Abstract
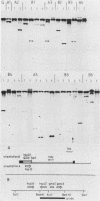
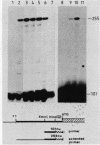
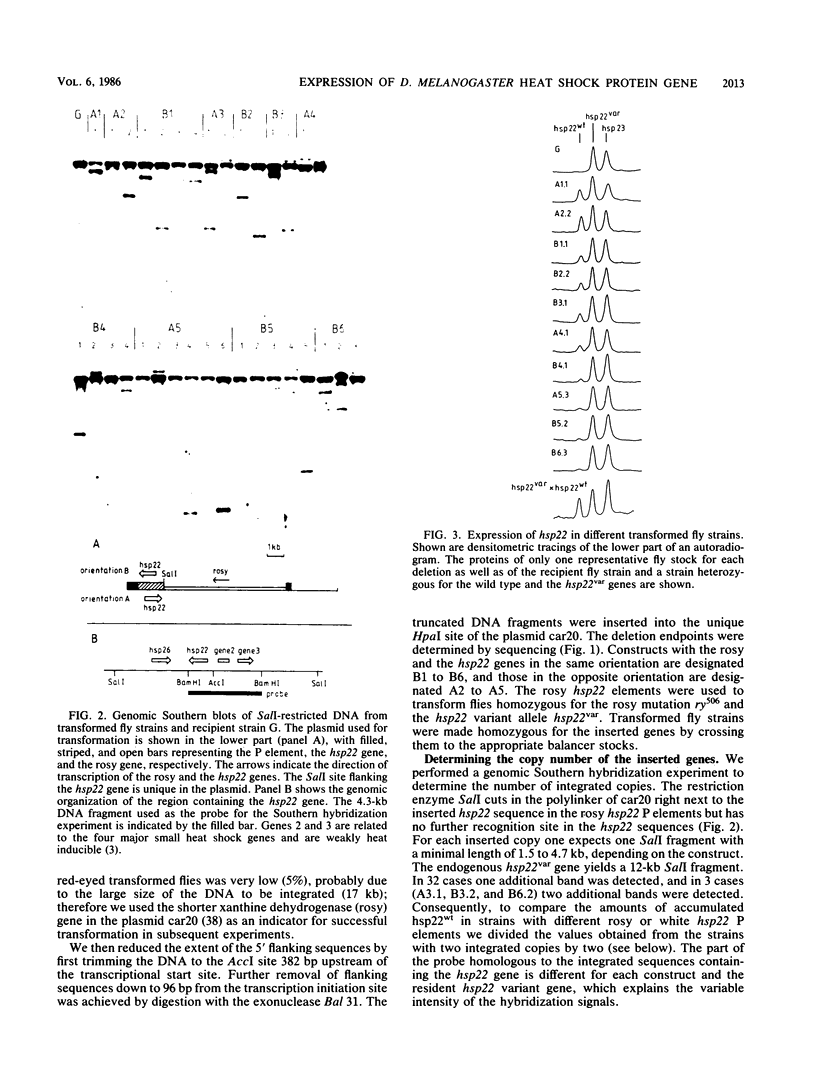
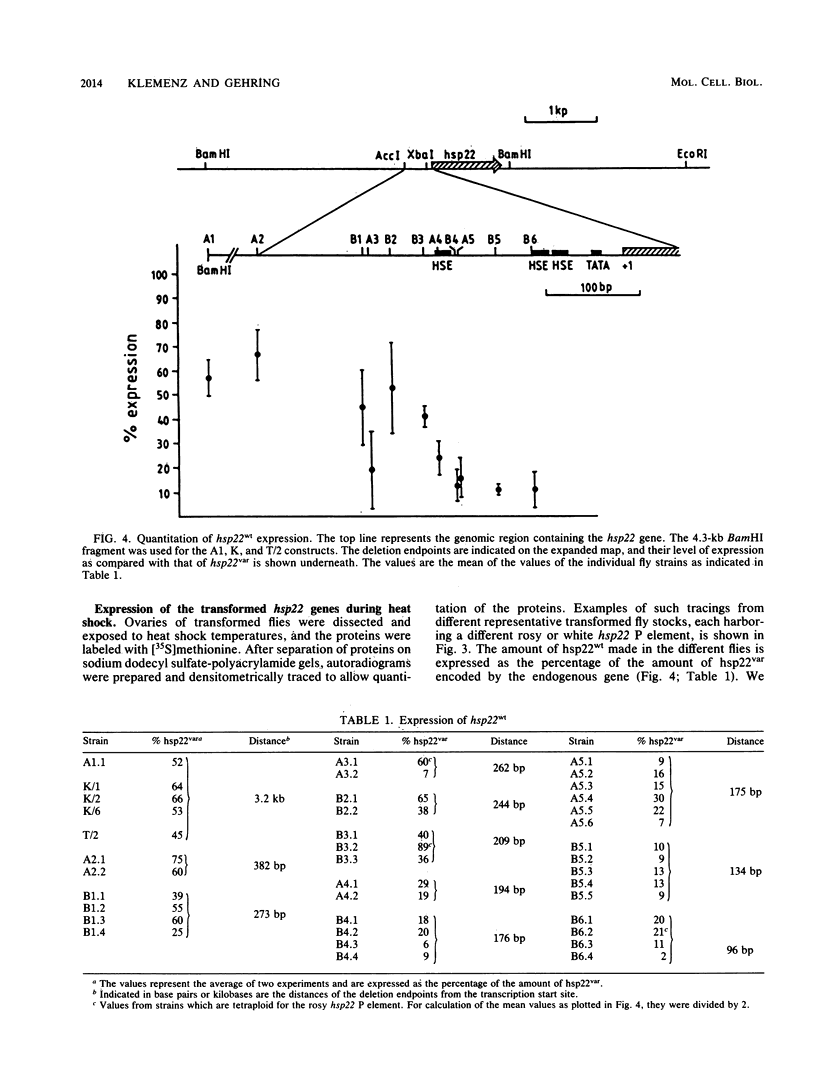
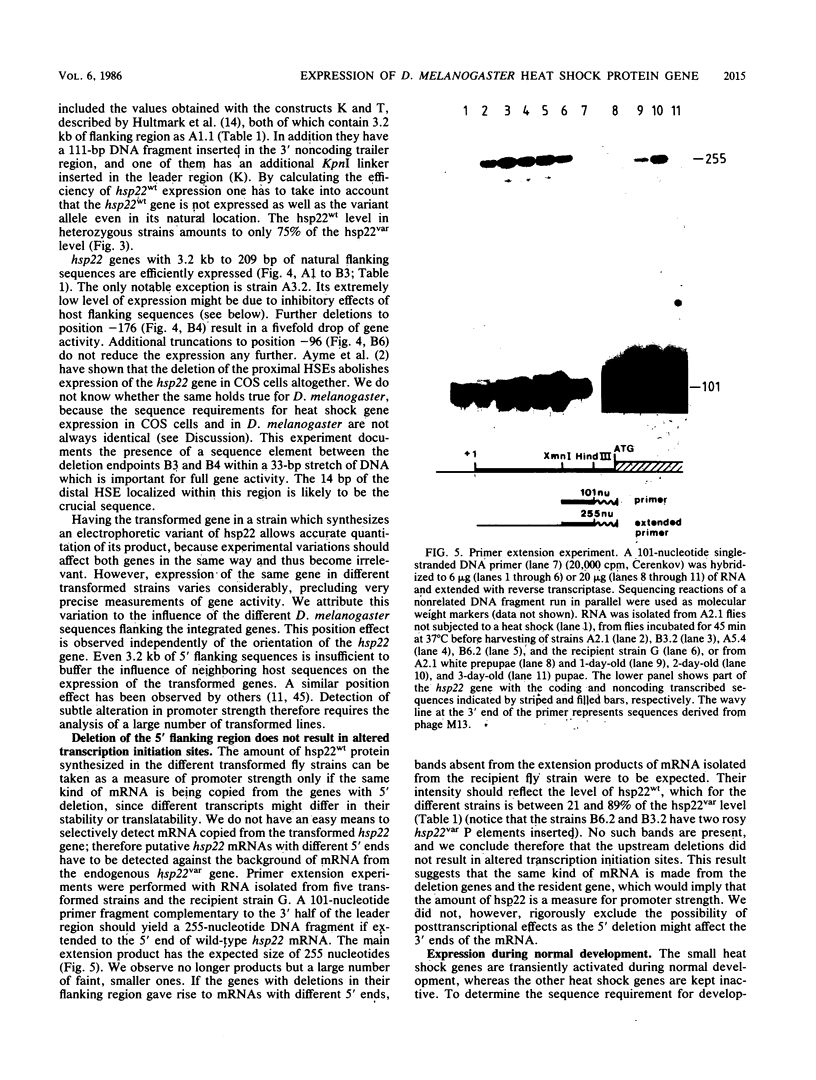
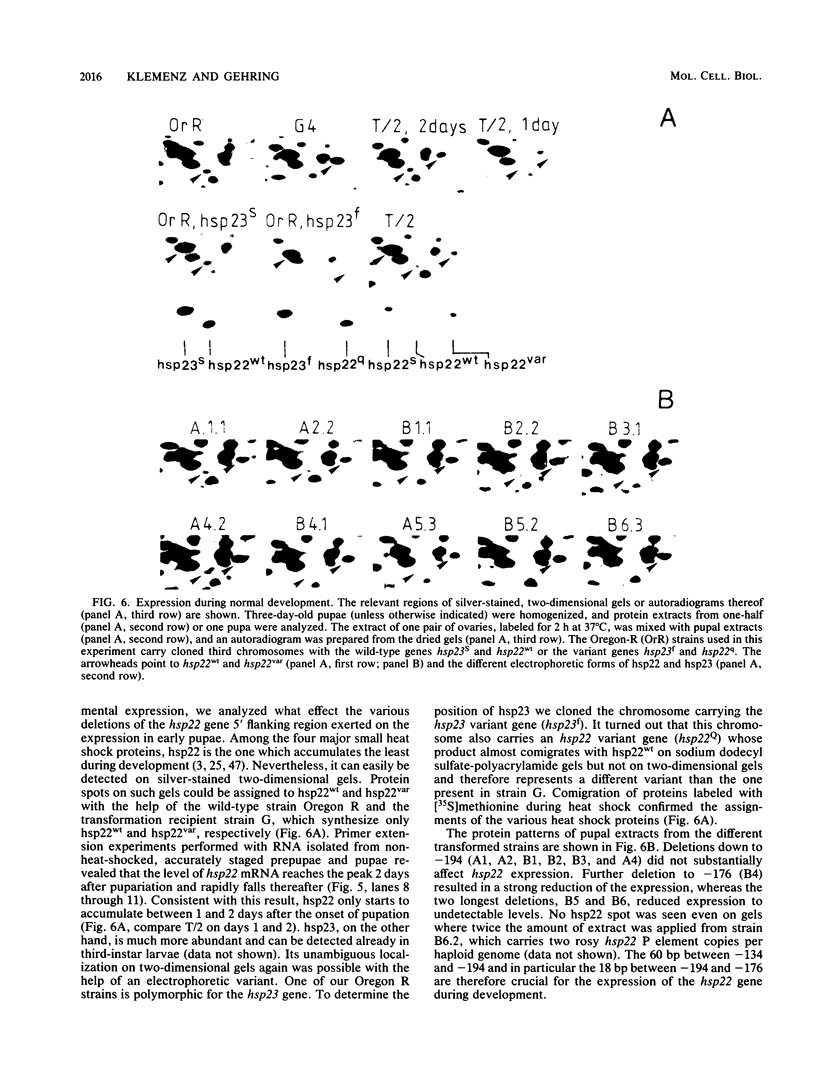
A 14-base-pair sequence element present in almost all Drosophila melanogaster heat shock genes has been implicated in the heat inducibility of transcription. The D. melanogaster gene encoding the smallest heat shock protein, hsp22, contains within its 5' flanking sequences three such repeats, two close to the transcription start site and a distally located third one 101 base pairs further upstream. Deletion analyses reveal that the 5' flanking sequences required for full expression of the hsp22 gene extend beyond the distal repeat. Deletion of the furthest upstream repeat results in a five to sixfold reduction of gene expression. The small heat shock genes are transiently expressed in the late third instar larval and early pupal stages without external stimulation. A deletion of 5' flanking sequences to position -194, which includes two nucleotides of the distal heat shock element, has no effect on the developmental expression, whereas removal of an additional 18 nucleotides, including 12 nucleotides of the distal heat shock element, severely reduces developmental expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin J., Mestril R., Lawson R., Klapper H., Voellmy R. The heat shock consensus sequence is not sufficient for hsp70 gene expression in Drosophila melanogaster. Mol Cell Biol. 1985 Jan;5(1):197–203. doi: 10.1128/mcb.5.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayme A., Southgate R., Tissières A. Nucleotide sequences responsible for the thermal inducibility of the Drosophila small heat-shock protein genes in monkey COS cells. J Mol Biol. 1985 Apr 20;182(4):469–475. doi: 10.1016/0022-2836(85)90233-5. [DOI] [PubMed] [Google Scholar]
