the third animal required only a single transfusion. Our earlier HOXB4-only animals required, on average, 6.7 transfusions while awaiting platelet engraftment. Therefore, the proposed expansion modality has allowed us to nearly eliminate the need for platelet therapy after CBT, a finding that offers potential for advancing post-transplant care. To put this into perspective, previously published studies involving human dCBT in the non-myeloablative setting have reported an average of 25.2 platelet transfusions required over the first 60 days following transplant.16 A subsequent study involving non-myeloablative single CBT combined with haploidentical CD34+ infusion reported a reduction in the number of transfusions required, to an average of 12.17 It should be noted that both of these prior studies involved reduced intensity conditioning, which is well documented to be associated with fewer transfusions than myeloablative conditioning. Thus it can be expected that these same transplant strategies in the myeloablative setting would result in an even higher demand for transfusions. For instance, Solh et al.16 reported that patients receiving myeloablative conditioning required an average of 23.2 platelet transfusions, compared with only 17.5 required by patients on reduced intensity conditioning regimens. Taking all of this into consideration, our findings are of even greater impact, as we have demonstrated not only a reduced transfusion requirement, but also complete transfusion independence in two of these animals, in a myeloablative setting.
In each of the animals followed, marking levels peaked during the first 3 weeks post-transplant before stabilizing (Figure 3a). It is suspected that the expansion technique described has a more profound impact on short-term repopulating cells than longer-term repopulating cells, thus explaining why marking tends to decrease and stabilize by around 60 days post-transplant. We have observed a similar pattern in previous transplant animals receiving bone marrow-derived10 and CB-derived9 CD34+ cells expanded with HOXB4. It is interesting to note that marking in L10162 is unique from the other two macaques (Figure 3a), in that YFP+ cells outnumber GFP+ cells in the granulocyte population. However, by noting the differences between expansion in the YFP+ arm and in the GFP+ (Delta) arm, we have observed that there is a degree of individual variability in each macaque's response to Delta ligand. The exact mechanism is unclear; however, recent reports indicate that there is a degree of randomness in Notch activation resulting in individual variation cycle-to-cycle and cell-to-cell;20 it is not unlikely that this variability extends animal-to-animal as well. Our observation that L10162 is the least responsive to Delta-induced Notch signaling likely accounts for the unexpectedly higher YFP marking observed in granulocytes in this animal. In addition, the marking pattern in L10162 confirms that Delta-1 exerts its influence primarily in lymphoid lineages and not myeloid lineages. This is emphasized in Figure 3b, where we illustrate that marking within individual lymphoid subsets in this animal increasingly favors GFP+ cells over time; GFP+ populations double or triple between 3- and 12-month post-transplant, whereas YFP+ population are stable or actually decline during this time frame.
In the human dCBT setting, it has been documented that lymphoid recovery can take as long as 9 months. This period is characterized by sustained decreased T- and B-cell counts as well as delayed recovery of thymopoiesis measured by T-cell receptor excision circles.21 In our macaques, we see recovery of CD3+ T cells within 3 months post-transplant and recovery of CD20+ B cells possibly as early as 1 month post-transplant (Figure 4a). Gene marking within these populations confirms that these cells are derived from the expanded graft. A shortening of the time to immune recovery translates clinically into a lower risk of infectious complications and more favorable long-term prognosis.
As part of post-transplantation follow-up, we regularly perform integration site analysis in these animals (Figure 5). Our findings indicate that cells expanded for 9 days in vitro with HOXB4 and Delta-1 are polyclonal in composition. Likewise, longitudinal analysis (thus far carried out through 9 months post-transplant in L10162 and 6 months post-transplant in K11053) shows no evidence of an increase in clonal dominance over time.
