Abstract
Immortalized cells are often used to model the behavior of osteogenic cells on orthopaedic and dental biomaterials. In the current study we compared the adhesive behavior of two osteosarcoma cell lines, MG-63 and Saos-2, with that of mesenchymal stem cells (MSCs) on hydroxyapatite (HA). It was found that osteosarcoma cells demonstrated maximal binding to fibronectin-coated HA, while MSCs alternately preferred HA coated with collagen-I. Interesting, the binding of MG-63 and Saos-2 cells to fibronectin was mediated by both α5 and αv-containing integrin heterodimers, whereas only αv integrins were used by MSCs. Cell spreading was also markedly different for the three cell types. Osteosarcoma cells exhibited optimal spreading on fibronectin, but poor spreading on HA disks coated with fetal bovine serum. In contrast, MSCs spread very well on serum-coated surfaces, but less extensively on fibronectin. Finally, we evaluated integrin expression and found that MSCs have higher levels of α2 integrin subunits relative to MG-63 or Saos-2 cells, which may explain the enhanced adhesion of MSCs on collagen-coated HA. Collectively our results suggest that osteosarcoma cells utilize different mechanisms than MSCs during initial attachment to protein-coated HA, thereby calling into question the suitability of these cell lines as in vitro models for cell/biomaterial interactions.
Keywords: hydroxyapatite, integrins, mesenchymal stem cells, bone, matrix
INTRODUCTION
Hydroxyapatite (HA), a calcium phosphate biomaterial, is known to be highly osseoconductive relative to many other implant materials [1]. Our laboratory has suggested that this is due, in part, to HA’s ability to adsorb adhesive proteins from the endogenous bone microenvironment. Our prior in vitro studies showed that greater amounts of fibronectin (FN) and vitronectin (VN), molecules that are abundant within blood, become adsorbed to HA, as compared with titanium, following coating with fetal bovine serum [2]. These adsorbed proteins provide a provisional matrix for attachment of osteogenic cells. In fact, in the absence of an adsorbed protein layer, HA is a poor substrate for cell adhesion and cell spreading [3,4].
Osteogenic cells typically bind to biomaterials through integrin-mediated mechanisms [5,6]. Integrins are heterodimeric glycoproteins composed of noncovalently-associated α and β subunits. Integrin specificity is determined by the combination of these subunits, as illustrated by α5β1’s specificity for FN, and α2β1’s selectivity for collagen or laminin. In contrast, αvβ3 receptors bind to many matrix molecules including FN, VN, bone sialoprotein and osteopontin. Following ligand binding and clustering of integrin receptors, aggregates of integrins and cytoskeletal-associated proteins are formed (e.g., focal adhesions), which in turn are associated with reorganization of the actin cytoskeleton. In addition to cytoskeletal restructuring, activated integrins stimulate signaling cascades that ultimately regulate many fundamental cell behaviors including cell proliferation, survival, motility and differentiation.
Upon placement of biomaterials in bone, mesenchymal stem cells (MSCs) are recruited from the bone marrow to the implant site, where they bind to the material surface and then differentiate into bone-forming osteoblasts [5,7,8]. Accordingly, an important goal of biomaterials research is to functionalize material surfaces with molecules that promote MSC attachment and osteoblastic differentiation. Enhanced biocompatibility has been associated with implant material surfaces that exhibit signaling properties similar to the endogenous extracellular matrix, thereby facilitating integration of the material within the host tissue [9]. Hence, studies aimed at defining the optimal matrix molecules for MSC adhesion are needed.
In many in vitro studies of cell/biomaterial interactions, immortalized cell lines have been used in place of primary cells like MSCs or bone-derived osteoblasts. This is done for primarily practical reasons. Immortalized cell lines, including those derived from human osteosarcomas, are easier to procure than primary cells, and they grow in vitro for an indefinite number of passages. Saos-2 and MG-63 are examples of osteosarcoma cell lines that have been widely used as model systems for elucidating osteogenic cell behavior on biomaterials. These cell lines are useful because they have the capacity to undergo osteoblastic differentiation in response to osteogenic chemical cues [10].
