Abstract
The failure to replace damaged body parts in adult mammals results from a muted growth response and fibrotic scarring. Although infiltrating immune cells play a major role in determining the variable outcome of mammalian wound repair, little is known about the modulation of immune cell signaling in efficiently regenerating species such as the salamander, which can regrow complete body structures as adults. Here we present a comprehensive analysis of immune signaling during limb regeneration in axolotl, an aquatic salamander, and reveal a temporally defined requirement for macrophage infiltration in the regenerative process. Although many features of mammalian cytokine/chemokine signaling are retained in the axolotl, they are more dynamically deployed, with simultaneous induction of inflammatory and anti-inflammatory markers within the first 24 h after limb amputation. Systemic macrophage depletion during this period resulted in wound closure but permanent failure of limb regeneration, associated with extensive fibrosis and disregulation of extracellular matrix component gene expression. Full limb regenerative capacity of failed stumps was restored by reamputation once endogenous macrophage populations had been replenished. Promotion of a regeneration-permissive environment by identification of macrophage-derived therapeutic molecules may therefore aid in the regeneration of damaged body parts in adult mammals.
Keywords: developmental biology, immunology, limb development, wound healing
Salamanders have the remarkable ability to regenerate complex structures such as limbs, tails, retina, and spinal cord, along with some sections of the heart and brain, during any stage of their life cycle (1–7). Salamanders are also able to perform scar-free repair of deep tissue wounds after injury (8). Although the cellular mediators and immunological signaling necessary for regeneration in the salamander have not been described, recent reports suggest that inflammation may influence the initiation and completion of wound healing and regeneration (9–14), as the cytokine microenvironment directly influences the time course of leukocyte infiltration, cellular proliferation, angiogenesis, and collagen remodeling of damaged tissues.
In mammals, fibrotic scarring is a major impediment to tissue regeneration (15), where cellular infiltration and immune signaling play a key role (16). Macrophages are an important source of both inflammatory and anti-inflammatory signals, arriving in mammalian wounds 48–96 h after injury, where they clear dead cells, release proinflammatory cytokines, and subsequently produce factors that dampen inflammation and stimulate angiogenesis, fibroblast migration, and replication (17). In mice, macrophage depletion or disruption of macrophage transcriptional regulation after skeletal muscle injury results in incomplete muscle repair and induction of fibrotic scarring (18, 19).
To investigate the role of macrophages during scar-free tissue repair in an efficiently regenerating model, we dissected the inflammatory response to injury in regenerating limbs of the axolotl aquatic salamander (Ambystoma mexicanum) at the cellular and molecular level. A distinctively dynamic signaling microenvironment during the early stages of axolotl limb regeneration was accompanied by early myeloid cell recruitment. We documented an absolute requirement for macrophages in the orchestration of postamputation immune microenvironment and blastema formation, providing direct evidence for the immunological control of successful regeneration in an adult vertebrate.
Results
Pro- and Anti-Inflammatory Signals Are Simultaneously Induced Following Axolotl Limb Amputation and Sustained Through Regeneration.
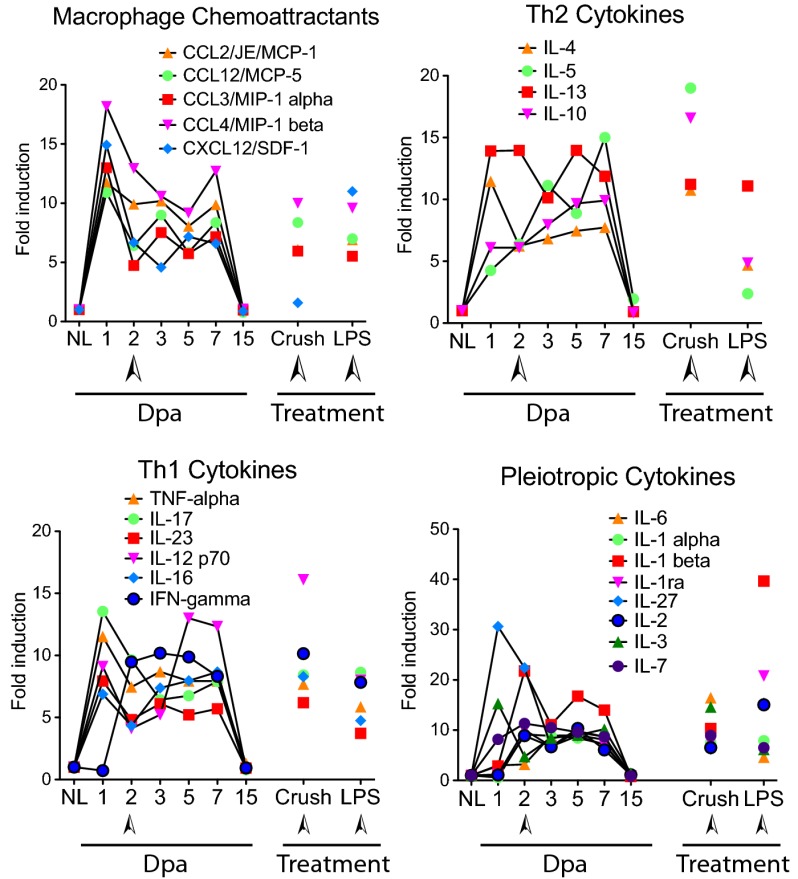
We screened the blastema of resected axolotl limbs with a cross-reactive mouse cytokine protein detection array, revealing a rapid induction of cytokines, chemokines, and inflammatory markers within 1 d of limb resection. Unexpectedly, we detected high levels of anti-inflammatory (Th2) cytokines (Fig. 1; Fig. S1), which are normally induced later in mammalian wound healing (20). The fibrotic responses normally associated with IL-4 and IL-13 induction were countered by increased expression of the antifibrogenic (Th1) cytokine IFN-γ (21). All cytokines and chemokines returned to normal baseline levels by 15 d postamputation (dpa; late blastema). Myeloid chemotactic molecules peaked within 1 dpa (Fig. 1), indicating that robust monocyte recruitment precedes limb regeneration. A comparative analysis of the inflammatory response induced by crush injury (necrotic model) and LPS injection (modeling bacterial infection) confirmed that many of the inflammatory cytokines and chemokines induced in mammalian wounding exhibit similar patterns of induction in the axolotl (Fig. 1; Fig. S1), as exemplified by the strong induction of IL-1β to LPS exposure (40-fold). Thus, we focused attention on the unusually early activation of anti-inflammatory signaling in the regenerating axolotl limb.
