Abstract
The natural killer group 2 membrane D (NKG2D) activating receptor plays crucial roles not only in host defense against tumors and viral infections, but also in autoimmune diseases. After NKG2D-mediated activation, Natural killer (NK) cells must be regulated to avoid potentially harmful reactivity. However, the negative regulation of these activated NK cells is poorly understood. Here, we reveal that the engagement of NKG2D by its ligand elicits not only target cell lysis, but also NK cell fratricide. Conventional mouse NK cells underwent cell death when cocultured with RMA cells expressing the NKG2D ligand retinoic acid early-inducible protein 1 (Rae-1), but not with RMA cells lacking MHC class I. NK cells from mice deficient for DAP10 and DAP12 or perforin did not undergo death, highlighting the importance of the NKG2D pathway for NK cell death. However, NKG2D does not transmit direct death signals in NK cells. Rather, the interaction between NKG2D and Rae-1 allowed NK cells to acquire tumor-derived Rae-1 by a membrane transfer process known as ”trogocytosis,” which was associated with clathrin-dependent NKG2D endocytosis. NK cells dressed with Rae-1 were lysed by neighboring NK cells through the NKG2D-induced perforin pathway in vitro and in vivo. These results provide the unique NKG2D function in negative regulation of activated NK cells.
Keywords: immunological synapse, intercellular membrane transfer, immune regulation
Natural killer (NK) cells are lymphocytes that mediate rapid response against virally infected cells and tumor cells without prior sensitization. Upon activation, NK cells proliferate and exert effector functions including perforin-mediated cytotoxicity against target cells and cytokine production such as IFN (IFN)-γ (1). In contrast to the pivotal role of NK cells in host defense, persistent expansion of activated NK cells in benign and malignant conditions is often associated with systemic autoimmune diseases (2–4). Therefore, NK cells themselves must be regulated after activation. However, the fate of activated NK cells has not been fully understood.
In IL-2–activated human NK cells, cross-linking stimulation of activating Fcγ receptor CD16 induces not only cytolytic function, but also suicide (5), indicating that activating receptor could transmit the death signal in NK cells under certain conditions. Additionally, a subpopulation of NK cells undergoes rapid apoptosis upon interaction with the NK-sensitive tumor cell line K562 (6, 7), although the molecular mechanism of NK cell death remains largely unknown. Given that K562 cells express natural killer group 2 membrane D ligand (NKG2DL) and are susceptible to NKG2D-mediated cytotoxicity (8), NKG2D as well as CD16 may also elicit activation-induced NK cell death.
NKG2D is an extensively characterized activating NK receptor (9). NKG2D is expressed on essentially all NK cells, and plays a crucial role in NK cell-mediated effector functions (10). Nevertheless, the involvement of NKG2D in activation-induced NK cell death remains unclear. NKG2D recognizes stress-induced self ligands on abnormal cells through a process known as “induced-self” recognition (11). These NKG2DLs include the retinoic acid early-inducible protein 1 (Rae-1), H60, and MULT1 in mice; as well as UL-16 binding proteins, and MHC class I (MHCI) chain-related molecules A (MICA) and B (MICB) in humans (10). Although NK cells had been considered to lyse cells that have lost MHCI expression through “missing-self” recognition (12), expression of NKG2DL renders cells susceptible to NK cytotoxicity, irrespective of MHCI expression (13). Upon recognition of NKG2DL, NKG2D recruits its adaptor proteins DNAX-activating protein 10 (DAP10) and DAP12 in conjunction with adhesion molecules at the cell–cell contact site called NK cell immunological synapse (NK-IS), which provides a platform for intracellular signal transduction leading to perforin degranulation (14–17).
Recently, unique functions of the NK-IS have been reported. We have demonstrated that upon NK-IS formation, NKG2D undergoes ligand-induced down-modulation for negative feedback regulation (18). In addition, upon NK-IS formation, human NK cells rapidly acquire MICA and MICB from tumor cells (19, 20). Such dynamic intercellular protein relocalization is mediated by the transfer of plasma membrane fragments from one cell to another during cell–cell contact; a process known as “trogocytosis” (21). The trogocytosed MICB masks NKG2D on NK cells and suppresses NKG2D-mediated NK cell functions (20). Given that the NKG2DL-expressing cells are susceptible to NK cytotoxicity by induced-self recognition, NK cells exogenously acquired NKG2DL might be lysed by other activated NK cells, called fratricide (22). However, this possibility has not previously been addressed, and the molecular mechanisms underlying NKG2D-mediated trogocytosis have not been previously defined.
We therefore asked whether the interaction between NKG2D and NKG2DL is involved in NK cell death. Furthermore, we investigated the possibility that NKG2D-mediated trogocytosis is a trigger of NK cell fratricide.
Results
NK Cell Death Occurs in an NKG2D-Dependent Manner.
