Abstract
Necrotizing enterocolitis (NEC) is a devastating disease of premature infants characterized by severe intestinal necrosis and for which breast milk represents the most effective protective strategy. Previous studies have revealed a critical role for the lipopolysaccharide receptor toll-like receptor 4 (TLR4) in NEC development through its induction of mucosal injury, yet the reasons for which intestinal ischemia in NEC occurs in the first place remain unknown. We hypothesize that TLR4 signaling within the endothelium plays an essential role in NEC development by regulating perfusion to the small intestine via the vasodilatory molecule endothelial nitric oxide synthase (eNOS). Using a unique mouse system in which we selectively deleted TLR4 from the endothelium, we now show that endothelial TLR4 activation is required for NEC development and that endothelial TLR4 activation impairs intestinal perfusion without effects on other organs and reduces eNOS expression via activation of myeloid differentiation primary response gene 88. NEC severity was significantly increased in eNOS−/− mice and decreased upon administration of the phosphodiesterase inhibitor sildenafil, which augments eNOS function. Strikingly, compared with formula, human and mouse breast milk were enriched in sodium nitrate—a precursor for enteral generation of nitrite and nitric oxide—and repletion of formula with sodium nitrate/nitrite restored intestinal perfusion, reversed the deleterious effects of endothelial TLR4 signaling, and reduced NEC severity. These data identify that endothelial TLR4 critically regulates intestinal perfusion leading to NEC and reveal that the protective properties of breast milk involve enhanced intestinal microcirculatory integrity via augmentation of nitrate–nitrite–NO signaling.
Keywords: neonatal inflammation, prematurity, infant formula, neonatal nutrition, sepsis
Necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in the premature infant and is gradually increasing in frequency (1). The defining pathological feature of NEC is the presence of patchy areas of ischemia and necrosis of the small and large intestine (2). Although prematurity is the leading risk factor for NEC development, breast milk administration has been identified as the most important protective strategy (3). Importantly, the mechanisms that lead to the acute development of intestinal necrosis in the premature intestine and factors within breast milk that may prevent NEC remain largely unexplored.
In seeking to understand the underlying biological mechanisms that lead to NEC, we and others have identified a critical role for the innate immune receptor toll-like receptor 4 (TLR4) in NEC pathogenesis, because mice deficient in TLR4 showed reduced mucosal inflammation and reduced intestinal necrosis in experimental NEC (4, 5). Microcirculatory perfusion of the premature intestine is primarily regulated by the vasodilator nitric oxide (NO), which is generated through the activity of endothelial NO synthase (eNOS) (6). Recent studies have shown that NO can also be generated through conversion from sodium nitrite within the diet through the activity of commensal microbes (7). Given that TLR4 is expressed on endothelial cells (8), we now hypothesize that TLR4 signaling within the endothelium plays an essential role in the pathogenesis of NEC by reducing blood flow to the small intestine through impaired eNOS signaling. We further hypothesize that breast milk protects against NEC development by augmenting eNOS signaling, enhancing intestinal perfusion, and thus limiting the deleterious effects of endothelial TLR4 activation and reducing NEC severity.
Results
TLR4 Signaling in Endothelium Is Required for Development of Experimental NEC.
To evaluate directly whether TLR4 signaling within the endothelium is important in NEC pathogenesis, we generated mice selectively lacking TLR4 on endothelial cells, by first generating Tlr4loxP mice (9), which were then bred with Angiopoietin receptor, (Tie2) mice transgene insertion 12, Richard A Flavell (B6.Cg–Tg[endothelial specific receptor tyrosine kinase (Tek)–cre]12Flv/J) to generate the homozygous TLR4Δendoth strain. TLR4Δendoth mice were found to be healthy and fertile and to produce offspring in accordance with expected Mendelian genetics. In parallel, we also generated mice in which TLR4 had been deleted from the intestinal epithelium, as we have recently described (9).