Fig. 1.
Cytokine regulation during the time course of regeneration. A cross-species cytokine protein array detects changes in cytokine/chemokine profiles in axolotl blastema at various time points after amputation relative to baseline expression in uninjured normal limb. Crush injury and LPS treatments were analyzed at 2 dpa after injury, relative to untreated limb. Arrowheads indicate 2 dpa time points. Each data point shows the mean from two separate experiments using pooled samples from six animals per time point/treatment. dpa, days postamputation; NL, normal limb.
Myeloid Cell Recruitment Is a Major Feature of Limb Regeneration.
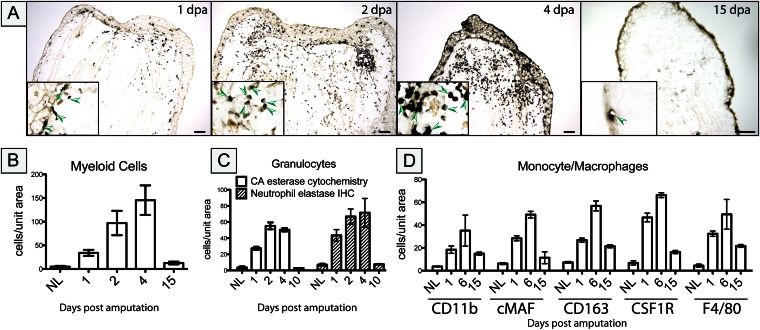
To monitor leukocyte subsets entering the regenerating limb blastema, we used enzyme cytochemistry to detect monocytes, macrophages, and granulocytes in regenerating axolotl tissue as early as 1 dpa, subsequently peaking between 4 and 6 dpa (Fig. 2; Fig. S2), before returning to baseline levels by 15 dpa (late blastema). Using naphthol AS-D chloroacetate (CA) esterase chemistry, neutrophil granulocytes were also specifically detected within the wound 1 dpa and persisted for up to 4–6 d (Fig. S2). Unexpectedly, macrophages detected with α-naphthyl acetate (NSE) staining were also increased within the 1-dpa blastema, located immediately around and within the developing wound epithelium. Macrophage numbers peaked at around 4–6 d and persisted in the regenerate up to the early redevelopment phase before returning to baseline numbers (Fig. 2; Fig. S3).
Fig. 2.
Myeloid cells accumulate in the regenerating limb blastema. (A) α-Naphthyl acetate (NSE) enzyme cytochemistry was used to detect myeloid cells at different time points after amputation. Cell numbers were determined by counting NSE-positive cells to detect myeloid cells (B), CA esterase cytochemistry to detect granulocytes (C), and immunocytochemistry to detect monocytes/macrophages (D), using Fiji image analysis software on at least three separate experiments. Cell numbers are expressed as the number of cells counted per unit area as the mean ± SEM. Representative sections are shown in Figs. S2 and S3. (Scale bars, 100 μm.)
To confirm the phagocytic capacity of macrophages detected within regenerating limb and deep tissue injuries, three independent labeling methods were used (Fig. S4). First, carbon-labeled phagocytes stained with Giemsa exhibited typical monocyte morphology within the blastema and wound epithelium (Fig. S4 A–C). Second, neutral red staining, shown to be highly selective for macrophages in young zebrafish (22), revealed positive cells accumulating in regenerating tissue (Fig. S4F). Neutral red staining cells also undertake phagocytosis of 0.7-µm-diameter fluorescent beads (Fig. S4J). Phagocytic cells could be tracked into deep tissue injury as early as 24 h (Fig. S4I) and were found in comparable locations throughout the regenerating limb as detected by macrophage-specific immunocytochemistry (Fig. S2). Third, 2 MDa dextran rhodamine was injected 24 h before limb resection to detect macropinocytic mononuclear phagocytes (23). Compared with tissue macrophages in uninjured limb and circulating monocytes in peripheral blood, macrophage levels were significantly enriched in the regenerating limb and injured tissue (Fig. S5). Flow cytometric isolation of the labeled populations from limb tissue showed the expected macrophage/monocyte morphology, staining positive for NSE chemistry (Fig. S5).