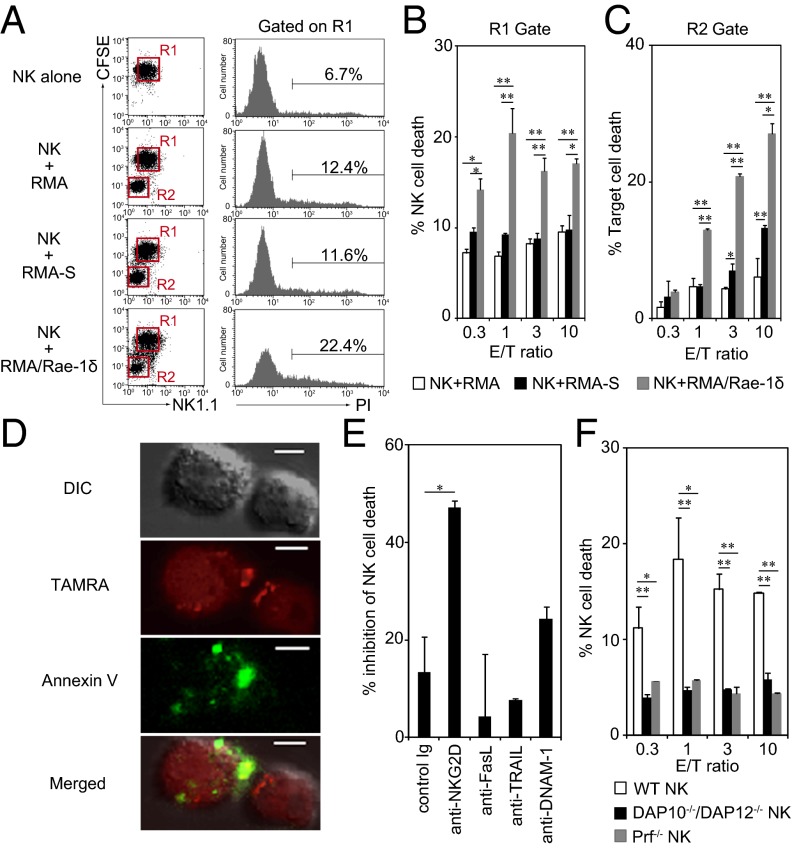
To investigate whether NKG2D and NKG2DL are involved in NK cell death, we addressed the relationship between NK cell cytotoxic activity and NK cell death. We used IL-2–activated C57BL/6 mouse NK cells as effector cells and three target cell lines: mouse T-cell lymphoma RMA cells (RMA), RMA lacking MHCI expression on their cell surface (RMA-S), and RMA stably expressing an NKG2DL, Rae-1δ (RMA/Rae-1δ). Consistent with previous reports (13, 23), NK cells effectively lysed both RMA-S and RMA/Rae-1δ but did not lyse parental RMA (Fig. S1). To address NK cell death, we labeled NK cells with 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) and measured the percentage of propidium iodide (PI)-positive cells in CFSE+ NK1.1+ NK cells cocultured with tumor cells. Surprisingly, within 2 h, more than 20% of NK cells died only when cocultured with RMA/Rae-1δ (Fig. 1 A and B). CFSE− NK1.1− RMA/Rae-1δ were lysed by NK cells in an effector/target (E/T) ratio-dependent manner (Fig. 1C), whereas effector NK cell death was independent of the E/T ratio (Fig. 1B). Confocal microscopy showed that 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (TAMRA)-labeled NK cells turned to be annexin V-positive after cocultured with RMA/Rae-1δ (Fig. 1D). This NK cell death was significantly inhibited by anti-NKG2D blocking mAb (Fig. 1E). These results suggest that NKG2D and Rae-1 interaction induces not only tumor cell death but also NK cell death. Furthermore, NK cells deficient for NKG2D adaptor molecules DAP10 and DAP12 (DAP10−/−/DAP12−/−) did not undergo cell death (Fig. 1F). Perforin-deficient (Prf−/−) NK cells did not undergo cell death (Fig. 1F), suggesting that NK cell death occurs through the NKG2D-induced perforin pathway. Neither death-inducing ligand such as Fas ligand and TNF-related apoptosis-inducing ligand (TRAIL) (24) nor another NK activating receptor DNAX accessory molecule 1 (DNAM-1) (25) is involved in this process (Fig. 1E). RMA-S are well known to be recognized as missing-self cells to be killed through perforin by NK cells (23). However, NK cells did not die (Fig. 1 A and B) when lysed RMA-S (Fig. S1 and Fig. 1C), excluding the possibility that NK cells simply lyse themselves via self-released perforin. We next addressed the possibility that NKG2D directly transmits death signals in NK cells; however, cross-linking of NKG2D induced IFN-γ production (Fig. S2A), but not NK cell death (Fig. S2B), indicating that NKG2D per se does not transmit death signals. Collectively, these results indicate that NK cell death is mediated by the NKG2D-induced perforin pathway, but NKG2D ligation alone is insufficient to cause NK cell death.
Fig. 1.
NK cell death is mediated through the NKG2D-induced perforin pathway. (A–C) NK cell death and target cell death in the same culture. NK cells were labeled with CFSE and then cocultured with RMA, RMA-S, or RMA/Rae-1δ at a 1:1 ratio (A) or the indicated ratios (B and C) for 2 h. Cells were stained with anti-NK1.1 mAb and propidium iodide (PI), and then percent cell death of R1-gated NK (PI+ CFSE+ NK1.1+ cells/CFSE+ NK1.1+ cells) and of R2-gated tumor (PI+ CFSE− NK1.1− cells/CFSE− NK1.1− cells) was calculated. Representative PI staining of NK cells (gray histograms) are shown in A. Percent NK cell death (B) and percent tumor cell death (C) are shown as means plus SDs of triplicates. *P < 0.05, **P < 0.01. (D) TAMRA-labeled NK cells cocultured with RMA/Rae-1δ were stained with FITC-conjugated annexin V and analyzed by confocal microscopy. (Scale bars, 5 μm.) (E) CFSE-labeled NK cells were treated with the indicated blocking mAb. Percent inhibition of cell death by mAbs was calculated by comparing the percent NK cell death in the presence of mAb with that in the absence of mAb. *P < 0.05; compared with control rat IgG. (F) NK cells from DAP10−/−/DAP12−/−, Prf−/−, or WT mice were cocultured with RMA/Rae-1δ, and percent NK cell death was analyzed. Data represent means plus SDs of triplicates. *P < 0.05, **P < 0.01; compared with WT NK. Similar results were obtained in three (A–C) or two (D–F) independent experiments.
