Abstract
The Polycomb Group (PcG) complex of transcriptional repressors is critical for the maintenance of stage-specific developmental gene expression, stem cell maintenance and for large-scale chromosomal dynamics. Functional deficiency of a single PcG gene can severely compromise PcG function, leading to developmental defects, embryonic lethality, or a number of malignancies. Despite the critical nature of PcG proteins, the mechanisms by which these complexes mediate their effects are relatively uncharacterized. Nearly all vertebrate PcG proteins lack inherent DNA binding capacity, making it unclear how they are targeted to Polycomb response element (PRE) sequences. Transcription factor YY1 is a functional ortholog of a Drosophila PcG protein, Pleiohomeotic (PHO), one of the few PcG proteins with specific DNA binding capability, and YY1 can recruit PcG proteins to specific DNA sequences. A small 25 amino acid YY1 domain (the REPO domain) is necessary and sufficient for recruitment of PcG proteins to DNA and for transcriptional repression. We show here that the YY1 REPO domain interacts with PcG protein Yaf2 and recruits Yaf2 to DNA. Interaction is lost when the YY1 REPO domain is deleted. In addition we show that Yaf2, when linked to a heterologous DNA binding domain, can recruit PcG proteins to DNA leading to transcriptional repression. When the Drosophila homolog of Yaf2 (dRYBP) is mutated, PcG recruitment to DNA is reduced. Taken together, our results suggest that Yaf2 serves as a molecular bridge between YY1 and other PcG complex proteins.
Keywords: POLYCOMB, YY1, REPRESSION, YAF2, DNA RECRUITMENT
Polycomb Group (PcG) proteins are important for normal embryogenesis and are implicated in pathological states such as cancer [Sparmann and van Lohuizen, 2006]. PcG proteins are best understood as regulators of gene expression where they act to silence target genes [Lund and van Lohuizen, 2004]. These proteins assemble into highly conserved complexes and inhibit transcription through mechanisms yet unknown [Otte and Kwaks, 2003; Levine et al., 2004]. Target genes of the PcG complex contain response elements (PREs) that bind the PcG proteins and are necessary for silencing [Mihaly et al., 1998; Ringrose et al., 2003; Ringrose and Paro, 2007]. These sites function as maintenance elements/memory elements that control on/off states of target genes. Functional deficiency of a single PcG gene can severely compromise PcG function resulting in either severe developmental defects or in embryonic lethality [Lewis, 1978; Simon et al., 1992; Muller and Kassis, 2006]. PcG proteins can regulate stem cell function and maintenance, and PcG dysfunction is associated with a number of malignancies [Valk-Lingbeek et al., 2004; Sparmann and van Lohuizen, 2006]. Despite the critical nature of PcG proteins, the mechanisms by which these complexes mediate their effects are relatively unknown. The potency of transcriptional silencing by PcG proteins emphasizes their necessity for development, and their importance in disease emphasizes the need to define the mechanisms that PcG proteins use to recognize PREs.
One potential mechanism for the recruitment of PcG proteins to PREs likely results from physical interactions with sequence-specific transcription factors that bind within PREs. In Drosophila, PcG proteins assemble into biochemically distinct Polycomb repressive complexes (PRC): PRC1 contains Polycomb (Pc), Polyhomeotic (Ph), Posterior sex combs (Psc), and Sex combs extra (Sce/dRing); PRC2 contains extra sex combs (Esc), Enhancer of zeste (E(z)), and Suppressor of zeste 12 (Su(z)12). However, these complexes lack sequence-specific binding activity suggesting that components outside these core complexes are responsible for recognizing sequences within PREs. Several proteins have been investigated as recruiting factors for the PcG proteins; YY1/PHO (see below), Pholike, Zeste, GAGA, Dsp1, and most recently, AEBP2 [Brown et al., 2003; Muller and Kassis, 2006; Kim et al., 2009]. Of these, the YY1/PHO proteins are the best-established links between PRC core complexes and PRE sequences.
YY1/PHO play important roles in proper targeting of PcG proteins in vivo. YY1 is the mammalian homolog of the Drosophila pleiohomeotic (PHO) protein [Brown et al., 1998]. These proteins share two regions of high homology: the C terminal region containing four C2H2-type zinc fingers (95%) and the internal REPO domain (82%). The high conservation in the zinc finger region is reflected in the DNA binding activity of the proteins. Well-characterized PRE sequences contain PHO (and YY1) sites and these are found to be required for silencing activity [Mihaly et al., 1998]. These PREs include, iab-2, iab-7, MCP, and the Ultrabithorax (UBX) PRE 1.6 [Brown et al., 1998; Fritsch et al., 1999; Shimell et al., 2000; Busturia et al., 2001; Mishra et al., 2001; Mahmoudi et al., 2003]. Silencing also depends on function of the PHO protein since pho mutants display loss of silencing and homeotic phenotypes [Girton and Jeon, 1994; Brown et al., 1998, 2003; Kwon et al., 2003; Fujioka et al., 2008]. In the absence of PHO, PcG proteins are lost from DNA at many, but not all genomic locations [Brown et al., 2003]. PHO has been linked to three PRCs. In the case of PRC1, PHO has been found to interact physically and functionally with the Pc protein [Mohd-Sarip et al., 2002, 2005; Kwon et al., 2003] and to the mouse RYBP protein and the Drosophila RYBP protein [Garcia et al., 1999; Bejarano et al., 2005]. PHO was also found to interact with the PRC2 complex protein, E(z) [Wang et al., 2004]. Finally PHO has been purified as part of a distinct complex, PhoRC, with methylated nucleosome binding activity [Klymenko et al., 2006]. Several studies indicate that PHO may require cofactors for optimal activity including Grainyhead or dSfmbt [Blastyak et al., 2006; Klymenko et al., 2006]. Some authors suggest that targeting may require several factors similar to an enhanceosome model. The sequence homology shared between YY1 and PHO strongly suggested that YY1 performs similar activity in mammals.
Based on the properties of PHO and the homology with YY1, we were interested in determining the function of YY1 in PcG complex recruitment to DNA. YY1 was found to silence a PcG-responsive reporter gene and recruit PcG proteins to this locus [Atchison et al., 2003; Srinivasan and Atchison, 2004]. YY1 corrected the lethal and homeotic phenotypes of Pho1/Pho1 and Pho1/Phocv mutants, respectively, arguing for a direct physiological role in PcG function [Atchison et al., 2003]. We subsequently set out to determine the YY1 sequences necessary and sufficient for its PcG function. Using a fly transgenic approach we found that deletion of a small 25 amino acid segment of similarity between YY1 and PHO (residues 201–225), abolished both PcG-dependent repression, and the ability of YY1 to recruit PcG proteins to DNA [Wilkinson et al., 2006]. Remarkably, this 25 amino acid domain, when tethered to the GAL4 DNA binding domain (DBD), was completely sufficient for PcG repression and recruitment of PcG proteins to DNA [Wilkinson et al., 2006]. We named this YY1 domain the REPO domain for its ability to REcruit POlycomb. However, the mechanism of interaction between the YY1 REPO domain and the PcG complexes remained undefined. In light of the importance of PcG function in development and disease, it is crucial to determine the molecular links between YY1 bound to DNA and the PcG complexes. We therefore, set out to identify the biochemical interactions that link the YY1 REPO domain with the PcG proteins in vivo.