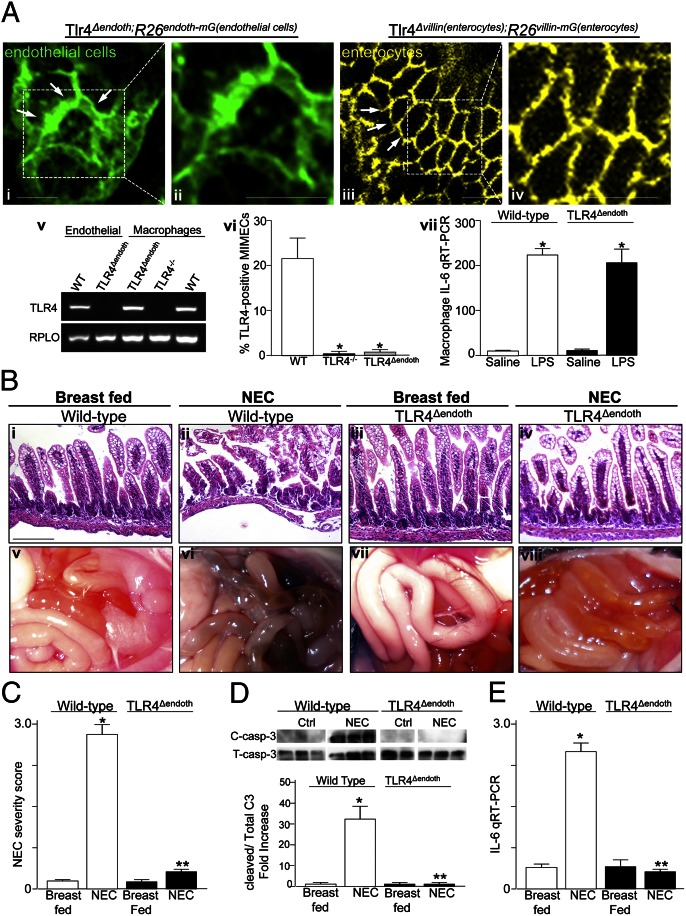
Because reliable anti-TLR4 antibodies are not available for immunohistochemistry, we confirmed that TLR4 was selectively removed from endothelial cells using alternate approaches. First, we generated a reporter strain, Tlr4Δendoth;R26endoth-mG mice, which indicated by GFP fluorescence that TLR4 had been deleted from endothelial cells. We first bred Gene trap ROSA 26, Philippe Soriano; targeted mutation 4, Liqun Luo (Gt) (ROSA)26Sortm4(ACTB-tdTomato,-EGFP) mice with Tlr4loxP mice to generate homozygous Tlr4loxP;R26mT/mG mice, which we then bred with Tie2–cre mice to generate the Tlr4Δendoth;R26endoth-mG strain. In the Tlr4Δendoth;R26endoth-mG strain, all endothelial cells lacking TLR4 express green fluorescence (green indicates staining of TLR4-deficient endothelial cells; Fig. 1 A, i and ii), which by whole-mount immunoconfocal analysis revealed a reticular network within the lamina propria of each villus. In a control experiment, we bred the Tlr4loxP;R26mT/mG mice with villin–cre mice [B6.SJL–Tg(Vil–cre)997Gum/J] to delete TLR4 from enterocytes [Tlr4Δvillin(enterocytes);R26villin-mG(enterocytes)], and we observed a markedly different pattern of GFP expression, which corresponded to the smooth outlines of the enterocyte membranes (yellow indicates staining of TLR4-deficient enterocytes; Fig. 1 A, iii and iv). We next purified mouse intestinal microvascular endothelial cells (MIMECs) according to the technique of Ogawa et al. (10) from wild-type, TLR4−/−, and TLR4Δendoth mice, along with peritoneal macrophages, and evaluated the expression of TLR4 on single cell suspensions by RT-PCR and flow cytometry. As shown in Fig. 1 A, v, both endothelial cells and peritoneal macrophages that were harvested from wild-type mice expressed TLR4, yet neither cell type expressed TLR4 when harvested from TLR4−/− mice. Importantly, macrophages, and not endothelial cells, that were harvested from TLR4Δendoth mice were found to express TLR4 by flow cytometry (Fig. 1 A, vi). Macrophages obtained from TLR4Δendoth mice also demonstrated a robust proinflammatory response after exposure to the TLR4 ligand lipopolysaccharide (LPS) (Fig. 1 A, vii), confirming that TLR4 is selectively deleted from endothelial cells and is preserved in macrophages.
Fig. 1.
Generation of mice lacking TLR4 within the intestinal endothelium and demonstration that TLR4 activation in the endothelium is required in the pathogenesis of NEC. (A, i–iv) Representative confocal micrographs showing expression of GFP in the ilea of Tlr4Δendoth;R26endoth-mG(endothelial cells) (i and ii) and Tlr4Δvillin(enterocytes);R26villin-mG(enterocytes) (iii and iv) mice. Green expression reveals endothelial cells (arrows, i and ii), and yellow indicates enterocytes (arrows, iii and iv) in which TLR4 has been deleted according to the breeding strategy described in Materials and Methods. Shown in ii and iv are magnifications corresponding to the regions outlined in i and iii, respectively. (Scale bars: 100 μm.) (v) RT-PCR showing expression of TLR4 in endothelial cells (MIMECs) and peritoneal macrophages from the indicated strain. (vi) Percent of MIMECs from the indicated strain that express TLR4 as assessed by flow cytometry. *P < 0.05 vs. wild-type (WT). (vii) qRT-PCR expression of IL-6 in peritoneal macrophages from the indicated strain after treatment with saline or LPS. *P < 0.05 vs. saline. (B) Representative histomicrographs of the terminal ileum (i–iv) and photographs (v–viii) of the intestines after fresh autopsy of newborn mice that were either breast-fed (i, iii, v, and vii) or induced to develop NEC (ii, iv, vi, and viii). (C) NEC severity score (mean ± SEM) in the strain and group indicated. (D) Measurement of apoptosis as the ratio of expression of cleaved caspase-3 to total caspase-3 by SDS/PAGE. (E) Expression of IL-6 by qRT-PCR (mean ± SEM) in wild-type or TLR4Δendoth mice that were either breast-fed or subjected to experimental NEC as indicated. *P < 0.05 vs. wild-type breast-fed controls; **P < 0.05 NEC in TLR4δendoth vs. wild-type mice. Results are representative of three separate experiments.