Cell Surface Expression of Rae-1 on NK Cells Is Induced After Coculture with RMA/Rae-1δ.
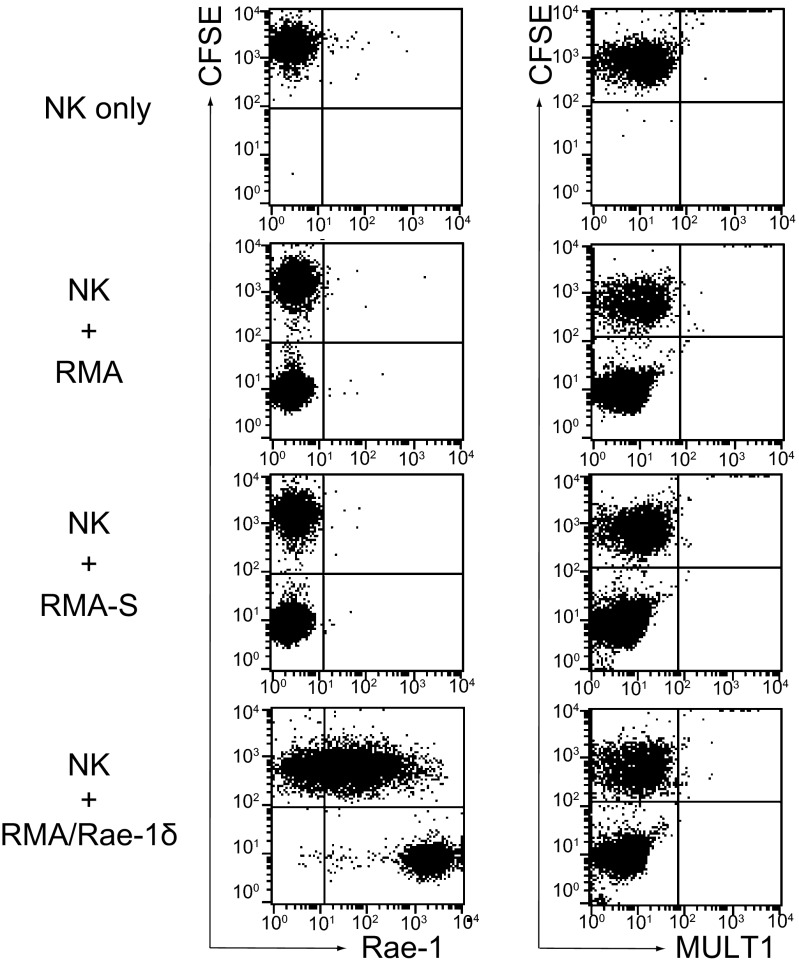
We hypothesized that NK cells themselves could turn to be target cells after interaction with RMA/Rae-1δ and to be lysed by perforin. Thus, we evaluated alterations of the cell surface expression of NKG2DL on NK cells. Although Rae-1 was not expressed on NK cells, cell surface expression was dramatically induced 2 h after coculture with RMA/Rae-1δ (Fig. 2). MULT1, another NKG2DL, was not expressed on these tumor cells, and MULT1 was not induced on NK cells (Fig. 2). Thus, upon coculture with RMA/Rae-1δ, NK cells turn to be Rae-1δ–positive, which may be recognized by other NK cells, and undergo fratricide.
Fig. 2.
Cell surface expression of NKG2DL on NK cells before and after coculture with RMA/Rae-1δ. CFSE-labeled NK cells were cocultured with tumor cells at a 1:1 ratio for 2 h. Then cells were stained with anti–Rae-1 mAb (Left) or anti-MULT1 mAb (Right). Representative data from three independent experiments are shown.
NK Cells Acquire Rae-1 from Target Cells via Trogocytosis.