We found that the YY1 REPO domain interacts with the PcG protein, Yaf2, and can recruit Yaf2 to DNA. In turn Yaf2 can recruit other PcG proteins to DNA, leading to transcriptional repression, and loss of the Drosophila Yaf2 homolog, dRYBP, results in reduced PcG recruitment. Our data are consistent with a model in which Yaf2 provides a bridging function between YY1/PHO and other PcG complex proteins.
MATERIALS AND METHODS
DROSOPHILA STRAINS
Drosophila strains were obtained from the Bloomington Stock center (dRYBP, stock number 14968) or as kind gifts from Nancy Bonini (ry506 and various balancer strains) and Jürg Muller (BGUZ).
PLASMID CONSTRUCTION
Various YY1 and Yaf2 expression constructs were prepared using PCR-based cloning techniques and verified by sequence analyses. Subcloning details are available on request. Constructs for Drosophila injections were prepared according to the protocols provided by Genetic Services, Inc. (Cambridge, MA).
YEAST TWO HYBRID SCREEN
Saccharomyces cerevisiae AH109, media dropout supplements, and plasmid vectors were from Clontech (Palo Alto, CA). Transformation of S. cerevisiae with plasmid vectors was performed according to the manufacturer’s protocol. Transformants were isolated on appropriate selective dropout media and passaged aseptically. All cultures were maintained at 30°C.
For the library screen, AH109 was transformed with the bait construct pGBKt7 expressing the REPO domain (YY1 201–226) cloned in frame with DNA binding residues (1–147) of the GAL4 transcription factor (GAL DBD) and maintained on Trp dropout medium. The pACT2 library containing mouse 17-day embryonic cDNA was transformed into the bait vector-transformed strain using the library scale protocol described by the manufacturer. Transformants were plated onto selective medium (Trp/Leu/Ade/His drop out). Colonies were passaged several times on selective medium. To recover prey plasmids, colonies were grown in selective medium (5 ml broth culture) and processed with the Yeastmaker plasmid isolation kit (Clontech). Plasmid DNA was transformed into DH5α cells under Ampr selection. Plasmids were sequenced using a primer corresponding the HA epitope tag of the pACT2 vector. Sequence data were subject to BLAST analysis against the nr/nt database using default parameters on the web interface (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
For mapping YY1–Yaf2 interactions, AH109 was transformed with pGADt7 prey constructs expressing the GAL4 activation domain (residues 768–881) cloned in frame with Yaf2 (full length, residues 1–179; N fragment, residues 1–101; or C fragment, residues 102–179) and maintained on Leu dropout medium. These strains were then transformed with pGBKt7 bait constructs containing full-length YY1, YY1 1–414Δ201–226, or YY1 201–226 fused to the GAL4 DBD where indicated. Cotransformants were isolated on Trp/Leu dropout medium and passaged onto selective medium (Trp/Leu/Ade/His dropout medium).
DROSOPHILA TRANSGENESIS
All fly strains were maintained at 25°C on commercially available medium. Transgenic injections of pRy-derived constructs were performed by Genetic Services, Inc. Transgene incorporation was determined by correction to ry+ phenotype. Transgene positive strains were crossed to balancer strains and maintained as balanced stocks.
BGUZ REPRESSION ASSAY
Processing of embryos for BGUZ transcriptional activity was as described previously [Muller, 1995; Atchison et al., 2003; Wilkinson et al., 2006]. Drosophila strains expressing full-length mouse Yaf2 (residues 1–179) contained the Yaf2 cDNA fused in frame with the GAL4 DBD (residues 1–147) under control of the hunchback promoter (hb-GALYaf2). Males from three independent transgenic Drosophila strains (representing transgene incorporation into each of the X, second, and third chromosomes) were crossed to BGUZ virgin females. Embryos from timed egg lays were fixed with formaldehyde at hour 6 after the conclusion of the egg lay. Fixed embryos were stained with X-gal to detect LacZ activity in embryonic tissues [Muller, 1995; Atchison et al., 2003].
CHROMATIN IMMUNOPRECIPITATION (ChIP) ASSAYS
Processing of Drosophila embryos for ChIP was essentially as described previously [Srinivasan and Atchison, 2004; Wilkinson et al., 2006]. Antibodies were kindly provided as follows: anti-PHO, Judy Kassis (NIH); anti-E(z), Vincent Pirotta (Rutgers). Antibodies were obtained also from commercial sources: anti-GAL DBD rabbit polyclonal (Santa Cruz sc-577), anti-Flag M2 mouse monoclonal (Sigma F-3165) anti-Polycomb rabbit polyclonal (Santa Cruz sc-25762), anti-H3AcK9 rabbit polyclonal (Upstate 06–942) and anti-H3me3K27 rabbit polyclonal (Upstate 07–449). Drosophila strains were crossed such that each embryo was predicted to contain one copy each of the BGUZ reporter element, hsp70-driven flag-tagged Yaf2, and either hsp70-driven GAL DBD or hsp70-driven GAL YY1 201–226. Embryos from crosses were heat shocked (37°C for 45 min) at 3 h after the start of the egg lay and maintained at 25°C until hour 6. At that time, the embryos were fixed with 2% formaldehyde, washed, and sonicated. Chromatin was estimated by absorbance at 260 nm.
Equal quantities of chromatin were diluted and immunoprecipitated using the indicated antibody. Immunoprecipitated chromatin was subject to crosslink reversal and detection by PCR using nested primers [Srinivasan and Atchison, 2004; Wilkinson et al., 2006].
RESULTS
THE YY1 REPO DOMAIN BINDS TO YAF2
A yeast two hybrid screen was used to identify potential ligands for the YY1 REPO domain. The YY1 REPO domain was cloned as a GAL4 DBD fusion construct into the bait vector, pGBKT7 (Clontech) and was used to screen a mouse 17-day embryonic library (Clontech) in yeast strain AH109. Colonies that were viable on selective medium (Trp/Leu/Ade/His dropout medium) were verified by several rounds of passaging. Plasmid DNA was recovered from each of the colonies and sequenced. Of the 20 unique clones obtained (see Supplementary Table), mouse Yaf2 was identified as a previously characterized YY1 binding partner. Yaf2 shares extensive homology with another protein, RYBP. RYBP was initially identified as an interacting partner for the mammalian PcG proteins Ring1A, Ring1B, M33, and YY1 [Garcia et al., 1999]. The Drosophila homolog of RYBP, dRYBP, was characterized as a PcG protein in its own right since it could repress transcription of Ubx in a PcG-dependent manner [Bejarano et al., 2005]. It is noteworthy that Yaf2 shares extensive homology with dRYBP as well as mammalian RYBP.
Similar to results obtained by Kalenik et al. [1997], the Yaf2 clone was missing the N terminus and consisted of residues 17–179 predicted from the mouse cDNA (accession number NM_024189). However, successful capture of this clone suggests that these missing residues are not required for interaction with the YY1 REPO domain. As Yaf2 interacts with the REPO domain, it represented a candidate for a bridge protein that links YY1 with the PcG complex. This bridge protein should interact with full-length YY1 and with the REPO domain fused to the GAL4 DBD, but should fail to interact with a YY1ΔREPO mutant.
