Abstract
Malaria control is reliant on the use of long-lasting pyrethroid-impregnated nets and/or indoor residual spraying (IRS) of insecticide. The rapid selection and spread of operationally significant pyrethroid resistance in African malaria vectors threatens our ability to sustain malaria control. Establishing whether resistance is operationally significant is technically challenging. Routine monitoring by bioassay is inadequate, and there are limited data linking resistance selection with changes in disease transmission. The default is to switch insecticides when resistance is detected, but limited insecticide options and resistance to multiple insecticides in numerous locations make this approach unsustainable. Detailed analysis of the resistance situation in Anopheles gambiae on Bioko Island after pyrethroid resistance was detected in this species in 2004, and the IRS program switched to carbamate bendiocarb, has now been undertaken. The pyrethroid resistance selected is a target-site knock-down resistance kdr-form, on a background of generally elevated metabolic activity, compared with insecticide-susceptible A. gambiae, but the major cytochrome P450-based metabolic pyrethroid resistance mechanisms are not present. The available evidence from bioassays and infection data suggests that the pyrethroid resistance mechanisms in Bioko malaria vectors are not operationally significant, and on this basis, a different, long-lasting pyrethroid formulation is now being reintroduced for IRS in a rotational insecticide resistance management program. This will allow control efforts to be sustained in a cost-effective manner while reducing the selection pressure for resistance to nonpyrethroid insecticides. The methods used provide a template for evidence-based insecticide resistance management by malaria control programs.
Malaria control activities in Africa have been scaled up during the last decade. Disease control is predominantly dependent on the distribution and use of pyrethroid-impregnated long lasting insecticide-treated nets (LLINs) and/or indoor residual spraying (IRS) of insecticides. The choice of insecticides for IRS is currently limited to four classes with only two modes of action. One of these insecticide classes, pyrethroids, is also the only class recommended for use by the World Health Organization (WHO) on LLINs. The recent rapid selection and spread of pyrethroid resistance in malaria vectors has stimulated the WHO to develop a Global Plan for Insecticide Resistance Management (1), encouraging countries to plan and implement insecticide resistance management strategies and to underpin these strategies with proper, timely entomological resistance monitoring and effective data management. This must be implemented in the short term at the same time medium- to long-term efforts are made to expand the available insecticide choice (2). To be effective, resistance management plans need to be closely aligned with local evidence supported by an effective monitoring system.
Here we detail how this process has been undertaken in Equatorial Guinea, resulting in a detailed Operational Plan for Insecticide Resistance Management, which is owned by the National Malaria Control Program, Ministry of Health and Social Welfare, Equatorial Guinea. The plan was formally adopted in 2012.
Bioko, the main island of Equatorial Guinea, has a population of ∼200,000 people. It is situated 30 miles off the coast of Cameroon, experiences high annual rainfall (∼2,000 mm/y), and has in recent years undergone major economic and infrastructural development as a result of offshore oil and gas production. Malaria is endemic, with Entomological Inoculation Rates of 281 and 787 infective bites per year recorded for Anopheles gambiae and Anopheles funestus, respectively, in 2002 (3), which was before the scaling-up of malaria control activities. Comprehensive malaria control interventions were introduced jointly by the Bioko Island Malaria Control Project (BIMCP) and the Ministry of Health and Social Welfare in 2004, with the aim of drastically reducing disease burden and, ultimately, eliminating malaria from the island. Serological markers suggest heterogeneity of effectiveness of malaria control activities across the island to date (4). The BIMCP is funded by a private sector consortium led by Marathon Oil Company. Malaria vector control, managed by Medical Care Development International, consists primarily of IRS of all houses on the island. The first round of IRS using a pyrethroid (deltamethrin) was carried out between March and July 2004, followed by two rounds per year of bendiocarb spraying from 2005 onward. In 2007, a mass distribution of LLINs [PermaNet (Vestergaard Frandsen) 2.0, containing 55 mg/m2 deltamethrin] was undertaken, providing one net per sleeping area. Although net coverage was initially high, numbers on the island declined rapidly as nets were redistributed by the recipient population.