To address whether NK cells become Rae-1–positive after Rae-1–expressing tumor cells in vivo, we inoculated wild-type (WT) C57BL/6 mice s.c. with B16/Rae-1ε (Fig. 3A) or RMA/Rae-1δ (Fig. 3B). Two weeks after the inoculation, we observed that DX5+ NK cells infiltrated into tumors, and that these NK cells become Rae-1–positive (Fig. 3 A and B). Because NK cells turn to be Rae-1–positive at 2 h coculture with RMA/Rae-1δ in vitro (Fig. 2), we next performed the short-term in vivo study. Specifically, we injected RMA/Rae-1δ into spleen of recombination activating gene-1–deficient (Rag-1−/−) mice that lack mature T and B lymphocytes, and found that splenic NK cells turned to be Rae-1–positive at 2 h after injection (Fig. 3C). We further aimed to elucidate the mechanism underlying Rae-1 induction on the NK cell surface in vitro. First, we investigated the kinetics of cell surface expression of Rae-1 on NK cells and found it to occur on both freshly isolated NK cells (Fig. S3) and IL-2–activated NK cells (Fig. 3D) within 10 min after coculture. Although Rae-1 transcript was very low but detectable in NK cells (Fig. S4) as well as a wide variety of normal cells (26, 27), the rapid appearance of Rae-1 (Fig. 3D) lead us to address the possibility that NK cells acquire Rae-1 from target cells, rather than through de novo synthesis. We and others have recently reported that NK cells have the ability to acquire cell surface proteins from target cells in a cell–cell contact-dependent manner, referred to as trogocytosis (21, 28, 29). Likewise, acquisition of Rae-1 expression occurred only with direct cell–cell contact and did not occur on NK cells cultured with RMA/Rae-1δ in Transwell plates (Fig. 3E). Notably, C57BL/6 mouse NK cells turned to be BALB/c allele Rae-1γ–positive when cocultured with RMA/Rae-1γ, reinforcing the fact that NK cells acquire tumor-derived Rae-1 (Fig. 3F). Confocal microscopy revealed that NK cells acquired Rae-1 from RMA/Rae-1δ in a cell–cell contact-dependent manner (Fig. 3G). We next evaluated whether NK cells acquire the naturally processed soluble form of NKG2DL, which has been reported in human, but not in mouse (10). Human NK cells also acquire MICA in a cell–cell contact-dependent manner, but not soluble MICA (Fig. S5), suggesting that NK cells acquire only the membrane-bound form of NKG2DL. Collectively, these results indicate that NK cells acquire tumor-derived Rae-1 via trogocytosis.
Fig. 3.
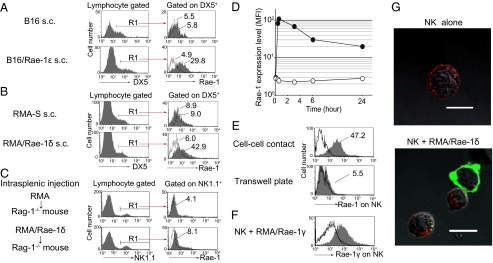
Rae-1 is transferred from RMA/Rae-1 to NK cells via trogocytosis. (A and B) B16 (5 × 105), B16/Rae-1ε (2 × 106) (A), RMA-S (5 × 105), or RMA/Rae-1δ (1 × 106) (B) were s.c. inoculated into C57BL/6 mice. Two weeks later, tumor-infiltrating lymphocytes were prepared, and Rae-1 expression on DX5+ NK cells was analyzed. Gray and dotted line histograms indicate anti–Rae-1 mAb and isotype control mAb staining, respectively. Numbers indicate the mean fluorescence intensity (MFI). (C) RMA or RMA/Rae-1δ (1 × 107) was injected into spleen of Rag-1−/− mice. After 2 h, Rae-1 expression on NK1.1+ splenocytes was analyzed as described in B. (D) CFSE-labeled NK cells were cocultured with RMA/Rae-1δ at a 1:1 ratio for different periods of time (10 min, 30 min, 3 h, 6 h, and 24 h). The MFI of anti–Rae-1 mAb staining (closed circles) or isotype control mAb staining (open circles) of CFSE+ NK cells is shown. (E) CFSE-labeled NK cells were cocultured with RMA/Rae-1δ for 2 h together (cell–cell contact) or separated by a semipermeable membrane (Transwell) on a 12-well plate. Then NK cells were stained with isotype control mAb (dotted line histograms), or anti–Rae-1 mAb (gray histograms). Solid line white histograms indicate anti–Rae-1 mAb staining of NK cells without coculture. (F) Cell surface expression of Rae-1γ on NK cells after coculture with RMA/Rae-1γ was analyzed by anti–Rae-1γ specific mAb (gray histogram) or isotype control mAb (dotted line histogram). Solid line white histogram indicates anti–Rae-1γ specific mAb staining of NK cells without coculture. (G) NK cells were stained with biotinylated anti-NK1.1 mAb, followed by DyLight 594-conjugated streptavidin. After 30 min of coculture with RMA/Rae-1δ, cells were stained with FITC-conjugated anti–Rae-1 mAb. Confocal microscopy imaging of NK cells alone (Left) and NK cells with RMA/Rae-1δ (Right) are shown. (Scale bars, 10 μm.) Representative data from three independent experiments are shown.
Trogocytosis of Rae-1 Is Coupled with Clathrin-Dependent NKG2D Endocytosis.
