Abstract
Most transient receptor potential (TRP) channels are regulated by phosphatidylinositol-4,5-biphosphate (PIP2), although the structural rearrangements occurring on PIP2 binding are currently far from clear. Here we report that activation of the TRP vanilloid 4 (TRPV4) channel by hypotonic and heat stimuli requires PIP2 binding to and rearrangement of the cytosolic tails. Neutralization of the positive charges within the sequence 121KRWRK125, which resembles a phosphoinositide-binding site, rendered the channel unresponsive to hypotonicity and heat but responsive to 4α-phorbol 12,13-didecanoate, an agonist that binds directly to transmembrane domains. Similar channel response was obtained by depletion of PIP2 from the plasma membrane with translocatable phosphatases in heterologous expression systems or by activation of phospholipase C in native ciliated epithelial cells. PIP2 facilitated TRPV4 activation by the osmotransducing cytosolic messenger 5′-6’-epoxyeicosatrienoic acid and allowed channel activation by heat in inside-out patches. Protease protection assays demonstrated a PIP2-binding site within the N-tail. The proximity of TRPV4 tails, analyzed by fluorescence resonance energy transfer, increased by depleting PIP2 mutations in the phosphoinositide site or by coexpression with protein kinase C and casein kinase substrate in neurons 3 (PACSIN3), a regulatory molecule that binds TRPV4 N-tails and abrogates activation by cell swelling and heat. PACSIN3 lacking the Bin-Amphiphysin-Rvs (F-BAR) domain interacted with TRPV4 without affecting channel activation or tail rearrangement. Thus, mutations weakening the TRPV4–PIP2 interacting site and conditions that deplete PIP2 or restrict access of TRPV4 to PIP2—in the case of PACSIN3—change tail conformation and negatively affect channel activation by hypotonicity and heat.
Keywords: structure, regulation, thermosensitivity
The transient receptor potential vanilloid 4 (TRPV4) is a nonselective cation channel that responds to osmotic (1–4), mechanical (5–7), and temperature stimulation (8), thereby contributing to many different physiological functions, including cellular (4, 9) and systemic volume homeostasis (10), vasodilation (11, 12), nociception (13), epithelial hydroelectrolyte transport (14), bladder voiding (15), ciliary beat frequency regulation (7, 16, 17), chondroprotection (18), and skeletal regulation (19). The osmotic (20) and mechanical (7, 16) sensitivity of TRPV4 depends on the activation of phospholipase A2 and subsequent production of the arachidonic acid metabolite 5′-6′-epoxyeicosatrienoic acid (EET), whereas the mechanism leading to temperature-mediated activation (observed only in intact cells) is not known at present (21). EET-independent TRPV4 activation by membrane stretch in excised patches from oocytes also has been reported (22), in apparent contradiction to early reports claiming lack of activation by membrane stretch (1). Several studies have characterized TRPV4 domains implicated in channel regulation by calmodulin (23, 24), protein kinase C and casein kinase substrate in neurons 3 (PACSIN3) (25), intracellular ATP (24) and inositol-trisphosphate receptor (16, 26). However, little is known about the domains relevant for TRPV4 activation by different stimuli, apart from the interaction between the TRPV4 activator 4α-phorbol 12,13-didecanoate (4α-PDD) and transmembrane domains 3 and 4 (27). Analysis of disease-causing mutations modifying channel activity that lay in regions close to the channel pore or within the ankyrin repeats (28) has contributed to our understanding of relevant protein domains as well.
Most transient receptor potential (TRP) channels are regulated by phosphatidylinositol phosphates, particularly by phosphatidylinositol-4,5-biphosphate (PIP2), the most abundant phosphoinositide in the inner leaflet of the plasma membrane (29, 30). In general terms, PIP2 has been proposed to modulate TRP channel gating and/or sensitivity to activating stimuli (29, 30). The interaction of PIP2 with TRPs involves protein regions characterized by the presence of several positively charged residues. Mutations of these positive residues (31–33) and manipulation of the PIP2 levels in intact cells (34) or in excised patches (33) have been the main tools used to evaluate PIP2-mediated channel regulation.
A recent report of the crystal structure of K+ channels with bound PIP2 provided the first atomistic description of a molecular mechanism by which PIP2 regulates channel activity (35, 36). PIP2 binding induces a large conformation change in the protein, expanding and bringing the cytosolic domains closer to the transmembrane domains (35). Whether PIP2 modulation of TRP channels involves similar conformational changes remains an open question.
Here we report that TRPV4 requires the interaction of PIP2 with a stretch of positive charges at the N-tail, before the proline-rich domain (PRD; residues 132–144), to be activated by hypotonicity and heat. We also demonstrate that the reported lack of channel response to heat in excised patches is fully recovered in the presence of PIP2, suggesting that TRPV4 is a bona fide thermosensitive channel. Finally, reduction of PIP2 levels or disruption of the interaction of PIP2 with the TRPV4 channel increased the fluorescence resonance energy transfer (FRET) signal between fluorescent probes on the TRPV4 cytosolic tails, consistent with a more compact cytosolic region. This is the first piece of evidence suggesting that, similar to PIP2-regulated K+ channels, interaction of PIP2 and the TRPV4 channel rearranges cytosolic domains.