During the first round of deltamethrin IRS in 2004, a large proportion of A. gambiae s.s. mosquitoes sampled from window exit traps were shown to possess the West African form of the kdr mutation (leu1014–phe), which confers dichlorodiphenyltrichloroethane resistance and a low level of cross-resistance to all pyrethroids through insecticide target-site insensitivity (5). Pyrethroid resistance assessed by WHO susceptibility tests and the kdr mutation were present in both the M and S forms of A. gambiae s.s. on Bioko. The presence or absence of metabolically conferred pyrethroid resistance was not assessed at this time, although data from neighboring Cameroon suggests that this form of pyrethroid resistance is now widespread in the A. gambiae complex (6, 7). The detection of pyrethroid resistance, and an apparent lack of response to the IRS treatment by A. gambiae compared with A. funestus, prompted a switch from deltamethrin to a carbamate insecticide from the second spray round onward. However, malaria indicator surveys carried out both prespraying in February to March 2004 and postspraying in the same months in 2005 showed a large reduction in the prevalence of malaria infection in children aged from 2 to <15 y, going from 46% [95% confidence interval (CI), 40–51%] to 31% [95% CI, 24–40%] (8), indicating that the deltamethrin spray round had a substantial epidemiological effect. Routine entomological surveys from 2004 to 2012 suggested that A. funestus had been eliminated or virtually eliminated from Bioko, leaving the vectors A. gambiae s.s. and Anopheles melas. Subsequent routine bioassays and PCR analysis for the kdr mutation showed that despite replacement of deltamethrin with bendiocarb for IRS during a 7-y period, the frequency of pyrethroid resistance and the kdr mutation remained high in A. gambiae s.s.
To determine retrospectively whether the presence of the kdr gene in A. gambiae compromised the operational effectiveness of deltamethrin IRS in Bioko in 2004, an analysis of ∼4,000 A. gambiae specimens caught in window traps after the spray round in 2004 was carried out. The aim was to determine whether sporozoite rates in kdr-positive mosquitoes were higher than in kdr-negative mosquitoes.
In addition, the poor alignment of kdr positivity with survival in WHO pyrethroid susceptibility tests suggested that multiple pyrethroid resistance mechanisms were circulating in the Bioko population of A. gambiae. Metabolically based resistance mechanisms were assessed using microarrays and quantitative (q)PCR in A. gambiae specimens collected from Bioko in 2011.
The retrospective sporozoite–kdr analysis of the A. gambiae specimens collected after the pyrethroid spray round in 2004, and the results of the metabolic resistance testing of A. gambiae samples collected in 2011, coupled with a reassessment of the mosquito density data from 2004 onward, has allowed us to develop an evidence-based operational resistance management program. This will underpin cost-effective maintenance of operational IRS activity for Equatorial Guinea and serve as a template that other malaria-endemic countries faced with similar insecticide resistance issues could follow.
Results and Discussion
Effect of Deltamethrin IRS on Mosquito Densities and Sporozoite Rates.
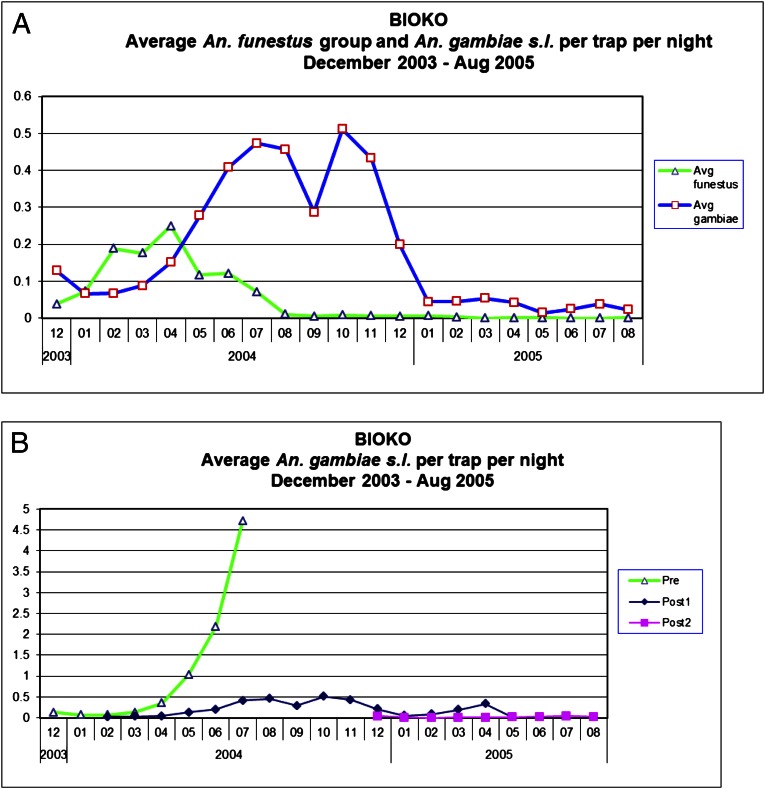
Data presented from window exit trap collections from sentinel sites in Bioko in 2004–2005 (5) have been widely interpreted as demonstrating that deltamethrin spraying rapidly controlled the A. funestus population but was ineffective against the A. gambiae population (Fig. 1A). We have now disaggregated the window trap data for A. gambiae (Fig. 1B) to show the actual numbers of mosquitoes caught pre- and postspray, as households with window traps were obviously not all sprayed simultaneously on the first day of the spray campaign but, rather, were treated progressively during the 3-mo treatment cycle, with the last houses sprayed in early July 2004. This reassessment of these data shows that deltamethrin had a similar effect on both A. funestus and A. gambiae, despite the high frequency of the kdr mutation in the A. gambiae. The average number of A. gambiae caught per trap per night rose steeply in the first half of 2004 in houses that had not yet been sprayed, in line with normal annual population density fluctuations linked to rainfall patterns. Collections from houses that had already been sprayed were substantially lower (Fig. 1B). Note that the natural peak in A. funestus numbers is not synchronous with that of A. gambiae, as the former breed in permanent breeding sites, whereas the latter are more dependent on transient breeding sites associated with recent rainfall.
