Abstract
Flight is a key adaptive trait. Despite its advantages, flight has been lost in several groups of birds, notably among seabirds, where flightlessness has evolved independently in at least five lineages. One hypothesis for the loss of flight among seabirds is that animals moving between different media face tradeoffs between maximizing function in one medium relative to the other. In particular, biomechanical models of energy costs during flying and diving suggest that a wing designed for optimal diving performance should lead to enormous energy costs when flying in air. Costs of flying and diving have been measured in free-living animals that use their wings to fly or to propel their dives, but not both. Animals that both fly and dive might approach the functional boundary between flight and nonflight. We show that flight costs for thick-billed murres (Uria lomvia), which are wing-propelled divers, and pelagic cormorants (Phalacrocorax pelagicus) (foot-propelled divers), are the highest recorded for vertebrates. Dive costs are high for cormorants and low for murres, but the latter are still higher than for flightless wing-propelled diving birds (penguins). For murres, flight costs were higher than predicted from biomechanical modeling, and the oxygen consumption rate during dives decreased with depth at a faster rate than estimated biomechanical costs. These results strongly support the hypothesis that function constrains form in diving birds, and that optimizing wing shape and form for wing-propelled diving leads to such high flight costs that flying ceases to be an option in larger wing-propelled diving seabirds, including penguins.
Keywords: adaptive landscape, energetics, flight performance, morphology
Flight is a key adaptation that has evolved independently on many occasions (1). Despite the apparent advantages of flying, the ability to fly has been secondarily lost in several groups. Because a major advantage of flight is reduced extrinsic mortality (2), one hypothesis for the evolution of flightlessness posits that gains in efficiency in other locomotory modalities, such as diving, offset mortality risks in relatively safe environments. The high energy demands of flight also may be disadvantageous, particularly in habitats with low productivity (3, 4). The restriction of some terrestrial flightless birds to remote, predator-free islands with low productivity supports this hypothesis (3, 4). The reasoning seems less tenable for flightless diving seabirds that often exploit highly productive waters but are vulnerable to predation by seals, whales, and sharks. Moreover, many species of penguin travel long distances between their breeding and feeding grounds on a journey that could be made far more quickly by flying than by walking and swimming (5). An alternative biomechanical hypothesis suggests that flightlessness evolved in these birds because of a tradeoff in the optimization of wing-propelled locomotion in different media. In short, as wings become more efficient for swimming they become less efficient for flying, and vice versa. At some point, adaptations to increase swimming efficiency lead to the evolution of wings that would require physiologically unsustainable flight costs. Species cannot cross this adaptive fitness valley without leaving flight behind.
Animals moving between different media face tradeoffs between maximizing function in one medium relative to the other (1, 6–9). Seabirds that dive and fly are excellent examples of animals facing tradeoffs in the optimization of locomotion for different media because they move between air, land, and water. Unlike diving beetles (family Dytiscidae), dippers (Cinclus spp.), and foot-propelled diving seabirds—the only other animals to routinely occupy all three media as adults—wing design in auks and other wing-propelled diving seabirds functions for both underwater and aerial locomotion (10–13). Extant flightless seabirds (penguins) evolved enhancements for underwater locomotion by reducing wingspan, enlarging wing bones, increasing body mass, optimizing muscle contraction rate for low-wingbeat frequencies, and augmenting myoglobin stores to increase dive endurance (1, 14, 15). In contrast, birds that both fly and dive, such as auks, are restricted by aerial flight demands for opposing adaptations (1, 12, 14).
We tested the biomechanical hypothesis for the evolution of flightlessness in seabirds by measuring the energy costs of flight and diving in two species of free-living, diving seabirds that are also able to fly: wing-propelled thick-billed murres (Uria lomvia) and foot-propelled pelagic cormorants (Phalacrocorax pelagicus). We predicted that murres would have elevated flight costs compared with nondiving birds, but would have low costs of swimming, although not as low as penguins, which have lost the ability to fly.
