Abstract
Functional near-infrared spectroscopy (fNIRS) is a non-invasive brain imaging method that uses light to record regional changes in cerebral blood flow in the cortex during activation. FNIRS uses portable wearable sensors to allow measurements of brain activation during tasking. In this study, fNIRS was used to investigate how the brain processes information from multiple sensory modalities during dynamic posturography. Fifteen healthy volunteers (9M/6F; ages 28 +/− 9 yrs) participated in the posturography study while undergoing fNIRS brain imaging. Four standard conditions from the sensory organization test (SOT) were performed and a bilateral fNIRS probe was used to examine the cortical brain responses from the frontal, temporal, and parietal brain regions. We found there was bilateral activation in the temporal-parietal areas (superior temporal gyrus, STG, and supramarginal gyrus, SMG) when both vision and proprioceptive information was degraded; forcing reliance on primarily vestibular information in the control of balance. This is consistent with previous reports of the role of these regions in vestibular control and demonstrates the potential utility of fNIRS in the study of cortical control of vestibular function during standing balance tasks.
Keywords: Functional Near-infrared Spectroscopy, Computerized Dynamic Posturography, Balance
Introduction
Maintenance of upright stance relies on the integration of sensory information about a person’s spatial orientation obtained from vestibular organs, cutaneous and proprioception receptors, and vision (Lee and Lishman, 1975; Magnusson et al., 1990; Nashner et al., 1982). Current theories suggest that during every day experiences, the relative information available from these channels must be continuously reweighted (Mahboobin et al., 2005; Mergner and Becker, 2003; Peterka and Loughlin, 2004). For example, when entering a dimly lit room, the postural control system must adjust to the loss of accurate visual input. Although models of sensory reweighting typically assign this integrative role to the central nervous system, direct evidence of the cortical structures involved with this is sparse.
In particular, because of technological restrictions, the role of brain activity in this multi-sensory integration process as it relates to standing postural control has not been directly studied. In general, neuroimaging techniques such as magnetic resonance imaging (MRI) or positron emission tomography (PET) require the patient’s head to remain motionless and to lie in the supine position. While FDG (18F-florodeoxy-glucose) based PET is unique in that the compound can be injected outside the PET scanner (e.g. during walking or balance (la Fougere et al., 2010; Shimada, 2012)) and later imaged, the long half-life of the PET compound (108-minutes) precludes good temporal resolution and prevents the sequential repeated measurements needed to quantify brain activity during the different phases of the SOT paradigm. Research with individuals who have peripheral and central vestibular disorders has implicated regions of the temporal and parietal cortex in multi-sensory integration including the inferior parietal lobe, superior temporal gyrus and supramarginal gyrus (Dieterich and Brandt, 2008). In addition, functional neuroimaging of caloric stimulation (Dieterich et al., 2003; Fasold et al., 2002), vestibular evoked myogenic potential (VEMP) stimulation (Schlindwein et al., 2008), and galvanic vestibular stimulation (Stephan et al., 2005) in healthy persons produces similar findings.