Results and Discussion
Possible Phosphoinositide-Interacting Site in the TRPV4 N-Tail Is Required for Channel Activation by Hypotonicity and Heat.
We screened TRPV4 for domains that may participate in the channel response to hypotonicity-induced cell swelling. (A schematic view of the channel molecule and all deletions/mutations generated is shown in Fig. S1.) We focused on the N-terminal tail because a TRPV4 SNP associated with hyponatremia, P19S, generates a channel with a reduced response to hypotonic cell swelling (37). We generated three deletions of different lengths: TRPV4-Δ1–30, TRPV4-Δ1–130, and TRPV4-Δ100–130.
We evaluated the sensitivity of TRPV4 to hypotonic conditions using whole-cell patch-clamp recordings and intracellular calcium imaging with Fura-2. Addition of a 30% hypotonic solution yielded large currents in cells transiently transfected with TRPV4-WT, but not in GFP-transfected cells (Fig. S2A). TRPV4-Δ1–130 and TRPV4-Δ100–130 responses to 30% hypotonicity were reduced to the levels recorded in GFP-transfected cells, whereas TRPV4-Δ1–30 displayed a normal response (Fig. 1A and Fig. S2B). Deletion of the first 30 residues greatly reduced the currents generated by 15% hypotonic solutions (Fig. S2D), mimicking the changes induced by the P19S polymorphism (37). Consistent with the electrophysiological experiments, expression of TRPV4-WT increased intracellular [Ca2+] in response to 30% hypotonicity, whereas the response of TRPV4-Δ100–130 was indistinguishable from that obtained in GFP-transfected cells (Fig. S2C).
Fig. 1.
Functional analysis of N-terminal truncations and mutations of TRPV4. (A) Mean current density measured at +100 and −100 mV in response to a 30% hypotonic shock in HEK-293 cells overexpressing TRPV4-WT, TRPV4-Δ1–30, TRPV4-Δ1–130, TRPV4-Δ100–130, and GFP. The number of cells recorded for each condition is shown. (B) Ramp current–voltage relationship of cationic currents recorded from HEK-293 cells transfected with TRPV4-WT or TRPV4-121AAWAA and exposed to 30% hypotonic shocks. (C) Mean current responses to isotonic and hypotonic stimuli in cells transfected with TRPV4-WT or TRPV4-121AAWAA. (D) Mean current responses to 4α-PDD stimulation in TRPV4-WT– or TRPV4-121AAWAA–expressing cells. (E) Calcium signals (Fura-2 ratio) obtained in HeLa cells transfected with GFP (n = 47), TRPV4-WT (n = 25), or TRPV4-121AAWAA (n = 59) and sequentially stimulated with 30% hypotonic solutions and 10 μM 4α-PDD. (F) Calcium signals obtained in HeLa cells transfected with TRPV4-WT (n = 335), TRPV4-121AAWAA (n = 318), or GFP (n = 254) and stimulated with warm solution (38 °C). *P < 0.05.
To evaluate whether differences in plasma membrane expression between WT and mutant TRPV4 proteins could explain the reduced responses of truncated TRPV4 proteins, we evaluated surface labeling of human embryonic kidney (HEK)-293 cells expressing TRPV4-WT and TRPV4-Δ1–130 proteins tagged with a V5 epitope in the first extracellular loop. Confocal microscopy imaging and quantification by ELISA revealed no apparent differences in membrane expression between TRPV4-WT and the protein with the longest truncation, TRPV4-Δ1–130 (Fig. S3).
To pin down the region within residues 100–130 required for the channel response to hypotonic cell swelling, we neutralized four positive charges within a sequence, 121KRWRK125, that has been proposed as a possible phosphoinositide (PI)-binding site (29). Cells transfected with TRPV4-121AAWAA displayed greatly reduced swelling-induced whole-cell cationic currents (Fig. 1 B and C). TRPV4-121AAWAA–generated currents in response to the synthetic agonist 4α-PDD (0.01–10 μM) were indistinguishable from TRPV4-WT currents (Fig. 1D). Sequential addition of a hypotonic solution and 10 μM 4α-PDD generated significant increases in intracellular Ca2+ levels in cells expressing TRPV4-WT, whereas cells expressing TRPV4-121AAWAA responded only to 4α-PDD (Fig. 1E).
TRPV4 is also activated by moderate heat (above 25 °C) (8, 21), although the mechanism of its temperature sensitivity is not fully understood (8). Ca2+ imaging on cells exposed to warm temperatures (38 °C) revealed a typical transient response in cells transfected with TRPV4-WT channels. Neutralization of the positive charges in TRPV4-121AAWAA decreased the Ca2+ response to the levels obtained in GFP-transfected cells (Fig. 1F).