To evaluate the role of endothelial TLR4 in the pathogenesis of NEC, we next subjected wild-type and TLR4Δendoth mice to an established model of NEC, consisting of 4 d of hypoxia and formula gavage (11). As shown in Fig. 1 B and C, NEC severity was significantly reduced in TLR4Δendoth mice compared with wild-type mice, as manifested by the relative preservation of the mucosal architecture (Fig. 1 B, i–iv), a reduction in the degree of intestinal ischemia and necrosis (Fig. 1 B, v–viii), reduced pathological severity score (Fig. 1C), decreased enterocyte apoptosis as determined by reduced expression of cleaved caspase 3 (Fig. 1D), and reduced expression of the proinflammatory cytokine IL-6 within the intestinal mucosa (Fig. 1E). Having identified a requirement for TLR4 signaling in the endothelium in NEC pathogenesis, we next sought to evaluate the mechanisms involved.
TLR4 Activation Within the Endothelium Impairs Perfusion of the Newborn Intestine in the Pathogenesis of NEC.
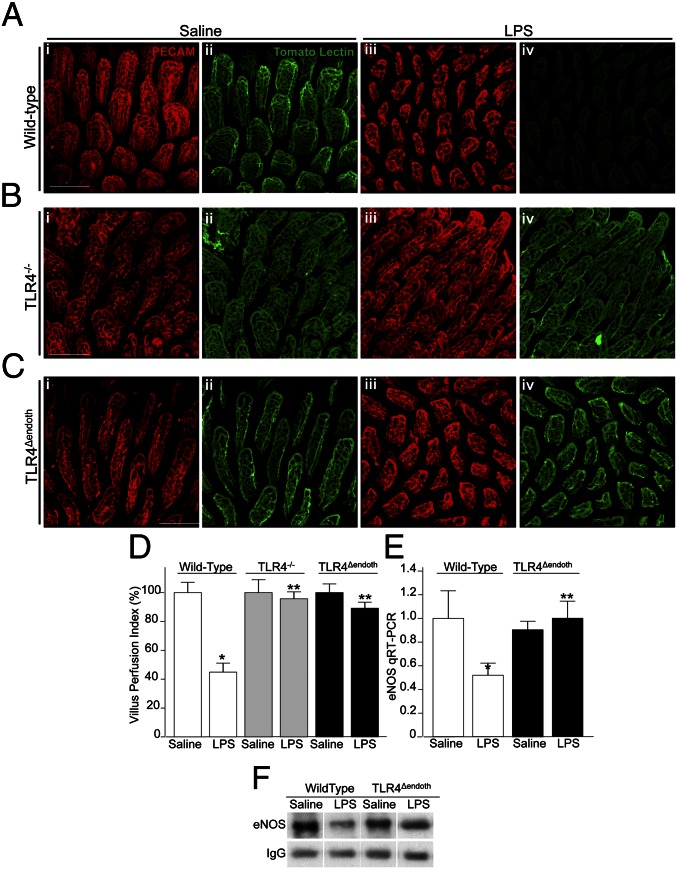
We next sought to investigate whether TLR4 activation in the endothelium could directly regulate blood flow into the intestinal microcirculation. To do so, we first performed intestinal microangiography in newborn mice, in which the distribution of a fluorescent tracer (tomato lectin) was assessed by whole-mount confocal microscopy on freshly harvested sections of the terminal ileum 5 min after intracardiac injection of tomato lectin (12). Tissues were costained with the endothelial marker platelet endothelial cell adhesion molecule 1 (PECAM-1) (CD31) to identify the architecture of the endothelial network within the lamina propria of the small intestine. Shown in Fig. 2 A–C are representative confocal images that were obtained in an en face orientation (lumen facing upward) from mice in the presence or absence of the TLR4 ligand LPS. As shown in Fig. 2 A, i–iv and D, in wild-type mice, LPS significantly reduced the perfusion of the villi as manifested by reduced fluorescence emission of tomato lectin (Fig. 2 A, ii vs. iv), despite overall preservation of the expression pattern of PECAM-1 (Fig. 2 A, i vs. iii). The administration of LPS to TLR4−/− mice did not reduce perfusion (Fig. 2 B and D), illustrating the importance of TLR4 signaling in the reduction in microvascular perfusion. Importantly, LPS did not impair perfusion of the intestinal microcirculation in TLR4Δendoth mice (Fig. 2 C and D), confirming the critical role of TLR4 signaling on endothelial cells in negatively regulating perfusion of the intestinal microcirculation.
Fig. 2.
TLR4 activation in the endothelium leads to reduced perfusion of the intestinal microcirculation and reduced expression of eNOS. (A–C) Representative confocal micrographs from whole-mount sections of terminal ileum from wild-type (A), TLR4−/− (B), or TLR4Δendoth mice (C) that were either treated with saline (i and ii) or LPS (iii and iv) 6 h before intracardiac injection with the fluorescent tracer tomato lectin. Whole mounts were immunostained with antibodies to PECAM-1 to assess the microvasculature of the intestinal mucosa (red images); the corresponding blood flow (Lucifer yellow) appears in green. (D) Villus perfusion index (VPI) as described in Materials and Methods (mean ± SEM). *P < 0.05 LPS vs. saline; **P < 0.005 LPS treated wild-type vs. LPS-treated TLR4−/− or TLR4Δendoth mice. (E) qRT-PCR (mean ± SEM) showing the expression of eNOS in wild-type or TLR4Δendoth mice treated with saline or LPS as indicated. *P < 0.05 LPS vs. saline; **P < 0.005 LPS-treated wild-type vs. TLR4Δendoth mice. (F) SDS/PAGE for eNOS expression in immunoprecipitates that had been pulled down with antibodies to eNOS from the terminal ileum of wild-type or TLR4Δendoth mice that were treated with saline or LPS; IgG is shown as a loading control. (Scale bars: 100 μm.)