Computerized posturography assessment involves a set of clinical tests used to assess posture and balance control. The sensory organization test (SOT, Neurocom, Inc) is a component of computerized dynamic posturography that assesses how people use different combinations of sensory feedback to maintain upright stance (Nashner and Peters, 1990). The SOT test is based on a series of sensory combinations involving the loss, or degradation, of accurate visual and/or proprioceptive feedback about the person’s orientation. Proprioceptive information about the angular position of the ankle joint is degraded by sway-referencing the standing support surface in the sagittal plane. In the clinical test, eye closure or sway-referencing of the visual enclosure compromises accurate visual feedback. By manipulating the sensory information through these sway referencing and light/dark conditions, the SOT protocol is used to systematically test the person’s ability (or inability) to compensate for the loss of sensory information and to maintain postural control. While in some cases balance problems may be due to uncompensated vestibular deficits, dynamic posturography specifically evaluates overall balance ability and provides information on potential fall risk (Furman and Whitney, 2000). In particular, multi-sensory integration dysfunction is often the cause of secondary balance problems associated with brain disorders including multiple sclerosis (Jackson et al., 1995), stroke (Bonan et al., 2004a; Bonan et al., 2004b; Ikai et al., 2003), Parkinson’s (Nocera et al., 2010; Toole et al., 1996) and Alzheimer’s (Suttanon et al., 2012). The SOT paradigm consists of a standard set of six conditions as described by Nashner and Peters (Nashner and Peters, 1990) where visual and proprioceptive information is altered or removed. Four of these conditions (SOT I, II, IV, and V) are designed to probe the interaction of vestibular and proprioceptive information in the presence or absence of visual information (e.g. eyes open/closed). The other two conditions (SOT III and VI) use a moving visual scene (visual sway-referencing) to examine the effect of conflicting visual information. In clinical practice, SOT I, II, IV, and V are used to evaluate vestibular disorders while the remaining two conditions have been suggested to be less reliable as clinical tools (Barin, 1992). In this study, only these four vestibular SOT conditions were examined due to both technical limitations and to limit subject fatigue associated with the length of the experiment needed to test all six conditions in pairwise combinations.
In this study, we used a novel brain imaging technique called functional near-infrared spectroscopy (fNIRS). FNIRS uses low levels of light to measure blood flow and blood oxygenation changes in the brain. Thus, fNIRS measures the hemodynamic response in the brain and provides similar information to functional magnetic resonance imaging (fMRI). Several previous studies (reviewed in (Steinbrink et al., 2006)) have shown close correspondence between fNIRS and fMRI signals with temporal and spatial (linear) correlations of up to R=0.98 (Huppert et al., 2006b) and R=0.86 (Huppert et al., 2006a), respectively. Unlike fMRI, fNIRS is a portable technique that uses fiber optic cables mounted in a wearable head cap. This lightweight head cap allows imaging of the brain even during ambulatory movement and has previously been used to record brain activity during cued stepping (Huppert et al., 2012), walking (Miyai et al., 2001; Suzuki et al., 2008), and balance (Karim et al., 2011) studies. The purpose of this study was to record changes in brain activity in healthy volunteer participants, using fNIRS during the four vestibular SOT conditions.
Methods
i. Experimental Subjects
Fifteen healthy, right-handed volunteers (9M/6F, aged 28 +/− 9 yrs) participated in this study. After providing informed consent, all subjects were screened for self-reported histories of vestibular, balance, or mobility impairments. This study was approved by the University of Pittsburgh Institutional Review Board protocol.
ii. Dynamic Posturography
All posturography was performed using a NeuroCom (Clackamas OR, USA) Equitest™ posturography platform (see figure 1A) while fNIRS data was recorded. FNIRS signals were recorded during testing of four postural conditions corresponding to SOT I (fixed floor – eyes open in light), SOT II (fixed floor – eyes open in dark), SOT IV (sway-referenced floor – eyes open in light), and SOT V (sway-referenced floor – eyes open in dark). Comparisons among these four conditions allow examination of subject balance and brain responses to the loss of accurate visual and proprioceptive feedback.