Here, we have shown that CB cells expanded with the combination of two HSC agonists (HOXB4 and Delta-1) leads to more rapid neutrophil recovery and a significantly reduced period of thrombocytopenia compared to cells expanded with HOXB4 and no Delta-1. The times to neutrophil and platelet recovery are also abbreviated compared with human clinical single or double unit conventional CBT with two of three animals requiring no transfusions in the post-transplant period. In addition, the demonstration that we are able to achieve such remarkable pre-infusion expansion and essential elimination of post-transplant transfusions offers hope that clinical CBT in adults can one day be successful utilizing a single unit instead of the double unit setting currently used. This offers the potential of significantly reducing the cost of this treatment, as well as avoiding the difficulties involved in procuring multiple units that fit required human leukocyte antigen matching criteria. Furthermore, expansion of hematopoietic long-term repopulating cells and improved engraftment is important for many fields of cell therapy, including gene therapy, HIV treatment, and stem cell transplant.
Though the macaque studies detailed here are not directly translatable to human studies, the results presented show that the combination of HOXB4 and Delta ligand offers potential as a means of expanding CB cells ex vivo. Translation to human studies will require alternate means of introducing HOXB4 (such as supplementation of media with a recombinant TAT-HOXB4 protein)22 and Delta-1 (such as coating of tissue culture plates with the immobilized form of Delta-1 currently used in clinical trials).23 Both of these strategies are currently being pursued for clinical studies and thus can be tested in both the preclinical and clinical setting in the future.
Materials and Methods
Experimental design. We implemented our previously designed nonhuman primate competitive repopulation model9 (Figure 1) to study HOXB4 + Delta-1–mediated expansion of CB cells in a clinically relevant setting,. In accordance with this model, we perform a cesarean section ~1 week before the due date and collect umbilical CB cells. The resulting CD34-enriched cells are split into two fractions; half are transduced with a HOXB4GFP γ-retroviral vector, expanded for 9 days on Delta-expressing OP9 cells (OP9-DL1), and then cryopreserved. The remaining cells are transduced with a HOXB4YFP γ-retroviral vector, expanded for 9 days on control (non-Delta expressing) OP9 cells, and then cryopreserved. By differentially marking the two populations with independent reporter genes (GFP or YFP), we can easily detect the presence of transplanted cells as part of our routine follow-up. The two fractions of cells are cryopreserved for at least 6 months until the infant reaches an appropriate weight. On the day of transplantation, cells are thawed, pooled, and transplanted into the myeloablated recipient by intravenous infusion.
Animal housing and care. Pig-tailed macaques (Macaca nemestrina) used in this study (n = 3) were housed at the University of Washington National Primate Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. All experimental procedures performed were reviewed and approved by the Institutional Animal Care and Use Committee. Beginning on day −3 before transplantation and continuing through day 56, animals received oral tacrolimus (FK-506) to prevent an immune response to the GFP contained within the vector. Serum levels were monitored daily and dosing was adjusted as necessary to maintain serum trough levels between 10 and 15 ng/ml. Animals were conditioned with fractionated, myeloablative total body irradiation of 1,100 cGy24 from a 6 MV X-ray beam of a single source linear accelerator located at the Fred Hutchinson Cancer Research Center South Lake Union Facility; irradiation was administered as a fractionated dose over the 2 days before cell infusion. During irradiation, animals were housed in a specially modified cage that provided unrestricted access for the irradiation while simultaneously minimizing excess movement. The dose was administered at a rate of 7 cGy/minute delivered as a midline tissue dose. G-CSF was administered daily from the day of cell infusion until the animals began to engraft; engraftment was considered to have occurred when the ANC was ≥500/μl for three consecutive days. Supportive care including antibiotics, electrolytes, fluids, and transfusions were given as necessary, and blood counts were analyzed daily to monitor hematopoietic recovery.
CB processing. Enrichment of CD34+ cells from umbilical CB has been described previously.9 Briefly, red cells were lysed in ammonium chloride red cell lysis buffer. Nucleated cells were incubated for 20 minutes with the 12.8 IgM anti-CD34 antibody, washed, and incubated for another 20 minutes with MACS IgM microbeads (Miltenyi Biotec, Auburn, CA). The cell solution was run through a magnetic column, allowing for enrichment of the CD34+ fraction to a purity of 80–99% CD34+ by flow cytometry.