In seeking to define how TLR4 signaling in the endothelium could regulate intestinal perfusion, we observed that LPS significantly reduced the expression of the vasodilatory molecule eNOS in endothelial cells from wild-type mice, whereas in TLR4Δendoth mice, eNOS message and protein levels were unchanged in the intestinal endothelium after LPS treatment, as confirmed by quantitative RT-PCR (qRT-PCR) (Fig. 2E) and SDS/PAGE (Fig. 2F). These findings raised the intriguing possibility that TLR4 regulation of eNOS activity in endothelial cells could account for the effects of TLR4 activation on the intestinal endothelium on intestinal perfusion, as will be explored further below.
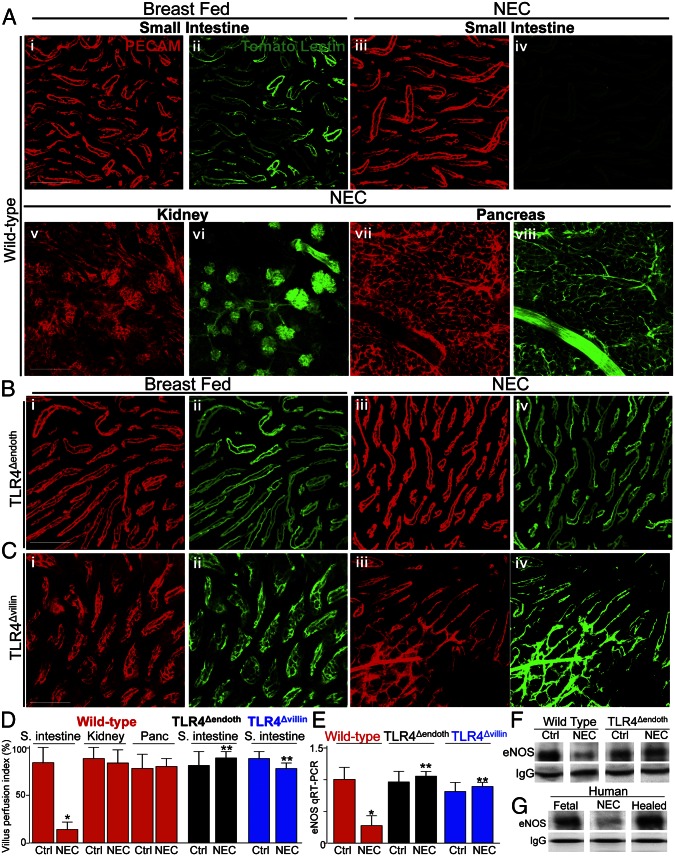
We next investigated whether TLR4 signaling within the endothelium could trigger a reduction in perfusion of the intestinal microcirculation and thus lead to the development of NEC. As shown in Fig. 3 A, i–iv and D, the perfusion of the intestinal microcirculation was significantly reduced in experimental NEC in wild-type mice, consistent with the ischemic appearance of the intestine that we observed in Fig. 1B and in agreement with recently published reports (12). It is noteworthy that the reduction in blood flow in NEC was restricted to the intestine, because microcirculatory blood flow was normal in the kidney and pancreas under conditions of NEC induction (Fig. 3 A, v–viii and D). By contrast, perfusion was not reduced in TLR4Δendoth mice that were subjected to experimental NEC and, in fact, was comparable to the breast-fed control mice of both strains (Fig. 3 B and D). Intestinal perfusion was maintained in TLR4Δvillin mice that were subjected to experimental NEC (Fig. 3 C and D), illustrating the important cross-talk between TLR4 signaling in the endothelium and intestinal epithelium in regulating intestinal perfusion in NEC. In assessing whether a TLR4-mediated reduction in vasodilatory eNOS within the endothelium could occur in the development of NEC, the expression of eNOS was reduced in the intestine of wild-type mice subjected to NEC but not in TLR4Δendoth or TLR4Δvillin mice (Fig. 3 E and F). The expression of eNOS was also significantly reduced in human intestinal tissue resected for NEC compared with fetal tissue at the same gestational age; these levels were reversed in intestine that was obtained from human tissue after NEC had resolved at the time of stoma closure (Fig. 3G). Together, these data strongly indicate that TLR4 signaling in the endothelium regulates perfusion of the microcirculation of the intestine in the pathogenesis of NEC and, further, demonstrate that TLR4 signaling in the endothelium leads to a reduction in eNOS.
Fig. 3.
