Abstract
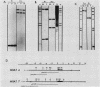
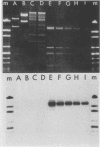
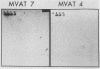
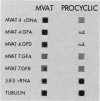
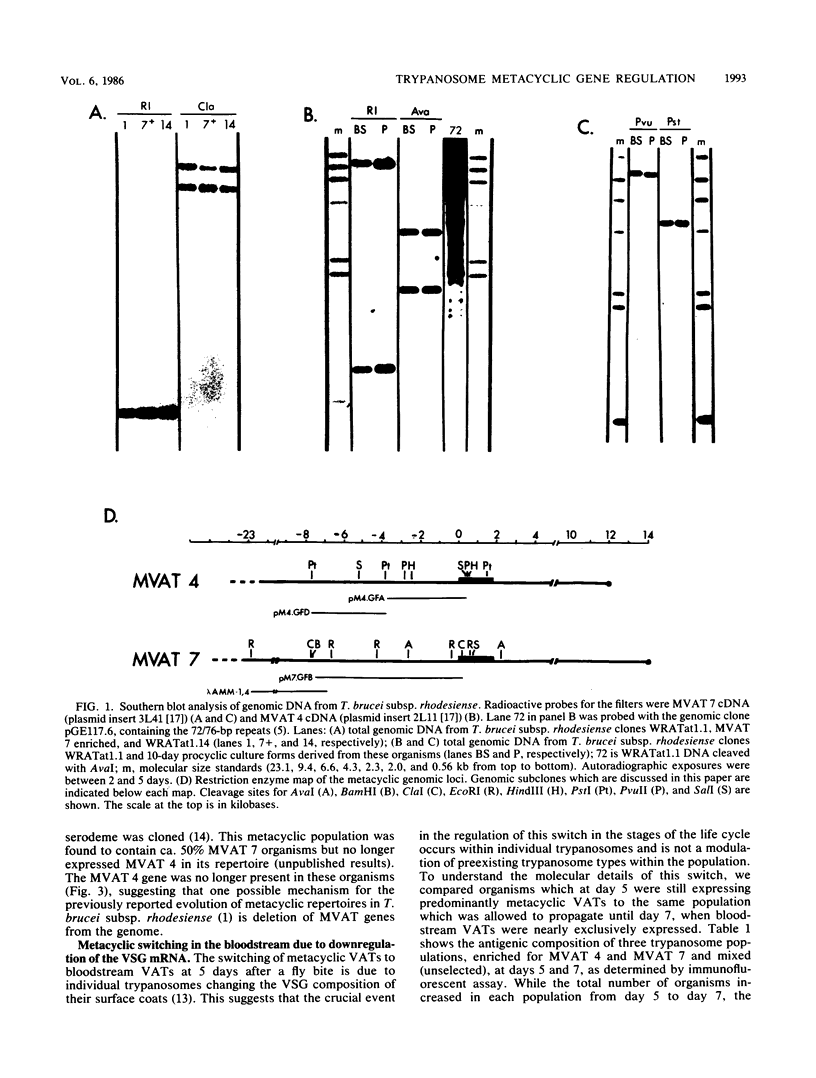
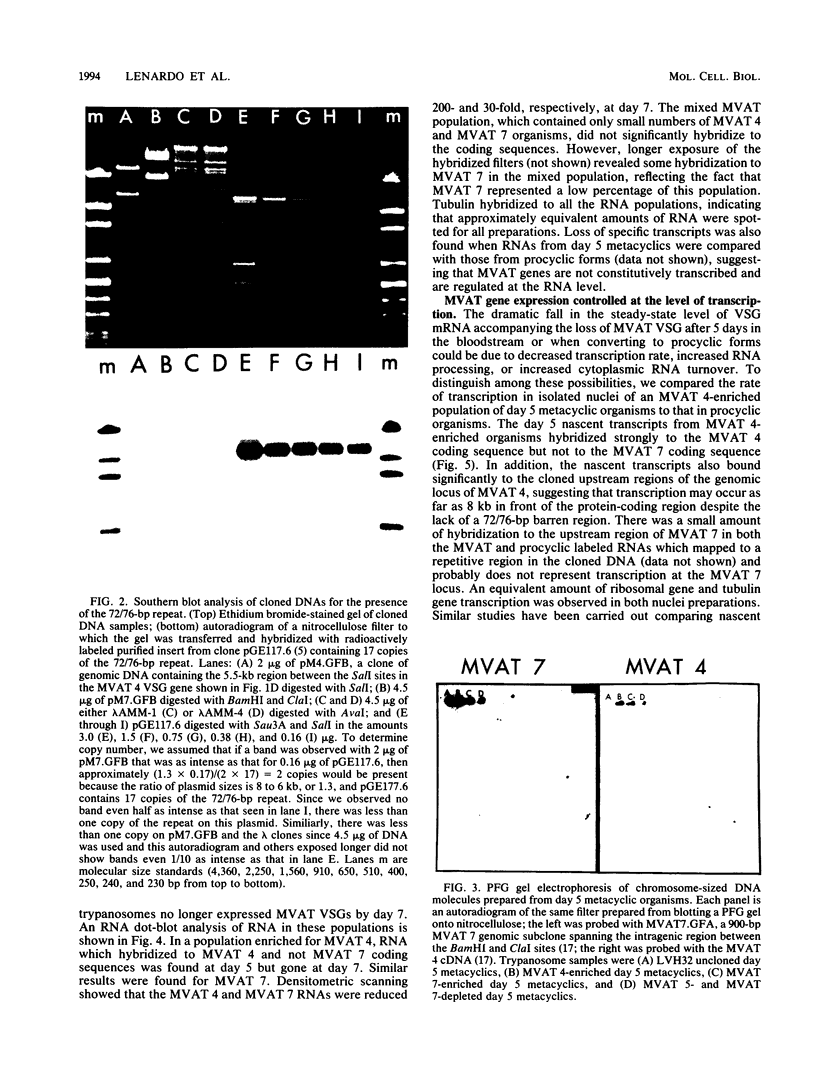
During the metacyclic stage in the life cycle of Trypanosoma brucei subsp. rhodesiense, the expression of variant surface glycoproteins (VSGs) is restricted to a small subset of antigenic types. Previously we identified cDNAs for the VSGs expressed in metacyclic variant antigen types (MVATs) 4 and 7 and found that these VSG genes do not rearrange when expressed at the metacyclic stage (M. J. Lenardo, A. C. Rice-Ficht, G. Kelly, K. Esser, and J. E. Donelson, Proc. Nathl. Acad Sci. USA 81:6642-6646, 1984). We now provide further evidence that these genes do not rearrange and demonstrate that their 5' upstream regions lack the 72 to 76-base-pair repeats which are considered the substrate for duplication and transposition events. Pulsed field gradient electrophoresis showed that the MVAT VSG genes were located on the largest chromosome-sized DNA molecules, and the lack of the MVAT 4 gene in one of two different serodemes suggested that one mechanism for the evolution of MVAT repertoires is gene deletion. When MVATs were inoculated into the bloodstream of a mammalian host by a bite from the insect vector, they rapidly switched into nonmetacyclic VSG types. We found that this switch was accomplished by a loss of MVAT RNA concomitant with the loss of metacyclic VSGs. Transcription studies with isolated metacyclic nuclei showed that the MVAT genes were expressed in situ from a single locus and were regulated at the level of transcription.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry J. D., Crowe J. S., Vickerman K. Instability of the Trypanosoma brucei rhodesiense metacyclic variable antigen repertoire. Nature. 1983 Dec 15;306(5944):699–701. doi: 10.1038/306699a0. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bernards A., van Harten-Loosbroek N., Borst P. Modification of telomeric DNA in Trypanosoma brucei; a role in antigenic variation? Nucleic Acids Res. 1984 May 25;12(10):4153–4170. doi: 10.1093/nar/12.10.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Bernards A., van der Ploeg L. H., Michels P. A., Liu A. Y., de Lange T., Kooter J. M. The control of variant surface antigen synthesis in trypanosomes. Eur J Biochem. 1983 Dec 15;137(3):383–389. doi: 10.1111/j.1432-1033.1983.tb07840.x. [DOI] [PubMed] [Google Scholar]
