Significance
The coding of olfactory information is based on the activity of odor receptors. The larval olfactory system of Drosophila contains 21 olfactory receptor neurons and a comparable number of odor receptors. Through a screen of >10,000 receptor–odorant combinations, we identify for each of 19 receptors an odorant that excites it strongly. These odorants elicited little cross-activation of other receptors under test conditions. Systematic analysis reveals dramatic diversity in the sensitivity and temporal dynamics of responses to cognate odorants. The odorants elicited diverse behavioral responses. The analysis provides a foundation for elucidating the circuitry that translates receptor responses into behavior.
Abstract
The ability of an animal to detect, discriminate, and respond to odors depends on the function of its olfactory receptor neurons (ORNs), which in turn depends ultimately on odorant receptors. To understand the diverse mechanisms used by an animal in olfactory coding and computation, it is essential to understand the functional diversity of its odor receptors. The larval olfactory system of Drosophila melanogaster contains 21 ORNs and a comparable number of odorant receptors whose properties have been examined in only a limited way. We systematically screened them with a panel of ∼500 odorants, yielding >10,000 receptor–odorant combinations. We identify for each of 19 receptors an odorant that excites it strongly. The responses elicited by each of these odorants are analyzed in detail. The odorants elicited little cross-activation of other receptors at the test concentration; thus, low concentrations of many of these odorants in nature may be signaled by a single ORN. The receptors differed dramatically in sensitivity to their cognate odorants. The responses showed diverse temporal dynamics, with some odorants eliciting supersustained responses. An intriguing question in the field concerns the roles of different ORNs and receptors in driving behavior. We found that the cognate odorants elicited behavioral responses that varied across a broad range. Some odorants elicited strong physiological responses but weak behavioral responses or weak physiological responses but strong behavioral responses.
The olfactory system of the Drosophila larva achieves remarkable function with minimal structure. It detects and responds to spatial and temporal gradients of odorants, transforming chemical information into navigation via an elegant repertoire of head sweeps, runs, and turns (1–3). Its sophisticated function is based on the activities of 21 olfactory receptor neurons (ORNs), which innervate the dorsal organ of the head and send axons to the antennal lobe of the brain (4). The activities of the ORNs are in turn based on the responses of odor receptors (Ors). Thus, to understand the molecular basis of larval olfactory navigation, it is necessary to understand the function of the receptors.
ORNs together express 25 members of the Or family of odor receptors and the Orco coreceptor (5–8). In each ORN, an Or and Orco together form a ligand-gated ion channel (9–11). Most ORNs express a single Or, although one ORN coexpresses Or94a and Or94b and another ORN coexpresses Or33b and Or47a (7). The significance of this coexpression remains speculative, but the response profiles of some coexpressed adult Ors are additive (12).
The responses of the larval Or repertoire to a limited odorant panel was previously examined in an in vivo expression system known as the empty neuron system (8, 13). With the use of this system, 21 of the larval Ors were found to be functional. However, studies of the larval Or repertoire have been limited not only in the number of odorants examined, but also in their consideration of receptor sensitivity, temporal dynamics, and roles in driving olfactory behavior.
An intriguing question in the biology of a sensory system concerns the equivalency of its primary sensory neurons in driving behavioral output. A priori, activation of different sensory neurons could drive equivalent behavioral responses, particularly in a simple sensory system. Alternatively, different neurons might drive different behavioral responses, particularly if connectivity and downstream processing are complex, as in the olfactory systems of mammals and adult flies (14–16). In Drosophila, much less is known about olfactory processing in the larva than in the adult.
One approach to examining the role of individual ORNs is to drive different individual neurons in a WT olfactory system with odorants, their natural stimuli, which activate them specifically.
Here we carry out a screen of all 21 functional larval Ors to a panel of ∼500 diverse odorants. For each of 19 receptors, we identify an odorant that excites it strongly. These odorants showed little cross-activation of other receptors in a physiological test. The receptors differed dramatically in sensitivity to their most effective odorants. The temporal dynamics of responses exhibit great variation as well, with some showing supersustained responses. The odorants drove behavioral responses that varied across a broad continuum. Some odorants drove weak physiological responses and strong behavioral responses, or strong physiological responses and weak behavioral responses.
Results
Screen for Odorants That Excite Each Or of the Drosophila Larva.
We wished to determine whether, for each ORN of the larval olfactory system, we could identify an odorant that excited the neuron strongly, and, if so, whether it activated the neuron selectively. Toward this end, we examined the 21 larval Ors that were previously found to be functional in the empty neuron system (5–8). In this system, individual Ors are expressed in a mutant neuron of the adult antenna that lacks an endogenous functional Or (8, 13). Odorant responses conferred by the ectopic expression of Ors correspond well to the activities of the ORN in which the receptor is endogenously expressed in a variety of cases, including receptors of Drosophila adults (13, 17) and larvae (8), and of mosquitoes (18).
We carried out a screen of 10,059 odorant–receptor combinations, testing a panel of 479 odorants (Fig. S1) against each of the 21 larval odorant receptors. The odorants were chemically diverse, including esters, acids, aldehydes, ketones, alcohols, pyrazines, aromatics, terpenes, and sulfur compounds, and were screened at a 10−2 dilution (Materials and Methods). The number of odorants used in this screen is much larger than in previous studies of the Drosophila Or repertoire, and there is little overlap with the odorant panels used previously (8, 19).
For 18 of the 21 odor receptors, we identified odorants that elicited strong responses, defined here as ≥150 spikes per second, which is approximately one half the maximal firing rate of this neuron (19), in this initial screen at the tested concentration. No responses of comparable magnitude were identified for Or2a, Or49a, and Or82a in this primary screen and in further testing; however, as Or82a was previously found to respond strongly and selectively to geranyl acetate (19), we added this odorant to our panel. Or2a and Or49a were excluded from further analysis. The other 18 receptors varied a great deal in the number of odorants to which they responded strongly in this primary screen. Some, such as Or45b and Or94b, gave responses of ≥150 spikes per second to a single odorant; at the other extreme, Or42a gave such strong responses to >50 odorants.
For each of these receptors, the odorants that elicited the strongest responses in the primary screen were tested in a secondary screen. If five or fewer odorants elicited responses ≥150 spikes per second from a particular receptor, all were tested against that receptor; otherwise, we selected the five odorants that elicited the strongest responses in the primary screen. In the secondary screen, the odorants were tested at lower concentrations, including 10−4 and 10−6 dilutions (3 ≤ n ≤6). From this secondary screen, we identified for each receptor the odorant that elicited the strongest response at lower concentrations (Fig. 1; also detailed later). For convenience, we refer to these odorants as “most effective” or “cognate” odorants, but we emphasize that these terms are not meant to imply that individual receptors have evolved to detect these odorants nor that they respond solely to them. Rather, the terms are used to designate the odorants identified among those tested in our screen as the most effective in activating each receptor.
