Abstract
The spatiochromatic properties of the red–green dimension of human colour vision appear to be optimized for picking fruit in leaves at about arms' reach. However, other evidence suggests that the task of spotting fruit from a distance might be more important. This discrepancy may arise because the task a system (e.g. human trichromacy) is best at is not necessarily the same task where the largest advantage occurs over the evolutionary alternatives (dichromacy or anomalous trichromacy). We tested human dichromats, anomalous trichromats and “normal” trichromats in a naturalistic visual search task in which they had to find fruit pieces in a bush at 1, 4, 8 or 12 m viewing distance. We found that the largest advantage (in terms of either performance ratio or performance difference) of normal trichromacy over both types of colour deficiency was for the largest viewing distance. We infer that in the evolution of human colour vision, spotting fruit from a distance was a more important selective advantage than picking fruit at arms' reach.
Keywords: colour blind, evolution, primate, trichromacy, polymorphism, visual search
1. Introduction
Humans tend to assume that our vision represents the world as it is; that, for example, an object appears red because that object is red. However, the colours that objects appear depend on the colour vision of the observer, and humans actually have a rare form of colour vision among all animals (e.g. Bowmaker, 1998; Jacobs, 1993). The early vertebrates were probably tetrachromatic and many fish, reptiles and birds remain so—meaning they have four types of cone receptor. Early mammals lost this multidimensional colour vision and became dichromatic, retaining only the cone classes sensitive to the longest and shortest wavelengths. An ancestor to African and Asian primates, with which we share our type of colour vision, reinvented full trichromatic vision from these dichromatic ancestors, so that we now have three types of cone—shortwave (S), mediumwave (M) and longwave (L)—two of which, L and M, remain similar to each other in many ways because they are the offspring of the same ancestral mammalian longwave cone. Most placental mammals have remained dichromatic, possessing S and L cones, while some groups have reduced to just L cones (and rods). The reason why primates bucked this trend has been the focus of a long-running debate. The main theories have suggested that the key selective advantage of trichromacy for primates lay in foraging, either for fruit (Allen, 1879; Mollon, 1989; Osorio & Vorobyev, 1996; Polyak, 1957; Regan et al., 1998, 2001; Riba-Hernandez, Stoner, & Osorio, 2004; Steward & Cole, 1989) or for young leaves (Dominy & Lucas, 2001; Sumner & Mollon, 2000a) among the ubiquitous forest background of mature leaves. However, foraging involves several distinct tasks. Here, we ask whether the key task might have been spotting food from a distance, or efficiently picking food once it is within reach (i.e. food detection or food selection; Lucas et al., 2003).
Parraga, Troscianko, and Tolhurst (2002) compared the spatiochromatic properties of calibrated natural images, including those of fruit on leafy backgrounds with the properties of the three early channels of human vision: the “LM” colour pathway, which compares L cone signals with M cone signals (this pathway is often called “red–green,” but this is slightly misleading); the “S-LM” pathway comparing S cone signals with pooled L and M signals (this pathway is often called “blue–yellow,” but this is highly misleading), and an achromatic pathway. It is the ability to compare L with M signals that was new in primate trichromacy, and Parraga et al. (2002) found that the spatiochromatic properties of this channel are optimized for encoding reddish or yellowish fruit or leaves on a background of foliage at relatively small viewing distances. In other words, our LM system is optimized for picking fruit or edible leaves, rather than for spotting them from a distance. Does it follow that picking food was the critical task driving the natural selection of primate trichromacy? We cannot necessarily make this inference because natural selection is not driven by what a phenotype might be best at, but by the advantage that phenotype offers over other competing phenotypes. Even though the LM pathway may be best at selecting food close up, the biggest advantage over dichromacy might still be at spotting fruit at a distance.
In fact, other authors have explicitly assumed that the biggest advantage lay in spotting food from a distance (Dominy, Lucas, Osorio, & Yamashita, 2001; Lucas et al., 2003; Regan et al., 1998, 2001; Sumner & Mollon, 2000a, 2000b). For example, Regan et al. and Sumner & Mollon modelled the task of finding fruit or young leaves in foliage for all plausible sensitivity functions that the L and M cones might take, in order to work out which combination of potential L and M cones would be optimal. It turned out that the optimal pair are very close to what we actually possess, supporting the argument that finding important objects amongst foliage was a driving force in the evolution of the LM colour channel. This approach did not incorporate the spatial properties of the system, unlike Parraga et al. (2002) but a component of the modelling—the estimation of quantum noise—did require the viewing distance of the stimuli to be specified. These studies explicitly assumed the key task was spotting food from a distance rather than picking food close up, and thus chose distances of about 10 m.
Behavioural evidence in favour of either task is circumstantial. South American monkeys have been the main focus of study because they are polymorphic in their colour vision (Mollon, Bowmaker, & Jacobs, 1984); within the same species, some individuals can have trichromacy, while others have dichromacy (we will return to this in the Discussion section). These species offer the opportunity of natural experiments, comparing the behaviour of trichromatic individuals with dichromatic individuals, either in zoos or in the wild. Caine and Mundy (2000) tested marmosets in visual search tasks in a naturalized captive setting. In one task, they scattered edible orange or green cereal balls in the cage, which required viewing from up to 6 m away, while in the other task the “targets” were placed on a tray among green wood shavings and viewed close up (<0.5 m). An advantage for trichromats was evident only for the longer distance task, but the backgrounds for the visual search were not matched across tasks (the primary aim of the study was to establish whether the trichromats had an advantage in any task rather than to formally test the distance manipulation).
