Abstract
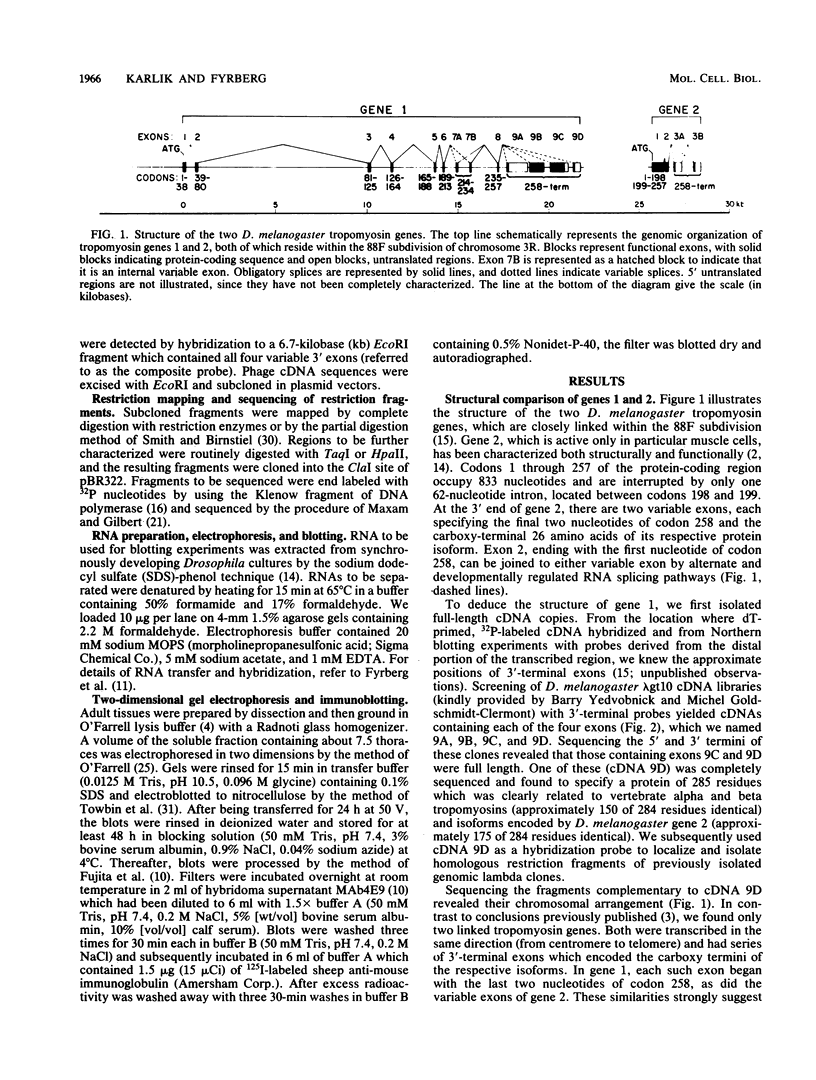
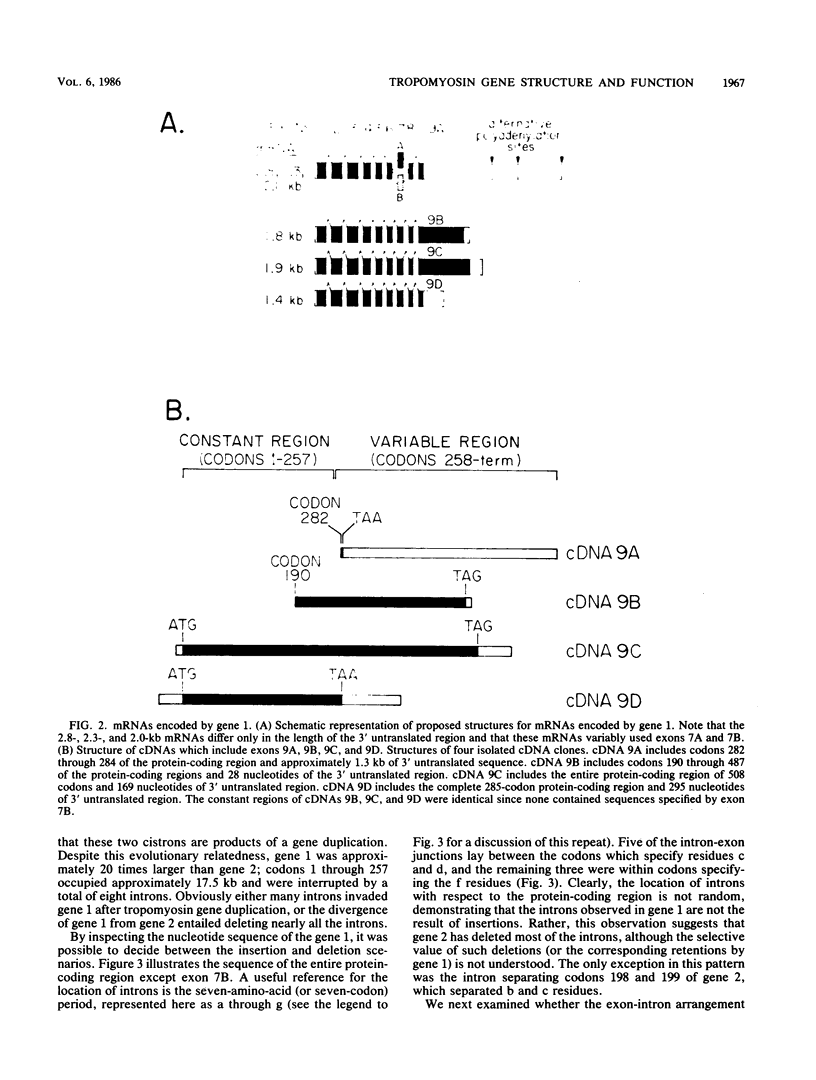
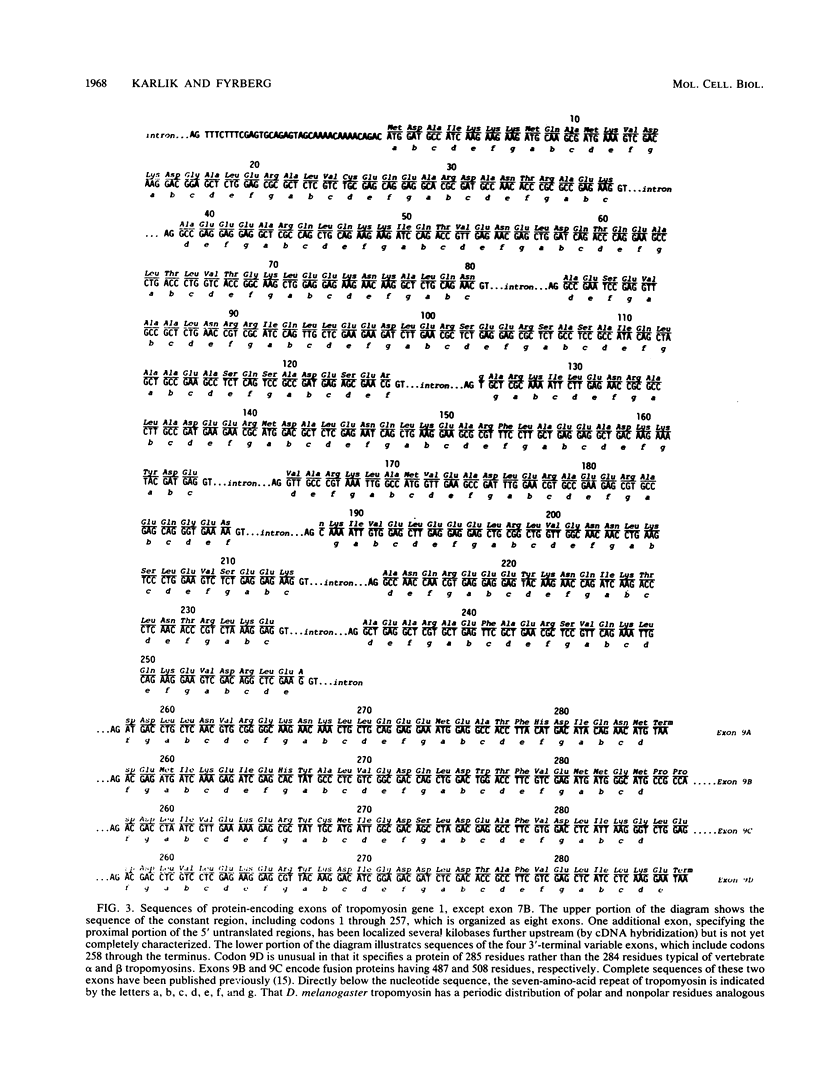
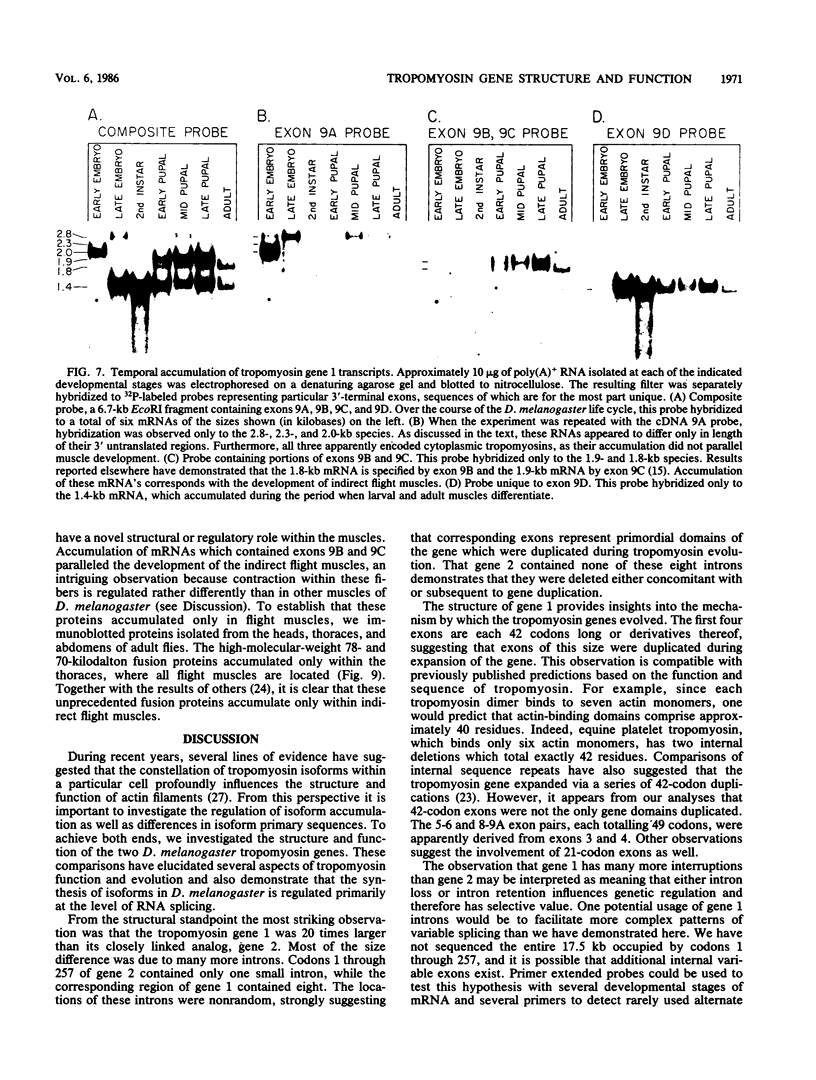
We compared the structure and function of the two Drosophila melanogaster tropomyosin genes. The most striking structural aspect was their size disparity. Codons 1 through 257 of gene 2 occupied 833 nucleotides and contained only one intron, whereas the corresponding region of gene 1 occupied 17.5 kilobases and was interrupted by eight introns. The intron-exon arrangement of gene 1 reflected evolutionary expansion of tropomyosin via 42- and 49-residue duplications, which are probably actin-binding domains. Functionally, gene 1 was considerably more complex than gene 2; it was active in both muscle and nonmuscle cell lineages, had at least five variable exons, and specified a minimum of five developmentally regulated isoforms. Two of these isoforms, which accumulated only in flight muscles, were unprecedented fusion proteins in which the tropomyosin sequence was joined to a carboxy-terminal proline-rich domain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Basi G. S., Boardman M., Storti R. V. Alternative splicing of a Drosophila tropomyosin gene generates muscle tropomyosin isoforms with different carboxy-terminal ends. Mol Cell Biol. 1984 Dec;4(12):2828–2836. doi: 10.1128/mcb.4.12.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautch V. L., Storti R. V. Identification of a cytoplasmic tropomyosin gene linked to two muscle tropomyosin genes in Drosophila. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7123–7127. doi: 10.1073/pnas.80.23.