Fig. 1.
Numbers of Anopheles caught in domestic window traps in Bioko in 2004–2005, disaggregated to show the effect of house spraying. (A) Graph reproduced from ref. 5. (B) The same data from Sharp et al., 2007, disaggregated into window-trap nights prespraying (green) and window trap nights postspraying (blue). The spray round was carried out between February and July 2004.
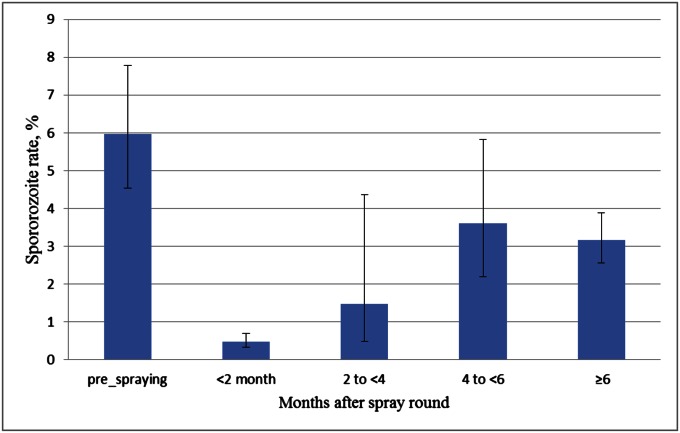
Before IRS was initiated, Plasmodium falciparum sporozoite positivity in A. gambiae was 6% (95% CI, 4.5–7.8%), dropping to 0.4% (95% CI, 0.3–0.5) immediately after spraying of a site, then rebounding partially to 3.1% (95% CI, 2.5–3.9%) at 6 mo after spraying of the island (Fig. 2).
Fig. 2.
The sporozoite rates in A. gambiae before and at different time intervals after spraying.
Status of Metabolically Based Resistance in A. gambiae.
Metabolically based pyrethroid resistance is increasingly being seen in A. gambiae and A. funestus from different parts of West Africa (9). To assess whether metabolic resistance is currently an issue in Bioko, larvae were collected from Malabo, Bioko, in 2011, reared to 3-d-old adults, and exposed to insecticide, and the survivors from these exposures were compared with the unexposed controls from the same collection and with an insecticide-susceptible West African colony of A. gambiae. Exposure to the WHO discriminating dosage of deltamethrin (0.05%) for 1 h resulted in 40% mortality, confirming that the Bioko population retained a high frequency of resistance to deltamethrin, despite 7 y of bendiocarb IRS. Exposure to the WHO discriminating dosage of bendiocarb (0.1%) for 1 h resulted in 100% mortality; hence, the exposure time was decreased to 10–15 min, giving 85% mortality to generate survivors for the microarray comparison. The bioassay data suggest that the Bioko population, unlike A. gambiae from much of West Africa, remains susceptible to bendiocarb despite the recent IRS selection pressure with this insecticide. The carbamate resistance elsewhere in West African A. gambiae is a result of a mutation in acetylcholinesterase, the target site for carbamates and organophosphates (10). The Gly119-Ser mutation in the acetylcholinesterase (ace) gene was assessed by PCR and was not present in the Bioko population. As this mutation is present at a high frequency in A. gambiae from Cameroon, where bendiocarb resistance has been documented (11), this suggests there is little gene flow between Bioko Island and the nearest mainland populations. The lack of gene flow is supported by studies on A. melas. Microsatellite and mitochondrial DNA analysis of 11 populations showed that this species had a high degree of genetic differentiation, with populations clustered into three distinct groups representing Western Africa, Southern Africa, and Bioko Island. Bayesian clustering provided no evidence for migration from the mainland to Bioko, although the data indicated that the Bioko and mainland populations were connected in the past, only becoming isolated during the last glaciation period (12). A similar study is underway for A. gambiae, with initial results indicating lower levels of genetic differentiation.