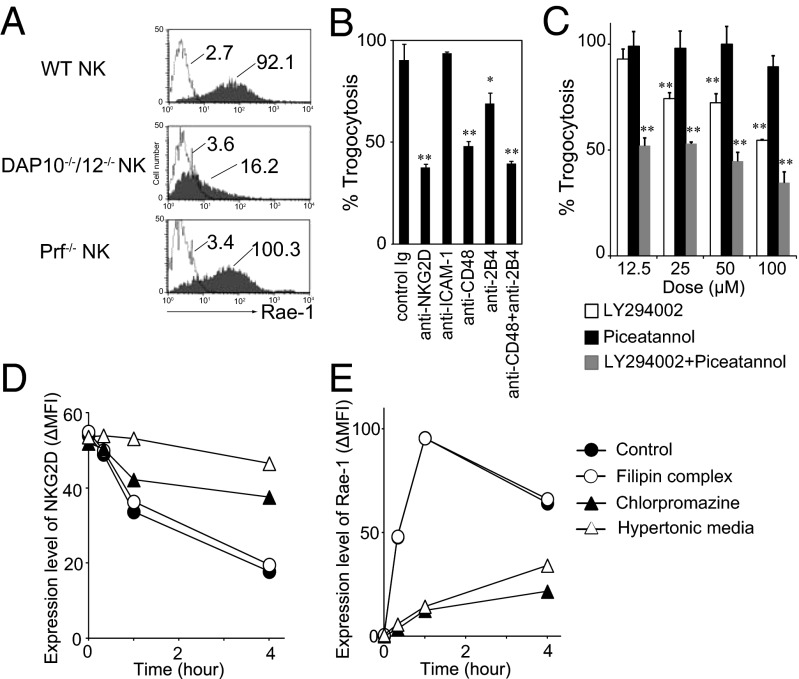
Upon ligation, NKG2D accumulates at the NK-IS, which provides a platform not only for signal transduction, but also for trogocytosis (20). Therefore, we examined the involvement of NK-IS components in trogocytosis. DAP10 and DAP12, which associate with NKG2D at the NK-IS (17), were involved in the trogocytosis of Rae-1 (Fig. 4A). Intercellular adhesion molecule-1 (ICAM-1), which is recruited to NK-IS (30), was not involved in the trogocytosis (Fig. 4B). At NK-IS, a coactivation receptor, 2B4 is engaged by CD48 on target cells (31). Both ligand and receptor is involved in the trogocytosis (Fig. 4B). We next investigated NKG2D signaling leading to trogocytosis by using chemical inhibitors listed in Fig. S6A. DAP10 and DAP12 recruit PI3-kinase (PI3K) and spleen tyrosine kinase (Syk), respectively (10). Combined inhibition of both pathways by LY294002 (PI3K inhibitor) with piceatannol (Syk inhibitor) markedly inhibited the trogocytosis, although LY294002 alone also inhibited at high dose (Fig. 4C). U0126 [mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) inhibitor] or PP2 (Src inhibitor) significantly inhibit the trogocytosis only at high dose (Fig. S6B).
Fig. 4.
Trogocytosis of Rae-1 is coupled with clathrin-dependent NKG2D down-modulation. (A) WT, DAP10−/−/DAP12−/−, or Prf−/− NK cells were cocultured with RMA/Rae-1δ for 1 h, and then Rae-1 acquisition by NK cells was analyzed as described in Fig. 3E. (B and C) WT NK cells were treated with the indicated blocking mAbs (B) or indicated inhibitors (C) for 30 min, and then cocultured with RMA/Rae-1δ as described in A. Percent trogocytosis was calculated as (MFI of anti–Rae-1 mAb staining in the presence of inhibitor)/(MFI of anti–Rae-1 mAb staining in the absence of inhibitor). Data represent means plus SDs of triplicates. *P < 0.05, **P < 0.01, compared with the absence of the inhibitor. (D and E) NK cells were cocultured with RMA/Rae-1δ in the presence or absence of the indicated inhibitors or in the medium containing 0.45 M sucrose for the indicated periods of time. Then cell surface expression of NKG2D and Rae-1 on NK cells was analyzed. Delta MFI was calculated by subtracting MFI of isotype control mAb staining from MFI of specific mAb staining. Similar results were obtained in three independent experiments.
Because we have previously demonstrated that ligand-induced down-modulation of NKG2D is mediated by clathrin-dependent endocytosis (18), we next examined the effect of various endocytosis inhibitors (32–34) on trogocytosis (Fig. 4 C and D). Both cytochalasin D (actin polymeization inhibitor) and methyl-β-cyclodextrin (broad endocytosis inhibitor) inhibited Rae-1 trogocytosis (Fig. 4 D and E and Fig. S6D). Interestingly, chlorpromazine (an inhibitor of clathrin-dependent endocytosis), but not filipin (an inhibitor of caveolae-dependent endocytosis), inhibited both NKG2D down-modulation and trogocytosis of Rae-1 (Fig. 4 D and E, and Fig. S6D). In addition, hypertonic media that block clathrin-coated pit formation (35, 36) showed both inhibitions (Fig. 4 D and E and Fig. S6D). These results suggest that NKG2D-mediated trogocytosis is coupled with clathrin-dependent NKG2D down-modulation.
NK Cells Eliminate Rae-1–Dressed NK Cells in Vitro and in Vivo.
To address the fate of Rae-1–dressed (Rae-1acq+) NK cells in vivo, we injected CFSE-labeled Rae-1acq+ NK cells or Rae-1acq− NK cells into spleen of Rag-1−/− mice. After 4 h, we found a significant decrease in the percentage of transferred Rae-1acq+ NK cells in spleen, suggesting that Rae-1acq+ NK cells are eliminated in vivo (Fig. 5 A and B). We next adoptively transferred Rae-1acq+ NK1.1− BALB/c NK cells into C57BL/6 mice or NK-depleted C57BL/6 mice that had been injected with anti-NK1.1 mAb. These Rae-1acq+ BALB/c NK cells were eliminated in C57BL/6 mice, but not in NK-depleted C57BL/6 mice (Fig. S7), suggesting that Rae-1acq+ NK cells are eliminated by NK cells in vivo. To further address whether Rae-1acq+ NK cells directly lysed by other activated NK cells, called fratricide, we next performed in vitro cytotoxicity assay using sort-purified Rae-1acq+ NK cells (Fig. S8A) as target cells on which Rae-1 remains until 24 h after trogocytosis (Fig. S8B). As we expected, Rae-1acq+ NK cells were lysed by WT NK cells, but not by NK cells from DAP10−/−/DAP12−/− or Prf−/− mice, in an E/T ratio-dependent manner, (Fig. 5 C and D). We next addressed whether cell surface expression of Rae-1 is sufficient for NK cell fratricide by using NK cells from Rae-1 transgenic mice (tg) (Fig. S9A) as target cells. Rae-1 tg NK cells are lysed by NK cells in an E/T ratio-dependent manner, but not by NK cells from DAP10−/−/DAP12−/− or Prf−/− (Fig. S9B). Overall, these results suggest that NK cell fratricide is mediated via the NKG2D-induced perforin pathway (Fig. 5E).