Since then, there has been no distance manipulation within the same study, and it has been surprisingly difficult for researchers to observe any significant behavioural differences between trichromatic and dichromatic monkeys in the wild. In a captive environment, the foraging advantage of trichromats has been replicated with tamarins finding simulated fruits in simulated leaves, but there was no distance manipulation (Smith, Buchanan-Smith, Surridge, Osorio, & Mundy, 2003b). In the wild, Smith et al. (2003b) found a hint that trichromatic moustached tamarins might be more likely to lead the troop into fruiting trees than dichromats, consistent with an advantage in spotting fruit, but this was not consistent and it was not the case that all troops of tamarins were led by trichromats (saddleback tamarins were mainly male-led, who are all dichromats). Similarly, Bunce, Isbell, Grote, and Jacobs (2011) found a hint that wild trichromatic titi monkeys found small patches of red or yellow fruit more often than their dichromatic conspecifics. However, the only significant observed advantage in wild monkeys has been capuchins selecting fruit at arms' reach (Melin et al., 2009), where trichromats accepted (i.e. ate) more of the fruit they picked, sniffed fruit less, and had shorter foraging sequences than dichromats did. However, this apparent advantage for picking fruit does not appear to translate into consuming more food overall in trichromatic capuchins or spider monkeys (Hiramatsu et al., 2008; Melin et al., 2009; Vogel, Neitz, & Dominy, 2007). Moreover, Hiramatsu et al. (2008) found that luminance, rather than colour, was the cue that best predicts foraging efficiency at grasping distance (for the fruits in their study at least). Additionally, olfaction and tactile cues can be available, reducing the importance of trichromacy (Hiramatsu et al., 2009; Lucas et al., 2003; Steward & Cole, 1989).
Thus, it remains unclear whether we should expect the greatest advantage of human-like trichromacy to be in the longer distance task of spotting food, or the close-up task of picking it. The aim of our experiment was to simulate the competition to find fruit between two primates (in this case humans) in a naturalistic visual search task of real fruit in real foliage. We tested “normal” trichromats, dichromats and anomalous trichromats who have less separation between the L and M cone sensitivity functions than do “normal” trichromats, which impairs their LM dimension of colour vision. We included anomalous trichromats both to boost “colour-deficient” participant numbers (dichromats are relatively rare, ∼2% of the male population), and because evolutionarily the competing state to what we now consider normal trichromacy has almost certainly been “anomalous” trichromacy as well as dichromacy. The task was to count the number of “ripe fruit” hanging in a real bush from a distance of 1, 4, 8 or 12 m (Figure 1). We used segments of yellow pepper as targets because they display spectral and thus chromatic properties representative of many fruit favoured by primates in a Ugandan rainforest (Figures 2 and 3). The task was performed simultaneously by a pair of participants, one dichromat or anomalous trichromat and one “normal” trichromat matched as closely as possible for age, sex, experience of visual experiments and knowledge of colour vision. Each pair performed the task simultaneously so that they were exposed to the identical trial sequence and lighting conditions (cloudy or sunny). This set-up also engendered some aspect of competition between the pair, simulating the competition for fruit of two primates simultaneously foraging in the same tree.
Figure 1.

The naturalistic visual search task. The upper panel shows two participants during a trial at 4 m with three targets. Both have removed their blindfolds and opened their eyes in order to search the shrub. The lower panel shows the shrub with the eight potential target positions marked with red circles. This trial contains two targets.
Figure 2.
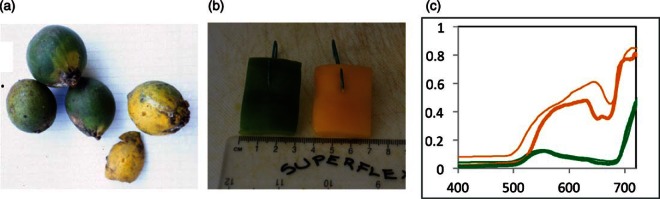
The stimuli. Using measurements from fruit in a Ugandan rainforest (e.g. panel a: samples of unripe, green, and ripe, yellow, Chrysophyllum albidum), we chose segments yellow pepper as targets and green pepper as distractors (b), because they have similar reflectance spectra (c) and chromatic properties (Figure 3). The “fruit” stimuli (b) subtended approximately 2, 0.5, 0.25 and 0.16 degrees of visual angle for distances 1, 4, 8, 12 m. In panel (c), thin orange and green lines are ripe and unripe Ugandan fruit Chrysophyllum albidum, orange and green thick lines are “ripe” and “unripe” peppers used as experimental stimuli.
Figure 3.