TLR4 signaling in the endothelium leads to impaired perfusion of the intestinal microcirculation in the pathogenesis of NEC and reduced expression of eNOS. (A–C) Representative confocal micrographs from whole mount sections of terminal ileum (A, i–iv; B; and C), kidney (A, v and vi), or pancreas (A, vii and viii), from wild-type (A), TLR4Δendoth (B), or TLR4Δvillin mice that were either breast fed (i and ii) or induced to develop NEC (A, iii–viii; B, iii and iv; and C, iii and iv). Mice were then subjected to intracardiac injection with the fluorescent tracer tomato lectin 5 min before they were killed. Whole mounts were immunostained with antibodies to PECAM-1 to assess the microvasculature of the indicated organ(red images); the corresponding blood flow (Lucifer yellow) appears in green. (D) VPI (mean ± SEM) as described in Materials and Methods. *P < 0.05 NEC vs. control; **P < 0.005 NEC wild-type vs. NEC TLR4Δendoth or TLR4Δvillin mice. (E) qRT-PCR (mean ± SEM) showing the expression of eNOS in wild-type, TLR4Δendoth, or TLR4Δvillin mice that were either breast-fed controls or induced to develop NEC as indicated. *P < 0.05 NEC vs. control; **P < 0.005 NEC in wild-type vs. TLR4Δendoth or TLR4Δvillin mice. (F and G) SDS/PAGE of immunoprecipitates of eNOS that had first been pulled down with antibodies to eNOS from intestinal MIMECs obtained from either the terminal ileum of wild-type or TLR4Δendoth mice that were either breast-fed controls or subjected to experimental NEC (F) or from the resected ileum from a fetus, from an infant with NEC, and a neonate 6 wk after the resolution of NEC at the time of stoma reversal (G). IgG is shown as a loading control. Results are representative of three separate experiments. (Scale bars: 100 μm.)
eNOS Deficiency Increases NEC Severity, Whereas TLR4 Activation Reduces eNOS Expression in Microvascular Endothelial Cells via Myeloid Differentiation Primary Response Gene 88.
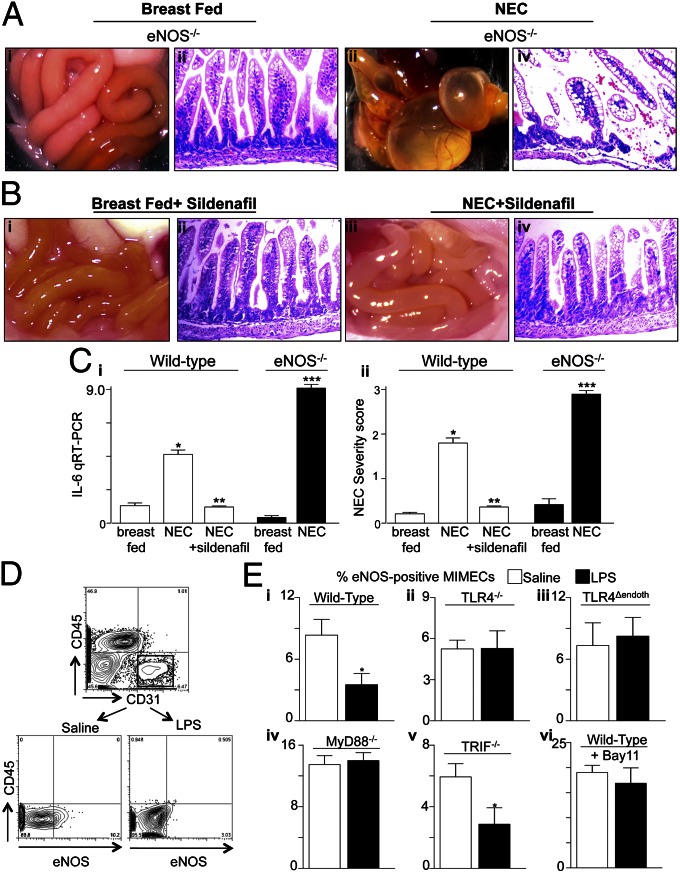
To define whether the TLR4-mediated eNOS deficiency may play a role in NEC pathogenesis directly, we subjected eNOS-deficient mice to experimental NEC. As shown in Fig. 4, eNOS-deficient mice developed an extremely severe form of NEC (Fig. 4 A, i–iv and C), which developed 1 d earlier in the model and was manifested by increased cytokine induction and mucosal disruption compared with wild-type counterparts with NEC (Fig. 4 A and C). By contrast, the daily supplementation of wild-type mice with the phosphodiesterase-5 inhibitor sildenafil, which leads to vasodilation by maintaining intraluminal NO activity (13), markedly reduced NEC severity (Fig. 4 B and C). We therefore next sought to define the mechanisms by which TLR4 reduces eNOS expression within MIMECs. As shown in Fig. 4 D and E, i–iii, treatment of mice with LPS significantly reduced eNOS expression in isolated endothelial cells compared with endothelial cells isolated from saline-treated mice, which was consistent with the in vivo findings shown in Fig. 2E. By contrast, LPS did not reduce the expression of eNOS in endothelial cells obtained from either TLR4−/− or TLR4Δendoth mice, confirming the importance of TLR4 in this process (Fig. 4 D and E, i–iii). TLR4 is known to signal through both Myeloid differentiation primary response gene 88 (MyD88) and Toll/interleukin-1 receptor domain-containing adapter-inducing interferon-β (TRIF), resulting in divergent downstream responses (14). As shown in Fig. 4 E, iv, LPS injection did not cause a reduction in eNOS expression in endothelial cells that were obtained from MyD88−/− mice but did cause a reduction in endothelial cells obtained from TRIF−/− mice (Fig. 4 E, v), indicating that MyD88 is responsible for the LPS-mediated reduction in eNOS expression, whereas TRIF is dispensable for it. In further support of the importance of MyD88 in mediating the TLR4 effects on eNOS in endothelial cells, inhibition of the MyD88 downstream molecule NF-κB by using (E)3-[(4-Methylphenyl)sulfonyl]-2-propenenitrile (BAY 11) (15) also prevented the reduction in eNOS expression in response to LPS (Fig. 4 E, vi). Perfusion of the newborn intestine may be regulated to a lesser degree by the vasoconstrictor endothelin-1 (ET-1) (16), which we found to be increased in response to TLR4 signaling in endotoxemia and NEC, in a reciprocal manner to that of eNOS (Fig. S1). Together, these studies illustrate that TLR4–MyD88 signaling is required for the reduction in eNOS expression in mesenteric endothelial cells.