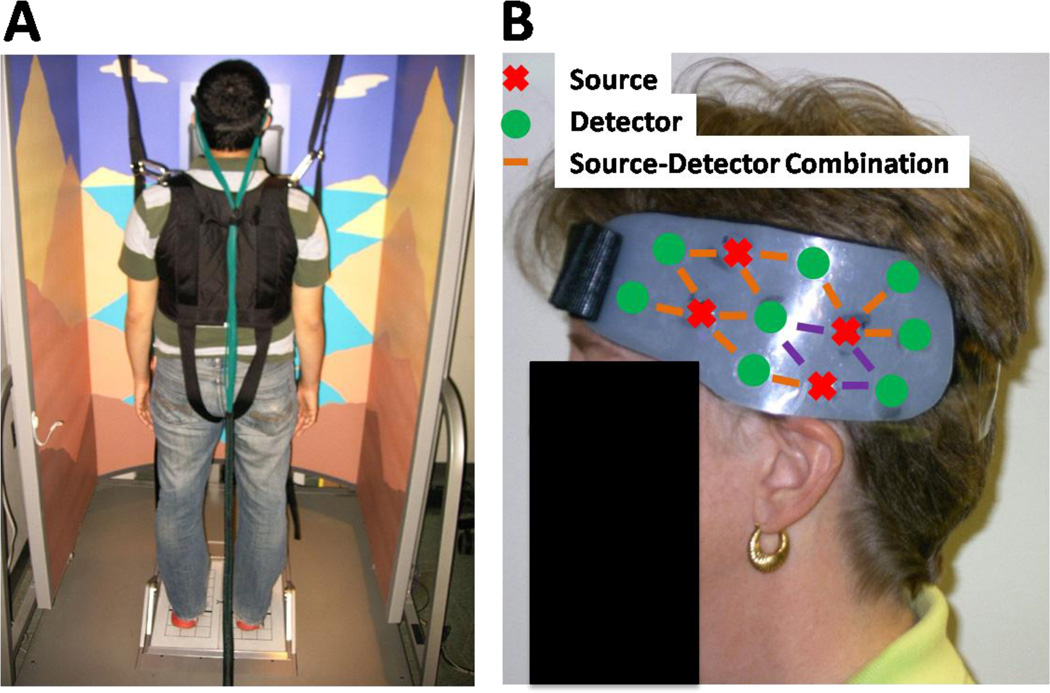
Figure 1.
Setup of fNIRS and dynamic posturography. (panel A) Subjects were harnessed into the Equitest™ system with fNIRS head cap attached. The fiber optic cables (green fibers) deliver light to and from the head cap and CW6 system. (panel B) View of the left side of the bilateral probe. Source-detector combinations in purple are a region of interest (ROI) used in figure 2.
During clinical posturography, each condition is tested separately. However for this fNIRS brain study, this paradigm was modified such that a pair of SOT conditions was tested sequentially in a blocked design. This design is therefore more consistent with standard functional testing in fMRI or fNIRS, which both provide relative measurements of changes in brain activity and require a statistical comparison between conditions acquired within the same scan. In this study, the postural conditions were paired into four comparisons as shown in Table 1. Each fNIRS scan consisted of an initial baseline condition (45 sec), a test condition (45 sec), and a repeat of the baseline condition (60 sec). For each condition-pair, test conditions had less sensory information than the corresponding baseline conditions. The effect of the transition from baseline to test condition was reversed during the final baseline condition.
Table 1.
Four sensory organization test (SOT) combinations used in this study. Each combination only degraded one sensory condition (test effect) when going from baseline to test condition while the other was kept constant. When the force platform did not move it was called ‘fixed’ while when it sway-referenced it was called ‘sway-referenced floor’ (SRF). When opaque goggles were used to blindfold the subject, it was called ‘dark’ while when this did not occur it was called ‘light.’
| Comparison | Baseline | Test | Accurate Sensory Feedback Modalities |
|---|---|---|---|
| A | Fixed-Dark (SOT II) | SRF-Dark (SOTV) | Baseline: Vestibular, Proprioception Test: Vestibular |
| B | Fixed-Light (SOTI) | SRF-Light (SOT IV) | Baseline: Vestibular, Proprioception, Vision Test: Vestibular, Vision |
| C | Fixed-Light (SOTI) | Fixed-Dark (SOT II) | Baseline: Vestibular, Proprioception, Vision Test: Vestibular, Proprioception |
| D | SRF-Light (SOT IV) | SRF-Dark (SOT V) | Baseline: Vestibular, Vision Test: Vestibular |
The comparison presentation order was randomized across subjects. For each subject the four scan series were presented twice making eight total scans. After every two scans, the participant was given a seated rest period for a minimum of two-minutes. The total participation time for this study was approximately 60 minutes including: subject consenting (5–10 minutes), instrumenting the subject with the fNIRS head cap (20–30 minutes), and posturography with breaks (26–30 minutes). For pairs 1 and 2, the Equitest™ posture data was recorded as three separate files due to a limitation of the Equitest™ system that did not allow for smooth transition from fixed to sway-referenced platform as this is not normally done in a clinical setting.