Mononuclear Phagocytes Regulate Regenerative Gene Expression Patterns.
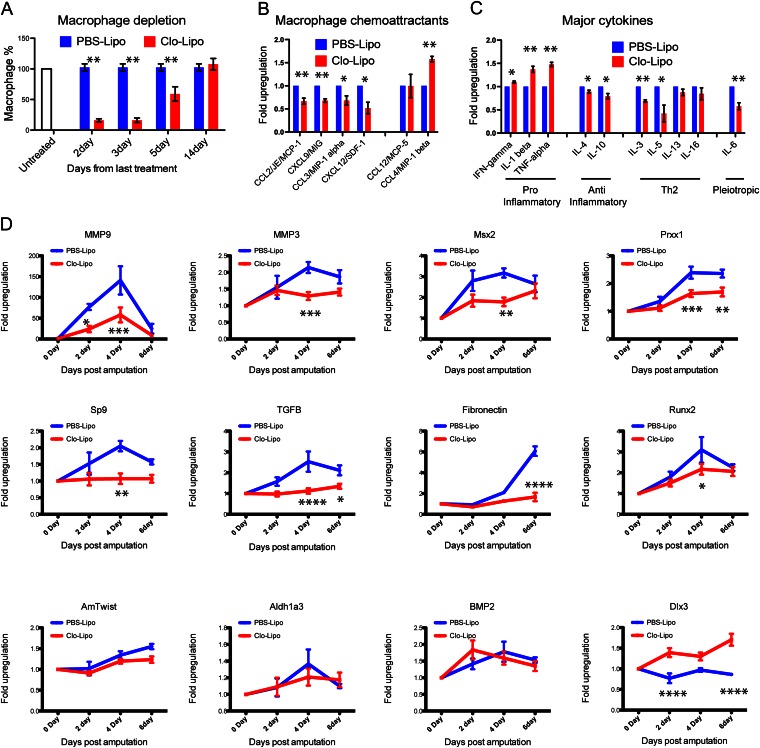
Phagocytic uptake of liposome-encapsulated clodronate (Clo-Lipo) is a well-established method for specific in vivo ablation of macrophages (24). This reagent was effective in specifically depleting circulating axolotl monocytes and tissue resident macrophages on i.v. injection, leaving the neutrophil numbers relatively unaffected (Figs. S5 and S6). Replenishment of mononuclear phagocytes begins 5–10 d after complete ablation (Fig. 3A; Fig. S5). By examining molecular profiles in the first week after amputation before macrophages return, we detected changes in macrophage chemoattractants (Fig. 3B), increased levels of inflammatory cytokines, and reduced levels of anti-inflammatory cytokines (Fig. 3C). A concomitant decrease in the expression of the ECM-degrading enzymes matrix metalloproteinase 9 and 3 (MMP9 and MMP3, respectively) was accompanied by a significant reduction in the expression of Msx2 and the blastemal marker genes Prrx1 and Sp9 (Fig. 3D). Macrophage depletion also caused a failure in the activation of Tgf-β1 and TGF target genes Runx2 and fibronectin. Down-regulation of TGF-β, fibronectin, and Msx2 was confirmed by histological examination (Fig. S7). TGF-β regulates various stages of mammalian wound healing and constitutes an important signaling pathway in salamander limb regeneration (25). The lack of the appropriate levels of TGF-β1 activation in macrophage-depleted limbs following amputation may therefore contribute to the failure to regenerate in these animals. Unexpectedly, macrophage depletion caused precocious expression of Dlx3, a homeobox ortholog of Drosophila distal-less, required for leg outgrowth in the fly (26) and strongly expressed in the wound epidermis of regenerating axolotl limb (27). Failure to up-regulate the activated wound epithelial marker WE3 in Clo-Lipo animals within 6 dpa suggested that the wound epithelium is not functional. Although BrdU incorporation was maintained in the wound epithelium, it was mainly undetectable in the underlying mesenchyme, and staining for the classic blastemal progenitor marker 22/18 confirmed the failure to activate mesenchymal progenitors (Fig. S7). Included among markers that were relatively unperturbed were Bmp2, normally associated with differentiation (28), and its downstream target AmTwist, a marker of dermal differentiation (29). Retinoic acid signaling, implicated in the patterning of the embryonic axolotl limb and the limb regenerate (30), was also unaffected, suggesting that the programs leading to proper proximodistal limb patterning in a more permissive environment are left intact. Thus, macrophage depletion before amputation disrupts specific gene pathways important for the progression from wound healing to regeneration within the first 6 d.
Fig. 3.
Macrophage depletion alters cytokine and chemokine induction after amputation and modulates the gene expression profiles of key regenerative genes. (A) Flow cytometric measurement of rhodamine-labeled macrophage populations in macrophage depleted (Clo-Lipo) vs. control (PBS-Lipo) animals. (B and C) Specific cytokine and chemokine profiles 4 dpa with and without macrophage depletion. Histograms show relative levels and were normalized to uninjured normal limb from the same animal. (D) Changes in gene expression profiles in the resected stump on macrophage ablation were assessed by quantitative RT-PCR, over the first 6 dpa. Plots show mean ± SEM of at least three independent experiments. *P ≤ 0.05; **P ≤ 0.01; **P ≤ 0.001, ****P ≤ 0.0001. n > 3. Primer sequences used in RT-PCR gene expression analysis are listed in Table S1.