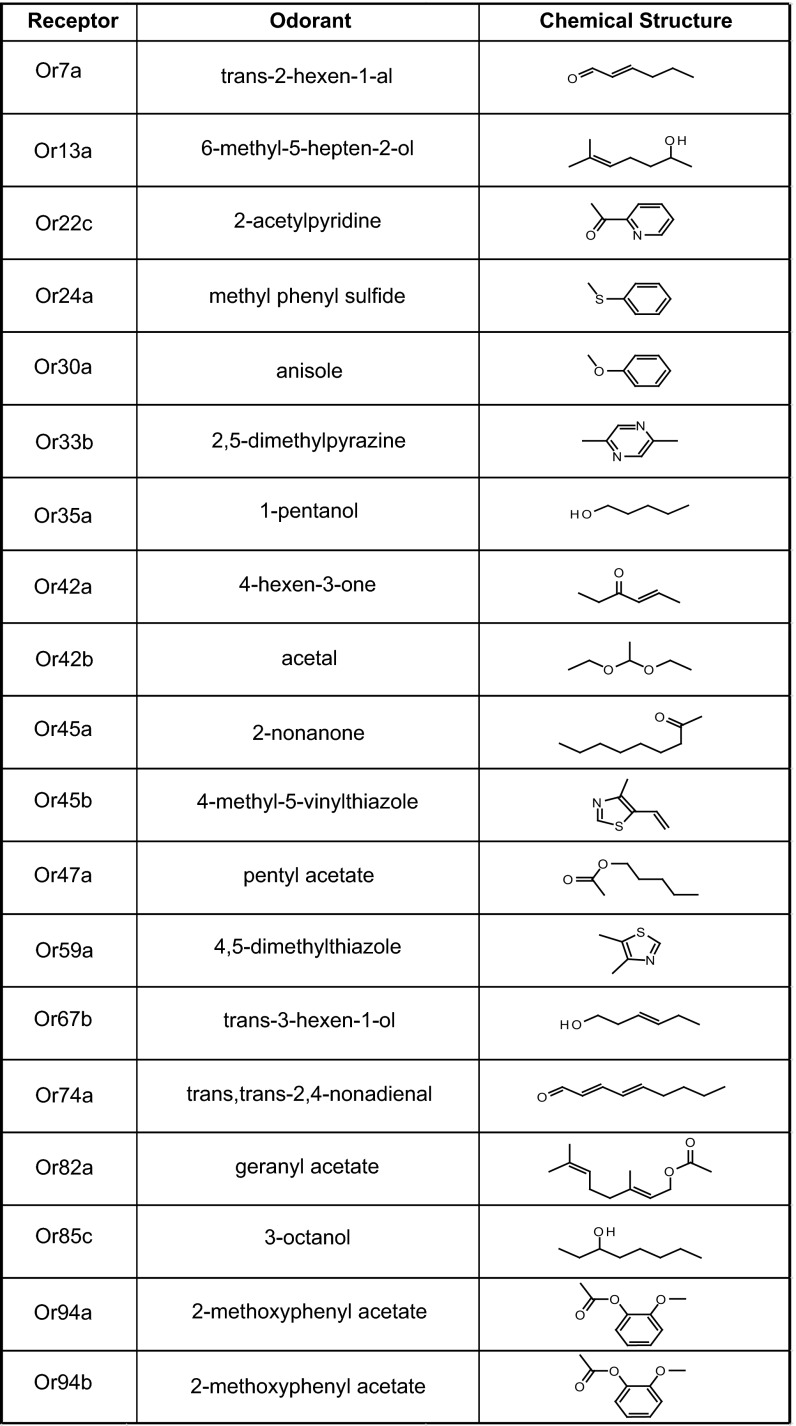
Fig. 1.
Cognate odorants for larval Ors. No strong odorants were identified for Or2a or Or49a. The same odorant was identified for Or94a and Or94b.
We note that the goal of the screening was not to characterize the coding properties of the receptors but to identify as economically as possible, from among a large odorant panel, a selected set of odorants that excite strongly each individual Or of the larva. Accordingly, the analysis here differs from most previous studies of the Or repertoire in that it considers in detail a set of 19 odorants, each identified by its strong activation of one member of the receptor repertoire.
The odorants are highly diverse, including a variety of aliphatic and aromatic compounds. They include alcohols, aldehydes, ketones, esters, a pyridine, a pyrazine, a terpene, and three sulfur-containing compounds. Many have been found in fruits, fungi, or yeast, and some play a role in insect chemical communication (20–24).
One odorant, 2-methoxyphenyl acetate, was independently identified for two different receptors, Or94a and Or94b. The Or94a and Or94b genes are closely related phylogenetically, they lie less than 1 kb apart in the genome, and they are coexpressed in the same larval ORN. The expression of two related receptors that respond strongly to the same odorant in the same neuron suggested the possibility that the two receptors play different roles in odor coding in the same ORN, a possibility that we consider later.
The independent identification of the same odorant for two closely related receptors raised the question of whether the same odorant would be identified if one particular receptor were independently screened twice against the panel of 479 odorants. The efficiency of the screen was of interest because the unprecedented number of receptor–odorant combinations examined (>10,000) allowed each combination to be tested only once (n = 1) in the primary screen, and we expected false-positive and false-negative findings. Accordingly, one receptor, Or7a, was screened twice, independently, against the entire odorant panel. We compared the set of 20 odorants that elicited the greatest response in the first repetition to the set of 20 that was identified in the second repetition, and found that 16 odorants were common to both sets. We conclude that, in the case of a few receptors, a more labor-intensive screen of the ∼500 odorants would likely have identified an odorant that activates it more strongly. Moreover, a screen of unlimited dimension would likely have identified many additional odorants of great interest. However, the screen described here of ∼10,000 odorant–receptor combinations was successful in identifying a set of odorants that strongly activate nearly each member of the larval Or receptor repertoire, providing a foundation for the analysis described here.
The Most Effective Odorants Elicit Strikingly Different Physiological Responses, Some Supersustained.
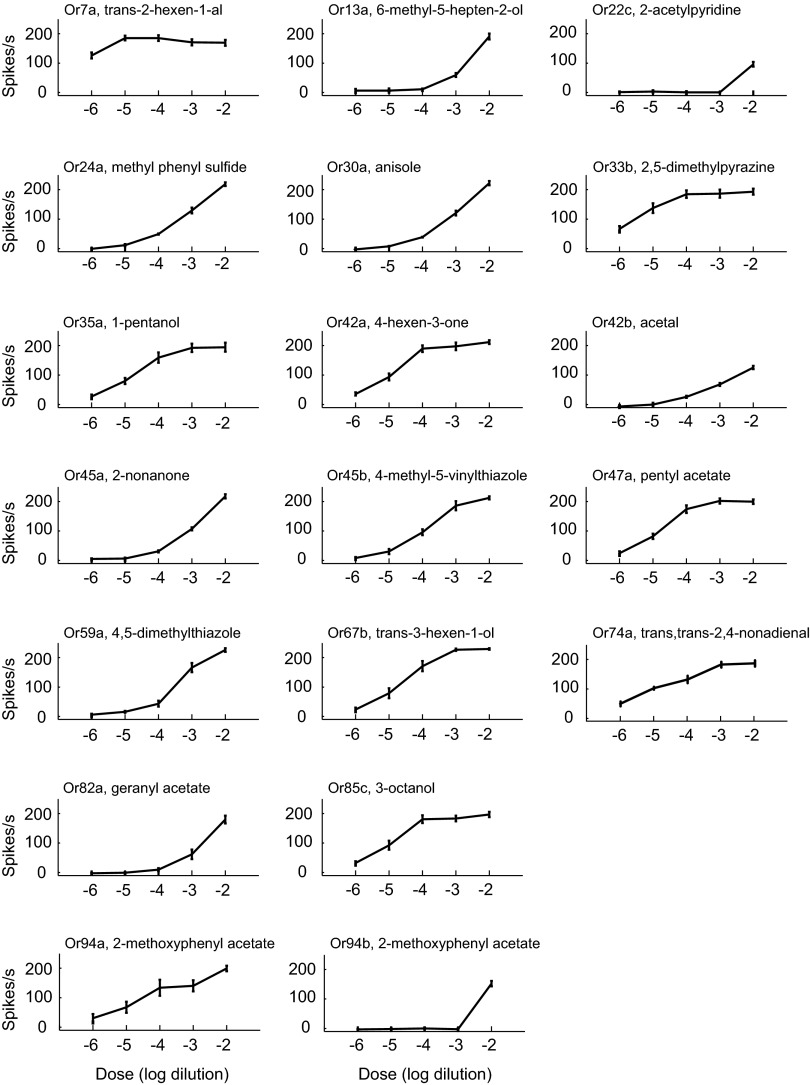
For each of the 19 receptor–odorant pairs, we systematically examined the responses across a broad range of concentrations (Fig. 2). The receptors showed dramatic differences in sensitivity to their cognate odorants. For example, the detection threshold of Or22c for its odorant was between a 10−3 dilution and a 10−2 dilution, whereas the threshold of Or7a for its odorant was at least four orders of magnitude lower. Thus, the receptors differ not only in the magnitudes of the responses to their cognate odorants at a 10−2 dilution, but also in their sensitivities to these odorants.