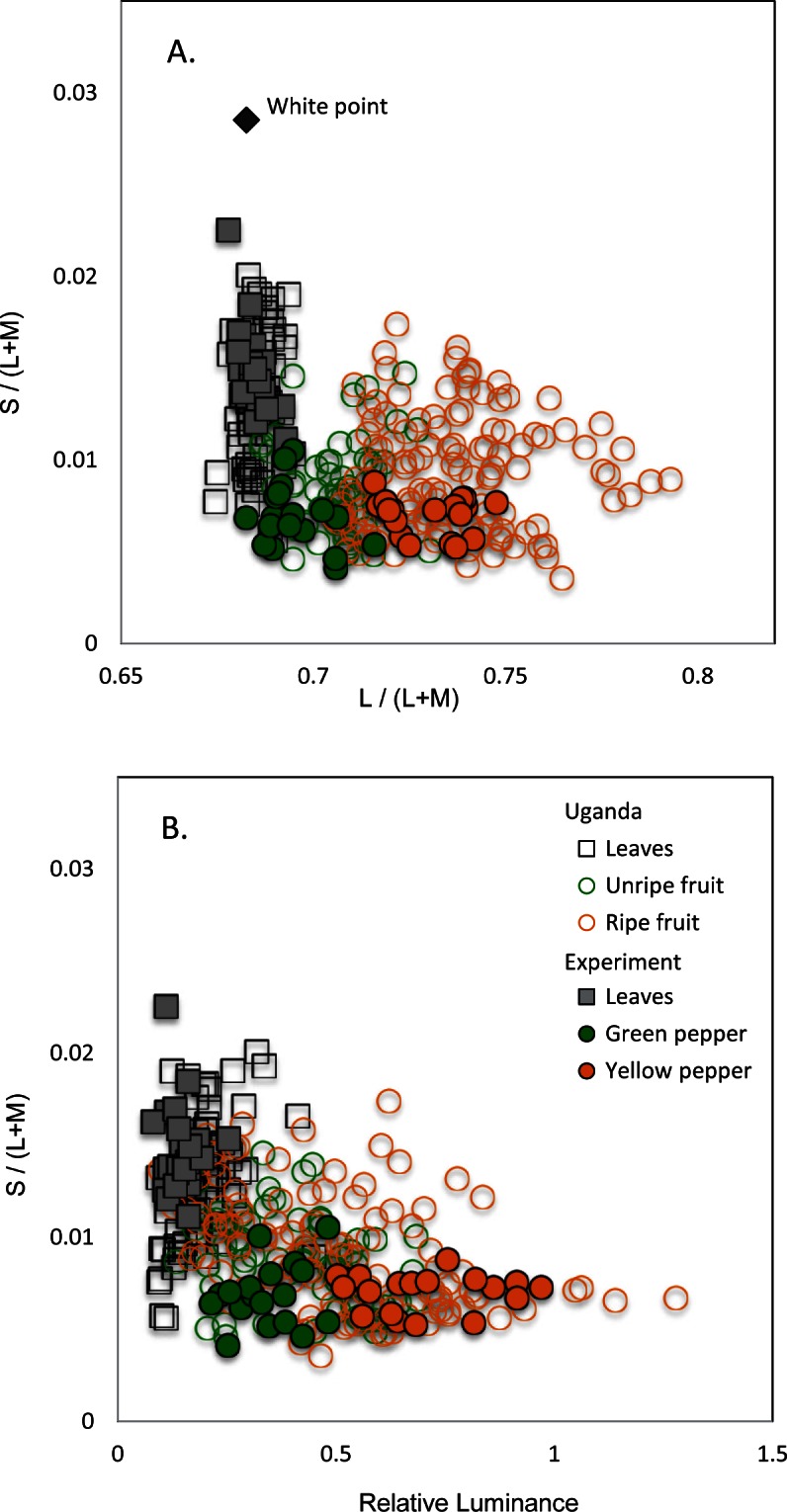
Chromatic properties of the stimuli. Segments of yellow/orange and green pepper used as targets and distractors overlap in their chromatic properties (orange and green filled circles) with some fruits eaten by primates in Uganda (unfilled circles). The leaves from the experimental bush (grey squares) also show similar properties to leaves in a Ugandan rainforest (open squares).
2. Results
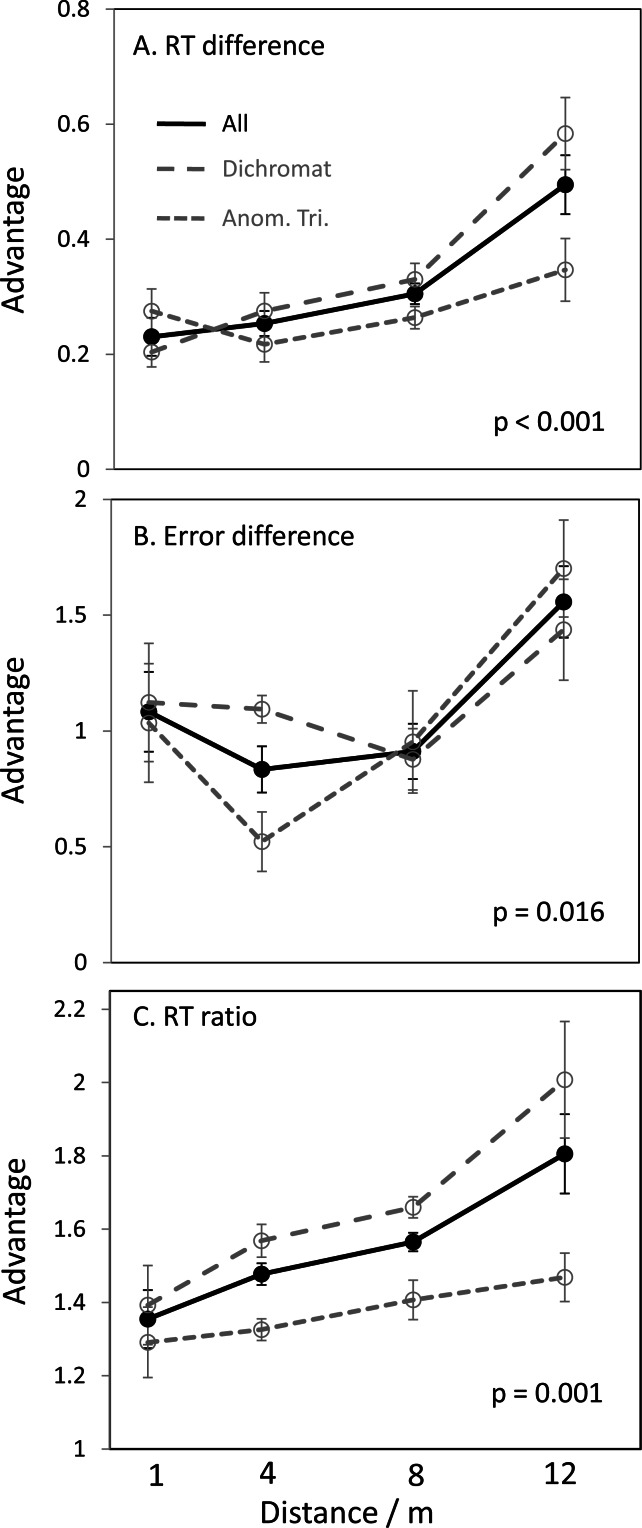
Normal trichromats made faster responses and fewer errors than their colour-deficient competitors, as expected (mean RTs 4.7 vs. 6.4 s; error score 0.17 vs. 0.54; all errors were “misses,” i.e. responding with a lower number than the actual number of targets). Our unit of analysis is the pairwise comparison between the members of each matched pair. The key question is how this advantage depended on viewing distance. As shown in Figures 4(a and b), the relative advantage increased with distance, both for response time and errors [One-way ANOVAs, F(3,21) = 9.7, p < 0.001 and F(3,30) = 4.0, p < 0.02; see the Methods section for subject numbers and other details]. In Figures 4(a and b), the difference in performance (RT or error) between the colour-deficient member and the colour-normal member of each pair was divided by the overall mean RT or error of that pair, in order to control for different overall performance and speed–accuracy trade-offs (range in mean RT, 3.4–12.8 s and in mean error score 0.15–0.57; the behaviour of each member was correlated, r = 0.76 for RT and r = 0.32 for errors, probably because they influenced each other in this regard, as would be expected, and/or because they had correlated age, experience and testing conditions).
Figure 4.
Advantage of normal trichromacy increases with distance. In panels (a and b), the difference in performance (RT or error) between the colour-deficient member and the colour-normal member of each pair was divided by the overall mean RT or error of that pair, in order to control for different overall performance and speed–accuracy trade-offs. In panel (c), the RT for the colour-deficient member of each pair in each condition is simply divided by the RT of the colour-normal member (which is a simpler analysis and also controls for differences in baseline speed with distance). This analysis could not be done reliably for error scores because there were many instances of dividing by zero or near-zero bins. The dashed and dotted lines show the subsets of results for dichromats and anomalous trichromats (each compared with their matched normal trichromat).
Figure 4(c) shows that the same pattern [F(3,21) = 5.6, p = 0.005] occurred for the mean ratio of response times of the colour-normal and colour-deficient members of each pair. The dashed and dotted lines in Figures 4(a–c) show the subsets of results for dichromats and anomalous trichromats (each compared with their matched normal trichromat), indicating that the effect on response time is greater for full dichromats [interaction F(3,18) = 3.4, p < 0.05], as would be expected. The same pattern is not evident in the error scores, but this could be a simple lack of power or a difference in speed accuracy strategy between groups. Note that the key comparison was between the colour-deficient and normal trichromatic member of each pair, not between dichromats and anomalous trichromats, because although the dichromats and anomalous trichromats were each matched in age and relevant experience to their control trichromats, dichromats and anomalous trichromats could not be matched in age or experience with each other. The mean age of the dichromats was 10 years older than that of the anomalous trichromats.