Early Macrophage Engagement Is Critical for Limb Regeneration.
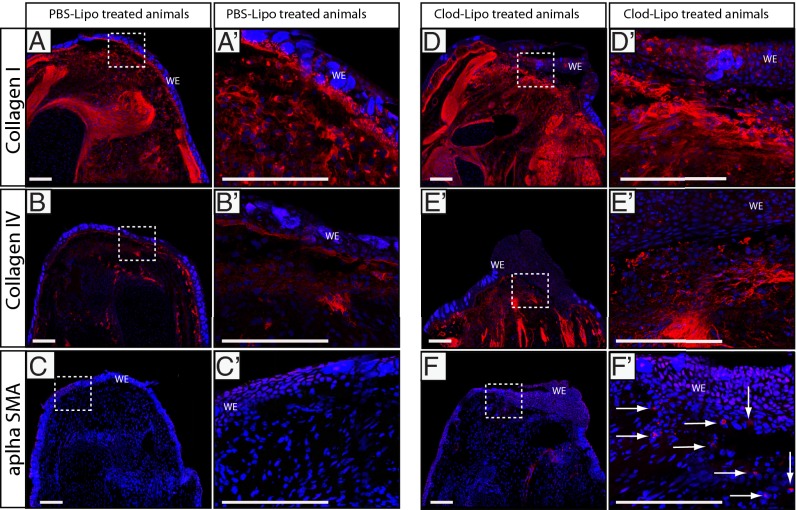
Whereas partial macrophage depletion with a single liposome injection before amputation delayed but did not block blastemal formation and limb outgrowth (Fig. S8), maximal macrophage depletion in both limb tissues and peripheral blood (three consecutive i.v. injections of clodronate liposomes over 4 d) before amputation (Fig. 4A) resulted in complete blockade of limb regeneration in all cases (n = 10; Fig. 4B). The fibrotic stumps that formed in the absence of macrophages contained permanent scar tissue still evident after 90 d (Fig. 4F). Some of these animals were followed for up to 150 d, and although full macrophage repopulation occurred within 2 wk after injection, limb stumps did not initiate blastema formation thereafter. The failed regenerates featured altered ECM components, with extensive collagen deposition and fibrosis in which thick mature type I collagen predominates, as shown by picosirius red staining and polarized light analysis (Fig. 4 G–J). Moreover, immunostaining revealed extensive collagen I, and collagen IV deposited directly below the wound epithelium at 20 and 6 dpa (Fig. 5 and Fig. S9). α-Smooth muscle actin (α-SMA) is an established marker for differentiated myofibroblasts displaying subepithelial localization (31–33) in several species. Macrophage-depleted animals showed increased numbers of α-SMA–positive cells compared with the normally low levels observed in control animals at 20 dpa (Fig. 5).
Fig. 4.
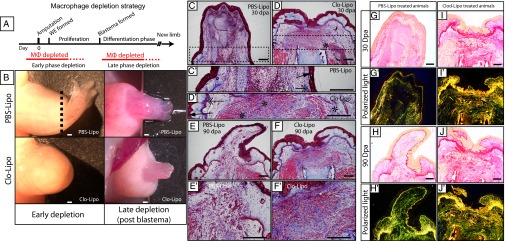
Blockade of limb regeneration depends on early macrophage depletion. (A) Macrophage depletion strategy: early phase depletion targets blastemal formation and late phase depletion targets the redevelopment phase. (B) Injection of PBS liposomes (PBS-Lipo) has no effect on normal limb regeneration (n = 10). Maximal macrophage ablation before amputation (early phase depletion) blocks limb regeneration in all animals (n = 10). Amputation plane is shown by a dotted line. Ablation of macrophages after blastemal formation (late phase depletion) allows regeneration to proceed to completion with a reproducible delay in defined regeneration stages (n = 5). (C) Control PBS-Lipo–injected animals enter paddle stage at 30 dpa and show weak collagen reactivity to trichrome staining (C′: dashed box marks inset showing high-magnification images). (D) Triple Clo-Lipo–treated animals do not initiate regeneration at 30 dpa and the failed regenerate (D′: high-magnification inset) features a complete basement membrane (arrow), a dense stratum compactum (double arrows), pronounced epidermal tongues (arrowhead), and extensive collagen deposition (asterisks). Extensive collagen deposition is still evident in Clo-Lipo stumps at 90 dpa (F and F′) relative to control PBS-Lipo regenerates (E and E′). (G–J) Picosirius red staining was used to analyze collagen fiber density. (I, I′, J, and J′) Macrophage depleted failed regenerates show dense thick collagen fiber deposition at 30 and 90 dpa compared with regenerating control limbs (G, G′, H, and H′). Thin fibers stain green and thicker fibers stain yellow/red under polarized light. (Scale bars, 100 μm.)
Fig. 5.
Extracellular matrix components are altered by macrophage depletion. At 20 dpa, Clod-Lipo–treated animals show increased (D and D′) collagen I and (E and E′) collagen IV deposition under the wound epithelium (WE) and (F and F′) an increased number of cells (marked with white arrows) staining positive for the myofibroblast marker α-SMA relative to control animals (A–C). Tiled confocal images taken at ×20 magnification. (Scale bars, 100 μm.)