Results and Discussion
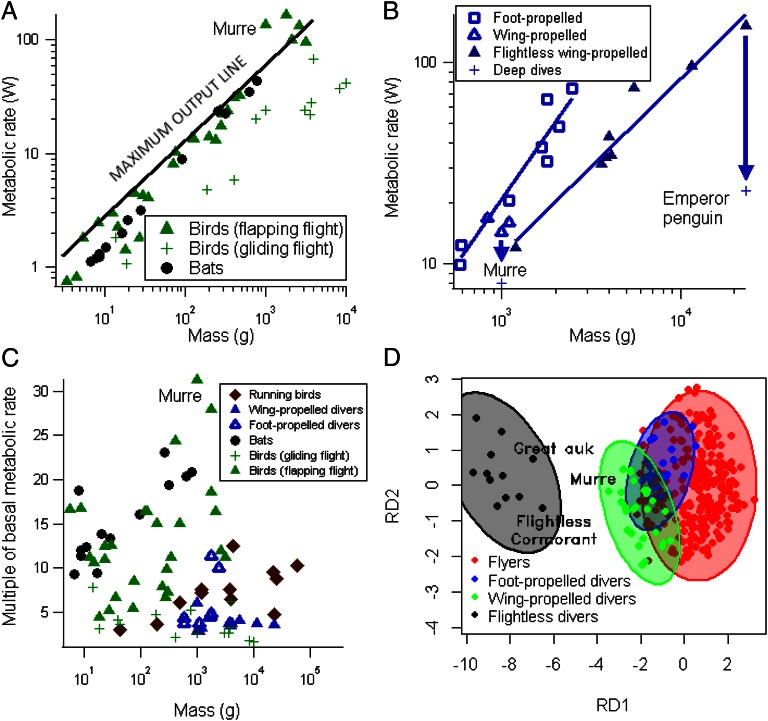
The compromises inherent in the tradeoffs in form for function in water and air were evident in murres, which have the highest wing loading (mass per unit area of the wing) of any flying bird. Our estimated flight cost for the 1-kg murre, 141 ± 18 W (146 W/kg or 0.83 J/Nm cost of transport; Fig. 1A and Figs. S1 and S2) surpassed the previous record of 135 W recorded in the 2.6-kg bar-headed goose (Anser indicus). Flight costs were also high for the 1.8-kg cormorant, 158 ± 51 W (87 W/kg or 0.70 J/Nm). The murre value is more than double that for a 980-g bird predicted by the maximum output line proposed to represent maximum aerobic capacity in a flying bird (16). Presumably, the apparent limit to aerobic capacity simply reflects the narrow range of body plans of species measured to date (Fig. 1A)—specifically, the absence of measurements from species that also swim with their wings underwater (16).
Fig. 1.
A comparison of flight costs and morphology across flying vertebrates, primarily birds. (A) A comparison of power output during flight across different bird species. The thick-billed murre value is indicated. The sustained maximum output limit proposed by Videler (16) is shown by the solid line. (B) Dive costs as a function of body mass for bird species. Thick-billed murre diving costs averaged across dive times. Average dive costs during deep diving of murres and emperor penguins also shown. Values were corrected to 13 °C to remove variation associated with temperature (26). Trend line for foot-propelled divers is significantly different from the trend line for penguins (analysis of covariance: F1,12 = 20.85, P = 0.0008). (C) Activity costs, as a multiple of basal metabolic rate, for sustained activity across bird and bat species. Flying thick-billed murre is indicated. Running metabolic rate represents maximal metabolic during sustained running, whereas swimming and flight metabolic rates represent levels that approximate minimal cost of transport. (D) Discriminant analysis of avian locomotory traits. Wing area is heavily loaded on the first axis (RD1), and body mass is heavily inversely loaded on the second axis (RD2). Murres (average of both Uria species) and the great auk are shown.