To verify that full-length Yaf-2 interacts with the YY1 REPO domain, we introduced the DNA encoding the missing N terminal amino acids into our clone by PCR based on the predicted nucleotide sequence. This construct was cloned into the pGADT7 prey vector and tested for interaction with full-length YY1, YY1 1–414Δ201–226 (REPO domain deletion), and the isolated YY1 REPO domain (YY1 residues 201–226) expressed from the pGBKT7 bait vector. These constructs were introduced into AH109 and cotransformants were isolated on permissive medium (Trp/Leu dropout medium). Cotransformed colonies were streaked onto selective medium (Trp/Leu/Ade/His dropout medium). Strains containing full-length YY1 or YY1 REPO domain bait plasmids were able to grow on selective medium when Yaf2 was expressed from the prey plasmid (Fig. 1). Thus, Yaf2 fulfills the predicted properties of a bridge protein in interacting with the REPO domain. Importantly, cultures containing the YY1 REPO domain deletion were unable to grow on selective medium. Empty vector controls containing either pGBKt7 or pGADt7 were also negative for growth on selective medium (Fig. 1). These data indicate that the YY1 REPO domain can interact with Yaf2 under cellular conditions, and that the REPO domain is necessary for interaction with Yaf2. Thus, Yaf2 fulfills the initial properties of a bridge molecule that links YY1 and the PcG complex. As an additional test, the YY1 REPO domain should be sufficient for recruiting Yaf2 to DNA.
Fig. 1.
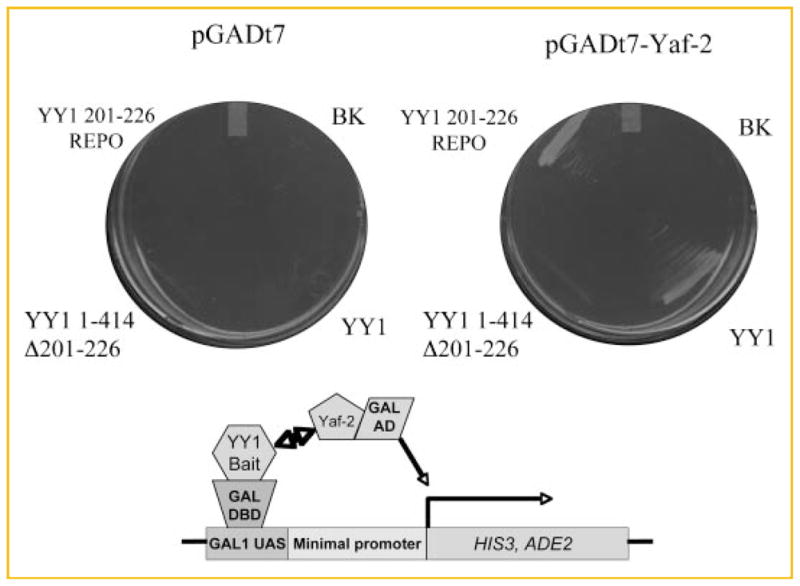
Yeast two hybrid detection of REPO–Yaf2 interactions. S. cerevisiae AH109 was transformed with the indicated bait and prey constructs. Cotransformants were passaged onto selective medium (Trp/Leu/Ade/His dropout medium). The plate on the left contains pGADt7 empty vector expressing the GAL4 activation domain (AD) and the plate on the right contains pGADt7 vector expressing the AD fused to full-length Yaf2 (residues 1–179, pGADt7-Yaf2). Bait constructs expressing GAL4 DBD fusions are as indicated: BK, pGBKt7 empty vector control; YY1, full-length YY1 1–414; YY1 1–414Δ201–226; YY1 201–226 REPO, YY1 residues 201–226. The scheme below diagrams the strategy used in the assay. Transcription of the nutritional markers HIS3 and ADE2 from interactions resulting from bait-prey binding interactions (two head arrow) is indicated.
THE YY1 REPO DOMAIN RECRUITS YAF2 TO DNA
If Yaf2 is a cellular ligand for the YY1 REPO domain, we predicted that this interaction would be observed at a promoter that is silenced in a PcG-dependent mechanism. The Drosophila BGUZ reporter was previously used to identify PcG-dependent silencing mechanisms [Muller, 1995; Atchison et al., 2003; Wilkinson et al., 2006]. BGUZ is a transgenic reporter that consists of a LacZ reading frame under control of the Ubx promoter and BXD enhancer. The construct also contains a multimerized Gal4 recognition sequence (Fig. 2A). To test our prediction, we generated a transgenic Drosophila strain that expressed flag-tagged Yaf2 protein under control of the heat shock-inducible promoter, hsp70. This strain was crossed to flies that contained the BGUZ reporter and either the GAL4 DBD (GAL) or the YY1 REPO domain fused to the GAL4 DBD (GALREPO) under control of hsp70. Thus, heat shock will induce expression of the flag-Yaf2 construct and the GAL constructs in the embryos simultaneously.
Fig. 2.
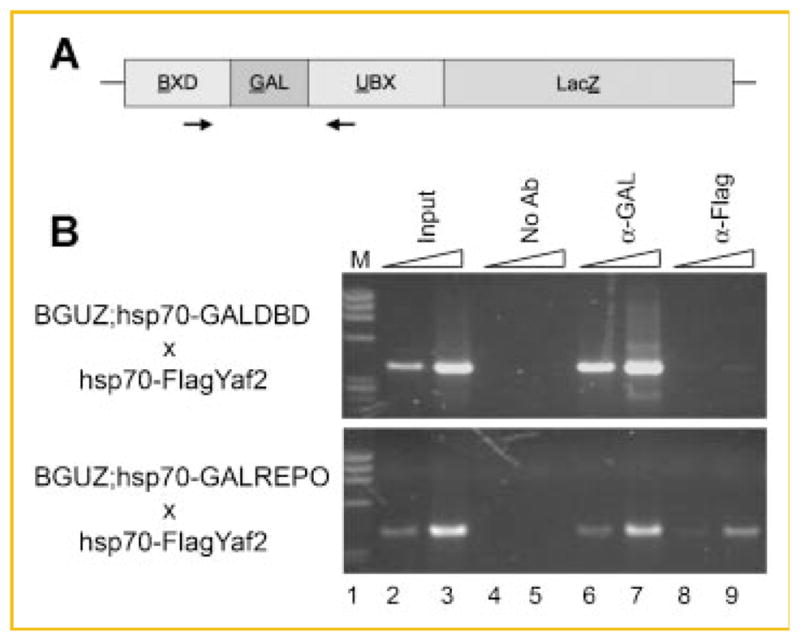
Yaf2 is bound to DNA in the presence of GALREPO. A: Diagram of the BGUZ transgenic reporter. The reporter consists of the LacZ coding sequence under control of the Ubx promoter and BXD enhancer elements. The multi-merized Gal4 recognition sequence is upstream from the Ubx promoter. The black arrows indicate the approximate positions of the PCR primers used in panel B. B: Agarose gel electrophoresis of PCR products detected from ChIP assays stained with ethidium bromide. The cross indicating the chromatin source is indicated on the left. The triangles indicate a 10-fold change in template concentration. Antibodies used for the immunoprecipitations are indicated above the appropriate lanes. M indicates molecular weight markers. Numbers indicate lanes referred to in the text.