iii. FNIRS Instrumentation
During fNIRS recordings, flexible fiber optic cables deliver low levels of light (<0.4W/cm2) to an arrangement of source positions on the scalp (see figure 1B). Each position contains two wavelengths of light (690nm and 830nm), which are used to separate absorption changes differentially due to oxy- and deoxy-hemoglobin. Based on data from previous modeling studies (Wang et al., 1995), the light emitting from a source position in the fNIRS head cap diffuses through the tissue and penetrates the outer 5–8mm of the cerebral cortex. Light is then detected as it exits the head using a discrete set of fiber optics that carry light back to photon detectors on the fNIRS instrument. Thus, the amount of light traveling between light source locations to detector positions is directly related to the absorption of the underlying tissue between measurement source-detector pair. During evoked brain activity, regional changes in blood flow to the active cortical region alter concentrations of oxy- and deoxy-hemoglobin, differentially changing the light absorption characteristics at different wavelengths due to the optical absorption profile differences in the two hemoglobin states. Changes in hemoglobin can be recovered from fNIRS measurements at multiple wavelengths using the modified Beer-Lambert law. By spatially arranging the optical sensors on the head, the location of the brain signal can be approximated as reviewed in Boas et al (Boas et al., 2004).
In this study, fNIRS data was recorded using a 32-channel continuous wave fNIRS instrument (CW6 real-time system; TechEn Inc; Milford, MA). The instrument uses two different wavelengths of light at 690 nm and 830 nm within the optical window, which allows changes of both oxy- and deoxy-hemoglobin to be recorded. The fNIRS bilateral head cap is made from plastic materials and Velcro and contained 8 sources and 16 detectors. The source-detector pairs were arranged in a nearest neighbor geometry with 3.2 cm source-detector spacing, creating 30 source-detector combinations. FNIRS data were sampled at 4 Hz. Custom acquisition software described in (Abdelnour and Huppert, 2009) allowed for real-time visualization of brain activity. The acquisition software allows for events to be manually marked by the operator throughout the task to indicate the transitions between SOT conditions. Although manual synchronization is somewhat suboptimal, the Equitest system that was used in this study was a clinical device that could not be modified for research purposes and does not come equipped with an output to automatically synchronize our system. In similar studies, we have observed that this means of synchronization has an error of no more than 2–3 sample points (500–750ms) which only represents about a 2% error in light of the long (45s) duration of the task.
v. Analysis
Statistical Analysis of Equitest™ Posturography Data
Subject center of pressure (COP) information in the anterior-posterior direction was measured using force plate data. The root-mean-square (RMS) of the COP from the mean COP and the average velocity (cumulative absolute displacement divided by time) of COP was calculated for the two baseline conditions and the test condition in each trial. Results from the two baseline conditions were averaged and subtracted from the test condition result yielding: the RMS difference and the velocity difference, respectively. RMS and velocity differences were analyzed separately using repeated measures AVOVA with condition pair as the within-subject variable (α=0.05 a priori).
FNIRS Data Analysis
The analysis of fNIRS data has been previously described in several previous papers (Huppert et al., 2009; Ye et al., 2009). In brief, analysis of fNIRS data was based on a spatial-temporal version of the general linear model (Ye et al., 2009). This approach is similar to the standard model used for the analysis of fMRI data via a canonical general linear model (e.g. (Friston, 2007)). A custom Matlab (R2011b; Mathworks, Natick MA) script was used to process the fNIRS data. Based on the onset times and identity of the stimulus events, a design matrix was constructed using the a gamma-variant function (Chen et al., 2005) as a model of the expected hemodynamic response. In addition, a series of discrete cosine transform terms (0–1/120 Hz) were used as nuisance regressors to remove slow drift. The model of light propagation in the head was used to model an inhomogeneous random-field in the model (Abdelnour and Huppert, 2011). A similar model had been previously proposed by Ye et al (Ye et al., 2009). In this work, we have used a probe-specific finite element diffusion model of light diffusion through the head (Abdelnour et al., 2010). Restricted maximum likelihood with a first-order autoregressive noise term was used to estimate the noise statistics (pre-whitening) and the linear model was solved using the Gauss-Markov equation (see (Friston, 2007)). Temporal analysis was performed on a per subject basis and the estimated weight coefficients and error models were then used for group-level statistics as described in (Abdelnour and Huppert, 2011).