3. Discussion
Our results show that in our naturalistic visual search task with human observers, the advantage of normal trichromacy over dichromacy or anomalous trichromacy increased with distance (even without the additional cues of smell and touch available to foraging monkeys). The advantage was greatest when the stimuli subtended no more than 0.2 degrees at the eye. This is likely to be because visual cues such as shape and the S/(L+M) dimension of colour vision decrease with distance for small targets. For very small stimuli, the absence of S cones in the very centre of the fovea (about the central 1/3 of a degree of visual angle), precludes information in the S/(L+M) pathway for fixated targets (known as “small field tritanopia”), although the effect of this is less when observers scan a scene, as in the present task (e.g. McCree, 1960).
Although we used fruit not young (reddish or yellowish) leaves as targets, we see no reason that our findings would not extend to such leaves, which are also important sources of food for some species of primate. Our results imply that the more important task for the natural selection of primate trichromacy may have been spotting food from a distance rather than picking it at arms' length. This in no way challenges the previous finding that the LM colour channel is optimal at the close-up task (Parraga et al., 2002). The biggest advantage of one phenotype over competing phenotypes does not have to arise for the task that the phenotype is best at. However, while the spatial-frequency sensitivity of the LM system undoubtedly makes it optimal at the close-up task, our results do raise the question of whether it has been optimized by that task. Rather, its optimality may have emerged as a side effect of the combined pressures of the longer distance task and other constraints, such as the random layout of L and M cones on the retina (Deeb, 2006) and the unavoidable fact that both colour resolution and achromatic resolution cannot be equally high because they are conflated in the most fine scale comparison of neighbouring cones.
One general puzzle is why it has been so difficult to observe any behavioural advantages for trichromats over dichromats in the wild, when they have been apparent as expected in experiments such as this one and those on captive tamarins and marmosets (Caine & Mundy, 2000; Smith et al., 2003b). Most studies in the wild have concentrated on observing monkeys picking fruit (e.g. Hiramatsu et al., 2008; Melin et al., 2009; Vogel et al., 2007), where our results predict the advantage is smallest, even without the extra tactile and olfactory cues available in the wild but not in our experiment. Unfortunately, it is very difficult to discern and quantify the longer distance task except by observing troop leadership, which is known to be influenced by other social factors which might mask any effect of colour vision (Smith, Buchanan-Smith, Surridge, & Mundy, 2003a).