Embryos that resulted from the above crosses were collected from a 1-h egg lay and processed for ChIP assay after heat shock. Equal mass of chromatin was immunoprecipitated with either anti-Gal4 DBD or anti-flag mouse M2 monoclonal antibodies, and immunoprecipitated DNA was detected by PCR after crosslink reversal. After heat-shock, both GAL DBD and GALREPO proteins bound efficiently to DNA as expected (Fig. 2B, compare top and bottom panels lanes 6 and 7). However, flag-Yaf2 bound to the BGUZ reporter only in the presence of the GALREPO protein but not the GAL DBD alone (Fig. 2B, lanes 8 and 9). Thus, Yaf2 can be selectively recruited to the BGUZ reporter in the presence of the REPO domain but is not detectable in its absence. The recruitment of Yaf2 to DNA by the REPO domain is an additional indicator that REPO–Yaf2 interactions represent a bridge between YY1 and the PcG complexes.
YAF2 RECRUITS POLYCOMB GROUP PROTEINS TO DNA
If the function of the YY1 REPO domain is to recruit Yaf2 to DNA, and Yaf2 serves to bridge YY1 to the PcG complex, we predicted that direct tethering of Yaf2 to the BGUZ reporter could obviate the need for interaction with REPO. To test this prediction, we assayed for recruitment of PcG complex proteins and associated histone modifications by direct tethering of Yaf2 to the BGUZ reporter via the GAL DBD (GALYaf2). Male flies from a transgenic Drosophila strain expressing GALYaf2 under control of the hsp70 promoter were crossed to BGUZ females and embryos were processed for ChIP as described above. Males from a transgenic strain expressing GAL DBD crossed to BGUZ females were used as a negative control. Both GAL DBD and GALYaf2 proteins bound efficiently to DNA after heat shock (Fig. 3, lanes 6 and 7). However, tethering of GALYaf2 to BGUZ resulted in a strong increase in Pc binding to DNA that was not observed with the GAL DBD alone (Fig. 3, compare top and bottom panels, lanes 8 and 9). We also observed a strong signal for H3 trimethyl-lysine 27 that remained unchanged, and we observed little change in the acetylated H3K9 mark (Fig. 3, lanes 10–13). Our results indicate that when Yaf2 is tethered to DNA, it is sufficient to bring PcG proteins to DNA in the absence of the REPO domain. These results are again, consistent with a model where Yaf2 functions as a bridge between the REPO domain and PcG proteins.
Fig. 3.
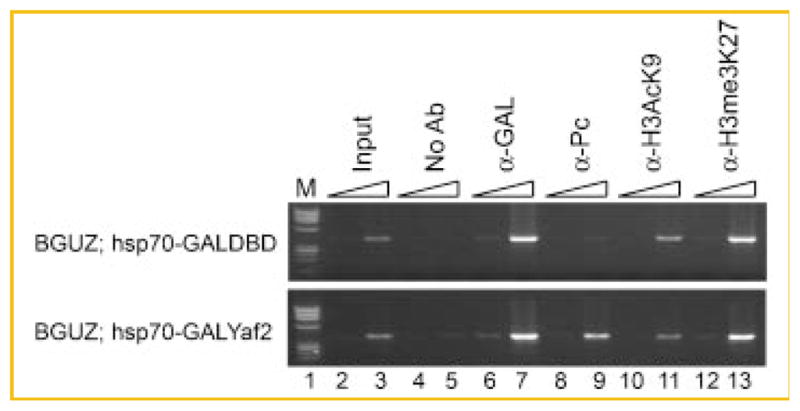
Yaf2 recruits PcG proteins to DNA. Agarose gel electrophoresis of PCR products detected by ChIP assay stained with ethidium bromide. The BGUZ reporter and PCR primer locations are as indicated in Figure 2. The cross indicating the chromatin source is indicated on the left. The triangles indicate a 10-fold change in template concentration. Antibodies used for the immunoprecipitation are indicated above the appropriate lanes. M indicates molecular weight markers. Numbers indicate lanes referred to in the text.
YAF2 SILENCES THE BGUZ REPORTER
In the previous experiment, Yaf2 was sufficient to recruit PcG proteins to DNA. Recruitment of PcG proteins is predicted to result in transcriptional silencing of the BGUZ reporter. To test this prediction, Yaf2 was expressed in early Drosophila embryos as a GAL DBD fusion (GALYaf2) under control of the hunchback (Hb) promoter. Hb drives anterior expression during the first 3 h of embryogenesis and this should lead to repressed LacZ activity from the BGUZ reporter due to repression by GALYaf2. Three independent transgenic strains were crossed to BGUZ flies and embryos from timed egg lays were fixed and stained for expression of LacZ (Fig. 4).
Fig. 4.
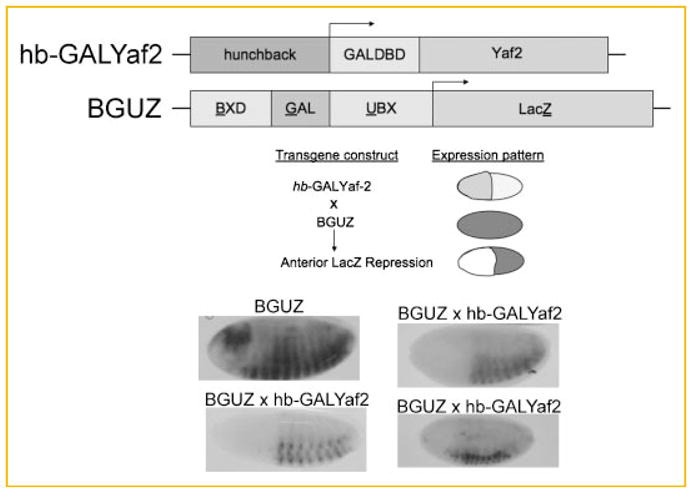
Transcriptional silencing of BGUZ by GALYaf2. A: BGUZ reporter transgene, hunchback-GALYaf2 effector transgene, and expression patterns of BGUZ, hunchback-GALYaf2, and the superimposed expression patterns in embryos. B: Drosophila embryos resulting from crosses with BGUZ females (1 h timed egg lay) fixed and stained with X-gal at hour 6. Crosses are indicated above each panel: BGUZ, BGUZ females × BGUZ males; other panels, BGUZ females crossed to distinct transgenic hunchback-GALYaf2 males.
As predicted, Yaf2 silenced the BGUZ reporter (Fig. 4). Anterior repression of staining was apparent in each of the transgene crosses consistent with the expression boundary of hb-GALYaf2. The pattern of repression was indistinguishable from that we previously observed with full-length YY1 [Atchison et al., 2003] or the isolated REPO domain [Wilkinson et al., 2006]. Thus, Yaf2 is able to silence transcription in the absence of the REPO domain if Yaf2 is targeted to DNA by a heterologous DBD. The ability of the tethered Yaf2 protein to silence transcription argues that a major activity of the REPO domain is to recruit the Yaf2 protein to DNA.