While it is possible that osteosarcoma cells represent a suitable model for studying osteoblastic differentiation on biomaterial surfaces, it isn’t yet clear that these cells mimic the behavior of MSCs during the initial phases of cell attachment to a biomaterial. It is well-established that the acquisition of a transformed/tumorigenic phenotype causes aberrant expression and/or activity of integrin receptors [11–14]. In prior studies from our laboratory, we found that Saos-2 osteosarcoma cells use different integrins than MSCs when attaching to serum-coated HA, and these cells also exhibit a divergent preference for selected matrix molecules [15]. In light of these results, the goal of the current study was to determine whether altered integrin-dependent attachment was a general feature of osteosarcoma cells. To this end, we performed a side-by-side comparison of mechanisms used by MSCs, Saos-2, and MG-63 cells to attach to protein-coated HA.
MATERIALS AND METHODS
Cell Culture
MG-63 and Saos-2 cells were obtained from American Type Culture Collection, and were maintained in ATCC Minimum Essential Eagle Media containing 10% fetal bovine serum (FBS) and supplemented with Amphotericin B and pen-strep. Human MSCs were harvested from bone marrow donations, with IRB approval. Briefly, MSCs were purified from the bone marrow using a Histopaque gradient [2] and then grown in Dulbecco’s Modified Eagles Media (DMEM) containing 10% fetal bovine serum (FBS), supplemented with amphotericin B and pen-strep. MSCs from passages 7 through 18 were used for the described experiments.
Cell Adhesion
HA disks were coated with FBS, 2% denatured bovine serum albumin (dBSA), 20 μg/ml of FN, or 20 μg/ml of collagen I overnight 4°C in a 24 well plate. dBSA coated HA disks served as the negative control. Immediately prior to adhesion assays, cells were labeled with cell tracker green dye (Molecular Probes) according to the vendor protocol, and then detached by trypsinization. Trypsin inhibitor was then added and cells were subsequently centrifuged, washed and resuspended in serum-free DMEM. Cells were seeded onto protein-coated HA disks and allowed to adhere for one hour at 37°C. A one hour time point for evaluating cell adhesion was selected for two reasons: 1) cells do not secrete a significant amount of matrix molecules within this time period (an event that could compromise the analyses of responses to the adhesion proteins adsorbed to HA), and 2) a one-hour time point is insufficient to allow cells to significantly change their integrin expression profile while adherent to HA, given that integrin synthesis is known to be relatively slow [16–18]. Following the one-hour adhesion interval, disks were washed several times with phosphate buffered saline (PBS) to remove non-adherent cells, and the adherent cells were lysed with 1% Triton X-100 in 50mM Tris buffer at pH 7.5. The degree of cell adhesion was quantified by measuring the fluorescence in the samples. Values were reported as fold increases over cell binding to dBSA.
Cell Morphology
HA disks were coated with selected proteins as described above. Cells were seeded onto the disks and allowed to adhere for one hour at 37°C. The samples were then washed with Tris-buffered saline (TBS), and the adherent cells were fixed with 3.7% formaldehyde. After fixation, cells were permeabilized with 0.2% Triton X-100, and then non-specific binding was blocked with 2% dBSA. The actin cytoskeleton was labeled with phalloidin, conjugated to Alexa 488 green fluorescent dye (Molecular Probes). Cells were visualized using a Leica fluorescent microscope.
Function Blocking Antibodies
Cells were labeled with cell tracker green, and detached by trypsinization as previously described. Cells were then incubated in suspension with anti-integrin function blocking antibodies for one hour at 37°C. The following antibodies were used for these studies; 1) anti-α5 integrin (Chemicon Int.), 2) anti-αv integrin (Chemicon Int.) and 3) a non-specific isotype control IgG (Chemicon Int.). All of the antibodies were used at a 20 μg/ml concentration. Following the incubation with function-blocking antibodies, the cells were seeded onto HA disks pre-coated with 20 μg/ml FN, and adhesion was quantified as previously described.
Western blot
Cells were grown on tissue culture plastic for 24 hours and then lysed in 50mM Tris buffer containing 1% Triton X-100, 20 μg/ml leupeptin, and 0.5mM phenylmethylsulfonyl fluoride (PMSF). Side-by-side lysates were prepared for MSCs, MG-63 and Saos-2 cells, and lysates were prepared on three separate days, from three independent batches of cells (to insure that any observed differences in protein expression were repeatable). The protein concentrations of the lysates were normalized through the use of a Bradford assay (Sigma). The lysates were then resolved by SDS-PAGE and transferred to PVDF membranes. Membranes were blocked in 5% milk dissolved in TBS containing 0.05% Tween-20 (TBST). The membranes were subsequently incubated with primary antibodies against fibronectin (Chemicon), vitronectin (Santa Cruz), α5 integrin (Santa Cruz), αv integrin (Chemicon), and α2 integrin (Chemicon), or with an antibody against β-actin. An HRP-conjugated secondary antibody was then added and proteins were visualized by enhanced chemiluminescence. The three independent batches of lysates for the three cell lines were subjected to Western blotting, and densitometry was used to quantify the visualized bands on each immunoblot. These data were normalized to the β-actin loading control band for each sample.