- Ayme A., Tissières A. Locus 67B of Drosophila melanogaster contains seven, not four, closely related heat shock genes. EMBO J. 1985 Nov;4(11):2949–2954. doi: 10.1002/j.1460-2075.1985.tb04028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W., Spierer P., Hogness D. S. Chromosomal walking and jumping to isolate DNA from the Ace and rosy loci and the bithorax complex in Drosophila melanogaster. J Mol Biol. 1983 Jul 25;168(1):17–33. doi: 10.1016/s0022-2836(83)80320-9. [DOI] [PubMed] [Google Scholar]
- Bienz M., Pelham H. R. Expression of a Drosophila heat-shock protein in Xenopus oocytes: conserved and divergent regulatory signals. EMBO J. 1982;1(12):1583–1588. doi: 10.1002/j.1460-2075.1982.tb01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Dudler R., Travers A. A. Upstream elements necessary for optimal function of the hsp 70 promoter in transformed flies. Cell. 1984 Sep;38(2):391–398. doi: 10.1016/0092-8674(84)90494-x. [DOI] [PubMed] [Google Scholar]
- Finley D., Ciechanover A., Varshavsky A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):43–55. doi: 10.1016/0092-8674(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Gehring W. J., Klemenz R., Weber U., Kloter U. Functional analysis of the white gene of Drosophila by P-factor-mediated transformation. EMBO J. 1984 Sep;3(9):2077–2085. doi: 10.1002/j.1460-2075.1984.tb02094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Goldberg A. L. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell. 1985 Jun;41(2):587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A., Posakony J. W., Maniatis T. Correct developmental expression of a cloned alcohol dehydrogenase gene transduced into the Drosophila germ line. Cell. 1983 Aug;34(1):59–73. doi: 10.1016/0092-8674(83)90136-8. [DOI] [PubMed] [Google Scholar]