Fig. 2.
Dose–response analysis for each Or and its most effective odorant (n = 6; SEM).
Or94a was more sensitive to 2-methoxyphenyl acetate than was Or94b by at least three orders of magnitude. The coexpression of these two receptors in an ORN may enhance the precision with which the neuron can evaluate the level of 2-methoxyphenyl acetate across a broad concentration range. It is also conceivable that the two receptors have evolved to perform different functions, perhaps Or94a in navigating toward a source of 2-methoxyphenyl acetate and Or94b in signaling when methoxyphenyl acetate levels have crossed a threshold and are so high as to represent potential toxicity. The ORN expressing both receptors may send a stronger signal to the CNS at high concentrations when both receptors are activated, although more complicated models are also possible. Another possibility is that Or94b has diverged to detect an odorant that was not tested in this study but has retained some affinity for 2-methoxyphenyl acetate.
The various receptors also differed markedly in that the responses of some did not reach saturation even at the highest concentrations tested, whereas others, such as Or7a and Or33b, reached saturation, in some cases at 10−4 dilutions or even lower doses.
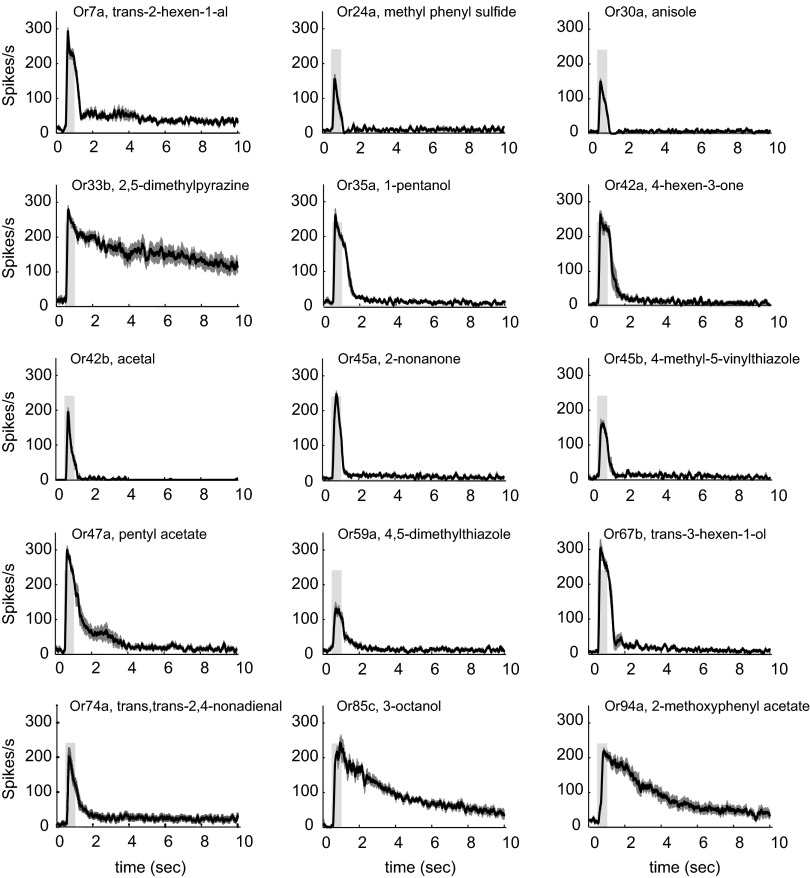
The temporal dynamics of ORN responses have been found to vary (25–28), but have not been systematically examined across an entire receptor repertoire. We found that the temporal dynamics differed strikingly among the strong odorant–receptor combinations (Fig. 3). We measured the temporal dynamics of the responses to a 0.5-s pulse of 10−4 dilutions, testing the 15 odorant–receptor combinations whose response thresholds were below a 10−4 dilution. Some combinations, such as (Or45a, 2-nonanone), elicited a phasic response that peaked and decayed quickly to baseline or to a level near baseline. By contrast, (Or33b, 2,5-dimethylpyrazine), (Or85c, 3-octanol), and (Or94a, 2-methoxyphenyl acetate) showed a more gradual decline toward the baseline. This identification of the long-lasting Or33b response provided a basis for a study showing that Or33b yields “supersustained” responses from a number of pyrazines, that the responses are odorant- and receptor-specific, and that those responses examined in detail do not arise solely because of adherence of the odorants to the tubing of the delivery system (29).
Fig. 3.
Temporal dynamics of responses of receptors to their most effective odorants. Peristimulus time histograms are shown for the 15 odorant–receptor combinations that yielded responses above background at 10−4 dilutions. The light gray bar indicates the 500-ms odorant pulse. SEM is indicated as gray bars (n = 6–8).
Some of the odorant–receptor combinations that showed the most conspicuously slow declines showed high sensitivity and saturation in the dose–response analysis of Fig. 2, such as (Or33b, 2,5-dimethylpyrazine) and (Or85c, 3-octanol). However, high sensitivity and saturation may not be sufficient conditions for such gradual declines, as evidenced by (Or42a, 4-hexen-3-one), which shows sensitivity and saturation but shows a relatively quick decline toward baseline.
Specificity of Strong Responses.
Having identified a set of odorants for the set of receptors, we next asked whether the strong responses among these odorant–receptor combinations were unique. First, does each member of the set of odorants strongly activate a unique receptor? Second, is each receptor strongly activated by a unique member of the odorant set? These questions were motivated in part by an interest in whether some of the strong odorants we identified were signaled via a unique information channel, and whether some of the receptors have evolved to signal the presence of cues of particular biological importance (30).