DROSOPHILA RYBP (YAF2) MUTANTS REDUCE PcG DNA BINDING
The bridging activity of Yaf2 is predicted to be important for tethering PcG proteins to DNA at appropriate genomic sites (i.e., PREs). A Drosophila gene was previously identified that is homologous to mammalian RYBP and Yaf2, dRYBP [Garcia et al., 1999; Bejarano et al., 2005]. Alignment of mammalian Yaf2, dRYBP, and mammalian RYBP (mRYBP) revealed substantial similarity among the three proteins [Bejarano et al., 2005]. Therefore, we predicted that haploinsufficiency of dRYBP would result in reduced occupancy of PcG proteins at endogenous PREs.
We assayed for binding of PHO, Pc, E(z), H3 trimethyl-lysine 27, and H3 acetyl-lysine 9 by ChIP at the PRED sequence in the dRYBP mutant line compared to flies with wild-type dRYBP levels (ry506). Interestingly, there was a clear reduction in binding of all PcG proteins in the dRYBP mutant compared to the wild-type controls (Fig. 5, lanes 6–11). These data indicate that dRYBP is important for normal recruitment of PcG proteins to DNA at PRE sequences.
Fig. 5.
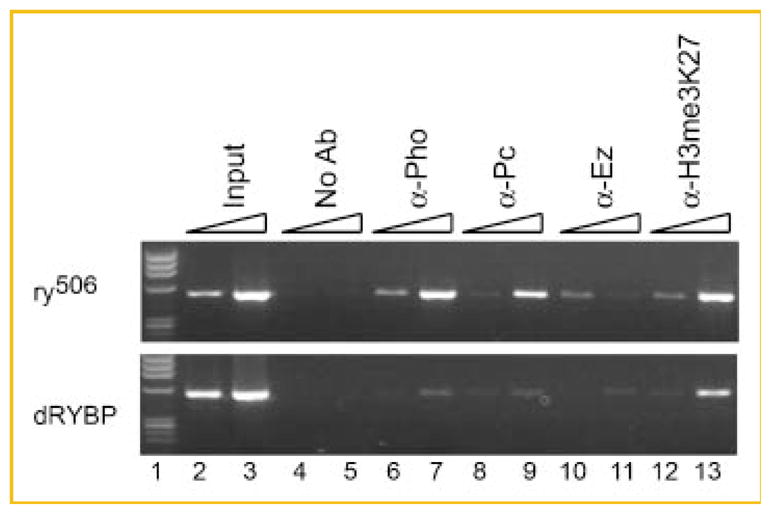
dRYBP mutation reduced PcG binding to PRED. Agarose gel electrophoresis of PCR products detected by ChIP assay stained with ethidium bromide. The strain indicating the chromatin source is indicated on the left. The triangles indicate a 10-fold change in template concentration. Antibodies used for the immunoprecipitation are indicated above the appropriate lanes. M indicates molecular weight markers. Numbers indicate lanes referred to in the text.
YAF2 RESIDUES 102–179 INTERACT WITH YY1 REPO DOMAIN
The Yaf2 protein was subject to an initial mapping study to identify structural components that mediate interactions with the REPO domain. Very little is known about the structure of the Yaf2 protein. Sequence analysis indicates a C2C2-type zinc finger composed of residues 25–42, otherwise no other structural information is apparent. Alignment of Yaf2 and mammalian RYBP indicates that RYBP has several amino acid insertions that are not represented in Yaf2 [Garcia et al., 1999; Bejarano et al., 2005]. A segment of amino acids (RYBP residues 112–144) with no homology to Yaf2 is flanked by regions with extensive homology with Yaf2. We used this insertion point (between Yaf2 residues 101 and 102) as an initial position to define the N terminal fragment (residues 1–101) and the C terminal fragment (residues 102–179) of the Yaf2 protein. Each of these fragments was assayed for its ability to interact with the isolated REPO domain using the yeast two hybrid strategy as described above (Fig. 1).
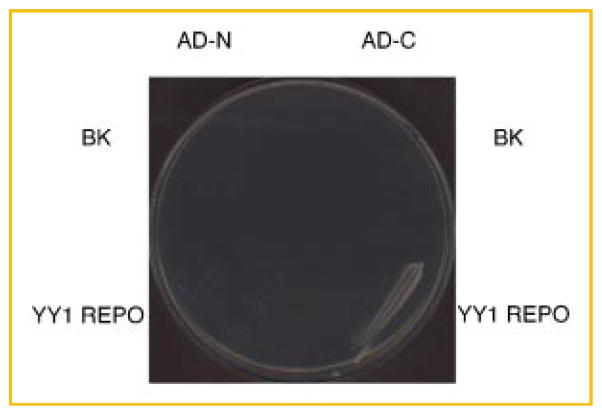
Cotransformation of the C terminal residues expressed as a GAL AD fusion (pGADt7-Yaf2-102-179, AD-C) and the REPO domain expressed as a GAL DBD fusion permitted growth on selective media (Trp/Leu/Ade/His dropout medium, Fig. 6). Cotransformation of the N terminal residues (pGADt7-Yaf2-1-101, AD-N) with either of the bait plasmids did not permit growth on selective medium. The empty vector controls (pGADt7 and pGBKt7, BK) likewise did not permit growth as above (Fig. 6 and data not shown). Together these results indicate that the C terminal fragment of Yaf2 (residues 102–179) mediates interactions with the REPO domain. The N terminal region and the C2C2-type zinc finger present within this region are not necessary for interactions with REPO.
Fig. 6.
Yeast two hybrid mapping of interactions between Yaf2 fragments and the YY1 REPO domain. S. cerevisiae AH109 was transformed with the indicated bait and prey constructs. Cotransformants were passaged onto selective medium (Trp/Leu/Ade/His dropout medium). The streaked cultures on the left contain pGADt7 expressing the GAL4 activation domain (AD) fused to Yaf2 residues 1–101 (AD-N) and the streaked cultures on the right contain pGADt7 expressing the AD fused to Yaf2 residues 102–179 (AD-C). These were cotransformed with bait constructs pGBKt7 empty vector (BK) or pGBKt7 expressing the GAL4 DBD fused to YY1 201–226 (YY1 REPO). Transcription of the nutritional markers HIS3 and ADE2 from interactions resulting from bait–prey binding interactions is as indicated in Figure 1. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Despite many years of investigation, little has been defined as to how PcG complexes are brought to PREs. Two notable aspects have hampered understanding this aspect of PcG biology. The first of these is the large size and poor homology of characterized PREs. Secondly, few PcG proteins contain sequence-specific binding activity. YY1/PHO proteins are the best characterized recruiting activities identified to date. However, the biochemical interactions between YY1/PHO and the PRC core complexes remained ill defined. Here, we present evidence that Yaf2 functions as a mediator of interactions between YY1/PHO and PRC core complexes. We have shown that (1) the YY1 REPO domain interacts with Yaf2, (2) Yaf2 fails to interact with the YY1ΔREPO mutant, (3) the REPO domain recruits Yaf2 to DNA, (4) Yaf2 can recruit PcG proteins to DNA, (5) Yaf2 can repress a PcG-dependent reporter transgene, and (6) mutation of the Drosophila Yaf2 homolog (dRYBP) results in reduced PcG recruitment to DNA. Coupled with published results showing that Yaf2 can physically interact with Ring1B and Ring1A, and co-localizes within nuclear speckles with PcG proteins Rae28/Mph1 and Ring1B [Ogawa et al., 2002; Kaneko et al., 2003], and that Drosophila dRYBP functions as a PcG protein [Bejarano et al., 2005], we believe that Yaf2 serves as a bridging protein between YY1 and the other PcG complex proteins.