Another possibility is that the colour properties of fruits consumed in the forests where these studies have taken place are not fully representative of the gamut of fruits eaten by our ancestors. It is difficult to do more than speculate here, but the chromaticity diagrams in Hiramatsu et al. (2008), who found no differences in feeding behaviour between dichromatic and trichromatic spider monkeys, show a higher ratio of cryptic fruits (those that stay green; see also De Araujo, Lima, & Pessoa, 2006) and fruits that turn dark brownish purple (as do many types of fig), and fewer orange or yellow fruit than are found in the diet of primates in Uganda (Sumner & Mollon, 2000a, 2000b). Cryptic fruits are detected from afar by smell or shape (and memory), and close up by shape, position, smell or feel (e.g. Hiramatsu et al., 2009; Melin et al., 2009), all of which dichromats can do at least as well as trichromats. Dark fruits have, by definition, a strong achromatic signal, which again dichromats can detect at least as well as trichromats (and possibly are better attuned to). However, viewed amongst foliage from a distance, dark fruits (unless in a clump) could easily be mistaken for shadow or gaps, so the use of achromatic cues in fruit foraging remains unclear (as does the extra cue of glossiness often present in dark fruits). It would be interesting to test human dichromats and trichromats with fruits showing these properties, such as green and red grapes. Note that our targets did also contain a strong luminance signal relative to the foliage background, and of course wild monkeys have a lifetime of practice in fruit picking which acts to diminish any behavioural differences between them.
Field studies have concentrated on polymorphic species of monkey where some individuals are trichromatic and some are dichromatic, both because this situation makes an ideal natural experiment and because the polymorphism is interesting and rare in itself. It arises through there being different alleles of the gene coding the L cone pigment, which give the pigment different sensitivities (akin to our L and M cones). If a monkey is homozygous for this gene, they can only have one type of L cone (in addition to S cones), and are thus dichromatic. But if they are heterozygous, and the alleles are expressed in different cones cells, then they possess two types of L cone (i.e. L and M cones) and can be trichromatic (Mollon et al., 1984). The polymorphic gene is on the X chromosome, which means that only females get the opportunity to be heterozygous and thus trichromatic. In many species, there are three alleles present in different frequencies, which means that approximately 60% of females are trichromatic (Jacobs, Neitz, & Neitz, 1993; Williams, Hunt, Bowmaker, & Mollon, 1992). Because trichromacy arises from heterozygosity, rather than from possessing a single advantageous allele, it cannot be simply passed on to offspring. In fact, there is about equal chance of a dichromatic or trichromatic mother having a trichromatic daughter (in-breeding avoidance can complicate this, and possibly make dichromats more likely to have trichromatic daughters; Surridge, Suarez, Buchanan-Smith, & Mundy, 2005).
However, rare alleles tend to be lost to populations if their presence provides no advantage (e.g. Surridge & Mundy, 2002), so it is assumed that to maintain the polymorphism there must be some advantage to trichromacy in the wild that translates into improved fitness (the potential advantage of having different types of dichromacy in a population has not been given any support; Riba-Hernandez et al., 2004). On the other hand, if this advantage was very large, there is the obvious question of why full trichromacy has not emerged in these species, as it did for Asian and African primates, and has done also for howler monkeys (Jacobs, Neitz, Deegan, & Neitz, 1996). One possibility is that the genetic event required to allow this is vanishingly rare and simply never happened in those species that remain polymorphic (Regan et al., 2001)—a gene duplication is required that puts two different alleles onto the same chromosome alongside working promoter regions and a mechanism for expressing these genes differentially in different cones. The other possibility is that the advantage of trichromacy is much subtler than originally expected when combined with social factors within a polymorphic population (e.g. Bunce et al., 2011; Smith et al., 2003a), and may also be offset by some advantages for dichromacy, such as breaking camouflage of cryptic insects (Melin, Fedigan, Hiramatsu, Sendall, & Kawamura, 2007; Regan et al., 2001; Saito et al., 2005). Either way, given that many species of monkey have successfully survived and diversified over millions of years in which the majority of individuals (and all the males) have been dichromatic (e.g. Surridge & Mundy, 2002), it should not be surprising that dichromats are not glaringly deficient at any crucial task in the wild that would dramatically harm their chances of survival. From this perspective, the advantage of trichromacy is bound to be quite subtle and hard to measure in free-ranging wild monkeys.
Lastly, some authors have suggested that the selective advantage of trichromacy might have been in signalling (Changizi, Zhang, & Shimojo, 2006). Many monkeys have red faces (and sometimes red sexual signals) or orange fur markings (Sumner & Mollon, 2003). More recent evidence suggests that such signalling post-dates the invention of trichromacy, and thus took advantage of trichromacy once it had arisen, rather than driving its natural selection initially (Fernandez & Morris, 2007). However, it may form part of the selection pressure maintaining it in current populations, as might predator detection (Smith, Buchanan-Smith, Surridge, & Mundy, 2005; Sumner & Mollon, 2003).
4. Conclusion
In conclusion, we found that in a naturalistic visual search task of finding food in foliage, humans with “normal colour vision” diverged in performance from dichromats or anomalous trichromats most for the longest viewing distance (12 m). This implies that in the natural selection of trichromacy, the task of spotting fruit from a distance was a greater driving force than picking fruit close up.