Flight costs for murres were also 33% higher than expected from biomechanical modeling based on the murre body plan (Fig. S1), implying low muscle or mechanical efficiency. The high energy costs exhibited by murres in flight suggest why most animals use their wing locomotion in a single medium. In addition to having the highest absolute cost of sustained flight (Fig. 1A), murres and cormorants also exhibit the highest flight costs in terms of multiples of basal metabolic rate (BMR; Fig. 1C). Because murres spend up to 5 h per day flying during reproduction (15), such a high level of sustained energy output (31× BMR) is remarkable; other vertebrates, except cormorants (∼28× BMR), do not exceed ∼25× BMR during intense activity (Fig. 1C).
In contrast to the high cost of flight, average daily energy expenditure of murres (25 ± 7 W or 5.7× BMR) and cormorants (23 ± 5 W or 4.1× BMR) during the chick-rearing period was well within the 7× BMR upper limit described for vertebrates (16). Thus, different mechanisms appear to limit maximum sustained energy expenditure over periods of days (e.g., internal properties related to heat dissipation and digestive or excretory abilities) compared with minutes (e.g., oxygen intake or use by muscles) (17). By altering time budgets, such that less time is spent doing energetically costly activities (flight), birds achieve an average daily energy expenditure that does not exceed the reported maximum and that is presumably limited by constraints that act over the scale of days, such as the amount of food that can be assimilated in a day.
Dive costs increase rapidly with body mass for flying divers, compared with nonflying divers (penguins; Fig. 1B). This fact, combined with the high flight costs of flying divers, likely explains the evolution of flightlessness in large, wing-propelled divers, such as penguins and extinct larger auks (15, 18). Flying, wing-propelled divers occupy a separate morphological space from other flying birds; foot-propelled divers occupy a space intermediate between flyers and wing-propelled divers (Fig. 1D and Fig. S3). Penguins are restricted to an entirely disjunct portion of the morphological space, well beyond the region occupied by flying, wing-propelled divers (Fig. 1D). The morphological differences in wing design are reflected in flight costs (Fig. S1). The space between flying and nonflying wing-propelled divers appears to represent a fitness valley in the adaptive landscape, with flying wing-propelled divers at the edge of the flying bird morphological space, supporting a biomechanical explanation for the origin of flightlessness in penguins. Within the auk family (Alcidae), the great auk (Pinguinus impennis) bounds the flightless edge of the valley, and murres bound the opposite, flighted edge of the valley (Fig. 1D). Some wing-propelled divers, such as albatrosses and shearwaters, and plunge divers, like boobies and gannets, which have long wings, overlap with the morphological space of nondiving flyers. The underwater performance of these species is likely limited in terms of energy economy or achievable depth.
We speculate that great auks and flightless cormorants (Phalacrocorax harrisi) are closer to the flying bird morphological space because of their more recent evolution (19). For instance, the humerus of the great auk is flattened compared with that of other auks, but the wing area still consists largely of primary feathers and the humerus is not nearly as large as that of penguins (Fig. S4). In penguins, the wing has flattened into a whale-like flipper. The great auk and its closest extant relatives, razorbills (Alca torda) and murres, shared a common ancestor roughly 20 Mya, whereas the penguin order (Sphenisciformes) split from their closest extant relatives, flying seabirds in the order Procellariiformes—an order that also includes flying, wing-propelled divers—over 80 Mya (9). The wing area of the great auk, ∼38 cm2, is similar to its closest flighted relatives, the razorbill (Alca torda; 42 cm2, 0.72 kg) and murres (46 cm2, 1.0 kg), although the great auk was much heavier (5 kg). Body size is more labile over evolutionary time than wing area—which in penguins has included remodeling the entire humerus—as exhibited by the rapid change in body size of flightless animals on recently colonized islands (3, 4, 10, 15).