Statistical analysis
For the graphical data shown in Figures 1 and 4, three independent experiments were performed, with each experiment performed in triplicate. Data are plotted as means + S.E.M., and statistical significance was evaluated by student’s t test (p<0.05). For the Western blots shown in Figures 3 and 5, three separate immunoblots were evaluated by densitometry, as described above. Data are graphed as mean densitometric units + S.E.M. for the three blots, and statistics were calculated using a student’s t-test (p<0.05).
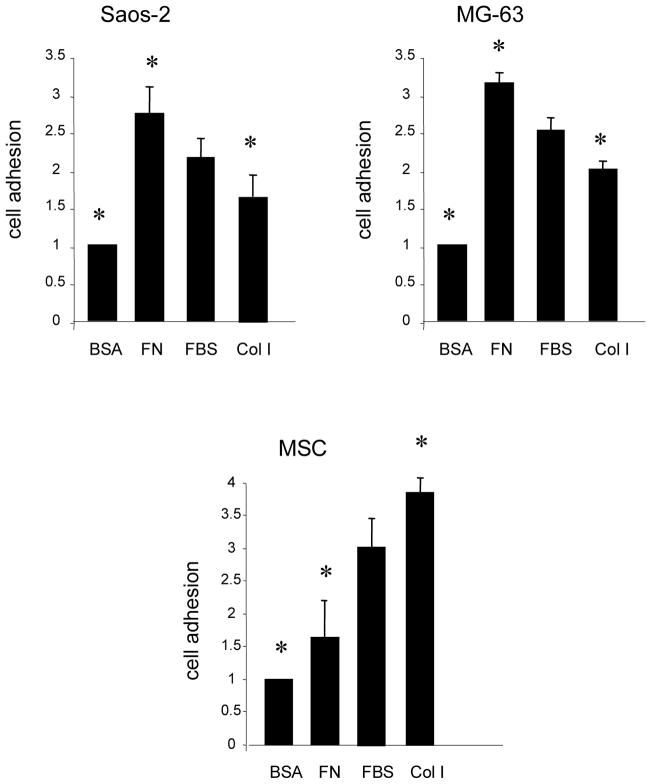
Fig. 1.
Osteosarcoma cells and MSCs bind optimally to different matrix molecules. Cells were pre-loaded with a fluorescent dye and then seeded onto HA disks precoated with FBS, FN, Col I or denatured BSA (a negative control). Cells were allowed to adhere for 1 hour, and were then evaluated for cell adhesion using a fluorometric-based assay. As shown, MG-63 and Saos-2 cells demonstrated greater attachment to FN coated disks than to FBS or col-I-coated disks. This contrasts with the behavior of MSCs, which adhere better to disks coated with FBS or col-I as compared with FN. Results are from 3 independent experiments, each performed in triplicate. * denotes significant difference from FBS-coated samples (p < 0.05). Bars = means plus S.E.M.
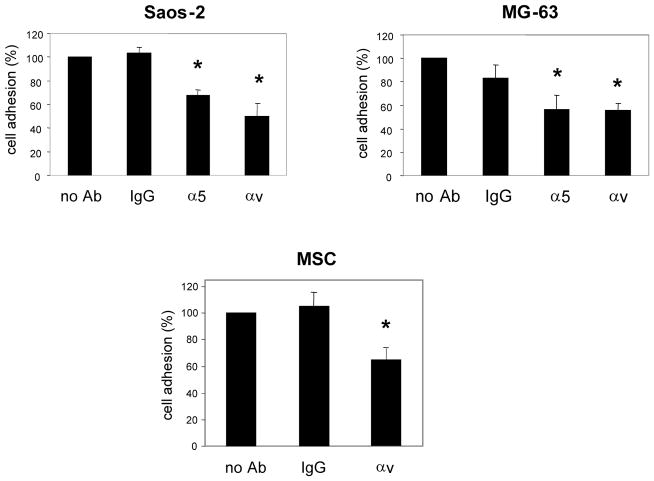
Fig. 4.