5. Methods
5.1. Participants
Colour-deficient participants were recruited through advert and tested using the Ishihara test (24 plate edition) and Nagel Anomaloscope to determine their type of colour vision. Following this screening, 12 participants with clearly defined colour deficiency (3 protanopes, 4 deuteranopes, 2 protanomalous and 3 deuteranomalous) performed the visual search task. Each was paired with control participant with normal trichromacy (tested using the Ishihara plates), matching as closely as possible for age, sex, experience of visual experiments and knowledge of colour vision. Each pair performed the task simultaneously so that they were exposed to the identical trial sequence in identical lighting conditions (cloudy or sunny). This set-up also engendered some aspect of competition between the pair, simulating the competition for fruit of two primates simultaneously foraging in the same tree. For the first three pairs of participants, accuracy was recorded, but not reaction time due to a technical error. One participant opened his eyes prematurely on some of the visual search trials, and so that pair was excluded from all analyses. Therefore, there were 16 participants (8 pairs) for the analysis of response time and 22 participants (11 pairs) for the analysis of error scores.
5.2. Visual search task
Participants had to count the number of “ripe fruit” hanging in a bush from a distance of 1, 4, 8 or 12 m (Figure 1). On each trial, the two participants were positioned at the appropriate distance and blindfolded while the experimenter positioned four “fruit” (see the Stimuli section below) amongst the leaves of the bush. Between zero and four of these stimuli were targets (“ripe fruit”) and the rest were distractors (“unripe fruit”).
When the stimuli were positioned, the experimenter instructed the participants to remove their blindfold keeping their eyes closed (compliance was monitored by the experimenter). Then, on the instruction “GO” the participants simultaneously opened their eyes and viewed the stimuli. Their task was to press one of the five keys (labelled 0, 1, 2, 3, 4) on a keyboard “as quickly and accurately as possible” to indicate the number of targets they could see. The keyboards were positioned on a trolley between the participants, which also contained the computer and could be moved to each distance as appropriate (Figure 1, lower panel). The side of the trolley taken by the colour-deficient participant was counterbalanced across pairs. RT was recorded (except for the first three participants) as well as the difference between the answer given and the correct answer (error score). While there was no barrier to entirely rule out participants seeing each other's responses, behaviour was watched by the experimenter. Furthermore, if a participant based their response on that of their competitor, by definition they would have longer reaction times, but consistently across distances. The effect this might have on results is to dilute any interaction between group and distance.
There were 40 trials per pair (10 per distance), taking approximately one hour to complete. On each trial, the number of each stimulus type and their positions, as well as the viewing distance, all followed a randomized sequence that ensured two trials with each number of targets (0–4) were conducted for each distance.
5.3. Stimuli
The aim was to use real fruit as target and distractor stimuli, positioned amongst real leaves. We located a suitable bush (Aucuba japonica) in an unused courtyard on the University campus, which could be viewed from between 1 and 12 m. We measured the reflected spectra (SpectroCal, Cambridge Research Systems) from 25 of the leaves in situ under natural overcast conditions in order to compare their chromaticity coordinates (Figure 3) with leaves measured in a Ugandan rainforest, also under overcast conditions, by Sumner and Mollon (2000a). These rainforest spectra are available online at the “Cambridge database of natural spectra” (http://vision.psychol.cam.ac.uk/spectra/). To calculate chromaticities, we used cone fundamentals from Stockman and Sharpe (2000) and corresponding scaling factors (http://www.cvrl.org).
To choose target and distractor stimuli, we measured the reflectance spectra of a range of fruits obtained from local supermarkets and specialist shops, in order to compare them with the fruit in a Ugandan forest (Sumner and Mollon (2000a, b). For this comparison, we used 10 fruit species (7 Ficus sp., 2 Celtis sp. and Chrysophyllum albidum) highly favoured by the primates in the Kibale forest, including monkeys and chimpanzees, from the “Cambridge database of natural spectra” (see Figure 2). To be used in our task, target and distractor fruit had to satisfy three conditions: (1) the chromaticities had to be representative of the rainforest fruit (to calculate chromaticities, we used a natural illuminant in overcast conditions from near the experimental bush for experimental stimuli, and from Uganda for Ugandan fruit, http://vision.psychol.cam.ac.uk/spectra/); (2) the stimuli had to be light enough to hang on the bush; (3) the stimuli must not change (e.g. change colour through oxidization) during the course of an experimental session. The fruit that best satisfied these criteria were green (“unripe”) and yellow/orange (“ripe”) peppers, which could be cut into equal-sized segments and hung on the bush with small green hooks. Although peppers are not indigenous to Africa, natural spectra are constrained by the pigments available to plants. Finding segments of pepper amongst leaves is a task highly representative of the chromatic visual search task facing primates in Uganda, which in turn is the environment most likely to represent that of our shared ancestors. Segments 3 × 5 cm were used (Figure 2), subtending the following degrees of visual angle for distances 1, 4, 8, 12: 1.7 × 2.3, 0.43 × 0.57, 0.21 × 0.29, 0.14 × 0.19.
Before performing the visual search trials, each participant was shown the yellow and green pepper segments intermingled on a plate and asked to categorize them into two piles. All participants did this without error. They were then informed that the yellow peppers, but not the green, were the targets for the visual search.
Acknowledgments
This paper is in memory of Tom Troscianko, who was an inspiration for this project. The results were first reported in the Symposium for Tom at ECVP 2012. We also thank Julian Partridge, David Tolhurst and Danny Osorio for discussion on this topic, Tom Margrain and Jon Erichsen for use of the Ishihara test and Nagel Anomaloscope, and Lynne Pritchard and Justin Savage for practical assistance with the portable task equipment. The study was funded by the School of Psychology, Cardiff University.
Contributor Information
Aline Bompas, School of Psychology, Cardiff University, Cardiff CF10 3AT, Wales, United Kingdom; e-mail: bompasa@cf.ac.uk.
Grace Kendall, School of Psychology, Cardiff University, Cardiff CF10 3AT, Wales, United Kingdom; e-mail: graci.kendall@hotmail.co.uk.