- Hiromi Y., Hotta Y. Actin gene mutations in Drosophila; heat shock activation in the indirect flight muscles. EMBO J. 1985 Jul;4(7):1681–1687. doi: 10.1002/j.1460-2075.1985.tb03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts R. B., Sage B., O'Connor J. D. Ecdysone titers during postembryonic development of Drosophila melanogaster. Dev Biol. 1977 Oct 1;60(1):310–317. doi: 10.1016/0012-1606(77)90128-2. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Primary sequence of the 5' flanking regions of the Drosophila heat shock genes in chromosome subdivision 67B. Nucleic Acids Res. 1981 Apr 10;9(7):1627–1642. doi: 10.1093/nar/9.7.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland R. C., Berger E. M. Synthesis of low molecular weight heat shock peptides stimulated by ecdysterone in a cultured Drosophila cell line. Proc Natl Acad Sci U S A. 1982 Feb;79(3):855–859. doi: 10.1073/pnas.79.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland R. C., Berger E., Sirotkin K., Yund M. A., Osterbur D., Fristrom J. Ecdysterone induces the transcription of four heat-shock genes in Drosophila S3 cells and imaginal discs. Dev Biol. 1982 Oct;93(2):498–507. doi: 10.1016/0012-1606(82)90137-3. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Karlik C. C., Coutu M. D., Fyrberg E. A. A nonsense mutation within the act88F actin gene disrupts myofibril formation in Drosophila indirect flight muscles. Cell. 1984 Oct;38(3):711–719. doi: 10.1016/0092-8674(84)90266-6. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Hultmark D., Gehring W. J. Selective translation of heat shock mRNA in Drosophila melanogaster depends on sequence information in the leader. EMBO J. 1985 Aug;4(8):2053–2060. doi: 10.1002/j.1460-2075.1985.tb03891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson R., Mestril R., Luo Y., Voellmy R. Ecdysterone selectively stimulates the expression of a 23000-Da heat-shock protein-beta-galactosidase hybrid gene in cultured Drosophila cells. Dev Biol. 1985 Aug;110(2):321–330. doi: 10.1016/0012-1606(85)90091-0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Varying patterns of protein synthesis in Drosophila during heat shock: implications for regulation. Dev Biol. 1980 Jun 15;77(2):463–479. doi: 10.1016/0012-1606(80)90488-1. [DOI] [PubMed] [Google Scholar]
- Mirault M. E., Southgate R., Delwart E. Regulation of heat-shock genes: a DNA sequence upstream of Drosophila hsp70 genes is essential for their induction in monkey cells. EMBO J. 1982;1(10):1279–1285. doi: 10.1002/j.1460-2075.1982.tb00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganelli C. M., Berger E. M., Pelham H. R. Transcription of Drosophila small hsp-tk hybrid genes is induced by heat shock and by ecdysterone in transfected Drosophila cells. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5865–5869. doi: 10.1073/pnas.82.17.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. What turns on heat shock genes? Nature. 1985 Oct 10;317(6037):477–478. doi: 10.1038/317477a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell. 1984 May;37(1):273–283. doi: 10.1016/0092-8674(84)90323-4. [DOI] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor contains a promoter-region-specific DNA-binding activity. Cell. 1984 Feb;36(2):357–369. doi: 10.1016/0092-8674(84)90229-0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Bienz M. A