Larger body size increases dive duration and efficiency (14, 15). Thus, loss of flight in diving birds appears to be followed by the rapid evolution of large body size, as shown by the presence of early giant penguins (20). Several observations support this scenario: (i) extant and fossil wing-propelled pursuit-diving birds are flightless above ∼1 kg and capable of flight below ∼1 kg (10, 15)—the largest auk and smallest penguin are both ∼1 kg; (ii) dive costs increase more rapidly with body size in flying birds than in flightless birds (Fig. 1B); and (iii) the flying, wing-propelled diving bird morphological space is strongly tilted along discriminant axis 1, which represents body mass (Fig. 1D), suggesting particularly strong morphological tradeoffs for heavy birds that use their wings for both flying and diving. Furthermore, along with extant and recently extinct flightless cormorants, auks, penguins, and ducks, a fifth lineage of flightless birds (order Hesperornithiformes) from the Cretaceous were large and showed a temporal progression from flying to flightlessness accompanied by the progression from foot-propelled to wing-propelled diving (21).
Metabolic costs for murres freely diving in 5.9 ± 0.3 °C water declined with dive depth and duration, with the best explanatory model being an exponentially declining function similar to that obtained for free-diving penguins: (3.64 ± 1.33) Σ[1 − e(−duration/1.23)] kJ (22). On average, murre dive costs were 27% lower than expected for a similarly sized foot-propelled diver, but 30% higher than expected for a similar-sized penguin (Fig. 1B). In contrast, foot-propelled cormorant dive costs were over threefold greater than expected for a similar-sized penguin (Fig. 1B). In general, flightless wing-propelled divers (penguins) have lower underwater energy costs and higher underwater efficiency than flying divers, whether wing or foot propelled (Fig. 1B). Presumably, “flying” underwater with long wings (murres) creates extra drag, whereas greater body mass (penguins) allows for insulation via adipose tissue rather than via a thick air layer in the feathers, reducing buoyancy costs in shallow water (23). In mammals, drag-based propulsion in semiaquatic animals is less efficient than lift-based propulsion in marine mammals (9, 24); foot-propelled avian divers use drag-based propulsion whereas wing-propelled divers rely on lift-based propulsion.
Unlike mammals and foot-propelled diving birds, many wing-propelled seabirds regularly exceed their calculated aerobic dive limit, the theoretical time limit for the exhaustion of oxygen stores during dives (25, 26). The calculated aerobic dive limit depends heavily on diving metabolic rate, which is usually measured in shallow dive tanks. Those calculations are problematic for deep-diving birds because metabolic rate likely decreases with dive depth due to reduced buoyancy, temperature, heart rate, and blood perfusion at depth (22, 23, 25–27). Using our exponentially declining model for oxygen consumption rate, and updating murre oxygen store estimates so that 90% of respiratory oxygen stores are used during the dive (as recently found in penguins) (25), leads to a calculated aerobic dive limit of 162 s, which is highly similar to the 150-s limit when surface pauses increase to allow for breakdown of accumulated lactate (25, 29). Thus, the low energy costs for deep-diving birds resolves the paradox that many deep dives exceed the calculated aerobic dive limit for birds using dive costs measured in shallow tanks (28, 29).
Large penguins have low metabolic costs during deep dives (21, 23), and our observation of hypometabolism in small, wing-propelled divers shows that the savings are not unique to large birds and may be characteristic of wing-propelled divers in general (Fig. 1B). Although mechanical costs during deep dives have been precisely measured and increase approximately linearly with dive depth (30–32), actual metabolic costs measured in the field decelerated as dive depth increased (Table 1). We suggest that physiological processes during the dive, such as oxygen store management and thermoregulation, are the dominant processes determining costs in wing-propelled divers diving to depths where buoyancy costs are minimal (22, 25, 27). In contrast, dive costs for foot-propelled divers are correlated with dive depth and duration, reflecting biomechanical costs that increase linearly with dive duration (Table 1). In foot-propelled divers, the leg muscles are separate from the body core, whereas in wing-propelled divers, the breast muscles encase the body core, enabling them to finely regulate body core temperature, muscle temperature, and oxygen availability in the muscles to minimize costs during deep dives (22, 25, 27). Progressive reductions in dive costs may explain why aquatic birds developed wing-propelled diving and finally flightlessness in response to foraging opportunities at increasing depths. In particular, high flight costs and low dive costs in auks illustrate why penguins, and other diving birds, have lost the ability to fly.