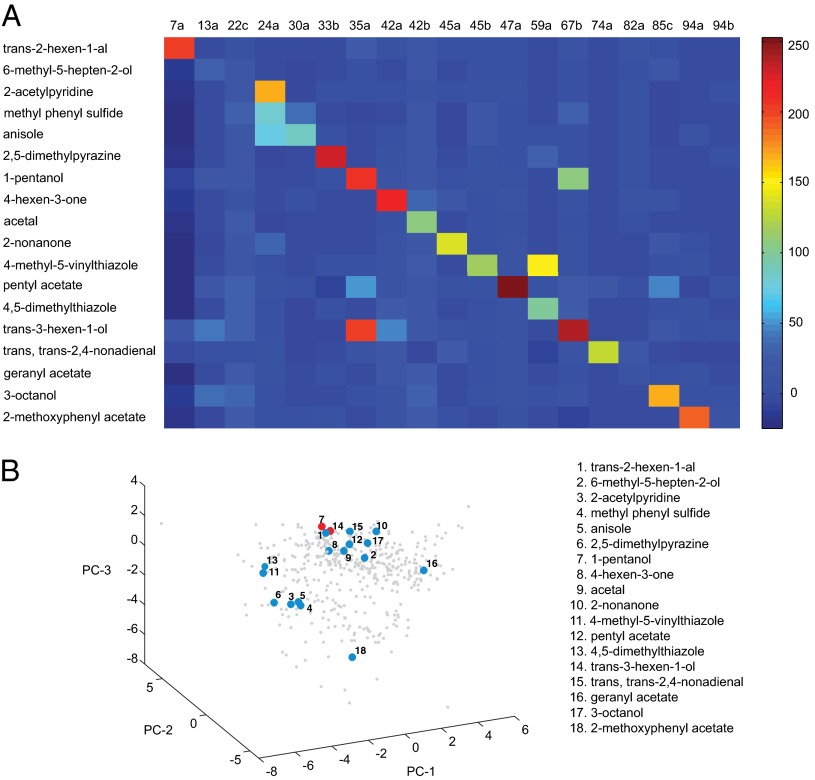
We tested the entire matrix of receptor–odorant combinations at odorant dilutions of 10−4, two orders of magnitude lower than the dose used in the initial screen. One reason for focusing on this lower dosage was that it might contribute valuable information about the responses of receptors to dosages experienced in the natural environment. The matrix reveals a diagonal pattern of activation (Fig. 4A and Dataset S1), reflecting responses of the receptors to their cognate odorants, with several notable exceptions.
Fig. 4.
Responses of receptors to the panel of most effective odorants. (A) Responses of each receptor to the panel of 18 odorants, each tested at a 10−4 dilution. Each value represents the mean response during a 0.5-s odor stimulation period. Spontaneous activity and response to solvent alone have been subtracted (6 ≤ n ≤10). (B) Mapping of the most effective odorants (large dots) and the other 461 odorants (small gray dots). Shown are the first three principal components (PCs) of the 32-dimensional odor space of Haddad et al. (31). Physicochemical descriptors were normalized. Variances explained by PC1, PC2, and PC3 are 17%, 13%, and 12%, respectively.
Some receptors, such as Or13a, Or22c, Or82a, and Or94b, yielded little or no response to their cognate odorants at 10−4 dilutions, consistent with the lack of responses that were observed at 10−4 dilutions in the dose–response analysis in Fig. 2. These are the four receptors that have the highest thresholds for their respective odorants. In two other cases, Or24a and Or59a, responses to the odorants are observed at 10−4 dilutions, but even stronger mean responses are observed to two other odorants: 2-acetylpyridine for Or24a and 4-methyl-5-vinylthiazole for Or59a. As the 10,059 odorant–receptor combinations were tested only once in the primary screen, it was expected that some strong odorants would elude identification as a result of stochastic variation in responses. It is also possible that some odorants that were not identified as the most effective odorant for a particular receptor elicit greater responses from the receptor at a 10−4 dilution than at a 10−2 dilution.
One receptor, Or35a, showed strong responses to two odorants at 10−4 dilutions: 1-pentanol and trans-3-hexen-1-ol (Fig. 4A). These odorants are structurally similar, as determined by mapping them in a 32-dimensional odorant space in which each dimension represents a different descriptor of chemical structure, such as aromaticity index (31). They mapped close to each other (Fig. 4B); the Euclidean distance between 1-pentanol and trans-3-hexen-1-ol was 1.83 arbitrary units (a.u.), whereas the mean distance between all pairwise combinations of the 18 odorants was 6.74 ± 2.1 a.u. (±SD). We note that 1-pentanol and trans-3-hexen-1-ol are also the two odorants that elicit the strongest responses from another receptor, Or67b. Finally, the map shows the distribution of all 479 odorants used in the screen and reveals that the most effective odorants are distributed broadly among them in odor space.
Strong Activators of Different Receptors Drive Behavioral Responses of Different Strengths.
Having identified odorants that strongly activated most of the individual receptors of the system, we wondered how many of the strong activators drive strong behavioral responses. We systematically tested each of the 18 odorants, initially by using a classic two-choice behavioral paradigm (32, 33). In this paradigm, ∼50 third-instar larvae are placed in the middle of an agarose Petri plate of 9-cm diameter. Two filter discs are placed diametrically opposed to one another, with one disk containing a drop of odorant diluted 10−2 and the other serving as a control. Larvae are allowed to migrate on the plate, and, after a 5-min test period, the number on each half is counted and a response index (RI) is calculated as RI = (S − C)/(S + C), where S is the number on the half of the plate containing odorant and C is the number on the half containing the control disk. If all larvae migrate to the side containing the odor, RI is equal to 1; if the larvae are indifferent to the odor, RI is equal to 0. We note that the doses used in such a behavioral assay are difficult to compare with those in the physiological assay as a result of differences in airflow, duration, and geometry.
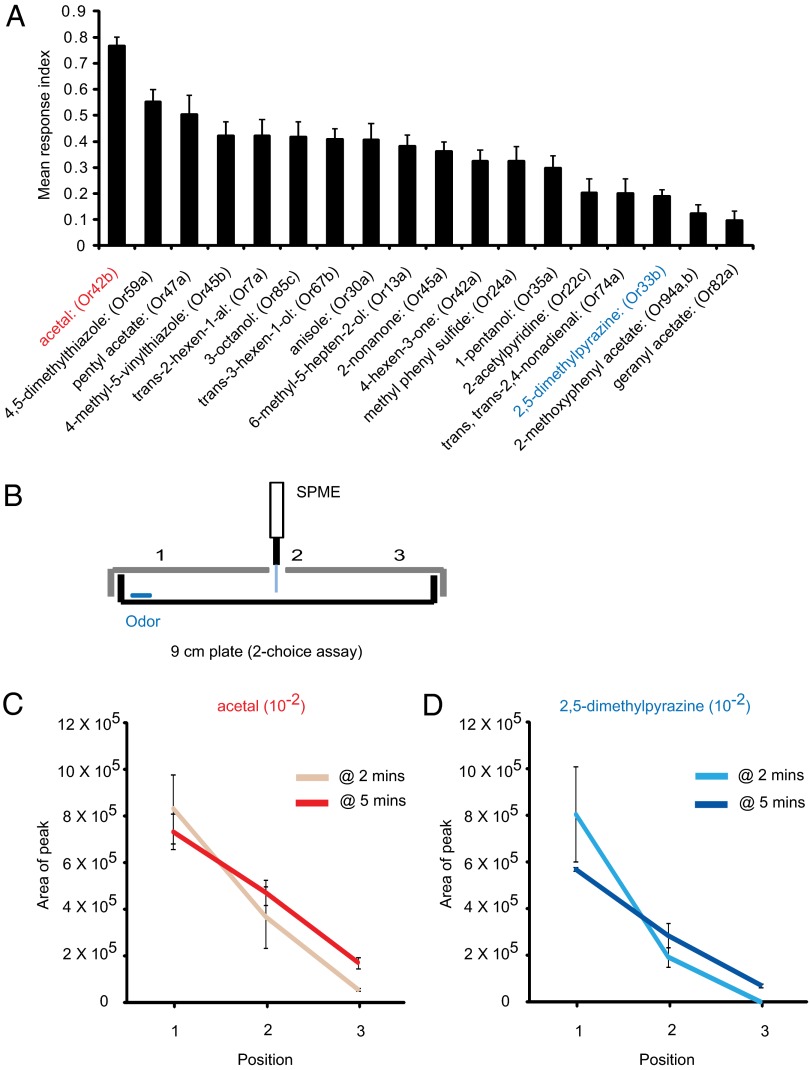
We found that the behavioral response indices varied widely across a continuum (Fig. 5A). Acetal, a strong activator of Or42b, drove the strongest response: 0.76 ± 0.03 (SEM; n = 8). At the other end of the continuum, geranyl acetate, an activator of Or82a, elicited little if any response: 0.09 ± 0.03 (SEM; n = 8). Odorants such as 2,5-dimethylpyrazine and 2-methoxyphenylacetate are capable of eliciting strong physiological responses from receptors across a broad range of concentrations (Or33b and Or94a, respectively; Fig. 2) but did not elicit strong behavioral responses in this behavioral test.
