Abstract
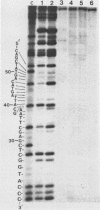
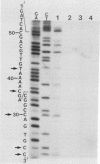
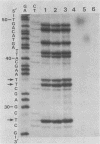
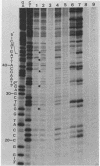
The substrate specificity of a calf thymus endonuclease on DNA damaged by UV ligh, ionizing radiation, and oxidizing agents was investigated. End-labeled DNA fragments of defined sequence were used as substrates, and the enzyme-generated scission products were analyzed by using DNA sequencing methodologies. The enzyme was shown to incise damaged DNA at pyrimidine sites. The enzyme incised DNA damaged with UV light, ionizing radiation, osmium tetroxide, potassium permanganate, and hydrogen peroxide at cytosine and thymine sites. The substrate specificity of the calf thymus endonuclease was compared to that of Escherichia coli endonuclease III. Similar pyrimidine base damage specificities were found for both enzymes. These results define a highly conserved class of enzymes present in both procaryotes and eucaryotes that may mediate an important role in the repair of oxidative DNA damage.
Full text
PDF

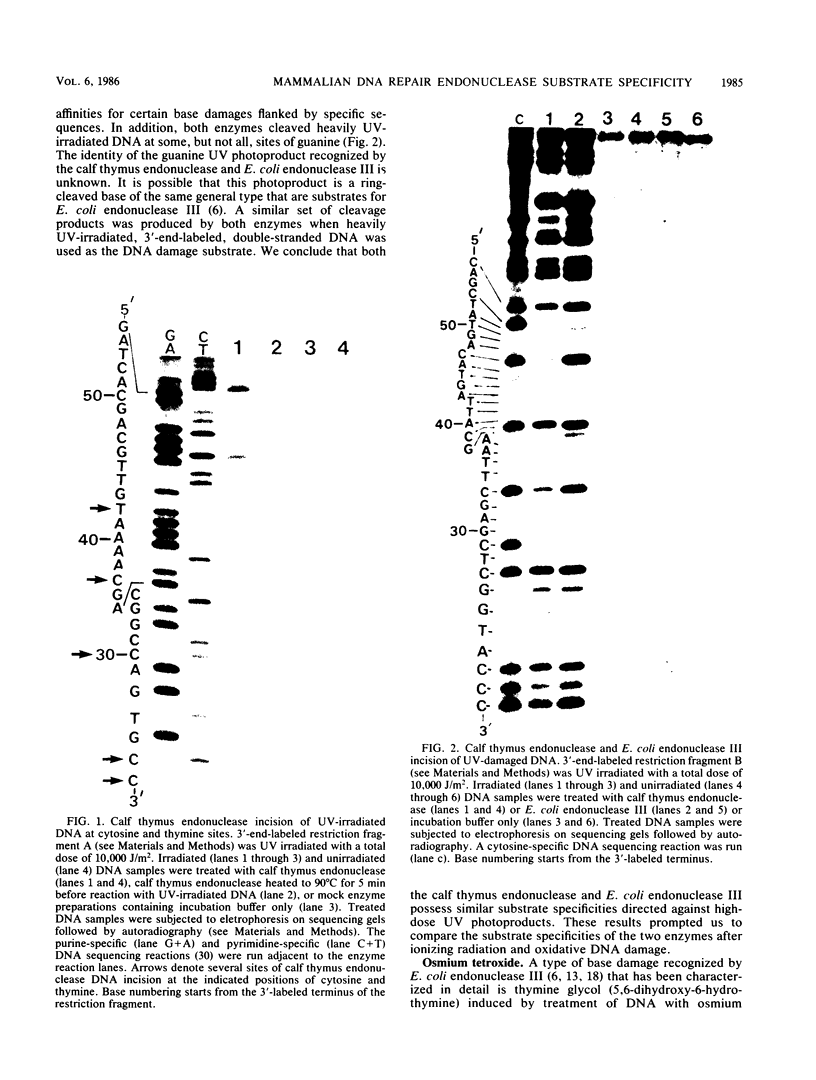

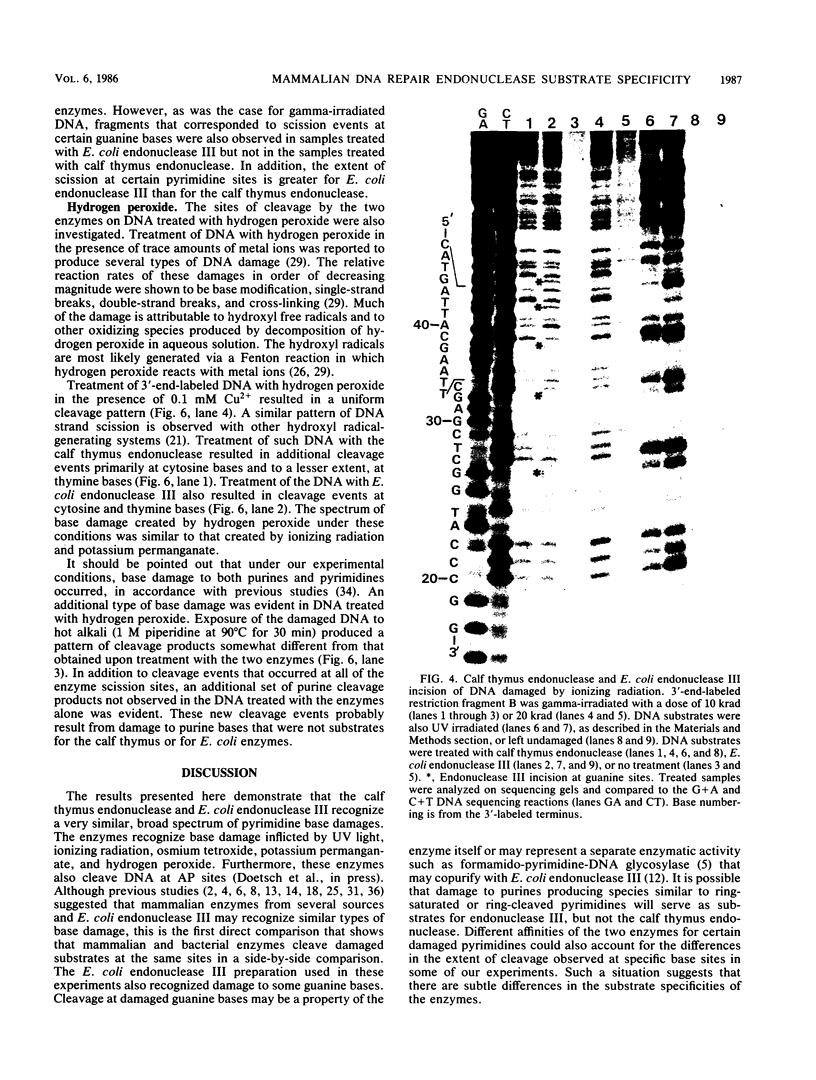


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Bacchetti S., Benne R. Purification and characterization of an endonuclease from calf thymus acting on irradiated DNA. Biochim Biophys Acta. 1975 May 16;390(3):285–297. doi: 10.1016/0005-2787(75)90349-4. [DOI] [PubMed] [Google Scholar]
- Breimer L. H. Enzymatic excision from gamma-irradiated polydeoxyribonucleotides of adenine residues whose imidazole rings have been ruptured. Nucleic Acids Res. 1984 Aug 24;12(16):6359–6367. doi: 10.1093/nar/12.16.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breimer L. H., Lindahl T. DNA glycosylase activities for thymine residues damaged by ring saturation, fragmentation, or ring contraction are functions of endonuclease III in Escherichia coli. J Biol Chem. 1984 May 10;259(9):5543–5548. [PubMed] [Google Scholar]
- Breimer L. H., Lindahl T. Thymine lesions produced by ionizing radiation in double-stranded DNA. Biochemistry. 1985 Jul 16;24(15):4018–4022. doi: 10.1021/bi00336a032. [DOI] [PubMed] [Google Scholar]
- Breimer L. H. Urea--DNA glycosylase in mammalian cells. Biochemistry. 1983 Aug 30;22(18):4192–4197. doi: 10.1021/bi00287a005. [DOI] [PubMed] [Google Scholar]