HOXB4 transduction. CD34-enriched cells were prestimulated for 48 hours before being plated on fibronectin-coated, non-tissue culture-treated plates for transduction. Transduction consisted of two, 4-hour exposures to virus-containing media (one exposure per day for two consecutive days). Cells were transduced at a multiplicity of infection of 0.3 (previously determined to cause minimal toxicity). The generation of Phoenix GALV-pseudotyped MSCV-HOXB4-ires-GFP or MSCV-HOXB4-ires-YFP viral vectors has been described previously.10,25,26 Virus titers were assayed on HT1080 cells, and titers were obtained in the range of 1 × 105 to 2 × 105 IU/ml. Vector supernatant was filtered through a 0.45-μm filter and frozen at −80 °C until used for transduction.
Delta-mediated expansion. OP9-DL1 cells were originally engineered by Schmitt et al.27 Preparation of OP9 cells for co-culture was started ~1 week before initiation of co-culture by thawing previously frozen stocks. OP9 and OP9-DL1 cells were cultured in Alpha MEM (minimum essential medium Eagle-Alpha modification) with 20% fetal bovine serum and 1% pen/strep, and were irradiated before co-culture. At the start of co-culture, OP9 and OP9-DL1 cells were ~75% confluent. At this time, the existing media was removed and replaced with CB expansion media, which consisted of Iscove's modified Dulbecco's medium, supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and thrombopoietin, Flt3 ligand, and stem cell factor, all at 100 ng/ml. Transduced CD34+ CB cells were added and maintained in co-culture for 9 days, with addition of fresh media and growth factors every 3–4 days.
Flow cytometry. Cells were prepared for flow cytometry following the instructions of the antibody manufacturer (Becton Dickinson, San Jose, CA). Flow cytometric data were collected using a Canto I (Becton Dickinson) and analyzed using FlowJo software (Tree Star, Ashland, OR). At least 10,000 events were collected for each sample. Parameters for analysis included expression of the reporter genes, GFP and YFP, as well as expression of the surface markers, CD3, CD4, CD8, CD13, CD14, CD20, CD34, and CD41. Non-transduced cells were used as a control for the gating of GFP+ and YFP+ cells, and isotype control antibodies were used as a control for gating of positive populations among antibody-labeled cells.
Colony-forming unit assays. To assess expansion of repopulating cells, colony-forming unit assays were carried out in MethoCult H4230 methylcellulose media (Stem Cell Technologies, Vancouver, British Columbia, Canada). These cultures were supplemented with 100 ng/ml erythropoietin, interleukin-3, interleukin-6, thrombopoietin, stem cell factor, G-CSF, and granulocyte-macrophage CSF. Plates were incubated at 37 °C for 12–14 days and subsequently inspected for colony generation; colonies comprised of >50 cells were considered viable.
Nebulization-mediated PCR for detection of retroviral integration sites. Integration of the HOXB4 vector was analyzed using an improved analytical technique involving nebulization-mediated PCR. Briefly, 300 ng to 3 μg DNA were nebulized with pressurized nitrogen for 60 seconds. Following fragmentation, segments of DNA were isolated and polished. Modified linkers were then ligated following standard procedures (454/Roche-GS 20 DNA Library Preparation Kit; 454 Life Sciences, Branford, CT). Sequential, nested exponential PCR reactions were used to amplify the vector–genome junction in double-stranded DNA segments.
Multiplex pyrosequencing of retroviral integration sites. DNA fragments ranging from 800–1,500 bp in length were then isolated by gel purification and shipped to the University of Illinois at Urbana-Champaign. Here, they were quality control checked and sequenced on the 454/Roche Titanium system (454 Life Sciences); FASTA format sequence reads were deposited on a secure server for downstream processing.