Table 1.
Comparison of models that estimate energy expenditure in watts (± SE) for several activities in thick-billed murres and pelagic cormorants
| At the colony | Flying | Water surface | Diving | Other | ΔAIC |
| Thick-billed murre | |||||
| 9.2 ± 3.1 | 141 ± 18 | 26 ± 6 | (1.01 ± 0.36) Σ[1 − e(−duration/1.23)] | 0.00 | |
| 11.4 ± 3.3 | 143 ± 18 | 29 ± 7 | (0.93 ± 0.37) Σ[1 − e(−duration/1.23)] | Wind = −16 ± 15 kJ × h/km | 1.10 |
| 9.7 ± 3.1 | 140 ± 18 | 28 ± 7 | (0.99 ± 0.37) Σ[1 − e(−duration/1.23)] | Mass loss = 2.57 ± 3.69 kJ × d/g | 1.45 |
| 8.6 ± 5.8 | 140 ± 18 | 27 ± 7 | (1.09 ± 0.44) Σ[1 − e(−duration/1.23)] | Temperature = −1.5 ± 8.1 kJ/°C | 1.89 |
| 7.2 ± 8.1 | 140 ± 15 | 27 ± 6 | (1.08 ± 0.26) Σ[1 − e(−duration/1.23)] | Sex | 1.92 |
| 7.5 ± 6.1 | 140 ± 18 | 24 ± 9 | (1.01 ± 0.37) Σ[1 − e(−duration/1.23)] | Mass = 0.30 ± 0.83 kJ/g | 1.85 |
| 9.2 ± 3.1 | 143 ± 19 | 27 ± 6 | (0.95 ± 0.39) ΣLn(depth) | 2.81 | |
| 8.9 ± 3.1 | 148 ± 18 | 28 ± 6 | 27 ± 12 | 3.09 | |
| 9.4 ± 3.3 | 158 ± 18 | 29 ± 6 | (3.1 ± 1.9) mechanical costs | 6.82 | |
| 165 ± 18 | All activities but flying = 16 ± 2 | 24.03 | |||
| 7.5 ± 3.3 | Away from the colony = 52 ± 4 | 31.47 | |||
| Pelagic cormorant | |||||
| 7.8 ± 2.5 | 168 ± 51 | 7.7 ± 12 | 66 ± 11 | 0.00 | |
| 6.4 ± 2.9 | 180 ± 52 | 3.4 ± 13 | 75 ± 15 | Mass loss = −0.18 ± 0.17 kJ × d/g | 0.92 |
| −4.4 ± 19.6 | 174 ± 56 | −4.0 ± 22.4 | 57 ± 18 | Mass = 0.16 ± 0.25 kJ/g | 1.50 |
| 8.2 ± 3.8 | 170 ± 55 | 10 ± 12 | 68 ± 13 | Temperature = −0.4 ± 2.1 kJ/°C | 1.81 |
| 11 ± 4.5 | 175 ± 68 | 11 ± 9 | 68 ± 12 | Wind = −4 ± 14 kJ × hr/km | 1.95 |
| 73 ± 12 | All activities but diving = 14 ± 2 | 7.12 | |||
| 8.4 ± 3.1 | Away from the colony = 54 ± 6 | 11.06 | |||
| 5.2 ± 5.1 | 167 ± 93 | 194 ± 72 | (0.15 ± 0.06) Σ[1 − e(−duration/0.89)] | 26.30 | |
| 5.1 ± 5.1 | 168 ± 9 | 53 ± 20 | (0.010 ± 0.004) ΣLn(depth) | 26.39 |
Models include different functional relationships between energy costs and dive depth or duration and terms for individual body mass, sex, average ambient air temperature, mass loss during the deployment period, and average wind speed. Water surface values for cormorants exclude preening. Models with ΔAIC values >2 are generally considered inferior.