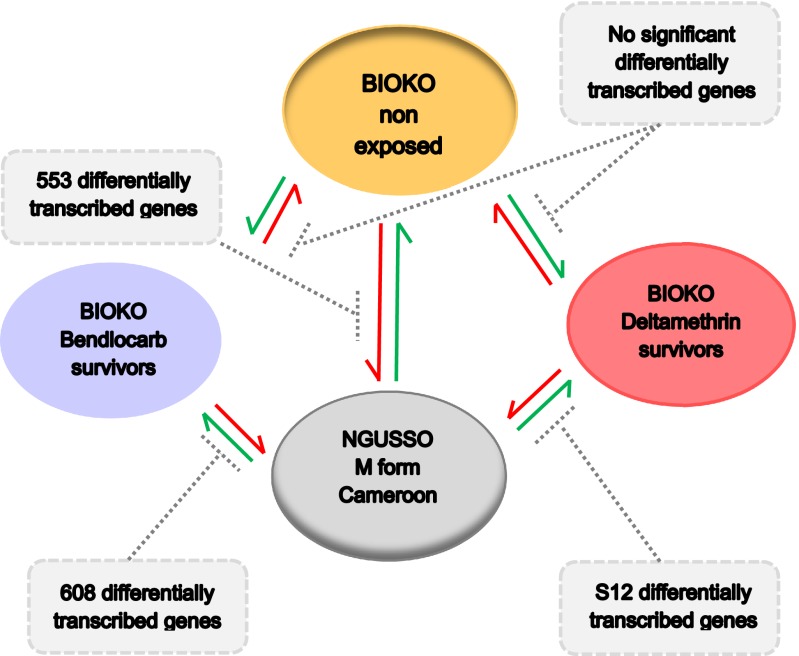
Replicate microarray analysis of insecticide treatment survivors, compared with unexposed control participants from the same collections, showed no significant differences in gene expression patterns. Hence, increased bendiocarb or deltamethrin pressure did not select for individuals with constitutively higher expression levels of metabolic genes associated with pyrethroid or carbamate resistance. Such differences would have been anticipated if a major metabolically based pyrethroid resistance mechanism was circulating in the Bioko population.
There were significant differences between all three Bioko microarray groups and the insecticide-susceptible Ngusso group (Fig. 3). Table 1 gives details of the main genes that were differentially expressed. These genes involve a raft of cytochrome P450s, GSTs, oxidative stress genes, and transporters. The pattern of up-regulation may reflect a general response to the harsher field conditions to which the larvae were exposed compared with the more controlled insectary conditions encountered by the Ngusso colony. Alternatively, they may reflect normal interstrain variability, although the 20–40-fold increases in oxidative stress gene expression in the field populations are outside the normal ranges we have seen from a large number of different Anopheles populations.
Fig. 3.
Whole-genome reciprocal dye swap pairwise microarray comparisons of A. gambiae populations collected from Bioko, unexposed or exposed to insecticides with a susceptible West African A. gambiae population.
Table 1.
Level of gene up-regulation from comparative microarray analysis in A. gambiae from Bioko compared with the susceptible Ngusso strain
| Transcript ID | Description (Blast2GO annotation) | Fold change |
||
| Ngusso vs. nonexposed | Ngusso vs. deltamethrin survivors | Ngusso vs. bendiocarb survivors | ||
| Detoxification genes | ||||
| AGAP012296-RA | CYP9J5: cytochrome P450 monooxygenase | 17 | 7.61 | |
| AGAP002113-RB | Cytochrome b5 | 4.00 | ||
| AGAP005992-RA | CYP302A1: cytochrome P450 monooxygenase | 3.26 | 3.59 | 3.25 |
| AGAP002863-RA | COEA6O: carboxylesterase | 2.59 | ||
| AGAP000284-RA | Cytochrome P450 | 2.39 | 2.21 | |
| AGAP002113-RC | Cytochrome b5 | 2.33 | ||
| AGAP004380-RA | Glutathione transferase GSTD12 | 2.30 | 2.52 | 2.35 |
| AGAP002429-RA | CYP315A1: cytochrome P450 monooxygenase | 2.27 | 2.22 | |
| AGAP005371-RA | COEBE2C: carboxylesterase | 2.06 | ||
| AGAP002416-RA | CYP4K2: cytochrome P450 monooxygenase | 2.01 | ||
| AGAP002417-RA | CYP4AR1: cytochrome P450 monooxygenase | 2.68 | ||
| AGAP002419-RA | CYP4D22: cytochrome P450 monooxygenase | 3.48 | 3.80 | |
| AGAP004164-RC | GSTD1_4: glutathione S-transferase | 2.23 | ||
| AGAP004383 RA | GSTD10: glutathione S-transferase | 2.97 | ||
| AGAP007480-RA | CYP6AH1: cytochrome P450 monooxygenase | 4.59 | 5.85 | |
| AGAP008209-RA | CYP6M1: cytochrome P450 monooxygenase | 2.54 | ||
| AGAP012295-RA | CYP9L1: cytochrome P450 monooxygenase | 10.82 | ||
| Cuticular genes | ||||
| AGAP006497-RA | CPR134: cuticle protein | 3.71 | ||
| AGAP003385-RA | CPR123: cuticle protein | 3.20 | ||
| AGAP003379-RA | CPR 117: cuticle protein | 3.08 | ||
| AGAP012795-RA | Cuticle protein putative | 2.10 | ||
| AGAP010906-RA | CPFL5: cuticular protein 5 from CPFL family | 4.28 | ||
| AGAP010908-RA | CPFL7: cuticular protein 7 from CPFL family | 2.80 | ||
| Oxidative stress | ||||
| AGAP006226-RA | Aldehyde oxidase | 46.13 | 41.74 | 35.40 |
| AGAP011054-RA | TPX2: thioredoxin-dependent peroxidase | 21.99 | 21.63 | 24.37 |
| ABC transporters | ||||
| AGAP011518-RA | ATP-binding cassette subfamily a member = ABCA1 | 4.37 | 3.51 | |
| AGAP010416-RA | ABC transporter | 2.01 | ||
| AGAP007504-RA | ATP-binding cassette subfamily a member | 2.68 | ||