We hypothesized that the sequence 121KRWRK125 may form a PI-binding site required for the PIP2–TRPV4 interaction to respond to hypotonic and heat stimulation. Different TRP protein sequences containing several positively charged amino acids have been proposed to interact with phosphoinositides, particularly PIP2 (29, 38). To examine the specificity of the neutralization of the 121KRWRK125-positive charges, we neutralized three positive charges of a nearby region, 114RHH116. Expression of TRPV4-114AAA produced hypotonicity and heat-induced Ca2+ increases similar to those obtained with TRPV4-WT (Fig. S4A). Taken together, the foregoing experiments suggest that residues 121KRWRK125 are critical for TRPV4 activation by hypotonicity and heat, but not necessary for channel activation by 4α-PDD.
Depletion of PIP2 Levels Prevents Channel Activation by Physiological Stimuli.
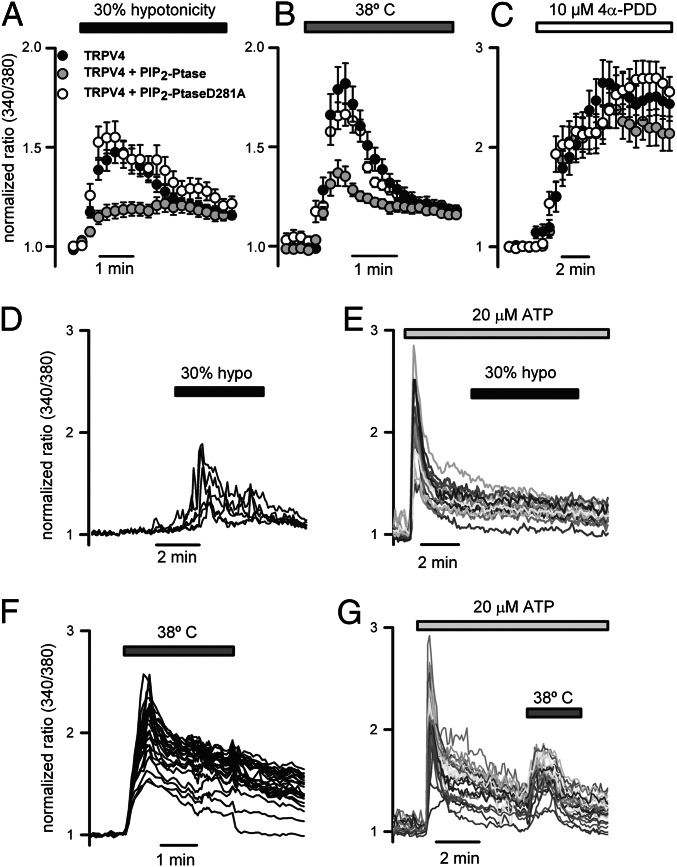
To assess whether deletion or mutation of residues 121KRWRK125 may be related to a PIP2-dependent mode of TRPV4 gating, we evaluated the impact of reduced PIP2 levels on channel activation. We used a rapamycin-induced translocatable 5-phosphatase to deplete PIP2 (39). The membrane-localized FK506 binding protein (FKBP)-rapamycin-binding protein (FRB) and the cytoplasmic enzyme construct FKBP-5-phosphatase were cotransfected with TRPV4-WT in HeLa cells. The addition of rapamycin to translocate the phosphatase to the plasma membrane locally depleted membrane PIP2 (Fig. S5 A–C) and prevented the increase of the Ca2+ signal after hypotonic cell swelling (Fig. 2A) and heat stimulation (Fig. 2B) without affecting the response to 0.1–10 μM 4α-PDD (Fig. 2C and Fig. S4B). Application of rapamycin to cells cotransfected with TRPV4 and a phosphatase-dead mutant (D281A) (39) did not affect the Ca2+ response to any of the stimuli tested (Fig. 2 A–C).
Fig. 2.
Effect of PIP2 depletion on TRPV4-mediated Ca2+ signals. HeLa cells were transfected with TRPV4-WT, TRPV4-WT + FRB, and PIP2-Ptase or the inactive phosphatase (D281A). (A–C) Average calcium signals (Fura-2 ratio) measured in the presence of the phosphatase translocation-inducing agent rapamycin (1 μM) in cells exposed to 30% hypotonicity (TRPV4, n = 302; V4+PIP2-Ptase, n = 229; V4+PIP2-PtaseD281A, n = 192) (A), heat (TRPV4, n = 150; V4+PIP2-Ptase, n = 146; V4+PIP2-PtaseD281A, n = 89) (B), and 4α-PDD (TRPV4, n = 278; V4+PIP2-Ptase, n = 233; V4+PIP2-PtaseD281A, n = 89) (C). (D–G) Representative intracellular Ca2+ signals obtained from mouse trachea ciliated cells exposed to a hypotonic solution in the absence (D) or presence (E) of 20 μM ATP, or exposed to heat (38 °C) in the absence (F) or presence (G) of 20 μM ATP. The percentage of ciliated cells responding to hypotonicity and heat was >90%. In the presence of ATP, these percentages were <5% for hypotonicity and >90% for heat.