Osteosarcoma cells use different integrin receptors than MSCs to adhere to fibronectin. MSCs, MG-63, and Saos-2 cells (labeled with cell tracker green) were preincubated with function blocking antibodies against α5 or αv integrins, or with a nonspecific IgG (IgG) as a control. Trials were also included in which no antibody was added (no Ab). The cells were then seeded onto FN-coated HA disks, and allowed to adhere for 1 hour at 37°C. The disks were then washed to remove non-adherent cells, and the number of adherent cells was quantified as previously described. Results represent the means and SEMs for 3 experiments performed in triplicate. * denotes significant difference from the no antibody samples (p < 0.05)
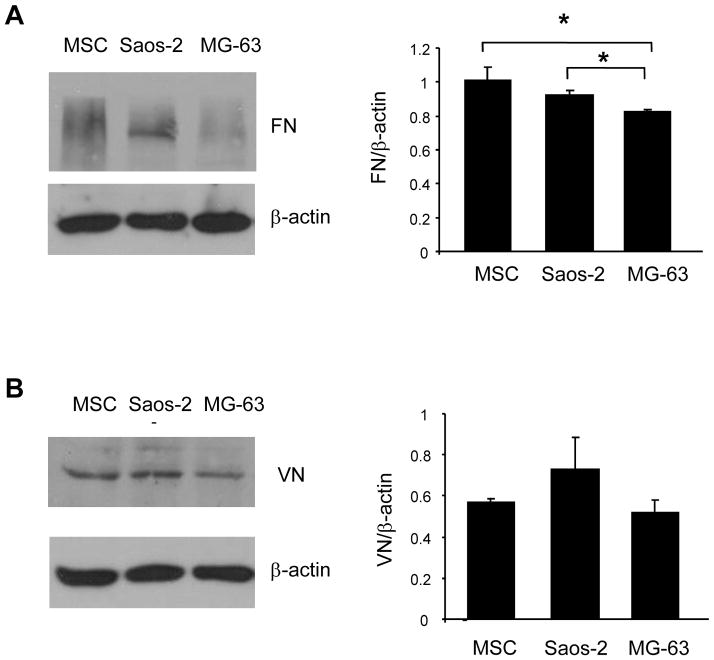
Fig. 3.
Osteosarcoma cells and MSCs synthesize FN and VN
Cells were grown overnight on tissue culture plastic and then lysed in buffer containing 1% TX-100. Lysates were resolved by SDS-PAGE, transferred to PVDF membrane and immunoblotted for either FN (A) or VN (B). All blots were stripped and re-probed with an antibody against βactin to control for protein loading. Western blots were performed using 3 independent harvests of cell lysates, and band intensities were quantified by densitometry. Values for integrin levels were normalized to the β-actin control bands. Graphs represent the means and SEMs; * denotes a statistically significant difference (p < 0.05).
Fig. 5.
Osteosarcoma cells and MSCs exhibit differential expression of integrin receptors. MSC, MG-63, and Saos-2 cell lysates were immunoblotted for α2 integrins (A), αv integrins (B) or α5 integrins (C). All blots were stripped and re-probed with an antibody against β-actin to control for protein loading. Western blots were performed using 3 independent harvests of cell lysates, and band intensities were quantified by densitometry. Values for integrin levels were normalized to the β-actin control bands. Graphs represent the means and SEMs; * denotes a statistically significant difference (p < 0.05).
RESULTS
Osteosarcoma cells and MSCs bind optimally to different matrix molecules
MSC, MG-63, and Saos-2 cells were monitored for adhesion to HA disks coated with either FN, FBS, collagen I or dBSA (binding to dBSA served as a negative control). Collagen I (col I) was included in this study because it is a major constituent of the organic bone matrix. HA disks were pre-coated with the various substrates, washed several times to remove loosely-bound proteins, and then cells were seeded onto the disks in serum-free media and allowed to adhere for one hour. As shown in Fig 1, MG-63 and Saos-2 cells demonstrated a similar preference for matrix molecules; maximal binding was observed on FN-coated HA, whereas binding was minimal on col I-coated HA. In contrast, the greatest degree of MSC attachment was observed on col I-coated HA, while MSC attachment was lowest on FN-coated HA. These data indicate that MSCs exhibit a markedly different preference for matrix molecules than osteosarcoma cells.