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst B. P. Two-dimensional gel patterns of protein synthesis before and after fertilization of sea urchin eggs. Dev Biol. 1976 Sep;52(2):310–317. doi: 10.1016/0012-1606(76)90248-7. [DOI] [PubMed] [Google Scholar]
- Cooper H. L., Feuerstein N., Noda M., Bassin R. H. Suppression of tropomyosin synthesis, a common biochemical feature of oncogenesis by structurally diverse retroviral oncogenes. Mol Cell Biol. 1985 May;5(5):972–983. doi: 10.1128/mcb.5.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik C. S., Rutter W. J., Fletterick R. Splice junctions: association with variation in protein structure. Science. 1983 Jun 10;220(4602):1125–1129. doi: 10.1126/science.6344214. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. Distribution of polymorphic forms of troponin components and tropomyosin in skeletal muscle. Nature. 1979 Apr 19;278(5706):714–718. doi: 10.1038/278714a0. [DOI] [PubMed] [Google Scholar]
- Egelman E. H. The structure of F-actin. J Muscle Res Cell Motil. 1985 Apr;6(2):129–151. doi: 10.1007/BF00713056. [DOI] [PubMed] [Google Scholar]
- Fujita S. C., Zipursky S. L., Benzer S., Ferrús A., Shotwell S. L. Monoclonal antibodies against the Drosophila nervous system. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7929–7933. doi: 10.1073/pnas.79.24.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Multiple tropomyosin polypeptides in chicken embryo fibroblasts: differential repression of transcription by Rous sarcoma virus transformation. Mol Cell Biol. 1984 Sep;4(9):1823–1833. doi: 10.1128/mcb.4.9.