We also analyzed whether phospholipase C (PLC)-induced depletion of PIP2 decreased TRPV4 channel activity in native cells. For that purpose, we used primary cultures of ciliated epithelial cells obtained from trachea and oviduct that express functional TRPV4 channels (7, 16, 17). Fig. 2D shows typical oscillatory Ca2+ signals generated by hypotonic solutions in tracheal ciliated epithelial cells. However, Ca2+ response to hypotonicity was abrogated after the activation of PLC with ATP, leading to the hydrolysis of PIP2 and generation of an inositol trisphosphate (IP3)-mediated Ca2+ signal (Fig. 2E). The heat response of epithelial cells was also reduced after the addition of ATP (Fig. 2 F and G). The reduction in hypotonicity- and heat-induced Ca2+ signals was not related to Ca2+-dependent inhibition of TRPV4, given that two consecutive stimuli elicited similar responses (Fig. S6 A and B). Similarly, the response of mouse ciliated oviductal cells to hypotonicity was reduced after the addition of ATP (Fig. S6 C and D). Although we could not directly assess whether PIP2 remained depleted at the time cells were challenged with TRPV4-activating stimuli, the absence of Ca2+ response to a second ATP stimulation within minutes of the first ATP application (Fig. S6E) may reflect a condition of PIP2 depletion.
Activity of PIP2-regulated channels typically decreases in excised inside-out patches and recovers after the addition of exogenous PIP2 (40). In those excised patches in which TRPV4 channel activity was present immediately after excision, channel activity decreased with time, and the addition of the water-soluble dioctanoylglycerol (diC8)-PIP2 (50–200 μM) or long acyl chain PIP2 (10 μM) did not recover initial channel activity (Fig. 3 A and C). The finding that PIP2 was not able to activate TRPV4 in excised patches might indicate loss of another, as-yet unidentified modulator required for channel activity after patch excision. Hypotonicity-mediated activation of TRPV4 in excised patches cannot be evaluated directly; instead, the osmotransducing cytosolic messenger EET has been used (20, 41). The addition of 1 μM EET in the presence of PIP2 activated TRPV4 in 71% of patches (Fig. 3 A and C); however, addition of EET in the absence of PIP2 activated only 20% of patches (Fig. 3 B and C), even though TRPV4 channel activity was demonstrated in the same patches using 4α-PDD (Fig. 3B).
Fig. 3.
Effect of PIP2 on TRPV4 channel activity in inside-out patches. (A) Two TRPV4 single-channel openings, which disappeared within seconds, were observed at +80 mV immediately after excision of a HeLa cell membrane patch (Top). The addition of diC8-PIP2 (50 μM) after complete channel rundown did not reactivate TRPV4 (Middle), whereas the addition of 5,6-EET (1 μM) in the presence of PIP2 activated TRPV4 (Bottom). (B) Recordings obtained at +80 mV in a patch sequentially exposed to EET (1 μM) and 4α-PDD (10 μM). (C) Mean open probability (NPo) calculated from control patches (2 min after excision) and in response to PIP2, EET, EET+PIP2, and 4α-PDD. The number of patches is indicated. The percentage of patches exhibiting TRPV4 activity was 20% for PIP2, 20% for EET, 71% for EET+PIP2, and 88% for 4α-PDD. (D and E) Single-channel recordings obtained from the same excised patch in response to 24 °C and 38 °C (warm) solutions in the presence (D) or absence (E) of 50 μM diC8-PIP2. (F) NPo calculated from consecutive 5-s recordings after exposure to warm solution and plotted against time (+PIP2, n = 11; −PIP2, n = 7; +PIP2 + 1 μM HC-067047 in pipette solution, n = 4; TRPV4-121AAWAA + PIP2, n = 5). *P < 0.05.
We next tested channel activation by heat in excised inside-out patches obtained from cells overexpressing TRPV4. In the presence of PIP2 TRPV4-WT channel activity was detected within seconds after application of warm solution (Fig. 3D), whereas in the presence of PIP2 and the TRPV4 blocker HC-067047 (42) or TRPV4-121AAWAA, no channel activity was elicited by heat (Fig. 3F). We discarded a shear stress-dependent component under our experimental conditions for heat activation of TRPV4 (Fig. S7A). In the absence of PIP2, and consistent with previous reports (8, 21, 43), no significant change in channel activity was elicited by heat (Fig. 3E). Fig. 3F shows mean channel activity in response to heat and plotted versus time after the addition of warm solution in the presence or absence of PIP2. The TRPV4 temperature coefficient (Q10) obtained from excised patches containing TRPV4-WT in the presence of PIP2 was 21 ± 5 (n = 3) (Fig. S7B), consistent with values obtained from previous TRPV4 whole-cell recordings (21, 43). Considered together, these experiments confirm that PIP2 is required for TRPV4 activation by physiological stimuli, most likely through action as an allosteric modulator. However, at present we do not have a comprehensive model incorporating all factors involved in TRPV4 gating to explain why TRPV4 gating by 4α-PDD is not affected by PIP2 depletion or why PIP2 is unable to activate TRPV4 on its own.
PIP2 Interacts with TRPV4 N-Tail.