Osteosarcoma cells and MSCs demonstrate differential cell spreading on matrix molecules
In order to evaluate the morphology of cells adherent to the various substrates, the attached cells were labeled with phalloidin. These studies revealed that maximal cell spreading of osteosarcoma cells was induced by different matrix molecules than those stimulating maximal spreading of MSCs. As shown in Fig 2, both of the osteosarcoma cell lines were well-spread on FN-coated HA, but were almost completed rounded on FBS. In contrast, MSCs were maximally spread on FBS. Cell spreading on col I appeared to be intermediate for the three cell types.
Fig. 2.
Osteosarcoma cells and MSCs demonstrate differential cell spreading on matrix molecules. Cells were allowed to adhere for 1 hour on HA disks pre-coated with pro-adhesive proteins. The cells were then fixed with formaldehyde, and the actin cytoskeleton was labeled using phalloidin conjugated to a green fluorescent dye. Maximal spreading of Saos-2 and MG-63 cells was observed on FN-coated HA disks, whereas only limited cell spreading was observed on disks coated with either col-I or FBS. The morphology of MSCs was very different from that of Saos-2 and MG-63 cells, in that MSCs appeared to spread better on FBS than on either collagen or FN.
Osteosarcoma cells and MSCs synthesize similar amounts of matrix molecules
We next tested whether osteosarcoma cells synthesize different amounts of matrix proteins than MSCs. Cells were grown for 24 hours on tissue culture plastic and then lysed using standard methods. Lysates were evaluated for FN or VN production by Western blotting (of note, we also attempted to blot for col I, but were unsuccessful in obtaining a convincing blot). Results from these experiments indicated that the three cell types produce similar amounts of VN (Fig 3). FN synthesis was also similar for MSCs and Saos-2 cells, however less FN was synthesized by MG-63 cells (Fig 3). These data suggest that the preference of osteosarcoma cells for adhesion to FN-coated HA (as shown in Fig 1) cannot be explained by increased synthesis of this matrix molecule by these cells. Moreover, in the cell adhesion assays performed in Fig 1, cells were allowed to adhere to protein-coated HA for one hour only (in serum-free media), a time interval which is generally insufficient to allow significant cellular secretion of matrix molecules.
Osteosarcoma cells use different integrin receptors than MSCs to adhere to fibronectin
We speculated that variable cell behavior on FBS and FN-coated HA may be due to differences in integrin receptor utilization. In a prior study, we determined that Saos-2 cells adhere to FBS-coated HA using both α5 and αv-containing integrin heterodimers, whereas MSCs utilize αv, but not α5. In the present study, we screened for integrins involved in cell adhesion to FN. Specifically, cells were preincubated with anti-integrin function blocking antibodies, and then monitored for cell adhesion to FN-coated HA. As shown (Fig 4), MG-63 and Saos-2 cell adhesion was significantly inhibited by function-blocking antibodies against both the αv and α5 integrin subunits, indicating the involvement of both of these integrins in binding to FN. As with osteosarcoma cells, MSCs clearly use αv-containing integrins to adhere to FN (Fig 4), however we previously reported that anti-α5 function-blocking antibodies do not block MSC adhesion to FN [15]. Thus, although MSCs express α5β1 integrins, this receptor does not appear to mediate MSC adhesion to either FBS- or FN-coated HA.
Osteosarcoma cells and MSCs exhibit differential expression of integrin receptors
We next examined levels of integrin expression in the three cell types. Western blot analyses of cell lysates revealed that the α2 integrin subunit was expressed at higher levels in MSCs than in the two osteosarcoma cell lines (Fig 5A), which may explain the higher degree of MSC adhesion to col I as compared with MG-63 and Saos-2 cells. MSCs also expressed higher levels of the αv integrin subunit (Fig 5B), whereas the expression of α5 integrins was equivalent in the three cell lines (Fig 5C). Thus, the two osteosarcoma cell lines exhibit differential expression of integrins as compared with MSCs. Importantly, the integrin expression profile, cell preference for matrix molecules, and cell spreading behavior, are strikingly similar for the two osteosarcoma cell lines, suggesting that there may be common alterations in osteoblastic cell behavior following tumorigenic transformation.