Processing of integration site data. DNA sequences isolated from nebulization-mediated PCR-based amplified vector long terminal repeat–chromosome junctions were processed as described previously.28,29,30 Using a stand-alone version of BLAT,31 the long terminal repeat proximal genomic sequences were aligned to the rhesus genome. Rhesus genome alignments were converted to the human genome position, and PERL programs were used to compare localized integration sites to various chromosomal features by using tables available from the University of California at Santa Cruz database as described previously.32
Ongoing follow-up. All three animals in this study are alive and healthy. L10162 is currently 16 months post-transplant; K11053 is currently 11 months post-transplant; Z11167 is currently 5 months post-transplant. For these animals, complete blood counts, blood chemistries, appetite, weight, and social behavior are equivalent to that of a healthy, non-transplanted macaque. Overall gene marking has stabilized in all three animals and persists at ~4% GFP and 4% YFP in L10162, 15% GFP and 6% YFP in K11053, and 8% GFP and 3% YFP in Z11167. We continue to monitor all animals monthly including complete blood counts, blood chemistries, gene marking, veterinary exam, and bacterial and fungal cultures. Every 3 months, animals undergo more intensive follow-up including subset stain panels and integration site analyses.
Acknowledgments
This work was supported by National Institutes of Health grants HL098489, HL084345, and AI080326. We thank Helen Crawford and Bonnie Larson for manuscript preparation; we also thank the University of Washington National Primate Research Center staff, members of the H.-P.K. laboratory, and the Fred Hutchinson Cancer Research Center Flow Cytometry Core Facility. H.-P.K. is a Markey Molecular Medicine Investigator and the recipient of the Jose Carreras/E.D. Thomas Endowed Chair for Cancer Research. The authors declared no conflict of interest.
References
- Anasetti C, Aversa F, Brunstein CG. Back to the future: mismatched unrelated donor, haploidentical related donor, or unrelated umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2012;18 suppl. 1:S161–S165. doi: 10.1016/j.bbmt.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW, Eurocord Group et al. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32:397–407. doi: 10.1016/j.exphem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, Attar E, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecora AL, Stiff P, Jennis A, Goldberg S, Rosenbluth R, Price P, et al. Prompt and durable engraftment in two older adult patients with high risk chronic myelogenous leukemia (CML) using ex vivo expanded and unmanipulated unrelated umbilical cord blood. Bone Marrow Transplant. 2000;25:797–799. doi: 10.1038/sj.bmt.1702222. [DOI] [PubMed] [Google Scholar]
- Shpall EJ, Quinones R, Giller R, Zeng C, Baron AE, Jones RB, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- Jaroscak J, Goltry K, Smith A, Waters-Pick B, Martin PL, Driscoll TA, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: results of a phase 1 trial using the AastromReplicell System. Blood. 2003;101:5061–5067. doi: 10.1182/blood-2001-12-0290. [DOI] [PubMed] [Google Scholar]
- Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- Watts KL, Nelson V, Wood BL, Trobridge GD, Beard BC, Humphries RK, et al. Hematopoietic stem cell expansion facilitates multilineage engraftment in a nonhuman primate cord blood transplantation model. Exp Hematol. 2012;40:187–196. doi: 10.1016/j.exphem.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XB, Beard BC, Beebe K, Storer B, Humphries RK, Kiem HP. Differential effects of HOXB4 on nonhuman primate short- and long-term repopulating cells. PLoS Med. 2006;3:e173. doi: 10.1371/journal.pmed.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojika S, Griffin JD. Notch receptors and hematopoiesis. Exp Hematol. 2001;29:1041–1052. doi: 10.1016/s0301-472x(01)00676-2. [DOI] [PubMed] [Google Scholar]
- Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106:2693–2699. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(-) cord blood cells. J Clin Invest. 2002;110:1165–1174. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney C, Ratajczak MZ, Laughlin MJ. Strategies to enhance umbilical cord blood stem cell engraftment in adult patients. Expert Rev Hematol. 2010;3:273–283. doi: 10.1586/ehm.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts KL, Delaney C, Humphries RK, Bernstein ID, Kiem HP. Combination of HOXB4 and Delta-1 ligand improves expansion of cord blood cells. Blood. 2010;116:5859–5866. doi: 10.1182/blood-2010-05-286062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solh M, Brunstein C, Morgan S, Weisdorf D. Platelet and red blood cell utilization and transfusion independence in umbilical cord blood and allogeneic peripheral blood hematopoietic cell transplants. Biol Blood Marrow Transplant. 2011;17:710–716. doi: 10.1016/j.bbmt.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118:6438–6445. doi: 10.1182/blood-2011-08-372508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballen K, Mendizabal AM, Cutler C, Politikos I, Jamieson K, Shpall EJ, et al. Phase II trial of parathyroid hormone after double umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2012;18:1851–1858. doi: 10.1016/j.bbmt.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- Vilas-Boas F, Fior R, Swedlow JR, Storey KG, Henrique D. A novel reporter of notch signalling indicates regulated and random Notch activation during vertebrate neurogenesis. BMC Biol. 2011;9:58. doi: 10.1186/1741-7007-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri A, Peffault de Latour R, Carmagnat M, Clave E, Douay C, Larghero J, et al. Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transpl Infect Dis. 2011;13:456–465. doi: 10.1111/j.1399-3062.2011.00632.x. [DOI] [PubMed] [Google Scholar]
- Krosl J, Austin P, Beslu N, Kroon E, Humphries RK, Sauvageau G. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat Med. 2003;9:1428–1432. doi: 10.1038/nm951. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B, Wu L, Yu M, Brashem-Stein C, Staats S, Flowers D, et al. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J Cell Sci. 2000;113 Pt 23:4313–4318. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- Watts KL, Beard BC, Wood BL, Kiem HP. Myeloablative irradiation in non-human primates. J Med Primatol. 2009;38:425–432. doi: 10.1111/j.1600-0684.2009.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonchuk J, Sauvageau G, Humphries RK. HOXB4 overexpression mediates very rapid stem cell regeneration and competitive hematopoietic repopulation. Exp Hematol. 2001;29:1125–1134. doi: 10.1016/s0301-472x(01)00681-6. [DOI] [PubMed] [Google Scholar]
- Pineault N, Abramovich C, Ohta H, Humphries RK. Differential and common leukemogenic potentials of multiple NUP98-Hox fusion proteins alone or with Meis1. Mol Cell Biol. 2004;24:1907–1917. doi: 10.1128/MCB.24.5.1907-1917.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- Beard BC, Dickerson D, Beebe K, Gooch C, Fletcher J, Okbinoglu T, et al. Comparison of HIV-derived lentiviral and MLV-based gammaretroviral vector integration sites in primate repopulating cells. Mol Ther. 2007;15:1356–1365. doi: 10.1038/sj.mt.6300159. [DOI] [PubMed] [Google Scholar]
- Beard BC, Keyser KA, Trobridge GD, Peterson LJ, Miller DG, Jacobs M, et al. Unique integration profiles in a canine model of long-term repopulating cells transduced with gammaretrovirus, lentivirus, or foamy virus. Hum Gene Ther. 2007;18:423–434. doi: 10.1089/hum.2007.011. [DOI] [PubMed] [Google Scholar]
- Trobridge GD, Miller DG, Jacobs MA, Allen JM, Kiem HP, Kaul R, et al. Foamy virus vector integration sites in normal human cells. Proc Natl Acad Sci USA. 2006;103:1498–1503. doi: 10.1073/pnas.0510046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ. BLAT–the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolchik D, Baertsch R, Diekhans M, Furey TS, Hinrichs A, Lu YT, University of California Santa Cruz et al. The UCSC Genome Browser Database. Nucleic Acids Res. 2003;31:51–54. doi: 10.1093/nar/gkg129. [DOI] [PMC free article] [PubMed] [Google Scholar]