Fig. 5.
Larval behavior in the two-choice small-format paradigm. (A) Cognate odorants elicit a range of behavioral responses. Odorants were tested at a 10−2 dilution. Each bar represents RI ±SEM (n = 8). Responses differ; for example, the response to 2,5-dimethylpyrazine differs from the responses to the eight odorants with the greatest RIs (ANOVA followed by Bonferroni post hoc test; P < 0.05). (B) Extraction of odorants for assessment of gradients. The SPME fiber was inserted at the indicated positions and odorants were extracted with SPME. The disk contains odor at a dilution of 10−2. (C and D) Assessment of gradients with GC-MS at 2 min and 5 min for acetal (C) and 2,5-dimethylpyrazine (D). Areas under GC-MS peaks are indicated to allow comparisons of levels of a particular odorant at different positions in the gradient (n = 3).
Thus, although 2,5-dimethylpyrazine elicited a greater physiological response from its receptor (Or33b) than acetal elicited from its receptor (Or42b) across a wide range of concentrations (Fig. 2), 2,5-dimethylpyrazine elicited a weaker behavioral response than acetal in this paradigm. Moreover, neither odorant elicited a strong physiological response from any other receptors at the concentration that was analyzed in detail (Fig. 4A). Finally, 2,5-dimethylpyrazine was tested behaviorally at a 10−4 dilution and again elicited a weak response, 0.11 ± 0.02 (n = 8); acetal at a 10−4 dilution again elicited a stronger response, 0.50 ± 0.03 (n = 8).
One possible explanation for the differences in behavioral responses is that different odorants may form gradients in the behavioral paradigm that differ in their stability over the course of the 5-min test period. We analyzed the relative levels of acetal at three different positions in the behavioral arena after 2 min and 5 min by using solid-phase microextraction (SPME) and GC-MS (Fig. 5B). We carried out the same analysis for 2,5-dimethylpyrazine.
Our results showed that a gradient of acetal was detectable after 2 min, that a gradient was also detectable after 5 min, and that the slopes of the acetal gradients were comparable at these two times (Fig. 5C). Likewise, 2,5-dimethylpyrazine showed a detectable gradient after 2 min and 5 min, and again their slopes did not differ dramatically at the two times (Fig. 5D). Thus, differences in the stabilities of the acetal and 2,5-dimethylpyrazine gradients seem unlikely to account solely for the difference in behavioral responses.
In the SPME/GC-MS analysis, the peak area values for the two odorants are similar (Fig. 5 C and D); however, odorants may differ in their adsorption to the SPME fibers, or to the agarose or plastic of the Petri plate, and we do not know whether the molecular gradients formed by the two odorants in the behavioral arena are similar. We note finally that there are pairs of odorants in our analysis, such as pentyl acetate and 2,5-dimethylpyrazine, that have similar vapor pressure (3.9 mm Hg, 3.9 mm Hg) and that elicit different response indices (0.50 ± 0.07, 0.19 ± 0.02; P < 0.001, two-sample t test).
Navigation Elicited by a Strong Activator Depends on its Cognate Receptor.
We wished to test further the in vivo functional significance of our physiological results. To extend the study, we used a different behavioral paradigm that permits analysis of larval navigation, with a special interest in determining whether an odorant that strongly activated a single receptor in our physiological analysis elicited navigation via that receptor.
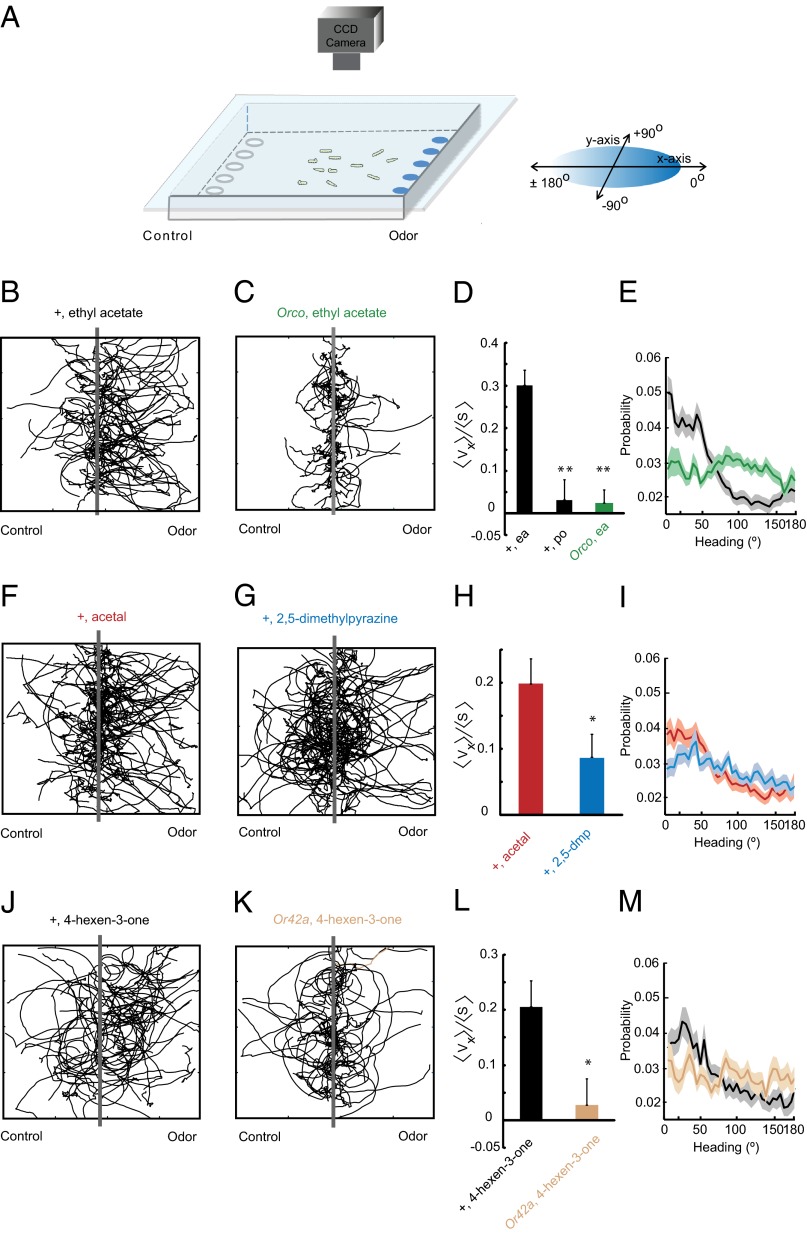
We developed a behavioral paradigm that allows tracking of the navigational trajectories of individual larvae. In this paradigm, a population of third-instar larvae is allowed to migrate toward an odor source on a square 22-cm × 22-cm agarose plate (Fig. 6A). Five filter discs containing odorant are placed at even intervals along one wall of the plate, and ∼20 larvae are placed along the midline of the plate parallel to the wall adjacent to the odorant. A CCD camera records their movement for 5 min, and their positions are analyzed as a function of time. This paradigm was inspired by earlier studies of larval navigational behavior (2, 34). Gradients formed in our assay may not be stable for long periods of time, an important feature of the system of Gershow et al. (2), but our paradigm uses an arena of the same dimensions and offers the virtue of simplicity.