To examine the role of macrophages in later stages of regeneration, we injected clodronate-loaded liposomes after midbud blastemal formation (three consecutive i.v. injections of clodronate liposomes days 10–13 after limb resection). Macrophage depletion at this later stage delayed but did not block regeneration, albeit with an apparent reduction in the superficial vascular network (Fig. S8). A role for macrophages in late phase tissue remodeling (34) is consistent with the relatively high level of conservation in the inflammatory signaling response that closely parallels the chemotactic events in mammalian wound healing (Fig. 1). However, the critical window for macrophage-mediated limb regeneration occurs early in blastemal formation.
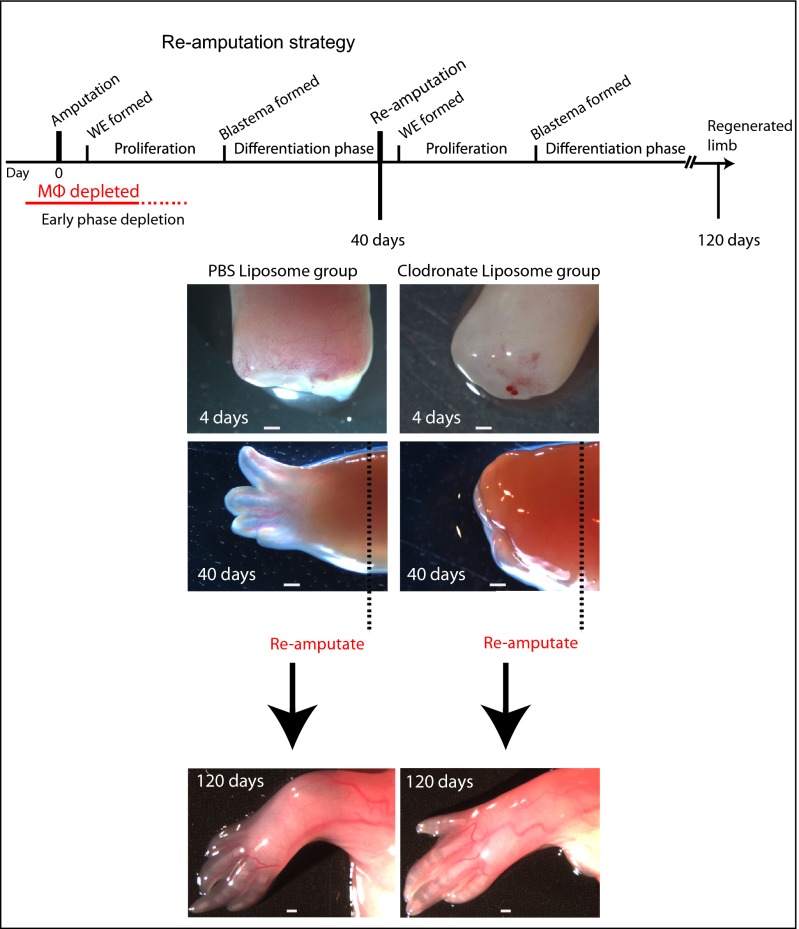
To determine whether the failure of limb regeneration caused by the initial absence of macrophages was permanent, we reamputated limb stumps that failed to regenerate with early clodronate treatment. Even after 150 d, resection of failed limb stumps at a more proximal position resulted in complete limb regrowth (Fig. 6), demonstrating that a viable regenerative program can be reactivated in the renewed presence of macrophages.
Fig. 6.
Reamputation of limbs stumps after clodronate-loaded liposome treatment and macrophage repopulation reactivates a functional regeneration program. After macrophages are replenished, limb regeneration is activated by reamputation at a more proximal position that includes transection of functional nerve (n = 6). WE, wound epithelium.
Discussion
In this study, we established an essential requirement for macrophages in orchestrating the early response to injury and the activation of subsequent limb regeneration in the salamander. Although macrophages are a major component of the mononuclear phagocyte system involved in clearance of tissue debris and host defense, our work adds to the emerging appreciation for the broader role of macrophages in tissue homeostasis and repair. Although wound healing and regeneration are thought to share common molecular pathways, the transition from wound healing to regeneration is likely to require a concerted gene program that integrates a permissive immunological signaling environment and the precise activation of both growth and developmental pathways. The unexpected early up-regulation of anti-inflammatory cytokines and precocious macrophage engagement after limb amputation may recapitulate developmental programs necessary for complete regeneration. Macrophages play a key role in embryonic tissue and organ morphogenesis by producing chemokines that in turn attract and/or activate other cell types (e.g., fibroblasts and progenitor cells) (35). Indeed, macrophage ablation disrupts amphibian development (36). The mammalian embryo retains the ability to repair wounds without scar formation, featuring a limited inflammatory response (37). In the 11.5 d postcoitum (dpc) mouse embryo, macrophages represent 3–10% of all cells and are particularly abundant in developing limb (35), consistent with a role in limb morphogenesis. Compared with adult macrophages by microarray, embryonic mouse macrophages are enriched in wound healing and angiogenesis signatures (38), which may support limb growth and development. Indeed, mouse embryos deficient in IL-10, a potent anti-inflammatory cytokine secreted by macrophages, lose their scar-free healing capacity (39). Thus, correct regulation of the inflammatory milieu may be a critical precondition for both embryonic and adult regeneration.