DISCUSSION
HA is a highly osseoconductive biomaterial that has numerous clinical advantages. When used as a coating for hard tissue implants, HA stimulates increased bone formation on implant surfaces, and the newly-synthesized bone is found in direct contact with the HA layer [1,19]. These features contribute to proper anchorage of orthopedic implants after surgery, a quality that has been shown to be associated with reduced rates of implant failure [20,21]. Our lab has suggested that at least one of the factors underlying HA’s high degree of osseoconductivity is the capacity of this material for adsorbing pro-adhesive proteins from body fluids within the bone microenvironment [22]. In vivo, orthopedic implants are bathed in blood, which contains an abundant amount of integrin-binding proteins such as FN, VN and fibrinogen [5]. Integrin-mediated cell attachment to these ligands enables cells to survive on the implant surface, and further contributes to the subsequent osteoblastic differentiation of MSCs [23]. Given the importance of integrins in cell/biomaterial interactions, as well as the current interest in functionalizing materials with biomimetic molecules, studies aimed at defining the mechanisms regulating osteogenic cell attachment are crucial.
The purpose of the present study was to examine whether osteosarcoma cell lines serve as good models for determining which matrix molecules best promote the attachment of osteogenic cells to HA. We find that MG-63 and Saos-2 osteosarcoma cells prefer FN coatings to FBS and col I, whereas MSCs bind best to col I and FBS. Furthermore, MG-63 and Saos-2 cells exhibit maximal cell spreading while adherent to FN, whereas MSC spreading is optimal on FBS. The mechanisms underlying the greater FN-binding capability of osteosarcoma cells, relative to MSCs, are not currently understood, but do not appear to involve the upregulation of integrin subunits involved in FN-binding; for example, αv and α5. MSCs actually express higher levels of αv than osteosarcoma cells, and equivalent levels of α5. Osteosarcoma preference for FN also does not seem to be due to increased secretion of FN from the cells themselves, given that MSCs synthesize equivalent amounts of FN as compared with Saos-2 cells, and more FN than MG-63 cells.
We speculate that the increased adhesion and spreading of osteosarcoma cells on FN may be related to the fact that these cells use different integrins than MSCs when adhering to FN (and also to FBS). In a prior study, we reported that MSCs used primarily αv-containing integrins, whereas Saos-2 cells used both αv and α5-containing integrins, to bind FBS-coated HA [15]. In the present study we find that the initial attachment of MG-63 and Saos-2 to FN-coated HA is similarly blocked by function-blocking antibodies against both αv and α5 integrin subunits, whereas only the anti-αv antibodies are effective against MSC adhesion to FN. Although α5 and αv-containing integrin heterodimers can both bind to FN, it is known that these diverse integrin species can direct different cell responses. Clearly MSCs express significant amounts of the α5β1 receptor, therefore it is intriguing that this receptor does not participate in MSC adhesion to FN. It is possible that the α5β1 integrins expressed by MSCs are in an inactive state, but become activated later in the osteogenic differentiation pathway. The expression of inactive cell surface α5β1 integrins is a common feature of some immune cell types [24–26]. Our results indicating that osteosarcoma cells have enhanced FN binding relative to MSCs, directed in part by α5β1, hint that α5β1 function may contribute to some aspect of the tumor cell phenotype. Alternately, increased α5β1 activity may reflect differences in the differentiation status of MG-63 and Saos-2 cells as compared with MSCs.
While α5β1 does not appear to mediate the initial attachment of MSCs to FN, signaling from this integrin species could play a role in the development and maintenance of the more mature osteoblastic phenotype. Function-blocking antibodies against α5β1 integrins have been reported to inhibit alkaline phosphatase activity [27] as well as the formation of mineralized nodules [28,29]. However, it should be noted that none of these prior studies was performed with MSCs; instead, osteoblast differentiation was assessed using MG-63 cells [27] and fetal calvarial osteoblasts as model systems [28,29]. Further studies are needed to determine if differentiating MSCs require the same integrin species for osteoblast-related functions as more mature osteoblastic cell types or osteosarcoma cells.
Interestingly, neither of the osteosarcoma cell lines was able to spread on FBS-coated HA, whereas MSCs spread very well on this substrate. In our prior studies we observed that FN and VN were deposited on HA surfaces following coating with FBS, and moreover, the adsorbed FN and VN molecules adopted conformations that were appropriate for binding to purified integrin receptors [2]. Several other investigators have also reported that HA adsorbs FN and VN from FBS [30,31]. Thus, the finding that osteosarcoma cells do not spread on FBS-coated HA, despite the presence of adsorbed FN and VN, suggests that there is some component within FBS that actively blocks osteosarcoma, but not MSC, cell spreading. Identification of this component could have therapeutic implications with regard to cancer metastasis.