- Brent T. P. Properties of a human lymphoblast AP-endonuclease associated with activity for DNA damaged by ultraviolet light, gamma-rays, or osmium tetroxide. Biochemistry. 1983 Sep 13;22(19):4507–4512. doi: 10.1021/bi00288a024. [DOI] [PubMed] [Google Scholar]
- Burton K., Riley W. T. Selective degradation of thymidine and thymine deoxynucleotides. Biochem J. 1966 Jan;98(1):70–77. doi: 10.1042/bj0980070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Cunningham R. P., Weiss B. Endonuclease III (nth) mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jan;82(2):474–478. doi: 10.1073/pnas.82.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Linn S. 5,6-Saturated thymine lesions in DNA: production by ultraviolet light or hydrogen peroxide. Nucleic Acids Res. 1982 Jun 25;10(12):3781–3789. doi: 10.1093/nar/10.12.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Linn S. DNA N-glycosylases and UV repair. Nature. 1980 Sep 18;287(5779):203–208. doi: 10.1038/287203a0. [DOI] [PubMed] [Google Scholar]
- Doetsch P. W., Chan G. L., Haseltine W. A. T4 DNA polymerase (3'-5') exonuclease, an enzyme for the detection and quantitation of stable DNA lesions: the ultraviolet light example. Nucleic Acids Res. 1985 May 10;13(9):3285–3304. doi: 10.1093/nar/13.9.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gates F. T., Linn S. Endonuclease from Escherichia coli that acts specifically upon duplex DNA damaged by ultraviolet light, osmium tetroxide, acid, or x-rays. J Biol Chem. 1977 May 10;252(9):2802–2807. [PubMed] [Google Scholar]
- Glickman B. W., Rietveld K., Aaron C. S. gamma-Ray induced mutational spectrum in the lacI gene of Escherichia coli: comparison of induced and spontaneous spectra at the molecular level. Mutat Res. 1980 Jan;69(1):1–12. doi: 10.1016/0027-5107(80)90171-2. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Villasante A., Corces V., Pellicer A. Activation of a c-K-ras oncogene by somatic mutation in mouse lymphomas induced by gamma radiation. Science. 1984 Sep 14;225(4667):1159–1162. doi: 10.1126/science.6474169. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Grunberg S. M., Haseltine W. A. Sites and structure of gamma radiation-induced DNA strand breaks. J Biol Chem. 1982 Oct 10;257(19):11750–11754. [PubMed] [Google Scholar]