It is notable that the major cytochrome P450s, Cyp6Z2, Cyp6P3, Cyp6M2, and Cyp9J32, which metabolize deltamethrin and are highly up-regulated in strains with very high levels of pyrethroid resistance in many parts of Africa, are not overexpressed in the Bioko populations. The pattern of gene up-regulation observed in the Bioko population may produce a marginal two- to threefold increase in tolerance to pyrethroids, accounting for the high levels of survival in the WHO susceptibility assays and lack of correlation between kdr and susceptibility assay survival, but this is not expected to produce the 20–100-fold levels of resistance typically observed in cases of metabolic resistance where operational control failure is suspected. Hence, this Bioko population resembles that seen in Kenya (13), rather than those resistant strains found in Benin, Cameroon, Malawi, Mozambique, and Zambia (14–18).
Effect of kdr on Malaria Transmission.
To assess whether mosquitoes with kdr target site resistance were more likely to be transmitting malaria, mosquitoes collected in window traps from the sentinel sites in 2004–2005 were analyzed for molecular form, kdr, and sporozoite positivity status. A total of 4,619 A. gambiae specimens were tested for P. falciparum, of which 119 (2.6%) were positive. Of the 4,195 mosquitoes with a valid test for molecular form of A. gambiae and P. falciparum, 1,495 (36%) were M-form, of which 2.1% were positive for P. falciparum, whereas 3.3% of the 2,700 S-form mosquitoes were P. falciparum-positive (Table 2). Of the 3,848 mosquitoes for which both kdr and P. falciparum results were available, 1,741 (45%) were homozygous negative for kdr (SS) and 1,564 (41%) were heterozygous for the kdr mutation, with the remaining 543 (14%) homozygous for kdr. Of the homozygous non-kdr, heterozygous kdr, and homozygous kdr, 2.4%, 4.4%, and 1.1%, respectively, were sporozoite-positive (Table 2).
Table 2.
Mosquito characteristics and sporozoite status of specimens caught in exit traps in Bioko in 2004
| Molecular form and kdr status | % (N) | Sporozoite rate | |
| % Pf (n) | Odds ratio [95% CI] | ||
| Total A. gambiae | 100 (4,500) | 2.6 (119) | |
| Kdr* | |||
| Homozygous susceptible (SS), % | 45 (1,741) | 2.4 (42) | 1 |
| Heterozygous (SRw), % | 41 (1,564) | 4.4 (69) | 1.9 [0.91–3.8], P = 0.090 |
| Homozygous resistant (RwRw), % | 14 (543) | 1.1 (6) | 0.45 [0.25–0.83], P = 0.01 |
| Molecular form | |||
| M form, % | 36 (1,495) | 2.1 (31) | 1 |
| S form, % | 64 (2,700) | 3.3 (88) | 1.6[0.98–2.6], P = 0.061 |
Seven specimens were heterozygous for the east African variant of kdr SRe, and 5 were ReRw (all sporozoite negative). For 640 specimens, no kdr status was available (all SEs adjusted for between cluster differences).
As kdr tends to be functionally recessive (19), the insecticide resistance phenotype of non-kdr and kdr heterozygotes is similar. Hence, the homozygote non-kdr and heterozygote kdr were combined into a single category. When the sporozoite-positive proportions for these combined groups were calculated separately for each of the molecular forms, sporozoite-positive fractions were lower for homozygous kdr than for the combined heterozygous and homozygous non-kdr group. This was the case both in the S form [1.2% vs. 3.0%; odds ratio (OR), 0.38; 95% CI, 0.22–0.64; P = 0.002; Table 2] and in the M form (0.9% vs. 3.5%; OR, 0.25; 95% CI 0.04–1.43; P = 0.11; Table 3). Adjusting for molecular type, the overall effect of kdr status on sporozoite positivity was highly significant (OR, 0.34; 95% CI, 0.22–0.54; P < 0.001) for homozygous kdr vs. the combined heterozygous and homozygous non-kdr group). The effect of S form vs. M form on sporozoite positivity, adjusted for kdr status, was OR, 1.15 (95% CI, 0.70–1.89; P = 0.58). There was no evidence that molecular form modified the effect of kdr on sporozoite positivity (test for interaction, P = 0.65).