Petroc Sumner, School of Psychology, Cardiff University, Cardiff CF10 3AT, Wales, United Kingdom; e-mail: sumnerp@cf.ac.uk.
References
- Allen G. The colour-sense: Its origin and development. London: Trubner & Co.; 1879. [DOI] [Google Scholar]
- Bowmaker J. K. Evolution of colour vision in vertebrates. Eye. 1998;12:541–547. doi: 10.1038/eye.1998.143. [DOI] [PubMed] [Google Scholar]
- Bunce J. A., Isbell L. A., Grote M. N., Jacobs G. H. Color vision variation and foraging behavior in wild Neotropical titi monkeys (Callicebus brunneus): Possible mediating roles for spatial memory and reproductive status. International Journal of Primatology. 2011;32(5):1058–1075. doi: 10.1007/S10764-011-9522-Y. [DOI] [Google Scholar]
- Caine N. G., Mundy N. I. Demonstration of a foraging advantage for trichromatic marmosets (Callithrix geoffroyi) dependent on food colour. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2000;267:439–444. doi: 10.1098/rspb.2000.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changizi M. A., Zhang Q., Shimojo S. Bare skin, blood and the evolution of primate colour vision. Biology Letters. 2006;2(2):217–221. doi: 10.1098/Rsbl.2006.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Araujo M. F. P., Lima E. M., Pessoa V. F. Modeling dichromatic and trichromatic sensitivity to the color properties of fruits eaten by squirrel monkeys (Saimiri sciureus) American Journal of Primatology. 2006;68(12):1129–1137. doi: 10.1002/Ajp.20312. [DOI] [PubMed] [Google Scholar]
- Deeb S. S. Genetics of variation in human color vision and the retinal cone mosaic. Current Opinion in Genetics & Development. 2006;16(3):301–307. doi: 10.1016/J.Gde.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Dominy N. J., Lucas P. W. Ecological importance of trichromatic vision to primates. Nature. 2001;410:363–366. doi: 10.1038/35066567. [DOI] [PubMed] [Google Scholar]
- Dominy N. J., Lucas P. W., Osorio D., Yamashita N. The sensory ecology of primate food perception. Evolutionary Anthropology. 2001;10(5):171–186. doi: 10.1002/evan.1031. [DOI] [PubMed] [Google Scholar]
- Fernandez A. A., Morris M. R. Sexual selection and trichromatic color vision in primates: Statistical support for the preexisting-bias hypothesis. American Naturalist. 2007;170(1):10–20. doi: 10.1086/518566. [DOI] [PubMed] [Google Scholar]
- Hiramatsu C., Melin A. D., Aureli F., Schaffner C. M., Vorobyev M., Kawamura S. Interplay of olfaction and vision in fruit foraging of spider monkeys. Animal Behaviour. 2009;77(6):1421–1426. doi: 10.1016/J.Anbehav.2009.02.012. [DOI] [Google Scholar]
- Hiramatsu C., Melin A. D., Aureli F., Schaffner C. M., Vorobyev M., Matsumoto Y., Kawamura S. Importance of achromatic contrast in short-range fruit foraging of primates. PLoS One. 2008;3(10) doi: 10.1371/journal.pone.0003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs G. H. The distribution and nature of colour vision among the mammals. Biological Reviews. 1993;68:413–471. doi: 10.1111/j.1469-185X.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Jacobs G. H., Neitz J., Neitz M. Genetic basis of polymorphism in the colour vision of platyrrhine monkeys. Vision Research. 1993;33:269–274. doi: 10.1016/0042-6989(93)90083-9. [DOI] [PubMed] [Google Scholar]
- Jacobs G. H., Neitz M., Deegan J. F., Neitz J. Trichromatic colour vision in New World monkeys. Nature. 1996;382:156–158. doi: 10.1038/382156a0. [DOI] [PubMed] [Google Scholar]
- Lucas P. W., Dominy N. J., Riba-Hernandez P., Stoner K. E., Yamashita N., Loria-Calderon E., Darvell B. W. Evolution and function of routine trichromatic vision in primates. Evolution. 2003;57(11):2636–2643. doi: 10.1111/j.0014-3820.2003.tb01506.x. [DOI] [PubMed] [Google Scholar]
- McCree K. J. Small field tritanopia and the effects of voluntary fixation. Optica Acta. 1960;7:317. doi: 10.1080/713826349. [DOI] [Google Scholar]
- Melin A. D., Fedigan L. M., Hiramatsu C., Hiwatashi T., Parr N., Kawamura S. Fig foraging by dichromatic and trichromatic Cebus capucinus in a tropical dry forest. International Journal of Primatology. 2009;30(6):753–775. doi: 10.1007/S10764-009-9383-9. [DOI] [Google Scholar]
- Melin A. D., Fedigan L. M., Hiramatsu C., Sendall C. L., Kawamura S. Effects of colour vision phenotype on insect capture by a free-ranging population of white-faced capuchins, Cebus capucinus. Animal Behaviour. 2007;73:205–214. doi: 10.1016/J.Anbehav.2006.07.003. [DOI] [Google Scholar]
- Mollon J. D. Tho she kneel'd in that place where they grew…. Journal of Experimental Biology. 1989;146:21–38. doi: 10.1242/jeb.146.1.21. [DOI] [PubMed] [Google Scholar]
- Mollon J. D., Bowmaker J. K., Jacobs G. H. Variations of colour vision in a New World primate can be explained by polymorphism of retinal photopigments. Proceedings of the Royal Society of London B. 1984;222:373–399. doi: 10.1098/rspb.1984.0071. [DOI] [PubMed] [Google Scholar]