Yaf2 interaction with the YY1 REPO domain and loss of interaction with the YY1ΔREPO mutant are key observations. The REPO domain is a highly conserved domain present in YY1, PHO, PHOlike, and YY2. Our previous investigation defined the REPO domain as necessary and sufficient for PcG recruitment to DNA and silencing of a PcG-dependent reporter gene [Wilkinson et al., 2006]. When tethered to DNA, the REPO domain is able to recruit PcG proteins to DNA resulting in H3 trimethylation of lysine 27. The YY1ΔREPO mutant fails to recruit PcG proteins to DNA, thus, functional integrity of the REPO domain is essential for the biochemistry of YY1 PcG recruitment. The interaction of Yaf2 with the REPO domain, and loss of interaction with YY1ΔREPO suggests that the role of Yaf2 is to mediate interactions between YY1 and the PcG complex.
Other investigators have observed Yaf2 and RYBP interaction with YY1 [Kalenik et al., 1997; Garcia et al., 1999], but these studies suggested that Yaf2 interacted with a distinct YY1 region. In these studies, Yaf2/RYBP binding to YY1 was argued to require the first and second zinc finger domains of YY1. Our mapping study is in disagreement with these results since we are unable to detect an interaction between Yaf2 and the YY1 REPO deletion protein that retains intact Zn finger domains. In addition, we found that the isolated REPO domain (lacking zinc finger domains) interacted with Yaf2 using two distinct methodologies (yeast two hybrid and ChIP). The reason for the discrepancy is not clear but may result from different methodologies employed (GST pull-down vs. ChIP and yeast two hybrid).
The ability of the YY1 REPO domain to recruit Yaf2 to DNA is an additional argument for the proposed bridging function of Yaf2. Since Yaf2 does not bind DNA itself, interactions with a sequence-specific binding protein are necessary for its localization to DNA. When GALREPO and flag Yaf2 were coexpressed, Yaf2 was recruited to the multimerized GAL4 binding sites in the BGUZ transgene. This recruitment was not observed with the GAL DBD alone. Thus, there is a strict requirement for the REPO domain to localize Yaf2 to DNA.
In addition, we found that when tethered to DNA via the GAL4 DBD, Yaf2 was able to recruit PcG proteins to DNA, resulting in transcriptional repression of a PcG-dependent reporter transgene, again arguing for a bridging function. Finally, mutation of the Drosophila Yaf2 homolog, dRYBP, resulted in reduced PcG recruitment. It should be noted that DNA binding by PHO was also reduced in the dRYBP mutant background arguing that PHO gains access to DNA perhaps most efficiently when it is part of a productive complex with Yaf2 and other PcG proteins.
A function of Yaf2 in PcG-mediated silencing is not unexpected. Yaf2 shares several highly conserved domains with the PcG protein, RYBP. RYBP can bind to the PcG proteins YY1, M33 (the mammalian homolog of Pc), and Ring1A/1B (homolog of Sce/dRing), as well as to ubiquinated histone H2A, a histone mark associated with PcG-mediated silencing [Garcia et al., 1999; Arrigoni et al., 2006]. The high structural conservation between Yaf2 and RYBP suggests that they may make common protein–protein interactions. As mentioned above, Yaf2 and RYBP do contact some of the same PcG proteins (such as Ring1B [Kaneko et al., 2003]), but Yaf2 and RYBP do not always mediate identical processes. In some circumstances, Yaf2 and RYBP were found to act antagonistically [Sawa et al., 2002; Stanton et al., 2006]. However our characterization of Yaf2 here is more consistent with Yaf2 sharing similar activity with RYBP. Yaf2 and RYBP have overlapping, but distinct expression profiles suggesting that these proteins could potentially bridge YY1 to distinct PcG complexes [Kaneko et al., 2003].
Yaf2 has not been extensively characterized, but a role in transcription regulation has been apparent. This protein was initially described as an interacting ligand for the YY1 protein [Kalenik et al., 1997], but subsequent work has identified additional transcription factors that bind Yaf2. The activities of the DNA-binding MYC proteins were modified by Yaf2 [Bannasch et al., 2001; Madge et al., 2003]. MycN transactivation was enhanced, but that of c-MYC was inhibited suggesting Yaf2 is multifunctional. Transactivation activity of human GA-binding protein (E4TF1/hGABP) was also enhanced by Yaf2 [Sawa et al., 2002]. These varied observations suggest a complex biology for the Yaf2 protein.
The interaction between Yaf2 and the YY1 REPO domain begins to define a biochemical link between the YY1 protein and PcG mediated silencing. YY1 can bind to mammalian PRC2 components EED and EZH2 [Satijn et al., 2001; Caretti et al., 2004] and interactions with the PRC2 complex argue for a role in recruiting the PcG methyltransferase activity [Cao et al., 2002; Kirmizis et al., 2004]. Jones and coworkers [Wang et al., 2004] observed a binding interaction between the REPO domain of the PHO protein and the E(z) protein by a GST pull-down strategy and ChIP analyses. This observation may represent a functional divergence between the PHO and the YY1 proteins, an undetected role for the Drosophila Yaf2 homolog, dRYBP, in their system, or suggest that the REPO domain may be capable of multiple contacts within the PcG complexes. However, the mouse homologs of E(z), EZH1 and EZH2, were not among the clones isolated from the library when screened with the YY1 REPO domain. This observation suggests that the isolated YY1 REPO domain is insufficient to interact with these proteins or requires the presence of Yaf2 for the interaction (see Supplementary Table). Thus, the role of Yaf2 in mediating potential interactions between YY1 and the PRC2 core complex need to be clarified. Equally intriguing is a potential interaction between YY1 and the PRC1 core complex.
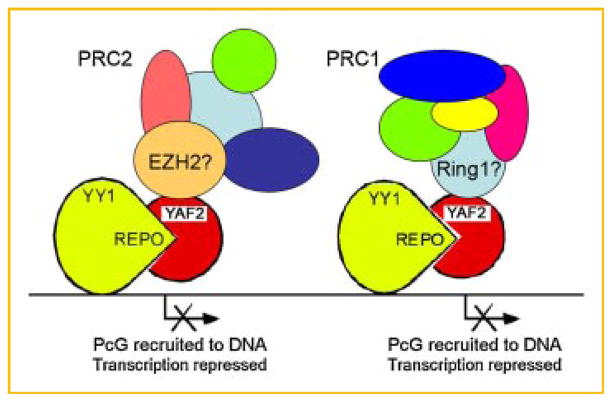
Several lines of evidence indicate that Yaf2 might bridge YY1/PHO and the PRC1 complex. PHO can form a complex with the Pc protein, a core component of PRC1, and recruit Pc to DNA suggesting a similar function for YY1 [Mohd-Sarip et al., 2002, 2005]. It is noteworthy that the Yaf2 homolog, mammalian RYBP, was identified based on its ability to interact with YY1 and the PRC1 core proteins Ring1A and 1B [Garcia et al., 1999]. Ring1A and 1B are homologs of the Drosophila Sce/dRing protein [Gorfinkiel et al., 2004]. The Ring1 proteins are bona fide members of the PRC1 complex and interact with the mammalian Pc homolog, M33 [Satijn et al., 1997; Schoorlemmer et al., 1997; Garcia et al., 1999]. Sce/dRing was found to be essential for silencing and required a wild-type pho background for activity [Fritsch et al., 2003; Bejarano et al., 2005]. Homozygous null dRYPB mutants showed progressive lethality (similar to the lethality of other PcG homozygotes) and enhanced the phenotypes of Sce/dRing mutations [Gonzalez et al., 2008]. These physical and functional interactions among PHO (DNA binding protein), dRYBP, and Sce/dRing (PRC1 core complex component) argue for a bridging role for dRYBP [Gonzalez et al., 2008]. Therefore, it is likely that Yaf2 functions to directly bridge the YY1 protein and the PRC1 core complex through interactions with the Ring proteins and the REPO domain. A model showing potential interactions between YY1 and PRC complexes mediated by Yaf2 is shown in Figure 7. Future studies will define the molecular mechanisms of these interactions.