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Tropomyosin is decreased in transformed cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5633–5637. doi: 10.1073/pnas.78.9.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlik C. C., Fyrberg E. A. An insertion within a variably spliced Drosophila tropomyosin gene blocks accumulation of only one encoded isoform. Cell. 1985 May;41(1):57–66. doi: 10.1016/0092-8674(85)90061-3. [DOI] [PubMed] [Google Scholar]
- Karlik C. C., Mahaffey J. W., Coutu M. D., Fyrberg E. A. Organization of contractile protein genes within the 88F subdivision of the D. melanogaster third chromosome. Cell. 1984 Jun;37(2):469–481. doi: 10.1016/0092-8674(84)90377-5. [DOI] [PubMed] [Google Scholar]
- Klenow H., Henningsen I. Selective elimination of the exonuclease activity of the deoxyribonucleic acid polymerase from Escherichia coli B by limited proteolysis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):168–175. doi: 10.1073/pnas.65.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Tropomyosin antibody: the specific localization of tropomyosin in nonmuscle cells. J Cell Biol. 1975 Jun;65(3):549–561. doi: 10.1083/jcb.65.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W. G., Cote G. P., Mak A. S., Smillie L. B. Amino acid sequence of equine platelet tropomyosin. Correlation with interaction properties. FEBS Lett. 1983 Jun 13;156(2):269–273. doi: 10.1016/0014-5793(83)80511-0. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B., Stewart G. R. A comparison of the amino acid sequences of rabbit skeletal muscle alpha- and beta-tropomyosins. J Biol Chem. 1980 Apr 25;255(8):3647–3655. [PubMed] [Google Scholar]