Fig. 4.
The severity of experimental NEC is increased in eNOS−/− mice and reduced after administration of sildenafil, and TLR4 reduces the expression of eNOS in isolated MIMECs in a MyD88-dependent manner. (A and B) Photographs of the intestines and histomicrographs obtained from eNOS−/− (A) or wild-type mice that had been administered sildenafil as described in Materials and Methods (B) that were either breast fed (i and ii) or induced to develop NEC (iii and iv). (C) qRT-PCR showing expression of IL-6 within the intestinal mucosa (i) or NEC severity score (ii) in wild-type or eNOS−/− mice under the condition indicated. *P < 0.05 wild-type NEC vs. breast fed; **P < 0.05 NEC plus sildenafil vs. NEC; ***NEC in eNOS−/− vs. NEC in wild-type mice. Results are representative of three separate experiments with at least 10 mice per experiment. (D and E) Mouse MIMECs were isolated from the ileum of mice based upon their staining for CD31+ CD45−. Mice had been treated 6 h prior with saline or LPS and were wild type, TLR4−/−, TLR4Δendoth, MyD88−/−, or TRIF−/− or had been pretreated with the NF-κB inhibitor BAY 11 as indicated. (D) Representative plot of sorted MIMECs in the presence of saline or LPS as shown. (E) Percent eNOS-positive MIMECs from the indicated strain treated with saline or LPS as indicated. *P < 0.05. Results are representative of three separate experiments. All values are mean ± SEM.
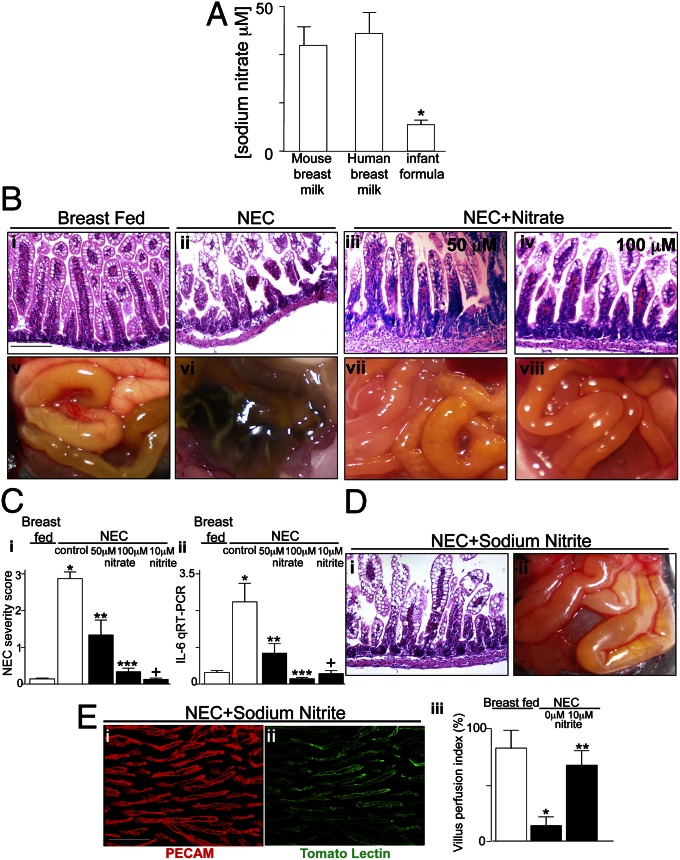
Breast Milk-Derived Nitrite Increases Perfusion of Intestinal Microcirculation and Reduces Severity of NEC.
We next sought to determine whether breast milk could protect against NEC development by restoring intestinal perfusion and, if so, whether the positive effects of breast milk occurred through protective effects on NO signaling. Previous authors have shown that the vasodilatory molecule NO is generated not only from the activity of eNOS (17)—which we have shown to be reduced upon TLR4 activation (Figs. 2–4)—but is also produced from the conversion of dietary nitrate (NO3−) to nitrite (NO2−) through the activity of enteral commensal bacteria (7). Strikingly, as shown in Fig. 5A, infant formula that is used to induce experimental NEC lacks sodium nitrate compared with mouse and human breast milk. These findings raised the exciting possibility that the lack of nitrate in the infant formula could limit the extent of available NO and thus lead to the development of NEC in conditions in which eNOS expression was reduced by TLR4 and, further, that the administration of exogenous sodium nitrate or its active metabolite nitrite could reverse the impairment in microperfusion of the intestine and attenuate NEC severity. To test these possibilities directly, newborn mice were administered sodium nitrate at two concentrations—including the concentration measured within breast milk—with each feed of the 4-d model, and the degree of NEC severity were assessed. As shown in Fig. 5, administration of sodium nitrate within the formula significantly reduced NEC severity (Fig. 5 B and C). Importantly, the administration of sodium nitrite—which is known to serve as a vasodilator upon conversion from nitrate—also significantly reduced the severity of NEC (Fig. 5 C, i and ii and D, i and ii). The reduction in NEC severity after the administration of nitrite correlated with a marked improvement in the degree of perfusion of the intestinal microcirculation, as shown in Fig. 5 E, i–iii, which was comparable to breast-fed mice (compare with Fig. 3 A and C), and had no effect when administered to the TLR4Δendoth mice (Fig. S2), in which eNOS expression was not reduced in NEC. These findings demonstrate that nitrate–nitrite–NO signaling can restore intestinal perfusion and attenuate the severity of NEC and also provide mechanistic insights to explain the protective effects of breast milk in this devastating disease.