To further characterize the interaction of PIP2 with the TRPV4 N-tail, we carried out limited proteolysis assays on the purified TRPV4 N-terminal region (residues 1–397), which includes the N-terminal tail and the ankyrin repeats. Papain digestion led to cleavage at four positions within the N-tail (Fig. 4A). Quantification of the bands thus obtained (Fig. 4 B–E) showed that proteolysis of the TRPV4 N-terminus is reduced in the presence of PIP2, but not in the presence of PI. PIP2-dependent proteolysis protection was not observed with the isolated TRPV4 ankyrin repeats (residues 136–397), the TRPV1 ankyrin repeats, or the TRPV4-121AAWAA N-terminal region (Fig. S8), ruling out nonspecific inhibition of papain by PIP2. Thus, these biochemical data support a direct interaction of PIP2 with the N-tail region of TRPV4-WT.
Fig. 4.
PIP2 binding to the TRPV4 N-tail. (A) Coomassie blue-stained SDS/PAGE showing protection from limited papain digestion by PIP2, but not by PI. Purified protein corresponding to residues 1–397 of human TRPV4 (10 μM) was digested with papain (38 nM) in the absence or presence of lipid (PI and PIP2 at 150 μM). The cleavage positions corresponding to each isolated band, determined by N-terminal sequencing, are indicated. (B–E) The four indicated bands were scanned, quantified, and plotted against digestion time. Significant changes were observed in the presence of PIP2 for bands 1 and 3 at all times, whereas band 4 showed significant differences at 45 and 60 min. Data are mean ± SD. n = 3. *P < 0.05 control vs. PIP2; **P < 0.01 control and PI vs. PIP2.
PACSIN3 Bin-Amphiphysin-Rvs (F-BAR) Domain Is Required for TRPV4 Channel Regulation.
The effect of neutralizing the positively charged residues, or depleting PIP2 levels, on channel activity resembled the response of TRPV4 when coexpressed with PACSIN3, with reduced channel response to hypotonicity and heat but normal response to 4α-PDD (25, 44). PACSIN3 belongs to a family of proteins containing a Bin-amphiphysin-Rvs (BAR) domain required to penetrate and remodel the plasma membrane (45, 46). PACSIN3 binds through its sarcoma (SRC) homology 3 (SH3) domain to the PRD of TRPV4 (44), in close proximity to the PI-binding site.
Two competing hypotheses are that binding of membrane-bound PACSIN3 to the PRD may either promote or physically block the interaction of the PI-binding site with membrane PIP2. To test these hypotheses, we generated a PACSIN3 lacking the F-BAR domain. A similar deletion in PACSIN1 renders the protein unable to interact with the lipids of the plasma membrane (47). The F-BAR domain of PACSIN3 is not required for interaction with TRPV4 (44). Accordingly, we detected interaction of PACSIN3-ΔF-BAR with TRPV4 (Fig. S9). Coexpression of TRPV4 with PACSIN3-ΔF-BAR, unlike coexpression with PACSIN3, did not reduce the whole-cell currents generated by hypotonic challenges (Fig. 5A). The channel response to 4α-PDD was not affected under any of the experimental conditions tested (Fig. 5B). These results are consistent with the hypothesis that PACSIN3 interferes with the interaction of TRPV4 with PIP2, an effect lost when a membrane-unbound PACSIN3-ΔF-BAR was used.
Fig. 5.
PIP2-dependent rearrangment of TRPV4 cytosolic tails. (A and B) Mean current density of hypotonicity-activated (A) or 4α-PDD–activated (B) currents recorded from HEK-293 cells transfected with GFP, TRPV4, TRPV4+PACSIN3, or TRPV4+PACSIN3-ΔF-BAR. (C) FRET efficiency was determined at the plasma membrane of HEK-293 coexpressing soluble YFP and CFP-fused TRPV4, CFP- and YFP-fused TRPV4-WT, CFP- and YFP-fused TRPV4-121AAWAA, CFP- and YFP-fused TRPV4-Δ1–130, or CFP- and YFP-fused TRPV4-WT coexpressed with either PACSIN3 or PACSIN3-ΔF-BAR. (D) FRET efficiencies between CFP- and YFP-fused TRPV4-WT determined at the plasma membrane in the absence or presence of tetracycline. TRPV4 constructs were transiently cotransfected in HEK-293 cells expressing a tetracycline-inducible 5-phosphatase. The number of cells recorded for each condition is shown. Data are mean ± SEM. *P < 0.05 vs. TRPV4-WT, one-way ANOVA and Bonferroni post hoc (A–C) or Student t test (D).
PIP2 Rearranges TRPV4 Cytosolic Tails.
Taken together, our findings support the involvement of PIP2 in TRPV4 gating by physiological stimuli. However, an important question remains that has not yet been resolved for any PIP2-modulated TRP channel: Does PIP2 binding affect the structural conformation of TRPV4? We approached this question by studying the impact of TRPV4 deletions and mutations on the conformation of cytosolic tails. For this purpose, we evaluated the proximity of the intracellular C-tails of CFP- and YFP-tagged TRPV4 proteins, which we assumed formed a random population of heteromeric channels, by FRET. We tagged C-tails, which remained unmodified in all of the TRPV4 deletions/mutations generated, to avoid possible FRET artifacts generated by the different lengths of the N-tails.