Finally, it is noteworthy that MSCs express greater levels of the α2 integrin subunit, relative to MG-63 and Saos-2 cells, which likely contributes to the high degree of adhesion to col-I exhibited by MSCs. Similarly, α2β1 integrins are more highly expressed by fetal rat calvarial osteoblasts as compared with R0S17/2.8 osteosarcoma cells [14]. The organic phase of bone is approximately 90% col-I, and therefore α2β1 function may be important for anchoring MSCs or pre-osteoblastic cells to bone during wound healing or bone-remodeling. However, the α2β1 integrin species is not only important for initial cell attachment, but also appears to play a crucial role in inducing osteoblastic differentiation in both MSCs [32,33], and the MC3T3-E1 preosteoblastic cell line [34–36]. Ligand binding to α2β1 stimulates phosphorylation (and therefore activation) of Focal Adhesion Kinase (FAK), which leads to the activation of the osteoblast-specific transcription factor, cbfa-1 (runx2), possibly through an ERK-dependent signaling cascade [34,36–38]. In turn, activation of cbfa-1 induces increased expression of genes such as osteocalcin and alkaline phosphatase and promotes matrix mineralization. Function-blocking antibodies specific for α2β1 inhibit osteoblast-specific gene expression and matrix mineralization [29,35,39]. Taken together these results suggest that functionalizing biomaterial surfaces with molecules that activate α2β1 receptors may provide a dual benefit by promoting both initial MSC attachment and osteoblastic differentiation.
CONCLUSIONS
The broad goal of the current study was to determine whether osteosarcoma cell lines represent valid cell systems for evaluating the behavior of osteogenic cells on hydroxyapatite biomaterials. Notably we find that two osteosarcoma cell lines, MG-63 and Saos-2, exhibit striking similarities in their preferences for matrix molecules, as well as expression and utilization of integrin receptors. However, the adhesive behavior and integrin expression profiles of the osteosarcoma cell lines are quite different from that of MSCs. These results are important because the development of biomaterial surfaces that optimally promote osteogenic cell attachment and survival is a crucial factor in successful therapeutic strategies for bone regeneration. Our results suggest that osteosarcoma cells are not ideal cell systems for modeling the behavior of MSCs.
Acknowledgments
The authors gratefully acknowledge support from NIAMS grant R01AR51539 (SLB), and a predoctoral NRSA from NIBIB (KMH).
References
- 1.Geesink RG. Clin Orthop Relat Res. 2002;395:53. doi: 10.1097/00003086-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Kilpadi KL, Chang PL, Bellis SL. J Biomed Mater Res. 2001;57:258. doi: 10.1002/1097-4636(200111)57:2<258::aid-jbm1166>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Sawyer AA, Hennessy KM, Bellis SL. Biomaterials. 2005;26:1467. doi: 10.1016/j.biomaterials.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Sawyer AA, Hennessy KM, Bellis SL. Biomaterials. 2007;28:383. doi: 10.1016/j.biomaterials.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Anselme K. Biomaterials. 2000;21:667. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 6.Siebers MC, ter Brugge PJ, Walbooners XF, Jansen JA. Biomaterials. 2005;26:137. doi: 10.1016/j.biomaterials.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Conget PA, Minguell JJ. J Cell Physiol. 1999;181:67. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 9.Ringe J, Kaps C, Burmester GR, Sittinger M. Naturwissenschaften. 2002;89:338. doi: 10.1007/s00114-002-0344-9. [DOI] [PubMed] [Google Scholar]
- 10.Tabb MM, Sun A, Zhou C, Grün F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, Forman BM, Blumberg B. J Biol Chem. 2003;278:43919. doi: 10.1074/jbc.M303136200. [DOI] [PubMed] [Google Scholar]