Fig. 6.
Larval navigation. (A) Paradigm containing a 22-cm × 22-cm square agarose Petri plate. Odorant is placed on discs at the right; paraffin oil diluent alone is placed on discs to the left. The chamber is sealed by placing a clear glass plate over the arena. Third-instar larvae are placed along the central y axis parallel to the line of odor discs. Movement of larvae is recorded with a CCD camera. (B and C) Trajectories of WT (B) and Orco (C) larvae in response to ethyl acetate, neat. Gray bars along the y axis indicate starting positions of larvae (n = 84 tracks were analyzed for CS WT in six assays; n = 81 tracks in six assays for Orco). (D) navigational indices of indicated genotypes to ethyl acetate (ea) and paraffin oil. (E) Relative probabilities of orientations along the trajectories of WT (black) and Orco (green). Directions refer to those illustrated in A. The probability indicated for each angle θ is the sum of the probabilities for θ and −θ. (F) Trajectories of WT larvae to acetal (F) and 2,5-dimethylpyrazine (G), both neat (n = 111 tracks for acetal in eight assays; n = 110 tracks for 2,5-dimethylpyrazine in eight assays). (H) Navigational indices of WT to acetal and 2,5-dimethylpyrazine. (I) Relative probabilities of orientation. (J and K) Trajectories of WT and Or42a; odorant is 4-hexen-3-one, diluted 10−2 in paraffin oil (n = 68 tracks in six assays for WT; n = 43 tracks in six assays for Or42a). (L) Navigational indices of indicated genotypes to 4-hexen-3-one. (M) Relative probabilities of orientation of WT and Or42a; odorant is 4-hexen-3-one.
As an initial test of the method, we used ethyl acetate, which was used by Gershow et al. (2), and confirmed that it attracts larvae in our assay (Fig. 6B). To quantify this attraction, we used the navigational index <vx>/<s> (2), in which the mean velocity of larvae in the x direction, <vx>, is divided by the mean crawling speed, <s>. Thus, the index is 1 if all larvae migrate uniformly toward the odor source and 0 if their movement is random. As a control we tested the Orco mutant, which lacks an essential coreceptor of Or genes, and found that <vx>/<s> was severely reduced, to the level elicited by the paraffin oil diluent alone (Fig. 6 C–E).
We then tested acetal and 2,5-dimethylpyrazine and found that both acted as attractants (Figs. 6 F–I). <vx>/<s> was greater for acetal (P < 0.05, t test), consistent with the greater attraction to acetal observed in the two-choice paradigm (Fig. 5A).
Having thus validated the paradigm in this manner, we then examined the navigation of larvae toward 4-hexen-3-one, an odorant that strongly activated a single receptor in our physiological analysis, Or42a, a receptor for which a mutant is available (Fig. 4A). We compared the behavior elicited by 4-hexen-3-one in the presence and absence of Or42a. The Or42a mutation had been backcrossed against our w Canton-S (wCS) strain 10 times. Both strains were tested behaviorally against a 10−2 dilution of 4-hexen-3-one. If 4-hexen-3-one strongly activates Or42a, and only Or42a, in vivo, as in the empty neuron, one would predict that the behavioral response to 4-hexen-3-one would be severely reduced in the Or42a mutant.
The Or42a mutant in fact showed a much lower navigational index (P < 0.05, two-sample t test) than the control strain (Figs. 6 J–M). These results are consistent with the notion that 4-hexen-3-one, a strong activator of Or42a in the empty neuron, also activates Or42a in its endogenous neuron. Moreover, it is striking that there is little if any residual behavioral response of the Or42a mutant to 4-hexen-3-one under these conditions, consistent with the low levels of physiological response from other receptors in the empty neuron (Fig. 4A).
Discussion
We have identified odorants that strongly activate nearly all the larval Ors. Some of these odorants activate individual receptors much more strongly than any odorants identified in earlier studies. It is clear that the receptor repertoire has evolved strong responses to a wide diversity of odorants distributed broadly in a chemically defined odor space.
The odorants considered here in detail were identified, from a collection of ∼500 chemically diverse odorants, by the strength of the responses they elicited from their respective receptors at successively decreasing concentrations. When the most effective odorants were tested against the entire receptor repertoire at a 10−4 dilution, responses were sparse (Fig. 4A). Thus, if these odorants have particular significance to the animal at low concentrations, the significance is likely conveyed by a very small number of ORNs.
There remain two Ors for which no strong activators are known, Or2a and Or49a. It is formally possible that these receptors require another component that is not present in our test system. However, we note that, before this screen, no strong activators were known for Or33b, and we identified a strong activator of Or33b by expanding the test panel to include a pyrazine, allowing an analysis of the coding of pyrazines (29). Strong activators of Or2a and Or49a might be identified by screening other kinds of odorants, such as long-chain pheromones, or by fractionation and analysis of complex odor sources from the larval environment, such as fermenting fruits or predators. Larvae of the cotton leafworm Spodoptera littoralis were recently found to respond to a sex pheromone of the adult stage (35). In Drosophila, the adult receptor Or56a, for which no ligand had previously been identified, was recently shown to be strongly and selectively activated by geosmin, an odorant emitted by microbes that are detrimental to the fly (30).
There is great variation in the dose–response relationships for the 19 receptor–odorant combinations. Some receptors are exquisitely sensitive to their most effective odorants, whereas others respond to their odorants only at high concentrations. Physicochemical properties of the odorants do not alone dictate the dose–response relationships: one odorant, 2-methoxyphenyl acetate, was identified as the odorant for two receptors, Or94a and Or94b, and it activated one at low concentrations and the other only at high concentrations. We do not know whether receptors that respond to their odorants only at high concentrations have evolved to encode these odorants, or whether they respond more sensitively to other unidentified odorants.
It is possible that the sensitivities of some receptors have evolved to reflect the concentrations of their odorants in natural environments. For example, the high sensitivity of Or7a to trans-2-hexen-1-al could reflect the importance of detecting this odorant in contexts in which it is scarce.
In our physiological screen, we initially tested odorants at 10−2 dilutions. For some purposes, it would be highly informative to adjust the doses to compensate for differences in the physicochemical properties of the odorants, such that, for each odorant, an equivalent number of molecules reached the antenna (27, 36). It is more difficult to compensate for differences in the coefficients that dictate how many molecules partition from the air into the cuticle and from the cuticle into the sensillum lymph, or to compensate for differences in transport to receptors at the ORN membrane. Given these difficulties, we have found it simplest to test dosages at standard dilutions, with the understanding that the results describe responses to odorants at defined dilutions and not to defined numbers of odorant molecules accessible to receptors.
Our analysis of temporal dynamics was likewise conducted at a standard test dilution, 10−4. A wide variety of temporal dynamics was observed. The results illustrate that the identity of a stimulus at a particular concentration in the natural environment can be encoded not only by the identity of the responding receptors but also by the temporal dynamics of their responses (25–27). This analysis also provides a foundation for further studies in which the stimulus intensities may be adjusted to compensate for properties of the odorants or their receptors.
Each of the odorants examined physiologically was also tested behaviorally. Despite differences in the physical and biological parameters of the physiological and behavioral paradigms, navigational behavior driven by one of the odorants, 4-hexen-3-one, was found to depend on the cognate receptor identified in the empty neuron system. Thus, 4-hexen-3-one elicits a strong physiological response from one and only one receptor in the empty neuron system, and 4-hexen-3-one drives a behavioral response if and only if that receptor is present in the larva. These results provide additional validation of the empty neuron system as a means of analyzing larval odor receptors.