The relative CFP and YFP fluorescence intensities in the plasma membrane were determined for every single cell and used to calculate FRET efficiencies in transiently transfected HEK-293 cells (Fig. 5C). TRPV4-WT generated a FRET ratio similar to that reported previously (48), whereas TRPV4-Δ1–130 and TRPV4-121AAWAA doubled the FRET ratio, indicating a more compacted tail conformation. Similarly, coexpression of TRPV4-WT with PACSIN3 markedly increased the FRET signal, an effect lost when coexpressed with PACSIN3-ΔF-BAR.
We reasoned that the increased FRET observed with mutant TRPV4 proteins or coexpression with PACSIN3 was related to the inability of TRPV4 to interact with membrane PIP2. To test this hypothesis, we studied how the reduced PIP2 levels affected the FRET efficiency of TRPV4-WT. We overexpressed CFP- and YFP-tagged TRPV4-WT channels in HEK-293 cells engineered with tetracycline-inducible expression of 5-phosphatase IV (33). Induction of this enzyme depleted PIP2 from the plasma membrane (Fig. S5D) and significantly increased the FRET ratio (Fig. 5D). This finding further supports the hypothesis that conditions that prevented the N-tail access to membrane PIP2 (by deletion/mutation of the PI-binding site or overexpression of PACSIN3) or depleted PIP2 from the plasma membrane rearranged the cytosolic TRPV4 tails into a more compacted conformation (i.e., increased FRET ratio). Thus, in the presence of PIP2 and an intact PI-binding site, the intracellular tails appeared to have an expanded conformation.
Conclusion.
Several conclusions can be drawn from this study. First, TRPV4, like many other TRP channels, is regulated by PIP2, a process involving binding of PIP2 to a PI-binding site (121KRWRK125) in the N-tail. It will be interesting to address whether PIP2 also inhibits TRPV4 activity in channels reconstituted in artificial lipid bilayers, as was recently demonstrated for TRPV1 (49). Second, PIP2 regulates channel activity in a stimulus-dependent manner. Third, TRPV4 is bona fide thermosensitive channel, providing that there is PIP2 to interact with the N-tail. Fourth, interaction of the TRPV4 PI-binding site with plasma membrane PIP2 favors an expanded conformation of the intracellular tails and channel activation by hypotonicity and heat. Conditions that reduce PIP2 levels (e.g., inducible phosphatase) or interfere the interaction of TRPV4 with PIP2 (e.g., mutations in the PI-binding site or coexpression with PACSIN3) promote a compacted tail conformation and prevent channel activation by hypotonicity and heat.
The present study suggests that, similar to PIP2-regulated K+ channels, the PIP2–TRPV4 channel interaction rearranges the cytosolic domains. Whether the intracellular tail rearrangement occurring on binding of PIP2 to TRPV4 facilitates the access of stimuli-generated messengers (e.g., EET) to their binding sites or favors the stimulus-dependent opening of the gates themselves remains unclear.
Materials and Methods
Cells and Transfection.
For electrophysiological and calcium imaging experiments, HeLa or HEK-293 cells were transiently transfected as described previously (16, 48). Primary cultures of trachea and oviduct ciliated cells were obtained as described previously (7, 17). Animals were maintained and experiments performed in accordance with guidelines issued by the Institutional Ethics Committee of the Universitat Pompeu Fabra.
Solutions.
Isotonic bath solutions used for imaging experiments contained 140 mM NaCl, 2.5 mM KCl, 1.2 mM CaCl2, 0.5 mM MgCl2, 5 mM glucose, and 10 mM Hepes (pH 7.3) with Tris. Bath solutions for whole-cell recordings contained 100 mM NaCl, 1 mM MgCl2, 6 mM CsCl, 10 mM Hepes, 1 mM EGTA, and 5 mM glucose (pH 7.3) with Tris. Osmolarity was adjusted to 310 mOsm using mannitol. The 30% and 15% hypotonic solutions (255 and 220 mOsm) were obtained by removing mannitol. Whole-cell pipette solution contained 20 mM CsCl2, 100 mM Cs acetate, 1 mM MgCl2, 0.1 mM EGTA, 10 mM Hepes, 4 mM Na2ATP and 0.1 mM NaGTP (300 mOsm; pH 7.25). Bath and pipette solutions for excised inside-out single-channel recordings contained 130 mM CsCl, 1 mM MgCl2, 1 mM Na2ATP, 0.034 mM CaCl2, 5 mM EGTA, 10 mM Hepes (310 mOsm; pH 7.25). When required, solutions were warmed using a water jacket device (Warner Instruments). All chemicals were obtained from Sigma-Aldrich, except diC8-PI and diC8-PI(4,5)P2 (Echelon Biosciences), HC-067047 (Tocris Biosciences), and Fura-2 (Invitrogen).
Electrophysiological and Ratiometric Ca2+ Recordings.
Patch-clamp whole-cell and single-channel currents were recorded at room temperature (∼24 °C) unless indicated otherwise, as described previously (16, 48). Cells and excised patches were perfused at 0.8 mL/min. Cytosolic Ca2+ signals, relative to the ratio (340/380) measured before cell stimulation, were obtained from cells loaded with 4.5 μM Fura-2AM as described previously (4).