- 11.Clover J, Gowen M. Bone. 1994;15:585. doi: 10.1016/8756-3282(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 12.Duan X, Jia SF, Zhou Z, Langley RR, Bolontrade MF, Kleinerman ES. Clin Exp Metastasis. 2004;21:747. doi: 10.1007/s10585-005-0599-6. [DOI] [PubMed] [Google Scholar]
- 13.Marco RA, Diaz-Montero CM, Wygant JN, Kleinerman ES, McIntyre BW. J Cell Biochem. 2003;88:1038. doi: 10.1002/jcb.10465. [DOI] [PubMed] [Google Scholar]
- 14.Tang CH, Yang RS, Liu CZ, Huang TF, Fu WM. Toxicon. 2004;43:11. doi: 10.1016/j.toxicon.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Kilpadi KL, Sawyer AA, Prince CW, Chang PL, Bellis SL. J Biomed Mater Res. 2004;68:273. doi: 10.1002/jbm.a.20043. [DOI] [PubMed] [Google Scholar]
- 16.Akiyama SK, Yamada KM. J Biol Chem. 1987;262:17536. [PubMed] [Google Scholar]
- 17.Heino J, Ignotz RA, Hemler ME, Crouse C, Massague J. J Biol Chem. 1989;264:380. [PubMed] [Google Scholar]
- 18.Lenter M, Vestweber D. J Biol Chem. 1994;269:12263. [PubMed] [Google Scholar]
- 19.Dumbleton J, Manley MT. J Bone Joint Surg Am. 2004;86A:2526. doi: 10.2106/00004623-200411000-00029. [DOI] [PubMed] [Google Scholar]
- 20.Soballe K, Mouzin OR, Kidder LA, Overgaard S, Bechtold JE. Acta Orthop Scand. 2003;74:239. doi: 10.1080/00016470310014139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karrholm J. J Pediatr Orthop B. 1997;6:91. [PubMed] [Google Scholar]
- 22.Hennessy KM, Clem WC, Phipps MC, Sawyer AA, Shaikh FM, Bellis SL. Biomaterials. 2008 doi: 10.1016/j.biomaterials.2008.04.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies JE. In vitro modeling of the bone/implant interface. Anat Rec. 1996;245:426. doi: 10.1002/(SICI)1097-0185(199606)245:2<426::AID-AR21>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Lévesque JP, Leavesley DI, Niutta S, Vadas M, Simmons PJ. J Exp Med. 1995;181:1805. doi: 10.1084/jem.181.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinashi T, Asaoka T, Setoguchi R, Takatsu K. J Immunol. 1999;162:2850. [PubMed] [Google Scholar]
- 26.Dastych J, Metcalfe DD. J Immunol. 1994;152:213. [PubMed] [Google Scholar]
- 27.Keselowsky BG, Wang L, Schwartz Z, Garcia AJ, Boyan BD. J Biomed Mater Res. 2007;80:700. doi: 10.1002/jbm.a.30898. [DOI] [PubMed] [Google Scholar]
- 28.Moursi AM, Globus RK, Damsky CH. J Cell Sci. 1997;110:2187. doi: 10.1242/jcs.110.18.2187. [DOI] [PubMed] [Google Scholar]
- 29.Schneider GB, Zaharias R, Stanford C. J Dent Res. 2001;80:1540. doi: 10.1177/00220345010800061201. [DOI] [PubMed] [Google Scholar]
- 30.Steele JG, Dalton BA, Johnson G, Underwood PA. Biomaterials. 1995;16:1057. doi: 10.1016/0142-9612(95)98901-p. [DOI] [PubMed] [Google Scholar]
- 31.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. J Biomed Mater Res. 2000;51:475. doi: 10.1002/1097-4636(20000905)51:3<475::aid-jbm23>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Mizuno M, Fujisawa R, Kuboki Y. J Cell Physiol. 2000;184:207. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 33.Mizuno M, Kuboki Y. J Biochem (Tokyo) 2001;129:133. doi: 10.1093/oxfordjournals.jbchem.a002824. [DOI] [PubMed] [Google Scholar]
- 34.Suzawa M, Tamura Y, Fukumoto S, Miyazono K, Fujita T, Kato S, Takeuchi Y. J Bone Miner Res. 2002;17:240. doi: 10.1359/jbmr.2002.17.2.240. [DOI] [PubMed] [Google Scholar]
- 35.Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. J Biol Chem. 1998;273:32988. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi Y, Suzawa M, Kikuchi T, Nishida E, Fujita T, Matsumoto T. J Biol Chem. 1997;272:29309. doi: 10.1074/jbc.272.46.29309. [DOI] [PubMed] [Google Scholar]
- 37.Tamura Y, Takeuchi Y, Suzawa M, Fukumoto S, Kato M, Miyazono K, Fujita T. J Bone Miner Res. 2001;16:1772. doi: 10.1359/jbmr.2001.16.10.1772. [DOI] [PubMed] [Google Scholar]
- 38.Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, Franceschi RT. J Biol Chem. 2000;275:4453. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- 39.Gronthos S, Simmons PJ, Graves SE, Robey PG. Bone. 2001;28:174. doi: 10.1016/s8756-3282(00)00424-5. [DOI] [PubMed] [Google Scholar]