Fig. 7.
Model of YY1 REPO–Yaf2–PcG interactions. YY1 bound to DNA is shown with the REPO domain interacting with Yaf2, and Yaf2 interacting with either PRC2 or PRC1. Candidate PRC2 and PRC1 interacting proteins EZH2 and Ring-1 are indicated respectively. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Supplementary Material
Acknowledgments
We want to acknowledge Judy Kassis (NIH) for kindly providing antibodies against the Drosophila PHO protein and Vincent Pirrotta (Rutgers) for kindly providing antibodies against the Drosophila E(z) protein. Additionally, we acknowledge Arindam Basu (University of Pennsylvania) for careful review of the manuscript and assistance with much of the work. This study was supported by NIH grant RO1 GM071830 (M.L.A.) and a faculty development grant awarded to F.W. by Philadelphia University.
Grant sponsor: NIH; Grant number: RO1 GM071830.
Abbreviations used
- PcG
Polycomb Group
- PRE
Polycomb Response Element
- REPO
Recruiter of Polycomb Domain
- PHO
pleiohomeotic
- RYBP
Ring1 and Yin Yang 1 binding protein
- YY1
Yin Yang 1
- Yaf2
Yin Yang 1 associated factor 2
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Arrigoni R, Alam SL, Wamstad JA, Bardwell VJ, Sundquist WI, Schreiber-Agus N. The Polycomb-associated protein Rybp is a ubiquitin binding protein. FEBS Lett. 2006;580:6233–6241. doi: 10.1016/j.febslet.2006.10.027. [DOI] [PubMed] [Google Scholar]
- Atchison L, Ghias A, Wilkinson F, Bonini N, Atchison ML. Transcription factor YY1 functions as a PcG protein in vivo. EMBO J. 2003;22:1347–1358. doi: 10.1093/emboj/cdg124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannasch D, Madge B, Schwab M. Functional interaction of Yaf2 with the central region of MycN. Oncogene. 2001;20:5913–5919. doi: 10.1038/sj.onc.1204747. [DOI] [PubMed] [Google Scholar]
- Bejarano F, Gonzalez I, Vidal M, Busturia A. The Drosophila RYBP gene functions as a Polycomb-dependent transcriptional repressor. Mech Dev. 2005;122:1118–1129. doi: 10.1016/j.mod.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Blastyak A, Mishra RK, Karch F, Gyurkovics H. Efficient and specific targeting of Polycomb group proteins requires cooperative interaction between Grainyhead and Pleiohomeotic. Mol Cell Biol. 2006;26:1434–1444. doi: 10.1128/MCB.26.4.1434-1444.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL, Fritsch C, Mueller J, Kassis JA. The Drosophila pholike gene encodes a YY1-related DNA binding protein that is redundant with pleiohomeotic in homeotic gene silencing. Development. 2003;130:285–294. doi: 10.1242/dev.00204. [DOI] [PubMed] [Google Scholar]
- Brown JL, Mucci D, Whiteley M, Dirksen ML, Kassis JA. The Drosophila Polycomb group gene pleiohomeotic encodes a DNA binding protein with homology to the transcription factor YY1. Mol Cell. 1998;1:1057–1064. doi: 10.1016/s1097-2765(00)80106-9. [DOI] [PubMed] [Google Scholar]
- Busturia A, Lloyd A, Bejarano F, Zavortink M, Xin H, Sakonju S. The MCP silencer of the Drosophila Abd-B gene requires both Pleiohomeotic and GAGA factor for the maintenance of repression. Development. 2001;128:2163–2173. doi: 10.1242/dev.128.11.2163. [DOI] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- Caretti G, Di Padova M, Micales B, Lyons GE, Sartorelli V. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 2004;18:2627–2638. doi: 10.1101/gad.1241904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch C, Beuchle D, Muller J. Molecular and genetic analysis of the Polycomb group gene Sex combs extra/Ring in Drosophila. Mech Dev. 2003;120:949–954. doi: 10.1016/s0925-4773(03)00083-2. [DOI] [PubMed] [Google Scholar]
- Fritsch C, Brown JL, Kassis JA, Muller J. The DNA-binding polycomb group protein pleiohomeotic mediates silencing of a Drosophila homeotic gene. Development. 1999;126:3905–3913. doi: 10.1242/dev.126.17.3905. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Yusibova GL, Zhou J, Jaynes JB. The DNA-binding Polycomb-group protein Pleiohomeotic maintains both active and repressed transcriptional states through a single site. Development. 2008;135:4131–4139. doi: 10.1242/dev.024554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E, Marcos-Gutierrez C, del Mar Lorente M, Moreno JC, Vidal M. RYBP, a new repressor protein that interacts with components of the mammalian Polycomb complex, and with the transcription factor YY1. EMBO J. 1999;18:3404–3418. doi: 10.1093/emboj/18.12.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girton JR, Jeon SH. Novel embryonic and adult homeotic phenotypes are produced by pleiohomeotic mutations in Drosophila. Dev Biol. 1994;161:393–407. doi: 10.1006/dbio.1994.1040. [DOI] [PubMed] [Google Scholar]
- Gonzalez I, Aparicio R, Busturia A. Functional characterization of the dRYBP gene in Drosophila. Genetics. 2008;179:1373–1388. doi: 10.1534/genetics.107.082966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorfinkiel N, Fanti L, Melgar T, Garcia E, Pimpinelli S, Guerrero I, Vidal M. The Drosophila Polycomb group gene Sex combs extra encodes the ortholog of mammalian Ring1 proteins. Mech Dev. 2004;121:449–462. doi: 10.1016/j.mod.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Kalenik JL, Chen D, Bradley ME, Chen SJ, Lee TC. Yeast two-hybrid cloning of a novel zinc finger protein that interacts with the multifunctional transcription factor YY1. Nucleic Acids Res. 1997;25:843–849. doi: 10.1093/nar/25.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Miyagishima H, Hasegawa T, Mizutani-Koseki Y, Isono K, Koseki H. The mouse YAF2 gene generates two distinct transcripts and is expressed in pre-and postimplantation embryos. Gene. 2003;315:183–192. doi: 10.1016/s0378-1119(03)00800-x. [DOI] [PubMed] [Google Scholar]
- Kim H, Kang K, Kim J. AEBP2 as a potential targeting protein for Polycomb Repression Complex PRC2. Nucleic Acids Res. 2009;37:2940–2950. doi: 10.1093/nar/gkp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmizis A, Bartley SM, Kuzmichev A, Margueron R, Reinberg D, Green R, Farnham PJ. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev. 2004;18:1592–1605. doi: 10.1101/gad.1200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymenko T, Papp B, Fischle W, Kocher T, Schelder M, Fritsch C, Wild B, Wilm M, Muller J. A Polycomb group protein complex with sequence-specific DNA-binding and selective methyl-lysine-binding activities. Genes Dev. 2006;20:1110–1122. doi: 10.1101/gad.377406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SH, Kim SH, Chung HM, Girton JR, Jeon SH. The Drosophila pleiohomeotic mutation enhances the Polycomblike and Polycomb mutant phenotypes during embryogenesis and in the adult. Int J Dev Biol. 2003;47:389–395. [PubMed] [Google Scholar]