Materials and Methods
Between July 15 and August 10, 2006, we captured 41 thick-billed murres at the Coats Island west colony, Nunavut, Canada, and equipped those birds with time-depth temperature recorders (TDRs). Between July 25 and August 14, 2012, we captured 22 pelagic cormorants at Middleton Island, Alaska, and equipped those birds with temperature-depth accelerometers. We simultaneously measured energy expenditure in the same birds using doubly labeled water and recorded weather. When murres injected with doubly labeled water and equipped with TDRs were compared with paired control murres equipped at the same time and breeding stage only with TDRs, there was no difference in maximum dive depth (t24 = 0.40, P = 0.69), average dive depth (t24 = 0.99, P = 0.33), time spent submerged (t24 = 0.71, P = 0.48), time spent flying (t24 = 0.32, P = 0.75), time spent at the colony (t24 = 0.35, P = 0.96), or time to switch-over (t24 = 1.83, P = 0.08). We concluded that the doubly labeled water injections had little impact on behavior.
To calculate activity-specific metabolic rate, we regressed energy expenditure against activity times for diving, flying, at the water surface, and on land. By using a multivariate approach and forcing the intercept to be zero (no energy was expended when no time elapsed), we overcame the problem of incorporating some of the slope value into the intercept. We subtracted the high cost of preening from cormorant activities. Because our depth recorders provided more details on time partitioning during the dive, we also considered three other models: (i) cost of diving was proportional to costs associated with buoyancy; (ii) cost of diving was proportional to total mechanical work during a dive; and (iii) cost of diving followed the oxygen depletion curve developed for deep-diving penguins. We compared the effectiveness of different models using Akaike’s information criterion, which penalizes models with increased numbers of parameters without improvement in fit. We compared the morphological space of flying birds and flightless divers by conducting a discriminant analysis on the first principal component of the log-transformed morphological traits (wing area, wingspan, body mass, and three functions derived from those parameters). See SI Materials and Methods for details of TDR deployments, calculations of energy expenditure, measurement of weather, the behavior of study birds relative to controls, morphological analyses, details of statistical modeling, and raw data.
Supplementary Material
Acknowledgments
We thank K. Ashcroft, M. Barrueto, P. Redman, A. Ronston, K. Woo, and P. Woodward for help in the field; C. Hambly and P. J. Thomson for technical assistance with isotope analysis; and V. Ellis for help with programming in R. J. Green, A. W. Diamond, and D. D. Roby provided many helpful comments on an earlier version of the manuscript; Y. Osa translated sections of his thesis; and G. Kaiser provided a copy of The Inner Bird morphology database. R. Armstrong at the Nunavut Research Institute and C. Eberl and M. Mallory at the Canadian Wildlife Service provided logistical support. Transportation was provided by the Polar Continental Shelf Project of Energy, Mines and Resources Canada. Funding was provided by Natural Sciences and Engineering Research Council (NSERC) Canada Graduate Scholarships M and Vanier (to K.H.E.); Northern Research Internship and Michael Smith Foreign Studies Supplement (to K.H.E.); Arctic Institute of North America Grant-in-Aid and Jennifer Robinson Scholarship; Association of Canadian Universities for Northern Studies Garfield Weston Scholarship; Society of Canadian Ornithologists/Bird Studies Canada Taverner and James Baillie Awards; American Museum of Natural History Frank M. Chapman Award; Canadian Wildlife Service Migratory Birds Division of Environment Canada; the University of Manitoba; and an NSERC Discovery Grant (to G.K.D.).