- Matsumura F., Lin J. J., Yamashiro-Matsumura S., Thomas G. P., Topp W. C. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Ultrastructural patterns of RNA synthesis during early embryogenesis of Drosophila melanogaster. Cell. 1976 Jun;8(2):305–319. doi: 10.1016/0092-8674(76)90014-3. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Stewart M., Smillie L. B. Sequence repeats in alpha-tropomyosin. J Mol Biol. 1975 Oct 25;98(2):281–291. doi: 10.1016/s0022-2836(75)80118-5. [DOI] [PubMed] [Google Scholar]
- Mogami K., Fujita S. C., Hotta Y. Identification of Drosophila indirect flight muscle myofibrillar proteins by means of two-dimensional electrophoresis. J Biochem. 1982 Feb;91(2):643–650. doi: 10.1093/oxfordjournals.jbchem.a133736. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paulin D., Perreau J., Jakob H., Jacob F., Yaniv M. Tropomyosin synthesis accompanies formation of actin filaments in embryonal carcinoma cells induced to differentiate by hexamethylene bisacetamide. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1891–1895. doi: 10.1073/pnas.76.4.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J. W. The Croonian Lecture, 1977. Stretch activation of muscle: function and mechanism. Proc R Soc Lond B Biol Sci. 1978 May 5;201(1143):107–130. doi: 10.1098/rspb.1978.0035. [DOI] [PubMed] [Google Scholar]
- Sanger J. W., Sanger J. M., Jockusch B. M. Differences in the stress fibers between fibroblasts and epithelial cells. J Cell Biol. 1983 Apr;96(4):961–969. doi: 10.1083/jcb.96.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]