synthetic heat-shock promoter element confers heat-inducibility on the herpes simplex virus thymidine kinase gene. EMBO J. 1982;1(11):1473–1477. doi: 10.1002/j.1460-2075.1982.tb01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin K., Davidson N. Developmentally regulated transcription from Drosophila melanogaster chromosomal site 67B. Dev Biol. 1982 Jan;89(1):196–210. doi: 10.1016/0012-1606(82)90307-4. [DOI] [PubMed] [Google Scholar]
- Southgate R., Ayme A., Voellmy R. Nucleotide sequence analysis of the Drosophila small heat shock gene cluster at locus 67B. J Mol Biol. 1983 Mar 25;165(1):35–57. doi: 10.1016/s0022-2836(83)80241-1. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell. 1983 Aug;34(1):47–57. doi: 10.1016/0092-8674(83)90135-6. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Topol J., Ruden D. M., Parker C. S. Sequences required for in vitro transcriptional activation of a Drosophila hsp 70 gene. Cell. 1985 Sep;42(2):527–537. doi: 10.1016/0092-8674(85)90110-2. [DOI] [PubMed] [Google Scholar]
- Vitek M. P., Berger E. M. Steroid and high-temperature induction of the small heat-shock protein genes in Drosophila. J Mol Biol. 1984 Sep 15;178(2):173–189. doi: 10.1016/0022-2836(84)90138-4. [DOI] [PubMed] [Google Scholar]
- Voellmy R., Goldschmidt-Clermont M., Southgate R., Tissières A., Levis R., Gehring W. A DNA segment isolated from chromosomal site 67B in D. melanogaster contains four closely linked heat-shock genes. Cell. 1981 Jan;23(1):261–270. doi: 10.1016/0092-8674(81)90290-7. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. L., Petri W., Meselson M. Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without heat shock. Cell. 1983 Apr;32(4):1161–1170. doi: 10.1016/0092-8674(83)90299-4. [DOI] [PubMed] [Google Scholar]