Footnotes
The authors declare no conflict of interest.
This paper was presented at the 25th International Ornithological Congress, Campos do Jordão, Brazil, August 28, 2010, and at the Pacific Seabird Group Meeting in Hakodate, Japan, February 22, 2009.
Data deposition: The data have been deposited in the Dryad database, http://dx.doi.org/10.506/dryad.23td2.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304838110/-/DCSupplemental.
References
- 1.Pennycuick CJ. 2008. Modelling the Flying Bird. Theoretical Ecology Series (Academic, New York), Vol 5. [Google Scholar]
- 2.Ricklefs RE. Evolutionary theories of aging: Confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. Am Nat. 1998;152(1):24–44. doi: 10.1086/286147. [DOI] [PubMed] [Google Scholar]
- 3.McNab BK. Energy conservation and the evolution of flightlessness in birds. Am Nat. 1994;144(4):628–642. [Google Scholar]
- 4.McNab BK, Ellis HI. Flightless rails endemic to islands have lower energy expenditure and clutch sizes than flighted rails on islands and continents. Comp Biochem Physiol A. 2006;145(3):295–311. doi: 10.1016/j.cbpa.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt-Nielsen K. Locomotion: Energy cost of swimming, flying, and running. Science. 1972;177(4045):222–228. doi: 10.1126/science.177.4045.222. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh ST, Lauder GV. Running on water: Three-dimensional force generation by basilisk lizards. Proc Natl Acad Sci USA. 2004;101(48):16784–16788. doi: 10.1073/pnas.0405736101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ropert-Coudert Y, et al. Between air and water: The plunge dive of the cape gannet Morus capensis. Ibis. 2004;146(2):281–290. [Google Scholar]
- 8.Roby DD, Ricklefs RE. Energy expenditure in adult least auklets and diving petrels during the chick-rearing period. Physiol Zool. 1986;59(6):661–678. [Google Scholar]
- 9.Kaiser GW. 2007. The Inner Bird (Univ of British Columbia Press, Vancouver) [Google Scholar]
- 10.Williams TW. The evolution of cost efficient swimming in marine mammals: Limits to energetic optimization. Phil Trans R Soc B. 1999;354(1380):193–201. [Google Scholar]
- 11.Wilson RP, et al. What grounds some birds for life? Movement and diving in the sexually dimorphic Galápagos cormorant. Ecol Monogr. 2008;78(4):633–652. [Google Scholar]
- 12.Sato K, et al. Stroke frequency, but not swimming speed, is related to body size in free-ranging seabirds, pinnipeds and cetaceans. Proc Biol Sci. 2007;274(1609):471–477. doi: 10.1098/rspb.2006.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaxter CB, et al. Influence of wing loading on the trade-off between pursuit-diving and flight in common guillemots and razorbills. J Exp Biol. 2010;213(Pt 7):1018–1025. doi: 10.1242/jeb.037390. [DOI] [PubMed] [Google Scholar]
- 14.Elliott KH, Shoji A, Campbell KL, Gaston AJ. Oxygen stores and foraging behavior of two sympatric, planktivorous alcids. Aquat Biol. 2010;8:221–235. [Google Scholar]
- 15.Gaston AJ. 2004. Seabirds: A Natural History (Yale Univ Press, New Haven, CT) [Google Scholar]
- 16.Videler JJ. 2005. Avian Flight (Oxford Univ Press, Oxford) [Google Scholar]
- 17.Hammond KA, Diamond JM. Maximal sustained energy budgets in humans and animals. Nature. 1997;386(6624):457–462. doi: 10.1038/386457a0. [DOI] [PubMed] [Google Scholar]