- Osorio D., Vorobyev M. Colour vision as an adaptation to frugivory in primates. Proceedings of the Royal Society of London B. 1996;263:593–599. doi: 10.1098/rspb.1996.0089. [DOI] [PubMed] [Google Scholar]
- Parraga C. A., Troscianko T., Tolhurst D. J. Spatiochromatic properties of natural images and human vision. Current Biology. 2002;12(6):483–487. doi: 10.1016/S0960-9822(02)00718-2. [DOI] [PubMed] [Google Scholar]
- Polyak S. The vertebrate visual system. Chicago, IL: University of Chicago Press; 1957. [Google Scholar]
- Regan B. C., Julliot C., Simmen B., Viénot F., Charles-Dominique P., Mollon J. D. Frugivory and colour vision in Alouatta seniculus, a trichromatic platyrrhine monkey. Vision Research. 1998;38:3321–3327. doi: 10.1016/S0042-6989(97)00462-8. [DOI] [PubMed] [Google Scholar]
- Regan B. C., Julliot C., Simmen B., Viénot F., Charles-Dominique P., Mollon J. D. Fruits, foliage and the evolution of the primate colour-sense. Philosophical Transactions of the Royal Society of London B. 2001;356:229–283. doi: 10.1098/rstb.2000.0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba-Hernandez P., Stoner K. E., Osorio D. Effect of polymorphic colour vision for fruit detection in the spider monkey Ateles geoffroyi, and its implications for the maintenance of polymorphic colour vision in platyrrhine monkeys. Journal of Experimental Biology. 2004;207(14):2465–2470. doi: 10.1242/jeb.01046. [DOI] [PubMed] [Google Scholar]
- Saito A., Mikami A., Kawamura S., Ueno Y., Hiramatsu C., Widayati K. A., Hasegawa T. Advantage of dichromats over trichromats in discrimination of color-camouflaged stimuli in nonhuman primates. American Journal of Primatology. 2005;67(4):425–436. doi: 10.1002/Ajp.20197. [DOI] [PubMed] [Google Scholar]
- Smith A. C., Buchanan-Smith H. M., Surridge A. K., Mundy N. I. Leaders of progressions in wild mixed-species troops of saddleback (Saguinus fuscicollis) and mustached tamarins (S-mystax), with emphasis on color vision and sex. American Journal of Primatology. 2003a;61(4):145–157. doi: 10.1002/Ajp.10117. [DOI] [PubMed] [Google Scholar]
- Smith A. C., Buchanan-Smith H. M., Surridge A. K., Mundy N. I. Factors affecting group spread within wild mixed-species troops of saddleback and mustached tamarins. International Journal of Primatology. 2005;26(2):337–355. doi: 10.1007/S10764-005-2928-7. [DOI] [Google Scholar]
- Smith A. C., Buchanan-Smith H. M., Surridge A. K., Osorio D., Mundy N. I. The effect of colour vision status on the detection and selection of fruits by tamarins (Saguinus spp.) Journal of Experimental Biology. 2003b;206(18):3159. doi: 10.1242/Jeb.00536. [DOI] [PubMed] [Google Scholar]
- Steward J. M., Cole L. What do color vision defectives say about everyday tasks? Optometry and Visual Science. 1989;66:288–295. doi: 10.1097/00006324-198905000-00006. [DOI] [PubMed] [Google Scholar]
- Stockman A., Sharpe L. T. The spectral sensitivities of the middle- and long-wavelength-sensitive cones derived from measurements in observers of known genotype. Vision Research. 2000;40(13):1711–1737. doi: 10.1016/S0042-6989(00)00021-3. [DOI] [PubMed] [Google Scholar]
- Sumner P., Mollon J. D. Catarrhine photopigments are optimised for detecting targets against a foliage background. Journal of Experimental Biology. 2000a;203:1963–1986. doi: 10.1242/jeb.203.13.1963. [DOI] [PubMed] [Google Scholar]
- Sumner P., Mollon J. D. Chromaticity as a signal of ripeness in fruits taken by primates. Journal of Experimental Biology. 2000b;203:1987–2000. doi: 10.1242/jeb.203.13.1987. [DOI] [PubMed] [Google Scholar]
- Sumner P., Mollon J. D. Colors of primate pelage and skin: Objective assessment of conspicuousness. American Journal of Primatology. 2003;59:67–91. doi: 10.1002/ajp.10066. [DOI] [PubMed] [Google Scholar]
- Surridge A. K., Mundy N. I. Trans-specific evolution of opsin alleles and the maintenance of trichromatic colour vision in Callitrichine primates. Molecular Ecology. 2002;11:2157–2169. doi: 10.1046/j.1365-294x.2002.01597.x. [DOI] [PubMed] [Google Scholar]
- Surridge A. K., Suarez S. S., Buchanan-Smith H. M., Mundy N. I. Non-random association of opsin alleles in wild groups of red-bellied tamarins (Saguinus labiatus) and maintenance of the colour vision polymorphism. Biology Letters. 2005;1(4):465–468. doi: 10.1098/Rsbl.2005.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel E. R., Neitz M., Dominy N. J. Effect of color vision phenotype on the foraging of wild white-faced capuchins, Cebus capucinus. Behavioral Ecology. 2007;18(2):292–297. doi: 10.1093/Beheco/Arl082. [DOI] [Google Scholar]
- Williams A. J., Hunt D. M., Bowmaker J. K., Mollon J. D. The polymorphic photopigments of the marmoset: spectral tuning and genetic basis. EMBO Journal. 1992;11:2039–2045. doi: 10.1002/j.1460-2075.1992.tb05261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]