The set of odorants used in this study differs from those used in most other studies in that they were selected by virtue of the strong physiological responses that each elicited from individual members of the receptor repertoire. Behavioral testing revealed a wide range of responses to the odorants in the classic two-choice paradigm. The strongest response was to acetal, which yielded a mean RI greater than 0.75, whereas the weakest response was to geranyl acetate, which elicited a mean RI less than 0.1. We note that an earlier study observed a repellent effect of geranyl acetate (8); we do not know the basis of this difference, but repellency has been found to be sensitive to larval age (37) and could also be sensitive to other factors that differed between the two studies (38). Aversive responses to CO2 are mediated by neurons of another larval organ, the terminal organ, that coexpress Gr21a and Gr63a (2, 39–42), and it is possible that other unidentified neurons also mediate airborne aversive responses.
The relationship between the physiological and behavioral responses elicited by the odorants is striking. Having screened almost 500 odorants for each receptor, and having carried out dose–response analysis with a strong odorant for each receptor, we identified odorants that elicit strong responses from their cognate receptors across a broad range of concentrations. However, some of these strong odorants, such as 2,5-dimethylpyrazine, elicit only a weak behavioral response, even when presented at concentrations that seem likely to exceed those found in the larva’s natural environment.
By contrast, the strongest response in the two-choice paradigm was to an odorant that produced one of the weakest responses in the physiological analysis. Acetal elicits a weaker physiological response than 2,5-dimethylpyrazine at every concentration tested, yet elicits stronger behavioral responses than 2,5-dimethylpyrazine in each of two behavioral paradigms tested.
Thus, some odorants appeared to elicit strong physiological responses but weak behavioral responses in our paradigms, whereas others elicited relatively weak physiological responses but strong behavioral responses. It is possible that these differences arise from differences in parameters of stimulus presentation, in the access of odorants via cuticle and lymph to receptors, or in the adaptation that the odorants elicit from ORNs. We note also the formal possibility that receptors not considered in this analysis contribute to the strong behavioral response to acetal.
Another possible interpretation concerns the functional organization of the system. The larval olfactory system is numerically much simpler than that of mammals or the adult fly, but little is known about how the information carried by individual larval ORNs contributes to behavior. One interpretation of our results is that the ORN expressing Or42b, which is activated by acetal, and the ORN expressing Or33b, which is activated by 2,5-dimethylpyrazine, do not belong to a single equivalence class; that is, they may play distinguishable roles in driving olfactory behavior. Although our results support a model in which many ORNs can activate an attraction response, their connectivity may not be functionally identical (43). Different ORNs may contribute differently to the modulation of the runs and turns of navigation behavior or to feeding decisions. Diverse odors in the natural environment of the larva provide diverse information about the quality of the environment. Differences among ORNs and their connectivity might provide a mechanism for translating different signals into different behavioral output. The present analysis of olfactory function in the periphery of this tractable model system may be useful in elucidating the circuitry by which such translation occurs.
Materials and Methods
Drosophila Stocks.
The stocks used for electrophysiology, including the Or22a-GAL4 flies, the 21 UAS-Or lines, and the ∆halo mutant flies, which lack a functional ab3A neuron, were described previously (5, 13, 17). Electrophysiological recordings were obtained from flies of either sex of genotype w;∆halo/∆halo;Or22a-GAL4/UAS-Or.
All behavioral experiments were performed on third instar larvae of either sex. The Canton-S (CS) line was used as a WT control in behavioral experiments. The mutant alleles of Or42a (stock no. 18758) and Orco1 (stock no. 23129) were obtained from the Drosophila Stock Center (Bloomington, IN). Mutant lines were backcrossed to a wCS line for 10 generations.
Electrophysiology.
All recordings were conducted as previously described (13, 17). For the screen of 10,059 odorant–receptor combinations, odorant stimuli were presented by using 3-mL syringes fitted with a 200-μL micropipette tip, each syringe containing 250 μL of an odorant diluted in paraffin oil (10−2 vol:vol) on a Whatman 55-mm filter paper disk (Millipore). Chemicals were of the highest purity available from SAFC, a subsidiary of Sigma-Aldrich.
For subsequent electrophysiological analysis, 50 μL of an odorant diluted 10−2, 10−3, 10−4, 10−5, or 10−6 in paraffin oil (vol:vol) were placed on Whatman 13-mm filter paper discs and inserted in Pasteur pipettes. These cartridges were prepared shortly before odor presentation and were never used more than three times each. All electrophysiological data were analyzed as described previously (13). Mean spontaneous activity and mean response to diluent alone were subtracted from each odor response for each receptor. To quantify response dynamics, spikes were sorted by using a custom MATLAB routine (MathWorks).
All data analysis was performed by using MATLAB. Physicochemical odor space was constructed by using a set of 32 optimized DRAGON descriptors (31) (DRAGON for windows, version 5.5, 2007; TALETE). Descriptors were normalized by their variance estimated for the full data set of 479 odorants. Principal-component analysis was performed by using the MATLAB routine “princomp.”
Behavior.
The two-choice Petri dish assay was conducted essentially as described previously (8, 32). Briefly, two filter paper discs were placed diametrically opposed to each other on a thin layer of 1.1% agarose (wt/vol) in a 10-cm Petri dish. Approximately 50 third-instar larvae were placed in the center of the dish and allowed 5 min to migrate, after which the RI was calculated.
The tracking assay was carried out on a layer of 1.5% agarose in a 22-cm × 22-cm square Petri dish. Five filter paper discs were placed equidistant from each other on two opposing sides of the dish. Approximately 20 third-instar larvae were placed in the center of the dish along a line parallel to the discs. Video microscopy of larvae within the experimental arena was performed by using dark-field illumination with red LEDs (850 nm, outside the range of larval phototaxis). Images were recorded at 2.3 frames per second by using a Monochrome CCD Firewire camera (Stingray F-504B; E0010066; Graftek Imaging) fitted with an IR long-pass 830-nm filter (LP830-30.5; Midwest Optical Systems) and an 8-mm focal length C-mount lens (M0814-MP2; Computar Lens). Each pixel in the captured image corresponded to a 0.119-mm × 0.119-mm square of the experimental arena.
Positions of larvae were extracted from video recordings by using custom routines written in MATLAB. The RI <Vx>/<s> was defined as the mean velocity of the larva in the x direction (<Vx>) divided by the mean crawling speed (<s>) as described previously (2).
SPME/GC-MS.
Commercially available SPME fibers [Stable Flex, 65 µm, poly(dimethylsiloxane)/divinylbenzene coating] suitable for volatile analysis (Supelco) were used for this study. To analyze odor gradients, experimental conditions including stimulus concentration and presentation methods were recreated for each SPME sampling. Gradients were allowed to form and measurements were performed at 2 min and at 5 min. At each time point, an SPME fiber was inserted into the headspace of the behavioral arena, always at the same height, at three different positions in the odor gradient (0 cm, 4 cm, and 8 cm from the odor source). Adsorption was allowed for 10 s.
SPME fibers were desorbed at 275 °C for 10 s in the injection port of a model QP2010S gas chromatograph/mass spectrometer (Shimadzu) with a DB-5ms GC column (30-m length, 0.25-mm i.d., 0.25-µm film thickness). The injection port was operated in splitless mode with a constant He flow of 1 mL/min. The initial oven temperature was 40 °C, held for 2 min, ramped at 35 °C min−1 to 275 °C, and then held at 275 °C for 2 min. The Shimadzu mass spectrometer was operated in the electron ionization mode at 70 eV, a source temperature of 250 °C, and interface temperature of 275 °C, with a continuous scan from m/z 45 to 300.