- Hentosh P., Henner W. D., Reynolds R. J. Sequence specificity of DNA cleavage by Micrococcus luteus gamma endonuclease. Radiat Res. 1985 Apr;102(1):119–129. [PubMed] [Google Scholar]
- Iida S., Hayatsu H. The permanganate oxidation of thymine. Biochim Biophys Acta. 1970 Jul 16;213(1):1–13. doi: 10.1016/0005-2787(70)90002-x. [DOI] [PubMed] [Google Scholar]
- Katcher H. L., Wallace S. S. Characterization of the Escherichia coli X-ray endonuclease, endonuclease III. Biochemistry. 1983 Aug 16;22(17):4071–4081. doi: 10.1021/bi00286a013. [DOI] [PubMed] [Google Scholar]
- Lesko S. A., Lorentzen R. J., Ts'o P. O. Role of superoxide in deoxyribonucleic acid strand scission. Biochemistry. 1980 Jun 24;19(13):3023–3028. doi: 10.1021/bi00554a029. [DOI] [PubMed] [Google Scholar]
- Levin D. E., Hollstein M., Christman M. F., Schwiers E. A., Ames B. N. A new Salmonella tester strain (TA102) with A X T base pairs at the site of mutation detects oxidative mutagens. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7445–7449. doi: 10.1073/pnas.79.23.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Massie H. R., Samis H. V., Baird M. B. The kinetics of degradation of DNA and RNA by H 2 O 2 . Biochim Biophys Acta. 1972 Jul 31;272(4):539–548. doi: 10.1016/0005-2787(72)90509-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nes I. F. Purification and properties of a mouse-cell DNA-repair endonuclease, which recognizes lesions in DNA induced by ultraviolet light, depurination, gamma-rays, and OsO4 treatment. Eur J Biochem. 1980 Nov;112(1):161–168. doi: 10.1111/j.1432-1033.1980.tb04997.x. [DOI] [PubMed] [Google Scholar]
- Rhaese H. J., Freese E., Melzer M. S. Chemical analysis of DNA alterations. II. Alteration and liberation of bases of deoxynucleotides and deoxynucleosides induced by hydrogen peroxide and hydroxylamine. Biochim Biophys Acta. 1968 Feb 26;155(2):491–504. [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoule R., Cadet J. Radiation-induced degradation of the base component in DNA and related substances--final products. Mol Biol Biochem Biophys. 1978;27:171–203. doi: 10.1007/978-3-642-81196-8_9. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Lancker J. L., Tomura T. Purification and some properties of a mammalian repair endonuclease. Biochim Biophys Acta. 1974 Jun 14;353(1):99–114. doi: 10.1016/0005-2787(74)90101-4. [DOI] [PubMed] [Google Scholar]