Table 3.
Sporozoite rate by kdr status for M and S molecular forms of A. gambiae s.s. caught in exit traps in Bioko in 2004
| Molecular form and Kdr-status | Sporozoite rate (Pf), % [95% CI] (N) | Odds ratio [95% CI] |
| M | ||
| SS or SR | 3.0 [2.4–3.9] (855) | 1 |
| RR | 1.2 [0.64–2.1] (430) | 0.38 [0.22–0.64], P = 0.002 |
| Overall | 2.4 [1.8–3.2] (1,285) | |
| S | ||
| SS or SR | 3.5 [2.1–5.9] (2,399) | 1 |
| RR | 0.9 [0.1–7.6] (110) | 0.25[0.04–1.43], P = 0.11 |
| Overall | 3.4 [2.0–5.9] (2,509) |
All SEs adjusted for clustering by sentinel site.
Mosquitoes being homozygous for kdr did not appear to compromise vector control. Indeed, given their lower sporozoite positivity in both M and S forms of A. gambiae, the pyrethroid-resistant kdr homozygotes may be transmitting less malaria than their susceptible counterparts. Sporozoite data implicate both M and S forms in transmission. Kdr heterozygote specimens were on average more likely to be positive relative to kdr-positive and kdr-negative homozygote specimens. This may require further investigation. However, an overall conclusion is that kdr status alone did not operationally reduce the effectiveness of pyrethroid IRS. Similarly a multivillage randomized control trial in Cote d’Ivoire showed that pyrethroid-treated LLIN efficacy was not adversely affected by kdr (20), although a study in Senegal suggested kdr had an effect on increased malaria transmission (21).
Operational Implications of the Resistance Data.
The decision taken by the BIMCP in 2004 to switch from pyrethroid IRS to carbamate was a sensible precaution after detecting high levels of kdr alleles in the A. gambiae population. The high densities of A. gambiae caught in window traps across the island in 2004 (5) appeared to confirm a lack of effectiveness of the pyrethroid spray round. In contrast, IRS, which was the only malaria control intervention in Bioko in 2004, had an evident epidemiologic effect, as demonstrated by community prevalence of infection surveys before and after the first round of deltamethrin treatment. Data presented in this study indicate that disaggregation of mosquito numbers by whether they were caught in a window trap before or after the locality in which it was situated was sprayed shows that densities of A. gambiae were reduced substantially in response to spraying (Fig. 1B). In addition, the very sharp reduction in sporozoite infectivity seen in A. gambiae mosquitoes caught in the period immediately after spraying at each site confirms the effectiveness of the IRS vector control intervention. Sporozoite rates subsequently rebounded, but at lower levels than before spraying (Fig. 2). Replacement of deltamethrin with bendiocarb continued to suppress the A. gambiae population (Fig. 1B). Although there is no evidence that bendiocarb resistance has been selected after 7 y of spraying, the continued low levels of malaria transmission, annual cost of insecticide, and requirement for two spray rounds per year, coupled with the need to introduce good insecticide resistance management practice, have resulted in a change of policy. In 2013, a strategy of alternating a longer-lasting pyrethroid formulation, which requires only a single round of treatment per year with a carbamate or organophosphate treatment, will be introduced. This is expected to maintain or enhance levels of disease control at reduced cost while limiting selection pressure on the insecticide resource.
Ongoing Monitoring Activities.
When deltamethrin is reintroduced, the operational control program will continue to monitor the insecticide resistance status of the local vector population, using routine WHO susceptibility assays. Any obvious changes in the resistance profile will trigger further investigation. The current program demonstrates the value of using rapid molecular assessment of local Anopheles vector resistance to inform operational decision making. If programs rely only on bioassay data, as demonstrated in Bioko in 2004, then insecticides may be prematurely removed from a country’s resistance management portfolio, further restricting an already limited choice of public health pesticides.
Although resistance monitoring should ideally be simplified to allow field personnel to fully assess the problem, with current technology this is not yet practical. Certain mutations, such as those conferring target site resistance (e.g., kdr and ace), can be screened for using simple PCR assays, but unfortunately, reliable DNA markers for the range of metabolic resistance genes that can be up-regulated are not yet available. Assessment of these requires RNA analysis via microarrays or qPCR. The use of synergists such as piperonyl butoxide (PBO) has been suggested as an intermediate step between bioassays and microarray analysis. Although this will give an indication of underlying metabolically based resistance, great care is needed when interpreting synergist data. The current study shows that the field population of A. gambiae from Bioko has broadly based increased metabolic activity compared with insecticide-susceptible insects. Preexposure of this field strain to the broad-spectrum synergist PBO before deltamethrin exposure significantly increased mortality in WHO susceptibility assays. This was expected, given the poor correlation between kdr and bioassay survival, but the microarray and infection data were still required to demonstrate that this type of low-level metabolic resistance had little or no operational significance.