- 18.Houston AI, Wood J, Wilkinson M. How did the Great Auk raise its young? J Evol Biol. 2010;23(9):1899–1906. doi: 10.1111/j.1420-9101.2010.02047.x. [DOI] [PubMed] [Google Scholar]
- 19.Pereira SL, Baker AJ. DNA evidence for a Paleocene origin of the Alcidae (Aves: Charadriiformes) in the Pacific and multiple dispersals across northern oceans. Mol Phylogenet Evol. 2008;46(2):430–445. doi: 10.1016/j.ympev.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Clarke JA, et al. Paleogene equatorial penguins challenge the proposed relationship between biogeography, diversity, and Cenozoic climate change. Proc Natl Acad Sci USA. 2007;104(28):11545–11550. doi: 10.1073/pnas.0611099104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez J. 2010. Late Cretaceous (Cenomanian) Hesperornithiformes from the Pasquia Hills, Saskatchewan, Canada. PhD dissertation (Carleton University, Ottawa) [Google Scholar]
- 22.Knower Stockard T, et al. Air sac PO2 and oxygen depletion during dives of emperor penguins. J Exp Biol. 2005;208(Pt 15):2973–2980. doi: 10.1242/jeb.01687. [DOI] [PubMed] [Google Scholar]
- 23.Wilson RP, Hustler K, Ryan PG, Burger AE, Noldeke EC. Diving birds in cold water: Do Archimedes and Boyle determine energetic costs? Am Nat. 1992;140(2):179–200. [Google Scholar]
- 24.Fish FE. Biomechanics and energetics in aquatic and semiaquatic mammals: Platypus to whale. Physiol Biochem Zool. 2000;73(6):683–698. doi: 10.1086/318108. [DOI] [PubMed] [Google Scholar]
- 25.Ponganis PJ, Meir JU, Williams CL. Oxygen store depletion and the aerobic dive limit in emperor penguins. Aquat Biol. 2010;8:237–245. doi: 10.1242/jeb.052233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen ES, Ricklefs RE. Foraging by deep-diving birds is not constrained by an aerobic diving limit: A model of avian depth-dependent diving metabolic rate. Am Nat. 2004;163(3):358–374. doi: 10.1086/381943. [DOI] [PubMed] [Google Scholar]
- 27.Niizuma Y, Gabrielsen GW, Sato K, Watanuki Y, Naito Y. Brünnich’s guillemots (Uria lomvia) maintain high temperature in the body core during dives. Comp Biochem Physiol A Mol Integr Physiol. 2007;147(2):438–444. doi: 10.1016/j.cbpa.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Enstipp MR, Grémillet D, Jones DR. The effects of depth, temperature and food ingestion on the foraging energetics of a diving endotherm, the double-crested cormorant (Phalacrocorax auritus) J Exp Biol. 2006;209(Pt 5):845–859. doi: 10.1242/jeb.02064. [DOI] [PubMed] [Google Scholar]
- 29.Croll DA, Gaston AJ, Burger AE, Konnoff D. Foraging behavior and physiological adaptation for diving in thick-billed murres. Ecology. 1992;73(1):344–356. [Google Scholar]
- 30.Lovvorn JR, Watanuki Y, Kato A, Naito Y, Liggins GA. Stroke patterns and regulation of swim speed and energy cost in free-ranging Brünnich’s guillemots. J Exp Biol. 2004;207(Pt 26):4679–4695. doi: 10.1242/jeb.01331. [DOI] [PubMed] [Google Scholar]
- 31.Watanuki Y, Niizuma Y, Gabrielsen GW, Sato K, Naito Y. Stroke and glide of wing-propelled divers: Deep diving seabirds adjust surge frequency to buoyancy change with depth. Proc Biol Sci. 2003;270(1514):483–488. doi: 10.1098/rspb.2002.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanuki Y, et al. Swim speeds and stroke patterns in wing-propelled divers: A comparison among alcids and a penguin. J Exp Biol. 2006;209(Pt 7):1217–1230. doi: 10.1242/jeb.02128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.