FRET Measurements.
FRET measurements were performed using a Leica TCS SP2 confocal microscope attached to an inverted microscope. FRET efficiencies were expressed as the increase in FRET donor CFP after bleaching of the FRET acceptor YFP (48).
Lipid Protection Assay.
Human TRPV4 ankyrin repeats (136–397) and N-tails (1–397) were cloned using NdeI and NotI into pET21-C6H (50). Recombinant proteins were produced and purified as described previously (51), except the size exclusion chromatography buffer was 10 mM Tris⋅HCl (pH 7.0), 300 mM NaCl, 10% glycerol, and 1 mM DTT for TRPV4 N-tails and 10 mM Tris⋅HCl (pH 7.0), 150 mM NaCl, and 1 mM DTT for TRPV4 ankyrin repeats.
Lipid protection assays by limited proteolysis were performed at 4 °C (on ice) in reaction buffer containing 180 mM NaCl, 20 mM Tris⋅HCl (pH 7.0), 1% glycerol, and 1 mM DTT for TRPV4-1-397 or 150 mM NaCl, 20 mM Tris⋅HCl (pH 7.0), and 1 mM DTT for TRPV4-136–397 and TRPV1-ankyrin repeat domain (ARD). Proteins were preincubated in the absence or presence of PI or PIP2 at 4 °C for 60 min and then digested with papain. Final concentrations of protein, lipid, and papain were 10 μM, 150 μM, and 38 nM, respectively. Digestion was stopped at 15, 30, 45, and 60 min by adding SDS sample buffer, after which samples were separated by SDS/PAGE and visualized by Coomassie blue staining. The gels were scanned, and signals were quantified with ImageJ.
Statistical Analysis.
Data are expressed as mean ± SEM (or mean ± SD in Fig. 4) of n experiments. Statistical analyses were performed with the Student unpaired t test or one-way ANOVA using SigmaPlot software.
Supplementary Material
Acknowledgments
We thank Dr. T. Voets (Catholic University of Leuven) for the gift of HEK-293 tetracycline-inducible phosphatase-expressing cells and Dr. M. Schaefer (University of Leipzig) for help with the initial FRET experiments. This work was supported by the Spanish Ministry of Science and Innovation (Grant SAF2012-38140, SAF2010-16725), Fondo de Investigación Sanitaria (Grant Red HERACLES RD12/0042/0014), Fondos Europeos de Desarrollo Regional (FEDER) Funds, Generalitat de Catalunya (Grant SGR05-266), and National Institutes of Health (Grant R01 GM081340). M.A.V. is the recipient of an Institució Catalana de Recerca i Estudis Avançats (ICREA) Academia Award, and U.A.H. is the recipient of a European Molecular Biology Organization (EMBO) Long-Term Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220231110/-/DCSupplemental.
References
- 1.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2(10):695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 2.Liedtke W, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103(3):525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wissenbach U, Bödding M, Freichel M, Flockerzi V. Trp12, a novel Trp related protein from kidney. FEBS Lett. 2000;485(2-3):127–134. doi: 10.1016/s0014-5793(00)02212-2. [DOI] [PubMed] [Google Scholar]
- 4.Arniges M, Vázquez E, Fernández-Fernández JM, Valverde MA. Swelling-activated Ca2+ entry via TRPV4 channel is defective in cystic fibrosis airway epithelia. J Biol Chem. 2004;279(52):54062–54068. doi: 10.1074/jbc.M409708200. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278(25):22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 6.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2003;100(Suppl 2):14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrade YN, et al. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol. 2005;168(6):869–874. doi: 10.1083/jcb.200409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Güler AD, et al. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22(15):6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández-Fernández JM, et al. Functional coupling of TRPV4 cationic channel and large conductance, calcium-dependent potassium channel in human bronchial epithelial cell lines. Pflugers Arch. 2008;457(1):149–159. doi: 10.1007/s00424-008-0516-3. [DOI] [PubMed] [Google Scholar]
- 10.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci USA. 2003;100(23):13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vriens J, et al. Modulation of the Ca2-permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97(9):908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 12.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97(12):1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 13.Alessandri-Haber N, Dina OA, Joseph EK, Reichling D, Levine JD. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci. 2006;26(14):3864–3874. doi: 10.1523/JNEUROSCI.5385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen DM. TRPV4 and the mammalian kidney. Pflugers Arch. 2005;451(1):168–175. doi: 10.1007/s00424-005-1456-9. [DOI] [PubMed] [Google Scholar]
- 15.Gevaert T, et al. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest. 2007;117(11):3453–3462. doi: 10.1172/JCI31766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandes J, et al. IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. J Cell Biol. 2008;181(1):143–155. doi: 10.1083/jcb.200712058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenzo IM, Liedtke W, Sanderson MJ, Valverde MA. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci USA. 2008;105(34):12611–12616. doi: 10.1073/pnas.0803970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muramatsu S, et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem. 2007;282(44):32158–32167. doi: 10.1074/jbc.M706158200. [DOI] [PubMed] [Google Scholar]