Materials and Methods
Monitoring of the IRS intervention is based on a system of 18 sentinel sites distributed around the island. Annual household malaria indicator surveys have been carried out since 2004. Entomological monitoring commenced in late 2003 with a system of window exit traps installed at 6 houses in each of the sentinel sites (5). Exit traps were emptied daily by householders and the contents preserved in specially prepared bottles, which were collected on a 28-d cycle. In 2009, the exit trap system was replaced by two weekly light-trap collections carried out at the sentinel sites because of the reduced numbers of insects in the traps after 5 y of IRS.
After initial sorting and morphological identification, specimens were shipped to the laboratories of the South African Medical Research Council in Durban, where a sample was regularly analyzed by PCR to determine species identification, A. gambiae molecular form, genotype mutation (kdr), and sporozoite status after removal of the abdomen. Results for each specimen were entered into a database, including date and location of capture, and residual material was preserved and stored for further subsequent analysis. To assess the effect of the kdr mutation on the effectiveness of the initial pyrethroid spray round, all specimens caught before and after the IRS in 2004 were recently analyzed retrospectively and were combined with specimen results that were obtained at the time.
Data Analysis.
For the A. gambiae mosquitoes caught in exit traps, the average number caught per trap per night for each calendar month was plotted for those caught before and those caught after spraying of the sentinel site. Sporozoite rates were calculated for mosquitoes caught prespraying and 0 to <2 mo, 2 to <4 mo, 4 to <6 mo, and 6 mo or longer after the spray round. Sporozoite rates were also calculated by kdr status (homozygous kdr, heterozygous kdr, and homozygous non-kdr) and by molecular form (M or S). As kdr is a functionally recessive trait, to assess the effect of kdr and molecular form on sporozoite positivity, odds ratios for mosquitoes being sporozoite-positive were calculated for kdr homozygotes vs. kdr heterozygote and kdr-negative homozygotes combined, unadjusted, and adjusted for molecular form, as well as for S form vs. M form, unadjusted and adjusted for kdr status, in a logistic regression model. All SEs were adjusted for clustering by sentinel site.
Microarray Analysis.
For microarray analysis, larval A. gambiae were collected in Malabo, Bioko, in 2011 and reared to 3-d-old adults. The population was split into three batches, which were either used as non-insecticide-exposed controls or exposed to the WHO discriminating dosages (22) of deltamethrin or bendiocarb for 1 h or 10–15 min, respectively. After exposure, all mosquito batches were held for 24 h with access to sugar solution before mortality was scored. PBO [4% (vol/vol) papers] preexposure for 1 h was undertaken in WHO testing kits immediately before deltamethrin exposure, as described earlier. A control of PBO exposure alone gave 0% mortality.
RNA extractions, cDNA synthesis, and labeling reactions were performed independently for each biological replicate. Total RNA was extracted from three replicate batches of 16–17 adult, female, 3-d-old mosquitoes from each treatment group, using a PicoPure RNA isolation kit (Arcturus) according to manufacturer's instructions. Replicates of the insecticide susceptible Ngusso M form A. gambiae colony were used as a susceptible control. Total RNA quantity and quality were assessed using a Nanodrop spectrophotometer (Nanodrop Technologies) before further use. RNA was amplified using a RiboAmp RNA amplification kit (Arcturus), according to the manufacturer's instructions. Amplified RNAs were checked for quantity and quality by spectrophotometry and agarose gel electrophoresis. Amplified RNA was reverse transcribed into labeled cDNA and hybridized to the array, as previously described (9). Each comparison was repeated three times with different biological samples. For each biological replicate, two hybridizations were performed in which the Cy3 and Cy5 labels were swapped between samples; hence, a total of six hybridizations were performed for each comparison. Labeled cDNA from the unexposed and insecticide survivors from the deltamethrin and bendiocarb treatments were cohybridized with the laboratory susceptible A. gambiae population.
Spots that failed to meet any of the following criteria in either channel were rejected: an intensity value of >300, signal-to-noise ratio >3, and greater than 60% of pixel intensity superior to the median of the local background ±2 SD. Normalization and statistical analyses of the data were performed using the Limma 1.9 software package for R 2.3.1, available from the CRAN repository (www.r-project.org). Background-corrected intensities from the red (R, Cy5) and the green (G, Cy3) channels were transformed to intensity log-ratios (M = logR/G) and their corresponding geometrical means [A = (logR + log G)/2]. Within each array, M-values were normalized as a function of A, using the Lowess (23) scatter plot smoothing function and scaled to equalize the median absolute value across all arrays to account for technical biases between replicate hybridizations. Mean expression ratios were submitted to a one-sample Student t test against the baseline value of one (equal gene expression in both samples) with a multiple testing correction (Benjamini and Hochberg false-discovery rate) (24). Genes showing both t test P values < 0.001 and ≥ twofold over- or underexpression were considered differentially expressed between comparisons. The expression data from these microarray experiments can be accessed at Vector base (www.vectorbase.org). A five-way comparison of the microarray data was undertaken, as shown in Fig. 3.