In mammalian tissue repair, macrophages replace neutrophils and participate in a variety of functions during the complex multistep process of wound resolution. Macrophages debride damaged tissue by releasing various proteases, growth factors, and cytokines that attract cells involved in the proliferation stage of repair, and their exit from the wound environment is associated with the end of the inflammatory phase and the onset of wound contraction (16). Conditional macrophage depletion studies have documented other functions during the early inflammatory phase of mammalian skin repair, where their ablation reduces the formation of vascularized granulation tissue, impairs epithelialization, and minimizes scar formation (34).
In contrast, early macrophage depletion in the axolotl did not affect epithelial wound closure after limb amputation but instead caused excessive fibroplasia, collagen deposition, and a complete block in blastemal formation. It is likely that the early arrival of macrophages into the regenerating axolotl blastema by 24 h after amputation, along with simultaneous induction of inflammatory and anti-inflammatory cytokines, is part of a distinct regenerative program. Macrophage depletion also blocked activation of the wound epithelial marker WE3, suggesting that the wound epithelium is nonfunctional in these animals: although cellular proliferation was maintained in the epithelium, underlying mesenchymal proliferation was not faithfully activated. Indeed, dedifferentiation markers Prrx1 (40), Sp9 (41), and 22/18 (42) were disregulated in the amputated limb by early ablation of macrophages, consistent with disruption of the early blastema, comprising dedifferentiated multipotent cells. Moreover, homeodomain transcription factor Msx2, which is normally activated in both healing and regeneration phases (43, 44) and implicated in repression of differentiation, was also decreased, whereas Dlx3, an early differentiation phase marker (27, 44), was up-regulated early in macrophage-depleted limb stumps. Notably, overexpression of Dlx3 in epidermal basal cells of transgenic mice disrupts normal skin differentiation by a blockade in cellular proliferation and the premature induction of basal cell maturation (45). Together, these findings indicate that one potential mechanism whereby macrophages permit regeneration is by promoting dedifferentiation and formation of the progenitor cell pool by either direct or indirect routes.
Macrophage-depleted axolotl limb stumps also failed to fully activate expression of TGF-β1, a pleiotropic growth factor and key regulator of embryonic development (46) and mammalian wound healing (46), as well as its target genes Runx2 and fibronectin. Inhibition of TGF-β1 signaling blocks successful limb regeneration in the salamander (25), and fibronectin forms part of the provisional matrix during scar-free repair (31) that may contribute to a regeneration permissive environment. The stunted activation of MMP9 and MMP3 on macrophage ablation is consistent with an essential role for matrix remodeling in successful limb regeneration (47) and implicates macrophages as a key regulator of matrix degrading enzymes. Other matrix components such as collagen were markedly altered in the absence of macrophages, leading to a fibrous cap. The regenerative blastema features mainly collagen type III (thin fibers), with a distinctive lack of collagen IV (48, 49), whereas collagen I (thick fibers) is normally down-regulated. Disruption of collagen production in macrophage-depleted limb stumps is presumably due to the activation of myofibroblasts, which are mainly absent in normal axolotl limb and scar-free skin regeneration (31), contributes to scarring in mammals (50, 51), and represents a major difference between fibrotic and scar-free repair in the mouse (32).
The role of macrophages in later stages of wound repair are reminiscent of macrophage depletion during mammalian skin tissue regrowth, which results in severe hemorrhage, endothelial cell apoptosis, vessel destabilization, and a failure in wound closure (34). Consistent with this role of macrophages, we observed a delay in limb redevelopment associated with an apparent reduction in surface vasculature when axolotls are depleted of macrophages after blastemal formation. The requirement for macrophages in successful salamander limb regrowth may therefore involve functionally distinct roles in successive stages of the regeneration program.
The presence of leukocytes in the newt blastema was first observed in 1961, when a contribution of mononucleated blood cells to multinucleated osteoclasts was proposed (52, 53). More recent studies in the axolotl reported the recruitment of neutrophils in deep tissue wounds but not in regenerating limbs (8, 31). By contrast, using a range of methods, the present study confirms the active involvement of macrophages within the regenerating axolotl limb. In an embryonic model of Xenopus tail regeneration, unregulated myeloid populations in the absence of T-regulatory cells have a negative effect on regenerative ability (54). Our findings indicate a positive role for macrophages in adult amphibian regeneration, reinforcing the importance of macrophage phenotypic states to the outcome of wound resolution and regeneration. Understanding the early regulation of expression patterns and timing of various extracellular matrix components by macrophage signaling is critical in identifying pathways permissive for appendage regeneration. Their early engagement in the secretion of anti-inflammatory cytokines and other factors promoting the efficient regeneration of axolotl limbs point to potential therapeutic strategies for preventing fibrotic scarring and promoting tissue regeneration in mammals following tissue injury.
Methods
All procedures using axolotls were performed in accordance with Monash University’s Animal Ethics Committee. Tissues were collected from normal or regenerating axolotl limbs, fixed in 4% (wt/vol) paraformaldehyde (PFA), embedded in paraffin or O.C.T. compound (Tissue-Tek), and analyzed by immunohistochemistry, histology, or cytology. Cytokine protein analysis was performed on homogenized tissue lysates and assayed using a mouse cytokine array kit (R&D Systems) according to the manufacturer’s instructions. Macrophage labeling was performed by uptake of 2 MDa dextran Rhodamine, 0.7 μM fluorescent beads, colloidal carbon, and neutral red dye. Macrophage depletion was performed using i.v. injection of clodronate-encapsulated liposomes. Macrophage isolation from tissue was performed using an enzymatic and mechanical GentleMACS (Miltenyi) cell dissociation protocol. Flow cytometric analysis was performed on fresh or fixed cells using commercial reagents. Flow cytometric analysis/sorting was performed on a LSRII (7 laser) flow cytometer or BD Influx cell sorter. Quantitative real-time PCR was performed using a Roche LC480, using SYBR green. Full methods and any associated references are available in SI Methods.