Prior to collection of the fNIRS data, a three-dimensional electromagnetic tracker (Polhemus, Colchester VT) was used to mark the location of the optical sensors relative to the nasion, inion, and earlobe fiducial locations. This registration information was then used to register the location of the optical sensors to an anatomical MRI head using a custom registration algorithm (Abdelnour and Huppert, 2011). In this study, the Colin27 MRI atlas (Holmes et al., 1998) was used based on previous work by Custo et al (Custo et al., 2006) which demonstrated that atlas-based registration was sufficient for modeling light paths through the head of healthy, normal, subjects. Based on the registration of the optical sensors to the atlas head, a finite-element model of light diffusion (Dehghani et al., 2008) was used as described in (Abdelnour et al., 2009). An image reconstruction model based on restricted maximum likelihood was used as described previously (Abdelnour and Huppert, 2009; Abdelnour and Huppert, 2011; Abdelnour et al., 2010).
Group-level analysis across the subjects was performed using a random-effects model of brain activity and simultaneous reconstruction of all subject's data in an image reconstruction as described elsewhere (Abdelnour and Huppert, 2011). The image reconstruction model was based on the cortical-surface model described in Abdelnour and Huppert (Abdelnour et al., 2009), which used wavelets to model the surface of the cortex of the brain. In brief, a group-level image is estimated, that is simultaneously consistent with all of the data from each subject using a Bayesian objective function based on maximum likelihood (Abdelnour et al., 2010). In the random-effects model (Abdelnour and Huppert, 2011), the Polhemus registration of the fNIRS cap from each subject is used to generate an individual optical forward model using the Colin27 atlas. The forward models from all the subjects are then collected into a single linear matrix operator such that the estimate of each subject’s brain activity is the sum of the group average and a random-effects perturbation term for that subject. Thus, instead of preforming 15 independent image reconstructions (one for each subject), the larger combined forward model is inverted in order to estimate an image of brain activity that is most simultaneously consistent with all subjects’ data. This approach was shown to be less susceptible to artifacts and errors introduced by outlier measurements in only a few subjects. Restricted maximum likelihood (ReML) is an empirical Bayesian method, which is used to provide stabilization of the inverse (image reconstruction) model and for simplified models is mathematically equivalent to an L-curve technique to optimize regularization (Abdelnour et al., 2010). This approach has been widely used in fMRI analysis (Cox, 1996; Friston, 2007) and in the implementation of the similar image reconstruction problem for electroencephalography (EEG) and magnetoencephalography (MEG) within the software SPM8 (Friston, 2007; Mattout et al., 2006). The use of ReML for fNIRS was described in Abdelnour et al (Abdelnour and Huppert, 2011). The ReML based random-effects image reconstruction method used in this work is very similar to the implementation of the random-effects MEG reconstruction model used in SPM8 (Mattout et al., 2006).