PCR was undertaken on DNA samples from individual A. gambiae adults to check for the presence of the Gly119-Ser mutation in the ace gene as described in ref. 10.
Footnotes
The authors declare no conflict of interest.
References
- 1.World Health Organization . Global Plan for Insecticide Resistance Management in Malaria Vectors. Geneva: World Health Organization; 2012. [Google Scholar]
- 2.Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL. The Innovative Vector Control Consortium: Improved control of mosquito-borne diseases. Trends Parasitol. 2006;22(7):308–312. doi: 10.1016/j.pt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Cano J, et al. Malaria vectors in the Bioko Island (Equatorial Guinea): Estimation of vector dynamics and transmission intensities. J Med Entomol. 2004;41(2):158–161. doi: 10.1603/0022-2585-41.2.158. [DOI] [PubMed] [Google Scholar]
- 4.Cook J, et al. Serological markers suggest heterogeneity of effectiveness of malaria control interventions on Bioko Island, equatorial Guinea. PLoS ONE. 2011;6(9):e25137. doi: 10.1371/journal.pone.0025137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharp B, et al. Malaria control by indoor residual spraying on the tropical island of Bioko, Equatorial Guinea. Malar J. 2007;6:52. doi: 10.1186/1475-2875-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etang J, et al. Insecticide susceptibility status of Anopheles gambiae s.l. (Diptera: Culicidae) in the Republic of Cameroon. J Med Entomol. 2003;40(4):491–497. doi: 10.1603/0022-2585-40.4.491. [DOI] [PubMed] [Google Scholar]
- 7.Wondji CS, et al. Impact of insecticide-treated bed nets implementation on the genetic structure of Anopheles arabiensis in an area of irrigated rice fields in the Sahelian region of Cameroon. Mol Ecol. 2005;14(12):3683–3693. doi: 10.1111/j.1365-294X.2005.02699.x. [DOI] [PubMed] [Google Scholar]
- 8.Kleinschmidt I, et al. Reduction in infection with Plasmodium falciparum one year after the introduction of malaria control interventions on Bioko Island, Equatorial Guinea. Am J Trop Med Hyg. 2006;74(6):972–978. [PubMed] [Google Scholar]
- 9.Ranson H, et al. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011;27(2):91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Djogbénou L, et al. Identification and geographic distribution of the ACE-1R mutation in the malaria vector Anopheles gambiae in south-western Burkina Faso, West Africa. Am J Trop Med Hyg. 2008;78(2):298–302. [PubMed] [Google Scholar]
- 11.Antonio-Nkondjio C, et al. Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaoundé (Cameroon): Influence of urban agriculture and pollution. Malar J. 2011;10:154. doi: 10.1186/1475-2875-10-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deitz KC, et al. Genetic isolation within the malaria mosquito Anopheles melas. Mol Ecol. 2012;21(18):4498–4513. doi: 10.1111/j.1365-294X.2012.05724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranson H, et al. Genetic mapping of genes conferring permethrin resistance in the malaria vector, Anopheles gambiae. Insect Mol Biol. 2004;13(4):379–386. doi: 10.1111/j.0962-1075.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- 14.Wondji CS, et al. Impact of pyrethroid resistance on operational malaria control in Malawi. Proc Natl Acad Sci USA. 2012;109(47):19063–19070. doi: 10.1073/pnas.1217229109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djouaka RF, et al. Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genomics. 2008 doi: 10.1186/1471-2164-9-538. 2008;9:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller P, et al. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 2008;4(11):e1000286. doi: 10.1371/journal.pgen.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chanda E, et al. Insecticide resistance and the future of malaria control in Zambia. PLoS ONE. 2011;6(9):e24336. doi: 10.1371/journal.pone.0024336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson BJ, et al. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: Sequential metabolism of deltamethrin revealed. Insect Biochem Mol Biol. 2011;41(7):492–502. doi: 10.1016/j.ibmb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34(7):653–665. doi: 10.1016/j.ibmb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Henry MC, et al. Protective efficacy of lambda-cyhalothrin treated nets in Anopheles gambiae pyrethroid resistance areas of Côte d’Ivoire. Am J Trop Med Hyg. 2005;73(5):859–864. [PubMed] [Google Scholar]
- 21.Trape JF, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: A longitudinal study Lancet Inf. Diseases. 2011;11(12):925–932. doi: 10.1016/S1473-3099(11)70194-3. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization 1998. Test procedures for insecticide resistance (WHO\CDS\CPC\MAL\98.12) Available at www.who.int/whopes/resistance/en/
- 23.Clevel WS, Devlin SJ. Locally Weighted Regression: An Approach to Regression Analysis by Local Fitting. J Am Stat Assoc. 1988;83(403):596–610. [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]