Supplementary Material
Acknowledgments
We thank members of the Rosenthal laboratory for technical advice and helpful discussions, Mark Davis for valuable assistance in establishing an axolotl colony, and Maik Fidel for animal support. This work was supported by Stem Cells Australia and a grant from the Australian Research Council (to N.A.R.). The Australian Regenerative Medicine Institute is supported by grants from the State Government of Victoria and the Australian Government. N.A.R. is a National Health and Medical Research Council Australia Fellow.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300290110/-/DCSupplemental.
References
- 1.Carlson BM. Principles of Regenerative Biology. New York: Elsevier/Academic Press; 2007. p. xix. [Google Scholar]
- 2.Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310(5756):1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- 3.Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol. 2002;3(8):566–574. doi: 10.1038/nrm881. [DOI] [PubMed] [Google Scholar]
- 4.Parish CL, Beljajeva A, Arenas E, Simon A. Midbrain dopaminergic neurogenesis and behavioural recovery in a salamander lesion-induced regeneration model. Development. 2007;134(15):2881–2887. doi: 10.1242/dev.002329. [DOI] [PubMed] [Google Scholar]
- 5.Ferretti P, Zhang F, O’Neill P. Changes in spinal cord regenerative ability through phylogenesis and development: Lessons to be learnt. Dev Dyn. 2003;226(2):245–256. doi: 10.1002/dvdy.10226. [DOI] [PubMed] [Google Scholar]
- 6.Singh BN, Koyano-Nakagawa N, Garry JP, Weaver CV. Heart of newt: A recipe for regeneration. J Cardiovasc Transl Res. 2010;3(4):397–409. doi: 10.1007/s12265-010-9191-9. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka EM, Reddien PW. The cellular basis for animal regeneration. Dev Cell. 2011;21(1):172–185. doi: 10.1016/j.devcel.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lévesque M, Villiard É, Roy S. 2010. Skin wound healing in axolotls: A scarless process. J Exp Zool 314(8):684–697. [DOI] [PubMed]
- 9.Godwin JW, Brockes JP. Regeneration, tissue injury and the immune response. J Anat. 2006;209(4):423–432. doi: 10.1111/j.1469-7580.2006.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahmy GH, Sicard RE. A role for effectors of cellular immunity in epimorphic regeneration of amphibian limbs. In Vivo. 2002;16(3):179–184. [PubMed] [Google Scholar]
- 11.Schotté OE, Sicard RE. Cyclophosphamide-induced leukopenia and suppression of limb regeneration in the adult newt, notophthalmus viridescens. J Exp Zool. 1982;222(2):199–202. doi: 10.1002/jez.1402220212. [DOI] [PubMed] [Google Scholar]
- 12.Sicard RE. Blood cells and their role in regeneration. II. Effects of putative immunological manipulations on circulating blood cell counts during regeneration. Exp Cell Biol. 1983;51(2):109–114. [PubMed] [Google Scholar]
- 13.Harty M, Neff AW, King MW, Mescher AL. Regeneration or scarring: An immunologic perspective. Dev Dyn. 2003;226(2):268–279. doi: 10.1002/dvdy.10239. [DOI] [PubMed] [Google Scholar]
- 14.Mescher AL, Neff AW. Limb regeneration in amphibians: Immunological considerations. ScientificWorldJournal. 2006;6(Suppl 1):1–11. doi: 10.1100/tsw.2006.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mescher AL, Neff AW. Regenerative capacity and the developing immune system. Adv Biochem Eng Biotechnol. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- 16.Koh TJ, DiPietro LA. Inflammation and wound healing: The role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187(5A):11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 18.Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578(Pt 1):327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruffell D, et al. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci USA. 2009;106(41):17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RHJ. Macrophages in skin injury and repair. Immunobiology. 2011;216(7):753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carradice D, Lieschke GJ. Zebrafish in hematology: Sushi or science? Blood. 2008;111(7):3331–3342. doi: 10.1182/blood-2007-10-052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim JP, Gleeson PA. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89(8):836–843. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 24.van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol. 2010;605:189–203. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]
- 25.Lévesque M, et al. Transforming growth factor: Beta signaling is essential for limb regeneration in axolotls. PLoS ONE. 2007;2(11):e1227. doi: 10.1371/journal.pone.0001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen SM, Brönner G, Küttner F, Jürgens G, Jäckle H. Distal-less encodes a homoeodomain protein required for limb development in Drosophila. Nature. 1989;338(6214):432–434. doi: 10.1038/338432a0. [DOI] [PubMed] [Google Scholar]
- 27.Mullen LM, Bryant SV, Torok MA, Blumberg B, Gardiner DM. Nerve dependency of regeneration: The role of Distal-less and FGF signaling in amphibian limb regeneration. Development. 1996;122(11):3487–3497. doi: 10.1242/dev.122.11.3487. [DOI] [PubMed] [Google Scholar]
- 28.Guimond J-C, et al. 2010. BMP-2 functions independently of SHH signaling and triggers cell condensation and apoptosis in regenerating axolotl limbs. BMV Dev Biol, 10:15.