- Campbell D. A., van Bree M. P., Boothroyd J. C. The 5'-limit of transposition and upstream barren region of a trypanosome VSG gene: tandem 76 base-pair repeats flanking (TAA)90. Nucleic Acids Res. 1984 Mar 26;12(6):2759–2774. doi: 10.1093/nar/12.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen A. W., Bakkeren G. A., Barry J. D., Michels P. A., Borst P. Characteristics of trypanosome variant antigen genes active in the tsetse fly. Nucleic Acids Res. 1985 Jul 11;13(13):4661–4676. doi: 10.1093/nar/13.13.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. S., Barry J. D., Luckins A. G., Ross C. A., Vickerman K. All metacyclic variable antigen types of Trypanosoma congolense identified using monoclonal antibodies. Nature. 1983 Nov 24;306(5941):389–391. doi: 10.1038/306389a0. [DOI] [PubMed] [Google Scholar]
- Cunningham I. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J Protozool. 1977 May;24(2):325–329. doi: 10.1111/j.1550-7408.1977.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Delauw M. F., Pays E., Steinert M., Aerts D., Van Meirvenne N., Le Ray D. Inactivation and reactivation of a variant-specific antigen gene in cyclically transmitted Trypanosoma brucei. EMBO J. 1985 Apr;4(4):989–993. doi: 10.1002/j.1460-2075.1985.tb03728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelson J. E., Rice-Ficht A. C. Molecular biology of trypanosome antigenic variation. Microbiol Rev. 1985 Jun;49(2):107–125. doi: 10.1128/mr.49.2.107-125.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman D. M., Lenardo M. J., Reddy L. V., Van der Ploeg L. H., Donelson J. E. The 5.8S ribosomal RNA gene of Trypanosoma brucei: structural and transcriptional studies. Nucleic Acids Res. 1985 May 24;13(10):3533–3549. doi: 10.1093/nar/13.10.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman D. M., Lenardo M. J., Reddy L. V., Van der Ploeg L. H., Donelson J. E. The 5.8S ribosomal RNA gene of Trypanosoma brucei: structural and transcriptional studies. Nucleic Acids Res. 1985 May 24;13(10):3533–3549. doi: 10.1093/nar/13.10.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser K. M., Schoenbechler M. J. Expression of two variant surface glycoproteins on individual African trypanosomes during antigen switching. Science. 1985 Jul 12;229(4709):190–193. doi: 10.1126/science.3892689. [DOI] [PubMed] [Google Scholar]