Fig. 5.
Sodium nitrate is enriched in breast milk and reduces the severity of experimental NEC. (A) Concentration (mean ± SEM) of sodium nitrate in mouse and human breast milk and the infant formula. (B) Representative histomicrographs (i–iv) of the terminal ileum and photographs of the intestines of newborn mice that were breast fed (i and v), induced to develop NEC (ii and vi), or induced to develop NEC after daily oral gavage with sodium nitrate at the doses indicated (iii, iv, vii, and viii). (C) NEC severity score (i) and qRT-PCR showing the expression of IL-6 in the intestinal mucosa (ii) of the indicated group. *P < 0.05, breast fed vs. NEC-control; **P < 0.001 NEC vs. NEC plus sodium nitrate (50 μM); ***P < 0.001 NEC vs. NEC plus sodium nitrate (100 μM); +P < 0.05 NEC vs. NEC plus sodium nitrite (10 μM). (D and E) Representative histomicrograph (D, i) and representative photograph of intestines at time of death (D, ii) of mice induced to develop NEC with daily administration of sodium nitrite (10 μM); whole-mount confocal images of terminal ileum of mice showing expression of PECAM-1 (E, i, red) and distribution of the fluorescent tracer tomato lectin (E, ii, green) to reveal intestinal perfusion quantified through the VPI (E, iii) as in Fig. 2. *P < 0.05 vs. breast fed; **P < 0.05 vs. NEC-0 μM nitrite. (Scale bars: 100 μm.)
Discussion
We now demonstrate that activation of endothelial TLR4 is required for the development of NEC, which occurs via a TLR4-mediated reduction in intestinal perfusion through reduced eNOS signaling. The present findings provide a unique extension to our recent studies showing that TLR4 signaling in the intestinal epithelium is required for NEC development (9) by addressing the critical question as to how intestinal ischemia in NEC occurs in the first place. Given the current observation that mice in which TLR4 had been deleted from the intestinal epithelium (TLR4Δvillin) also had normal intestinal perfusion when subjected to experimental NEC, these findings raise the intriguing possibility that TLR4 signals first within the intestinal epithelium—where bacteria rich in TLR4 ligands are first encountered—which is then followed by activation within the endothelium leading to NEC. In support of this paradigm, we have demonstrated that TLR4 activation within the epithelium promotes bacterial translocation in two ways: by disrupting the intestinal barrier through increased enterocyte apoptosis and reduced mucosal repair (5, 11) and through direct transcellular passage in which TLR4 mediates the uptake of TLR4 ligands by enterocytes (18). The subsequent activation of TLR4 within the endothelium then results in the impaired perfusion of the subjacent intestinal microcirculation through a reduction in eNOS, which leads to intestinal necrosis and the development of NEC. In further support of this model, the microcirculatory perfusion of remote organs, including the kidney and pancreas, were unaffected in experimental NEC, indicating the importance of the local effects of bacterial translocation on impairing perfusion of the nearest (i.e., intestinal) vascular bed. The present study may also serve to further explain the unique susceptibility of the premature infant for the development of NEC, given the observation by ourselves and others that TLR4 expression within the premature intestine is significantly elevated compared with that in full-term infants (9, 19, 20), which we now show to be deficient in eNOS (Fig. 3). Together, these results highlight how NEC develops through exaggerated TLR4 signaling in the premature newborn intestine.
One of the most important findings of the present study is the explanation that breast milk may exert its protective effects against NEC in part through its previously unrecognized vasodilatory effects by virtue of its rich levels of nitrate that are markedly deficient in standard infant formula (Fig. 5). NO has been shown to be generated from the conversion of the nitrate anion (NO3−) from sources in the diet—in this case from breast milk—to nitrite (NO2−) through the activity of enteral commensal bacteria (21). Nitrite is subsequently reduced to form NO through reactions with various nitrite reductase systems, which provide a major source of NO that bypasses the dysfunctional NO synthase (22). The present studies therefore serve to extend the explanation of the beneficial benefits of breast milk in NEC—which have previously been attributed to rather nonspecific immunomodulatory effects and to the rich concentration of growth factors—by identifying a unique effect on perfusion of the newborn mesentery through the generation of NO. Although future studies will be required to determine the relative role of the mucosal vs. circulatory effects of breast milk in the premature infant, as well as to define the exact metabolic processes by which sodium nitrate is converted to NO within the newborn intestinal tract, the present findings strongly support the conclusions that a lack of nitrate in normal infant formula is a predisposing factor to the development of NEC and that nitrate within breast milk protects against NEC development.