- 29.Satoh A, Bryant SV, Gardiner DM. Regulation of dermal fibroblast dedifferentiation and redifferentiation during wound healing and limb regeneration in the Axolotl. Dev Growth Differ. 2008;50(9):743–754. doi: 10.1111/j.1440-169X.2008.01072.x. [DOI] [PubMed] [Google Scholar]
- 30.Monaghan JR, Maden M. Visualization of retinoic acid signaling in transgenic axolotls during limb development and regeneration. Dev Biol. 2012;368(1):63–75. doi: 10.1016/j.ydbio.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seifert AW, Monaghan JR, Voss SR, Maden M. Skin regeneration in adult axolotls: A blueprint for scar-free healing in vertebrates. PLoS ONE. 2012;7(4):e32875. doi: 10.1371/journal.pone.0032875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seifert AW, et al. Skin shedding and tissue regeneration in African spiny mice (Acomys) Nature. 2012;489(7417):561–565. doi: 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levesque M, Villiard E, Roy S. Skin wound healing in axolotls: A scarless process. (Translated from eng) J Exp Zoolog B Mol Dev Evol. 2010;314(8):684–697. doi: 10.1002/jez.b.21371. [DOI] [PubMed] [Google Scholar]
- 34.Lucas T, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184(7):3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 35.Nucera S, Biziato D, De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol. 2011;55(4-5):495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
- 36.Smith SJ, Kotecha S, Towers N, Mohun TJ. Targeted cell-ablation in Xenopus embryos using the conditional, toxic viral protein M2(H37A) Dev Dyn. 2007;236(8):2159–2171. doi: 10.1002/dvdy.21233. [DOI] [PubMed] [Google Scholar]
- 37.Wilgus TA. Regenerative healing in fetal skin: A review of the literature. Ostomy Wound Manage. 2007;53(6):16–31. [PubMed] [Google Scholar]
- 38.Rae F, et al. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol. 2007;308(1):232–246. doi: 10.1016/j.ydbio.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg. 2000;35(6):866–872. doi: 10.1053/jpsu.2000.6868. [DOI] [PubMed] [Google Scholar]
- 40.Satoh A, makanae A, Hirata A, Satou Y. Blastema induction in aneurogenic state and Prrx-1 regulation by MMPs and FGFs in Ambystoma mexicanum limb regeneration. Dev Biol. 2011;355(2):263–274. doi: 10.1016/j.ydbio.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Satoh A, Graham GM, Bryant SV, Gardiner DM. Neurotrophic regulation of epidermal dedifferentiation during wound healing and limb regeneration in the axolotl (Ambystoma mexicanum) Dev Biol. 2008;319(2):321–335. doi: 10.1016/j.ydbio.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 42.Gordon H, Brockes JP. Appearance and regulation of an antigen associated with limb regeneration in Notophthalmus viridescens. J Exp Zool. 1988;247(3):232–243. doi: 10.1002/jez.1402470306. [DOI] [PubMed] [Google Scholar]
- 43.Carlson MR, Bryant SV, Gardiner DM. Expression of Msx-2 during development, regeneration, and wound healing in axolotl limbs. J Exp Zool. 1998;282(6):715–723. doi: 10.1002/(sici)1097-010x(19981215)282:6<715::aid-jez7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 44.Campbell LJ, et al. Gene expression profile of the regeneration epithelium during axolotl limb regeneration. Dev Dyn. 2011;240(7):1826–1840. doi: 10.1002/dvdy.22669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morasso MI, Markova NG, Sargent TD. Regulation of epidermal differentiation by a Distal-less homeodomain gene. J Cell Biol. 1996;135(6 Pt 2):1879–1887. doi: 10.1083/jcb.135.6.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 47.Vinarsky V, Atkinson DL, Stevenson TJ, Keating MT, Odelberg SJ. Normal newt limb regeneration requires matrix metalloproteinase function. Dev Biol. 2005;279(1):86–98. doi: 10.1016/j.ydbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Rao N, et al. Proteomic analysis of blastema formation in regenerating axolotl limbs. BMC Biol. 2009;7:83. doi: 10.1186/1741-7007-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asahina K, Obara M, Yoshizato K. Expression of genes of type I and type II collagen in the formation and development of the blastema of regenerating newt limb. Dev Dyn. 1999;216(1):59–71. doi: 10.1002/(SICI)1097-0177(199909)216:1<59::AID-DVDY8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 50.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 51.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127(3):526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 52.Hay ED, Fischman DA. Origin of the blastema in regenerating limbs of the newt Triturus viridescens. An autoradiographic study using tritiated thymidine to follow cell proliferation and migration. Dev Biol. 1961;3:26–59. doi: 10.1016/0012-1606(61)90009-4. [DOI] [PubMed] [Google Scholar]
- 53.Fischman DA, Hay ED. Origin of osteoclasts from mononuclear leucocytes in regenerating newt limbs. Anat Rec. 1962;143:329–337. doi: 10.1002/ar.1091430402. [DOI] [PubMed] [Google Scholar]
- 54.Fukazawa T, Naora Y, Kunieda T, Kubo T. Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development. 2009;136(14):2323–2327. doi: 10.1242/dev.033985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.