- Esser K. M., Schoenbechler M. J., Gingrich J. B. Trypanosoma rhodesiense blood forms express all antigen specificities relevant to protection against metacyclic (insect form) challenge. J Immunol. 1982 Oct;129(4):1715–1718. [PubMed] [Google Scholar]
- Kimmel B. E., Samson S., Wu J., Hirschberg R., Yarbrough L. R. Tubulin genes of the African trypanosome Trypanosoma brucei rhodesiense:nucleotide sequence of a 3.7-kb fragment containing genes for alpha and beta tubulins. Gene. 1985;35(3):237–248. doi: 10.1016/0378-1119(85)90002-2. [DOI] [PubMed] [Google Scholar]
- Lanham S. M. Separation of trypanosomes from the blood of infected rats and mice by anion-exchangers. Nature. 1968 Jun 29;218(5148):1273–1274. doi: 10.1038/2181273a0. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Rice-Ficht A. C., Kelly G., Esser K. M., Donelson J. E. Characterization of the genes specifying two metacyclic variable antigen types in Trypanosoma brucei rhodesiense. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6642–6646. doi: 10.1073/pnas.81.21.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A. Y., Van der Ploeg L. H., Rijsewijk F. A., Borst P. The transposition unit of variant surface glycoprotein gene 118 of Trypanosoma brucei. Presence of repeated elements at its border and absence of promoter-associated sequences. J Mol Biol. 1983 Jun 15;167(1):57–75. doi: 10.1016/s0022-2836(83)80034-5. [DOI] [PubMed] [Google Scholar]
- Pays E., Houard S., Pays A., Van Assel S., Dupont F., Aerts D., Huet-Duvillier G., Gomés V., Richet C., Degand P. Trypanosoma brucei: the extent of conversion in antigen genes may be related to the DNA coding specificity. Cell. 1985 Oct;42(3):821–829. doi: 10.1016/0092-8674(85)90278-8. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Cornelissen A. W., Michels P. A., Borst P. Chromosome rearrangements in Trypanosoma brucei. Cell. 1984 Nov;39(1):213–221. doi: 10.1016/0092-8674(84)90207-1. [DOI] [PubMed] [Google Scholar]