Fig. 5.
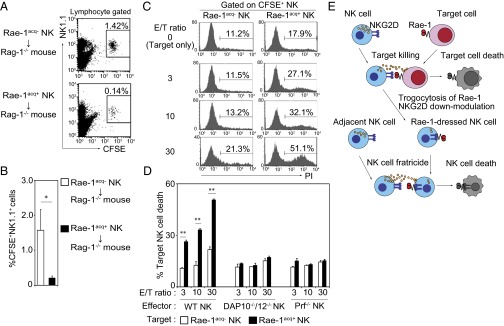
Rae-1–dressed NK cells are efficiently lysed by other NK cells both in vitro and in vivo. (A and B) CFSE-labeled NK cells (5 × 106) were cocultured with RMA or RMA/Rae-1δ for 15 min. Then, these cells were injected into spleen of Rag-1−/− mice (n = 4, each group). After 4 h, splenocytes were stained with anti-NK1.1 mAb, and the percentage of CFSE+ NK1.1+ cells in the lymphocyte gate was calculated. (C and D) CFSE-labeled NK cells were cocultured with RMA or RMA/Rae-1δ for 15 min, and then CFSE+ NK cells were sort purified as shown in Fig. S8A. These NK cells were used as target cells and cocultured with effector WT NK cells for 2 h. Percent cell death of target NK (PI+CFSE+ cells/CFSE+ cells) was calculated. Data are presented as the means plus SDs of triplicates. *P < 0.05, **P < 0.01, compared with Rae-1acq− NK cells. Representative data from two independent experiments are shown. (E) Model for NK cell fratricide via NKG2D-mediated trogocytosis. Interaction between NKG2D on NK cells and Rae-1 on target cells results in target cell lysis and trogocytosis of Rae-1 by NK cells. NK cells dressed with tumor-derived Rae-1 are lysed by other NK cells via the NKG2D-induced perforin pathway.
Discussion
In this study, we revealed a unique pathway for NK cell fratricide: NK cells acquire NKG2DL from target tumor cells via trogocytosis and are subsequently lysed by other NK cells through the NKG2D-induced perforin pathway (Fig. 5E). Our findings may explain the previously observed phenomena that NK cells undergo death when they interact with tumor cells (6, 7). We further showed that trogocytosis of Rae-1 is coupled with clathrin-dependent NKG2D endocytosis.
Trogocytosis was first observed 40 y ago on mouse T cells, which acquire MHCII from B cells (37). However, the molecular mechanism and physiological relevance of trogocytosis remained unknown for a long period. Recently, it was revealed that the trogocytosis of MHCII by T-cell receptor (TCR) requires the driving force of clathrin-independent TCR internalization, which is dependent on small GTPases TC21 and Rho G-mediated phagocytic machinery (38, 39). Unlike TCR trogocytosis, NKG2D trogocytosis may require the clathrin-dependent NKG2D internalization. In contrast to rapid internalization of NKG2D, the trogocytosed ligand Rae-1 remains on the NK cell surface at a substantial level for at least 24 h (Fig. S8B). Therefore, tumor-experienced NK cells probably lose NKG2D-mediated effector function and are subsequently lysed by other NK cells. Indeed, sort-purified Rae-1–dressed NK cells alone do not die (Fig. 5C; E/T ratio of 0), and these NK cells were lysed by newly added NK cells in an E/T ratio-dependent manner (Fig. 5C), suggesting that Rae-1–dressed NK cells do not attack each other. NK cells that committed fratricide may sequentially acquire Rae-1 and turn to be target cells, generating a negative feedback loop. When NK cells were cocultured with RMA/Rae-1δ, NK cells died most at E/T ratio of 1 (Fig. 1B). The trogocytosis and fratricide may occur most efficiently at E/T ratio of 1. A previous study showed that human NK cell death occurred in an E/T ratio-independent manner when cocultured with K562 (6). It is possible that trogocytosis-mediated NK cell fratricide may be one of the mechanisms of the NK cell death.