- 19.Masuyama R, et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008;8(3):257–265. doi: 10.1016/j.cmet.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Vriens J, et al. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA. 2004;101(1):396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe H, et al. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277(49):47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 22.Loukin S, Zhou X, Su Z, Saimi Y, Kung C. Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. J Biol Chem. 2010;285(35):27176–27181. doi: 10.1074/jbc.M110.143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem. 2003;278(29):26541–26549. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- 24.Phelps CB, Wang RR, Choo SS, Gaudet R. Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem. 2010;285(1):731–740. doi: 10.1074/jbc.M109.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’hoedt D, et al. Stimulus-specific modulation of the cation channel TRPV4 by PACSIN 3. J Biol Chem. 2008;283(10):6272–6280. doi: 10.1074/jbc.M706386200. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Elias A, Lorenzo IM, Vicente R, Valverde MA. IP3 receptor binds to and sensitizes TRPV4 channel to osmotic stimuli via a calmodulin-binding site. J Biol Chem. 2008;283(46):31284–31288. doi: 10.1074/jbc.C800184200. [DOI] [PubMed] [Google Scholar]
- 27.Vriens J, Owsianik G, Janssens A, Voets T, Nilius B. Determinants of 4 alpha-phorbol sensitivity in transmembrane domains 3 and 4 of the cation channel TRPV4. J Biol Chem. 2007;282(17):12796–12803. doi: 10.1074/jbc.M610485200. [DOI] [PubMed] [Google Scholar]
- 28.Verma P, Kumar A, Goswami C. TRPV4-mediated channelopathies. Channels (Austin) 2010;4(4):319–328. doi: 10.4161/chan.4.4.12905. [DOI] [PubMed] [Google Scholar]
- 29.Nilius B, Owsianik G, Voets T. Transient receptor potential channels meet phosphoinositides. EMBO J. 2008;27(21):2809–2816. doi: 10.1038/emboj.2008.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohacs T. Phosphoinositide regulation of non-canonical transient receptor potential channels. Cell Calcium. 2009;45(6):554–565. doi: 10.1016/j.ceca.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300(5623):1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- 32.Brauchi S, et al. Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc Natl Acad Sci USA. 2007;104(24):10246–10251. doi: 10.1073/pnas.0703420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilius B, et al. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 2006;25(3):467–478. doi: 10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukacs V, et al. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27(26):7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen SB, Tao X, MacKinnon R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature. 2011;477(7365):495–498. doi: 10.1038/nature10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell. 2011;147(1):199–208. doi: 10.1016/j.cell.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian W, et al. A loss-of-function nonsynonymous polymorphism in the osmoregulatory TRPV4 gene is associated with human hyponatremia. Proc Natl Acad Sci USA. 2009;106(33):14034–14039. doi: 10.1073/pnas.0904084106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gamper N, Rohacs T. Phosphoinositide sensitivity of ion channels, a functional perspective. Subcell Biochem. 2012;59:289–333. doi: 10.1007/978-94-007-3015-1_10. [DOI] [PubMed] [Google Scholar]
- 39.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314(5804):1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohács T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8(5):626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe H, et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 42.Everaerts W, et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA. 2010;107(44):19084–19089. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278(34):32037–32046. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- 44.Cuajungco MP, et al. PACSINs bind to the TRPV4 cation channel: PACSIN 3 modulates the subcellular localization of TRPV4. J Biol Chem. 2006;281(27):18753–18762. doi: 10.1074/jbc.M602452200. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q, et al. Molecular mechanism of membrane constriction and tubulation mediated by the F-BAR protein Pacsin/Syndapin. Proc Natl Acad Sci USA. 2009;106(31):12700–12705. doi: 10.1073/pnas.0902974106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plomann M, Wittmann JG, Rudolph MG. A hinge in the distal end of the PACSIN 2 F-BAR domain may contribute to membrane-curvature sensing. J Mol Biol. 2010;400(2):129–136. doi: 10.1016/j.jmb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Plomann M, Mörgelin M, Schael S. The PACSIN proteins and their role in mebrane trafficking. In: Aspenström P, editor. The Pombe Cdc15 Homology Proteins. Austin, TX: Landes Biosciences; 2009. pp. 39–48. [Google Scholar]
- 48.Arniges M, Fernández-Fernández JM, Albrecht N, Schaefer M, Valverde MA. Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J Biol Chem. 2006;281(3):1580–1586. doi: 10.1074/jbc.M511456200. [DOI] [PubMed] [Google Scholar]
- 49.Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat-sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013;77(4):667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin X, Touhey J, Gaudet R. Structure of the N-terminal ankyrin repeat domain of the TRPV2 ion channel. J Biol Chem. 2006;281(35):25006–25010. doi: 10.1074/jbc.C600153200. [DOI] [PubMed] [Google Scholar]
- 51.Inada H, Procko E, Sotomayor M, Gaudet R. Structural and biochemical consequences of disease-causing mutations in the ankyrin repeat domain of the human TRPV4 channel. Biochemistry. 2012;51(31):6195–6206. doi: 10.1021/bi300279b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.