Highly pure chemicals were used in our studies, and single peaks were obtained for each odorant tested. The peaks were quantified using the real-time analysis software GCMSsolution, version 2.70′, (Shimadzu). Samples were run in triplicates and integrated areas were plotted in Excel (Microsoft).
Supplementary Material
Acknowledgments
We thank Zina Berman and Paul Graham for many forms of support, Kathleen Prudic for advice on solid-phase microextraction/GC-MS and Frédéric Marion-Poll for comments on the manuscript. This work was supported by grants from the National Institutes of Health (NIH; to J.R.C.); C.M. and T.E. were supported by a Whitehall Foundation Research Award (to T.E.). A.D.T.S. and M.G. were supported by an NIH Pioneer Award to A.D.T.S. and a Charles King Trust Postdoctoral Fellowship to M.G.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306976110/-/DCSupplemental.
References
- 1.Luo L, et al. Navigational decision making in Drosophila thermotaxis. J Neurosci. 2010;30(12):4261–4272. doi: 10.1523/JNEUROSCI.4090-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gershow M, et al. Controlling airborne cues to study small animal navigation. Nat Methods. 2012;9(3):290–296. doi: 10.1038/nmeth.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez-Marin A, Stephens GJ, Louis M. Active sampling and decision making in Drosophila chemotaxis. Nat Commun. 2011;2:441. doi: 10.1038/ncomms1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramaekers A, et al. Glomerular maps without cellular redundancy at successive levels of the Drosophila larval olfactory circuit. Curr Biol. 2005;15(11):982–992. doi: 10.1016/j.cub.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Kreher SA, Kwon JY, Carlson JR. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46(3):445–456. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15(17):1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Fishilevich E, et al. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr Biol. 2005;15(23):2086–2096. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59(1):110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato K, et al. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452(7190):1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 10.Wicher D, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452(7190):1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 11.Smart R, et al. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem Mol Biol. 2008;38(8):770–780. doi: 10.1016/j.ibmb.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Ray A, van Naters Wv, Shiraiwa T, Carlson JR. Mechanisms of odor receptor gene choice in Drosophila. Neuron. 2007;53(3):353–369. doi: 10.1016/j.neuron.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobritsa AA, van der Goes van Naters WM, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37(5):827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 14.Su CY, Menuz K, Carlson JR. Olfactory perception: Receptors, cells, and circuits. Cell. 2009;139(1):45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr Biol. 2009;19(16):R700–R713. doi: 10.1016/j.cub.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RI, Mainen ZF. Early events in olfactory processing. Annu Rev Neurosci. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]
- 17.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117(7):965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 2010;464(7285):66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125(1):143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 20.Mattheis JP, Fan X, Argenta LC. Interactive responses of gala apple fruit volatile production to controlled atmosphere storage and chemical inhibition of ethylene action. J Agric Food Chem. 2005;53(11):4510–4516. doi: 10.1021/jf050121o. [DOI] [PubMed] [Google Scholar]
- 21.Tabata J, De Moraes CM, Mescher MC. Olfactory cues from plants infected by powdery mildew guide foraging by a mycophagous ladybird beetle. PLoS ONE. 2011;6(8):e23799. doi: 10.1371/journal.pone.0023799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rezanka T, Prell A, Sigler K. Identification of odorous compounds from nine fermentor-cultivated Streptomyces strains. Folia Microbiol (Praha) 2008;53(4):315–318. doi: 10.1007/s12223-008-0049-3. [DOI] [PubMed] [Google Scholar]
- 23.Leal WS. Chemical ecology of phytophagous scarab beetles. Annu Rev Entomol. 1998;43:39–61. doi: 10.1146/annurev.ento.43.1.39. [DOI] [PubMed] [Google Scholar]
- 24.Byrne KJ, Swigar AA, Silverstein RM, Borden JH, Stokkink E. Sulcatol: Population aggregation pheromone in the scolytid beetle, Gnathotrichus sulcatus. J Insect Physiol. 1974;20(10):1895–1900. doi: 10.1016/0022-1910(74)90096-1. [DOI] [PubMed] [Google Scholar]
- 25.Raman B, Joseph J, Tang J, Stopfer M. Temporally diverse firing patterns in olfactory receptor neurons underlie spatiotemporal neural codes for odors. J Neurosci. 2010;30(6):1994–2006. doi: 10.1523/JNEUROSCI.5639-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Bruyne M, Clyne PJ, Carlson JR. Odor coding in a model olfactory organ: The Drosophila maxillary palp. J Neurosci. 1999;19(11):4520–4532. doi: 10.1523/JNEUROSCI.19-11-04520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martelli C, Carlson JR, Emonet T. Intensity invariant dynamics and odor-specific latencies in olfactory receptor neuron response. J Neurosci. 2013;33(15):6285–6297. doi: 10.1523/JNEUROSCI.0426-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner SL, et al. Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature. 2011;474(7349):87–91. doi: 10.1038/nature10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montague SA, Mathew D, Carlson JR. Similar odorants elicit different behavioral and physiological responses, some supersustained. J Neurosci. 2011;31(21):7891–7899. doi: 10.1523/JNEUROSCI.6254-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stensmyr MC, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151(6):1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 31.Haddad R, et al. A metric for odorant comparison. Nat Methods. 2008;5(5):425–429. doi: 10.1038/nmeth.1197. [DOI] [PubMed] [Google Scholar]
- 32.Monte P, et al. Characterization of the larval olfactory response in Drosophila and its genetic basis. Behav Genet. 1989;19(2):267–283. doi: 10.1007/BF01065910. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues V, Siddiqi O. Genetic analysis of chemosensory pathway. Proc Ind Acad Sci Section B. 1978;87(7):147–160. [Google Scholar]
- 34.Louis M, Huber T, Benton R, Sakmar TP, Vosshall LB. Bilateral olfactory sensory input enhances chemotaxis behavior. Nat Neurosci. 2008;11(2):187–199. doi: 10.1038/nn2031. [DOI] [PubMed] [Google Scholar]
- 35.Poivet E, et al. The use of the sex pheromone as an evolutionary solution to food source selection in caterpillars. Nat Commun. 2012;3:1047. doi: 10.1038/ncomms2050. [DOI] [PubMed] [Google Scholar]
- 36.Andersson MN, Schlyter F, Hill SR, Dekker T. What reaches the antenna? How to calibrate odor flux and ligand-receptor affinities. Chem Senses. 2012;37(5):403–420. doi: 10.1093/chemse/bjs009. [DOI] [PubMed] [Google Scholar]
- 37.Cobb M, Bruneau S, Jallon JM. Genetic and developmental factors in the olfactory response of Drosophila melanogaster larvae to alcohols. Proc Biol Sci. 1992;248(1322):103–109. doi: 10.1098/rspb.1992.0048. [DOI] [PubMed] [Google Scholar]
- 38.Zhou S, Campbell TG, Stone EA, Mackay TF, Anholt RR. Phenotypic plasticity of the Drosophila transcriptome. PLoS Genet. 2012;8(3):e1002593. doi: 10.1371/journal.pgen.1002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faucher C, Forstreuter M, Hilker M, de Bruyne M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol. 2006;209(pt 14):2739–2748. doi: 10.1242/jeb.02297. [DOI] [PubMed] [Google Scholar]
- 40.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007;104(9):3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. Molecular and cellular organization of the taste system in the Drosophila larva. J Neurosci. 2011;31(43):15300–15309. doi: 10.1523/JNEUROSCI.3363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445(7123):86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 43.Swarup S, Huang W, Mackay TF, Anholt RR. Analysis of natural variation reveals neurogenetic networks for Drosophila olfactory behavior. Proc Natl Acad Sci USA. 2013;110(3):1017–1022. doi: 10.1073/pnas.1220168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.