NK cells and cytotoxic T lymphocytes (CTLs) were considered to be perforin resistant (40, 41), whereas perforin-mediated fratricide of CTLs was frequently observed (42–44). Therefore, besides Fas ligand, perforin may also play an important role for activation-induced cell death, in particular fratricide. Fratricide is proposed to contribute to memory T-cell homeostasis (45). For instance, CTLs acquire MHCI from their targets (42), and this trogocytosis is considered to promote self-recognition by CTLs, which may be involved in down-regulating the immune response (46). Likewise, we propose that NKG2D trogocytosis-mediated NK cell fratricide could contribute to maintenance of NK cell homeostasis. If tumor cells actively give their NKG2DL to NK cells for immune escape, the perturbation of NKG2D trogocytosis-mediated NK cell fratricide may enhance NK cell-mediated antitumor immunity. However, it may also cause NK cell-mediated host injury. Given that trogocytosis of Rae-1 requires NKG2D signal leading to generation of the pulling force, NK cells may intentionally acquire tumor-derived Rae-1 for the purpose of activation-induced regulation. Although the specific inhibition of trogocytosis is impossible at this moment, identification of a specific molecule that regulates trogocytosis would reveal the physiological relevance of trogocytosis.
Materials and Methods
Mice.
C57BL/6 and BALB/c female mice (6 wk old) were obtained from CLEA Japan. Toshiyuki Takai (Tohoku University, Sendai, Japan) kindly provided DAP10 and DAP12 double-deficient mice. Perforin-deficient mice and Rag-1–deficient mice were obtained from The Jackson Laboratory. Rae-1ε transgenic mice were generated as previously described (47). These mice were maintained under specific pathogen-free conditions and used according to the guidelines of the Tohoku University Institutional Animal Care and Use Committee.
Isolation of Mouse NK Cells.
NK cells were prepared from mouse splenocytes as described previously (28). In brief, splenocytes were incubated with anti-CD4 mAb (clone GK1.5) and anti-CD8 mAb (clone 53-6.7), followed by magnetic beads coated with goat anti-mouse Ig and goat anti-rat Ig Abs (Qiagen). Then CD4-positive cells, CD8-positive cells, and surface Ig-positive cells were removed by magnetic cell sorting. Subsequently, NK cells were positively selected by using phycoerythrin (PE)-labeled DX5 (anti-CD49b) mAb (BioLegend) and anti–PE-conjugated MACS beads (Miltenyi Biotec). Purified NK cells were cultured in growth medium supplemented with recombinant human IL-2 (rhIL-2; 1,000 units/mL; Wako) for 5 d.
Cell Lines.
James C. Ryan (University of California, San Francisco) kindly provided the RMA (H-2b-positive) lymphoma cells and the RMA-S (H-2b-deficient) cells. RMA cells stably expressing Rae-1γ (RMA/Rae-1γ) or Rae-1δ (RMA/Rae-1δ), and B16 melanoma stably expressing Rae-1ε (B16/Rae-1ε), were established as previously described (13).
Trogocytosis and NK Fratricide Assay.
CFSE-labeled IL-2–activated NK cells (1 × 105 per well) and Rae-1–expressing target cells (1 × 105 per well) were cocultured in a 96-well flat-bottom plates for indicated periods at 37 °C. Cells were stained with anti–Rae-1 mAb (186107; R&D Systems), and acquisition of Rae-1 by CFSE+ NK cells was analyzed by FACSCanto II (BD Biosciences). As for the NK fratricide assay, the cytotoxic activity against sort-purified Rae-1–dressed NK cells was analyzed.
Further details for materials and experimental procedures are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Lewis Lanier for helpful suggestions, Chika Takahashi and Madoka Itabashi for technical assistance, and Hiromi Yoshida for cell sorting. This work was supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (to K.O., M.K., and M.N.); and Grants-in-Aid for Scientific Research from the Ministry of Health, Labor, and Welfare of Japan [H22-meneki-ippan-004 (to K.O.) and 005 (to M.N.)].
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300140110/-/DCSupplemental.
References
- 1.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, et al. Chronic natural killer cell lymphocytosis: A descriptive clinical study. Blood. 1994;84(8):2721–2725. [PubMed] [Google Scholar]
- 3.Morice WG, Leibson PJ, Tefferi A. Natural killer cells and the syndrome of chronic natural killer cell lymphocytosis. Leuk Lymphoma. 2001;41(3-4):277–284. doi: 10.3109/10428190109057982. [DOI] [PubMed] [Google Scholar]
- 4.Zhang R, Shah MV, Loughran TP., Jr The root of many evils: Indolent large granular lymphocyte leukaemia and associated disorders. Hematol Oncol. 2010;28(3):105–117. doi: 10.1002/hon.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortaldo JR, Mason AT, O’Shea JJ. Receptor-induced death in human natural killer cells: involvement of CD16. J Exp Med. 1995;181(1):339–344. doi: 10.1084/jem.181.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taga K, et al. Target-induced death by apoptosis in human lymphokine-activated natural killer cells. Blood. 1996;87(6):2411–2418. [PubMed] [Google Scholar]
- 7.Jewett A, Bonavida B. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J Immunol. 1996;156(3):907–915. [PubMed] [Google Scholar]
- 8.Boissel N, et al. BCR/ABL oncogene directly controls MHC class I chain-related molecule A expression in chronic myelogenous leukemia. J Immunol. 2006;176(8):5108–5116. doi: 10.4049/jimmunol.176.8.5108. [DOI] [PubMed] [Google Scholar]
- 9.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3(10):781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 10.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235(1):267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diefenbach A, Raulet DH. Strategies for target cell recognition by natural killer cells. Immunol Rev. 2001;181:170–184. doi: 10.1034/j.1600-065x.2001.1810114.x. [DOI] [PubMed] [Google Scholar]