In summary, we provide direct evidence for a unique role for TLR4 within the endothelium in the regulation of perfusion of the intestinal microcirculation in the pathogenesis of NEC. Further, the increased abundance of nitrate in breast milk as opposed to infant formula, and the increased perfusion of the intestinal microcirculation and protection from NEC that was obtained when formula was supplemented with sodium nitrate, provide unique insights into the protective mechanisms of breast milk against this disease. Together, these findings provide unique insights into the TLR4-mediated regulation of perfusion in the pathogenesis of NEC and open doors for its possible therapy.
Materials and Methods
Assessment of Perfusion of Intestinal Microcirculation in Newborn Mice.
Intestinal perfusion was assessed in newborn mice in an adaptation of the technique of Besner and colleagues (12). In brief, newborn mice were injected with 1 mg/mL fluorescein-labeled tomato lectin (Vector Laboratories; 10 μL/g) for 5 min, and the terminal ileum was freshly harvested, then stained with the endothelial cell marker PECAM-1 (BD Biosciences), and evaluated by whole-mount confocal microscopy as described (23) to evaluate PECAM-1 and tomato lectin fluorescent emission. Images were compiled by using Imaris (Version 7.4; Bitplane AG). In parallel, sections of freshly harvested kidney or pancreas were processed as described above. Perfusion was quantified by using the villus perfusion index (VPI) as the sum of tomato lectin fluorescence within the mucosal vasculature divided by the volume of the mucosal vasculature within the imaged tissue as described.
Statistical Analysis.
All experiments were repeated at least in triplicate. For experiments of endotoxemia, at least 3 mice per group were assessed; for experiments of NEC, >10 mouse pups per group were included, and strain-specific litter-matched controls were included in all cases. Statistical analysis was performed by using ANOVA, χ2, or two-tailed Student t test for comparisons as appropriate using SPSS software (Version 13.0). In all cases, statistical significance was accepted at P < 0.05 between groups.
Supplementary Material
Acknowledgments
D.J.H. is supported by The Hartwell Foundation and National Institutes of Health (NIH) Grants R01GM078238 and R01DK08752; D.J.H. and T.R.B. are supported by NIH Grant P50GM053789; M.T.G. is supported by NIH Grants R01HL098032, R01HL096973, and P01HL103455; the Institute for Transfusion Medicine; and the Hemophilia Center of Western Pennsylvania.
Footnotes
Conflict of interest statement: M.T.G. holds a patent for the use of nitrite salts in cardiovascular diseases and consults with Aires Pharmaceuticals.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219997110/-/DCSupplemental.
References
- 1.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominguez KM, Moss RL. Necrotizing enterocolitis. Clin Perinatol. 2012;39(2):387–401. doi: 10.1016/j.clp.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Gephart SM, McGrath JM, Effken JA, Halpern MD. Necrotizing enterocolitis risk: State of the science. Adv Neonatal Care. 2012;12(2):77–87. doi: 10.1097/ANC.0b013e31824cee94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jilling T, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177(5):3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leaphart CL, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179(7):4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 6.Marletta MA. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993;268(17):12231–12234. [PubMed] [Google Scholar]
- 7.Gladwin MT, Tejero J. Nitrite-NO bailout for a NOS complex too big to fail. Nat Med. 2011;17(12):1556–1557. doi: 10.1038/nm.2591. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim A, et al. Anti-inflammatory and anti-angiogenic effect of long chain n-3 polyunsaturated fatty acids in intestinal microvascular endothelium. Clin Nutr. 2011;30(5):678–687. doi: 10.1016/j.clnu.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Sodhi CP, et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. 2012;143(3):708–718. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa H, et al. Mechanisms of endotoxin tolerance in human intestinal microvascular endothelial cells. J Immunol. 2003;170(12):5956–5964. doi: 10.4049/jimmunol.170.12.5956. [DOI] [PubMed] [Google Scholar]
- 11.Afrazi A, et al. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J Immunol. 2012;188(9):4543–4557. doi: 10.4049/jimmunol.1103114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X, Radulescu A, Zorko N, Besner GE. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology. 2009;137(1):221–230. doi: 10.1053/j.gastro.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kloner RA. Pharmacology and drug interaction effects of the phosphodiesterase 5 inhibitors: Focus on alpha-blocker interactions. Am J Cardiol. 2005;96(12B):42M–46M. doi: 10.1016/j.amjcard.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140(6):1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce JW, et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272(34):21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 16.Yanagisawa M, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 17.Förstermann U, Sessa WC. Nitric oxide synthases: Regulation and function. Eur Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neal MD, et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176(5):3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 19.Good M, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci USA. 2012;109(28):11330–11335. doi: 10.1073/pnas.1200856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfs TG, et al. Localization of the lipopolysaccharide recognition complex in the human healthy and inflamed premature and adult gut. Inflamm Bowel Dis. 2010;16(1):68–75. doi: 10.1002/ibd.20995. [DOI] [PubMed] [Google Scholar]
- 21.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 22.Sparacino-Watkins CE, Lai YC, Gladwin MT. Nitrate-nitrite-nitric oxide pathway in pulmonary arterial hypertension therapeutics. Circulation. 2012;125(23):2824–2826. doi: 10.1161/CIRCULATIONAHA.112.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokomizo T, et al. Whole-mount three-dimensional imaging of internally localized immunostained cells within mouse embryos. Nat Protoc. 2012;7(3):421–431. doi: 10.1038/nprot.2011.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.