- Levine SS, King IF, Kingston RE. Division of labor in polycomb group repression. Trends Biochem Sci. 2004;29:478–485. doi: 10.1016/j.tibs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lund AH, van Lohuizen M. Polycomb complexes and silencing mechanisms. Curr Opin Cell Biol. 2004;16:239–246. doi: 10.1016/j.ceb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Madge B, Geisen C, Moroy T, Schwab M. Yaf2 inhibits Myc biological function. Cancer Lett. 2003;193:171–176. doi: 10.1016/s0304-3835(02)00696-1. [DOI] [PubMed] [Google Scholar]
- Mahmoudi T, Zuijderduijn LM, Mohd-Sarip A, Verrijzer CP. GAGA facilitates binding of Pleiohomeotic to a chromatinized Polycomb response element. Nucleic Acids Res. 2003;31:4147–4156. doi: 10.1093/nar/gkg479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaly J, Mishra RK, Karch F. A conserved sequence motif in Poly-comb-response elements. Mol Cell. 1998;1:1065–1066. doi: 10.1016/s1097-2765(00)80107-0. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Mihaly J, Barges S, Spierer A, Karch F, Hagstrom K, Schweinsberg SE, Schedl P. The iab-7 polycomb response element maps to a nucleosome-free region of chromatin and requires both GAGA and pleiohomeotic for silencing activity. Mol Cell Biol. 2001;21:1311–1318. doi: 10.1128/MCB.21.4.1311-1318.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd-Sarip A, Cleard F, Mishra RK, Karch F, Verrijzer CP. Synergistic recognition of an epigenetic DNA element by Pleiohomeotic and a Polycomb core complex. Genes Dev. 2005;19:1755–1760. doi: 10.1101/gad.347005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd-Sarip A, Venturini F, Chalkley GE, Verrijzer CP. Pleiohomeotic can link polycomb to DNA and mediate transcriptional repression. Mol Cell Biol. 2002;22:7473–7483. doi: 10.1128/MCB.22.21.7473-7483.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J. Transcriptional silencing by the Polycomb protein in Drosophila embryos. EMBO J. 1995;14:1209–1220. doi: 10.1002/j.1460-2075.1995.tb07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Kassis JA. Polycomb response elements and targeting of Polycomb group proteins in Drosophila. Curr Opin Genet Dev. 2006;16:476–484. doi: 10.1016/j.gde.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Ishiguro K, Gaubatz S, Livingston DM, Nakatani Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science. 2002;296:1132–1136. doi: 10.1126/science.1069861. [DOI] [PubMed] [Google Scholar]
- Otte AP, Kwaks TH. Gene repression by Polycomb group protein complexes: A distinct complex for every occasion? Curr Opin Genet Dev. 2003;13:448–454. doi: 10.1016/s0959-437x(03)00108-4. [DOI] [PubMed] [Google Scholar]
- Ringrose L, Paro R. Polycomb/Trithorax response elements and epigenetic memory of cell identity. Development. 2007;134:223–232. doi: 10.1242/dev.02723. [DOI] [PubMed] [Google Scholar]
- Ringrose L, Rehmsmeier M, Dura JM, Paro R. Genome-wide prediction of Polycomb/Trithorax response elements in Drosophila melanogaster. Dev Cell. 2003;5:759–771. doi: 10.1016/s1534-5807(03)00337-x. [DOI] [PubMed] [Google Scholar]
- Satijn DP, Gunster MJ, van der Vlag J, Hamer KM, Schul W, Alkema MJ, Saurin AJ, Freemont PS, van Driel R, Otte AP. RING1 is associated with the polycomb group protein complex and acts as a transcriptional repressor. Mol Cell Biol. 1997;17:4105–4113. doi: 10.1128/mcb.17.7.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satijn DP, Hamer KM, den Blaauwen J, Otte AP. The polycomb group protein EED interacts with YY1, and both proteins induce neural tissue in Xenopus embryos. Mol Cell Biol. 2001;21:1360–1369. doi: 10.1128/MCB.21.4.1360-1369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa C, Yoshikawa T, Matsuda-Suzuki F, Delehouzee S, Goto M, Watanabe H, Sawada J, Kataoka K, Handa H. YEAF1/RYBP and YAF-2 are functionally distinct members of a cofactor family for the YY1 and E4TF1/hGABP transcription factors. J Biol Chem. 2002;277:22484–22490. doi: 10.1074/jbc.M203060200. [DOI] [PubMed] [Google Scholar]
- Schoorlemmer J, Marcos-Gutierrez C, Were F, Martinez R, Garcia E, Satijn DP, Otte AP, Vidal M. Ring1A is a transcriptional repressor that interacts with the Polycomb-M33 protein and is expressed at rhombomere boundaries in the mouse hindbrain. EMBO J. 1997;16:5930–5942. doi: 10.1093/emboj/16.19.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimell MJ, Peterson AJ, Burr J, Simon JA, O’Connor MB. Functional analysis of repressor binding sites in the iab-2 regulatory region of the abdominal-A homeotic gene. Dev Biol. 2000;218:38–52. doi: 10.1006/dbio.1999.9576. [DOI] [PubMed] [Google Scholar]
- Simon J, Chiang A, Bender W. Ten different Polycomb group genes are required for spatial control of the abdA and AbdB homeotic products. Development. 1992;114:493–505. doi: 10.1242/dev.114.2.493. [DOI] [PubMed] [Google Scholar]
- Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- Srinivasan L, Atchison ML. YY1 DNA binding and PcG recruitment requires CtBP. Genes Dev. 2004;18:2596–2601. doi: 10.1101/gad.1228204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SE, McReynolds LJ, Evans T, Schreiber-Agus N. Yaf2 inhibits caspase 8-mediated apoptosis and regulates cell survival during zebrafish embryogenesis. J Biol Chem. 2006;281:28782–28793. doi: 10.1074/jbc.M603348200. [DOI] [PubMed] [Google Scholar]
- Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004;118:409–418. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Wang L, Brown JL, Cao R, Zhang Y, Kassis JA, Jones RS. Hierarchical recruitment of polycomb group silencing complexes. Mol Cell. 2004;14:637–646. doi: 10.1016/j.molcel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Wilkinson FH, Park K, Atchison ML. Polycomb recruitment to DNA in vivo by the YY1 REPO domain. Proc Natl Acad Sci USA. 2006;103:19296–19301. doi: 10.1073/pnas.0603564103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.