- 12.Kärre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55(3):221–228. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 13.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci USA. 2001;98(20):11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8(9):713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dustin ML, Long EO. Cytotoxic immunological synapses. Immunol Rev. 2010;235(1):24–34. doi: 10.1111/j.0105-2896.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3(12):1150–1155. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 17.Giurisato E, et al. Phosphatidylinositol 3-kinase activation is required to form the NKG2D immunological synapse. Mol Cell Biol. 2007;27(24):8583–8599. doi: 10.1128/MCB.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogasawara K, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18(1):41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 19.McCann FE, Eissmann P, Onfelt B, Leung R, Davis DM. The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. J Immunol. 2007;178(6):3418–3426. doi: 10.4049/jimmunol.178.6.3418. [DOI] [PubMed] [Google Scholar]
- 20.Roda-Navarro P, Vales-Gomez M, Chisholm SE, Reyburn HT. Transfer of NKG2D and MICB at the cytotoxic NK cell immune synapse correlates with a reduction in NK cell cytotoxic function. Proc Natl Acad Sci USA. 2006;103(30):11258–11263. doi: 10.1073/pnas.0600721103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4(9):815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 22.Su MW, Walden PR, Golan DB, Eisen HN. Cognate peptide-induced destruction of CD8+ cytotoxic T lymphocytes is due to fratricide. J Immunol. 1993;151(2):658–667. [PubMed] [Google Scholar]
- 23.van den Broek MF, Kägi D, Zinkernagel RM, Hengartner H. Perforin dependence of natural killer cell-mediated tumor control in vivo. Eur J Immunol. 1995;25(12):3514–3516. doi: 10.1002/eji.1830251246. [DOI] [PubMed] [Google Scholar]
- 24.Smyth MJ, et al. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42(4):501–510. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Shibuya A, et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4(6):573–581. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 26.Cerwenka A, et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12(6):721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 27.Cédile O, et al. The NKG2D ligands RAE-1δ and RAE-1ε differ with respect to their receptor affinity, expression profiles and transcriptional regulation. PLoS ONE. 2010;5(10):e13466. doi: 10.1371/journal.pone.0013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama M, et al. Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells. Proc Natl Acad Sci USA. 2011;108(45):18360–18365. doi: 10.1073/pnas.1110584108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roda-Navarro P, Reyburn HT. Intercellular protein transfer at the NK cell immune synapse: Mechanisms and physiological significance. FASEB J. 2007;21(8):1636–1646. doi: 10.1096/fj.06-7488rev. [DOI] [PubMed] [Google Scholar]
- 30.Textor S, et al. NF-κ B-dependent upregulation of ICAM-1 by HPV16-E6/E7 facilitates NK cell/target cell interaction. Int J Cancer. 2011;128(5):1104–1113. doi: 10.1002/ijc.25442. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, et al. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity. 2009;31(1):99–109. doi: 10.1016/j.immuni.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamaze C, Fujimoto LM, Yin HL, Schmid SL. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997;272(33):20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- 33.Morcavallo A, et al. Insulin and insulin-like growth factor II differentially regulate endocytic sorting and stability of insulin receptor isoform A. J Biol Chem. 2012;287(14):11422–11436. doi: 10.1074/jbc.M111.252478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12(3):468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 35.Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108(2):389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12(8):517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 37.Cone RE, Sprent J, Marchalonis JJ. Antigen-binding specificity of isolated cell-surface immunoglobulin from thymus cells activated to histocompatibility antigens. Proc Natl Acad Sci USA. 1972;69(9):2556–2560. doi: 10.1073/pnas.69.9.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martínez-Martín N, et al. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35(2):208–222. doi: 10.1016/j.immuni.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dopfer EP, Minguet S, Schamel WW. A new vampire saga: The molecular mechanism of T cell trogocytosis. Immunity. 2011;35(2):151–153. doi: 10.1016/j.immuni.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Liu CC, et al. Resistance of cytolytic lymphocytes to perforin-mediated killing. Induction of resistance correlates with increase in cytotoxicity. J Exp Med. 1989;169(6):2211–2225. doi: 10.1084/jem.169.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balaji KN, Schaschke N, Machleidt W, Catalfamo M, Henkart PA. Surface cathepsin B protects cytotoxic lymphocytes from self-destruction after degranulation. J Exp Med. 2002;196(4):493–503. doi: 10.1084/jem.20011836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang JF, et al. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286(5441):952–954. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 43.Su MW, et al. Fratricide of CD8+ cytotoxic T lymphocytes is dependent on cellular activation and perforin-mediated killing. Eur J Immunol. 2004;34(9):2459–2470. doi: 10.1002/eji.200425096. [DOI] [PubMed] [Google Scholar]
- 44.Leisegang M, et al. MHC-restricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors. J Clin Invest. 2010;120(11):3869–3877. doi: 10.1172/JCI43437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callard RE, Stark J, Yates AJ. Fratricide: A mechanism for T memory-cell homeostasis. Trends Immunol. 2003;24(7):370–375. doi: 10.1016/s1471-4906(03)00164-9. [DOI] [PubMed] [Google Scholar]
- 46.Trambas CM, Griffiths GM. Delivering the kiss of death. Nat Immunol. 2003;4(5):399–403. doi: 10.1038/ni0503-399. [DOI] [PubMed] [Google Scholar]
- 47.Ogasawara K, Benjamin J, Takaki R, Phillips JH, Lanier LL. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol. 2005;6(9):938–945. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.