Results
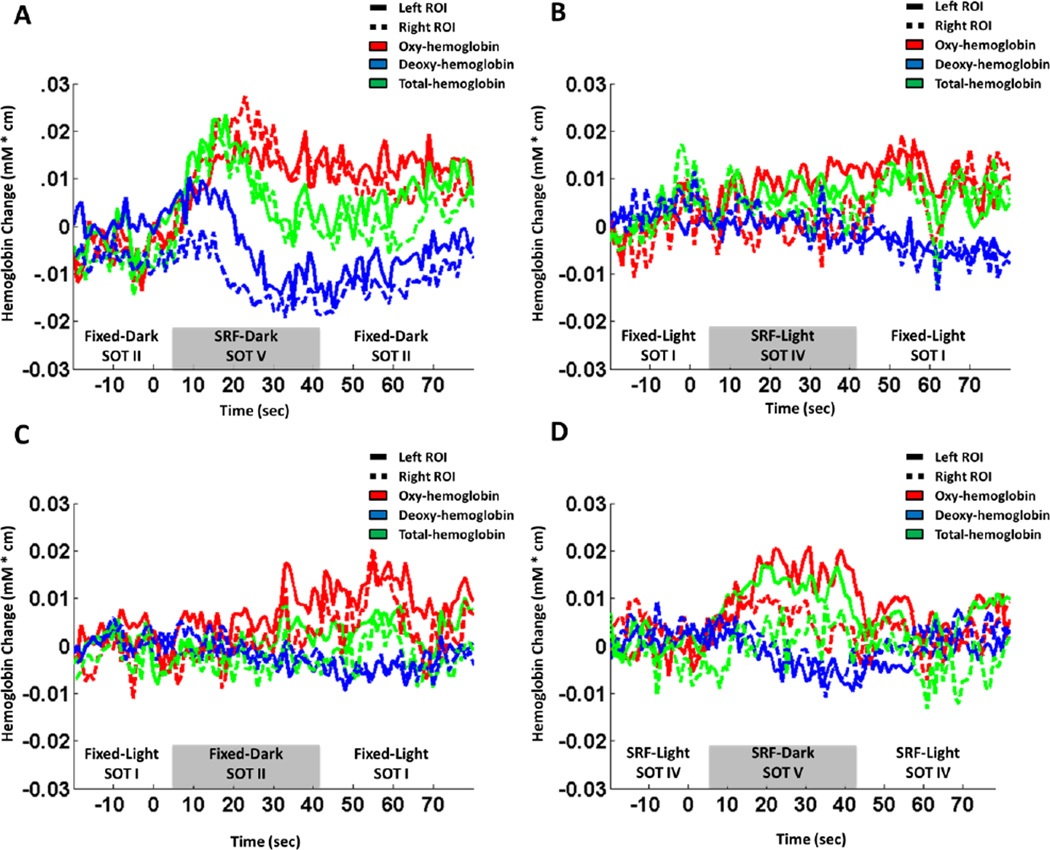
Functional NIRS responses were measured during the four pairs of SOT sensory conditions (Table 1). Figure 2 shows the average fNIRS temporal responses over all fifteen subjects from the channels located within a region-of-interest over the left and right temporal-parietal (see figure 1B) regions of the fNIRS probe. This region-of-interest was selected as the channels within 3 cm of the superior-temporal gyrus on either side of the head (channels shown in figure 1B). The trials from the fixed-dark (SOT II) to SRF-dark (SOT V) (Figure 2A) and SRF-light (SOT IV) to SRF-dark (SOT V) (Figure 2D) produced the largest change in brain signals. Both of these comparisons included the SOT V condition in which visual and proprioceptive feedbacks were degraded and participants were forced to rely primarily on vestibular information. As shown in Figure 2A and 2D, for these two comparisons the fNIRS data showed a typical hyperemic (increased blood flow) response in which both oxy- and total-hemoglobin rise following the transition from the baseline to test conditions. In these comparisons, deoxy-hemoglobin also decreased, which is consistent with the characteristic fMRI BOLD response (Steinbrink et al., 2006). The trials that evaluated changing from fixed-light (SOT I) to SRF-light (SOT IV) (Figure 2B) and fixed-light (SOT I) to fixed-dark (SOT II) (Figure 2C) showed much weaker responses in the same regions of interest. In these two test comparisons, only one of the three sensory inputs is degraded (either vision or proprioception), which allowed the participants to use both vestibular and the other remaining input to control balance.
Figure 2.
The ensemble-averaged fNIRS time course for all four SOT combinations in this study in two (right and left, see figure 1) temporal-parietal source-detector combinations. These source detector combinations sample a region over the superior temporal gyrus (STG).
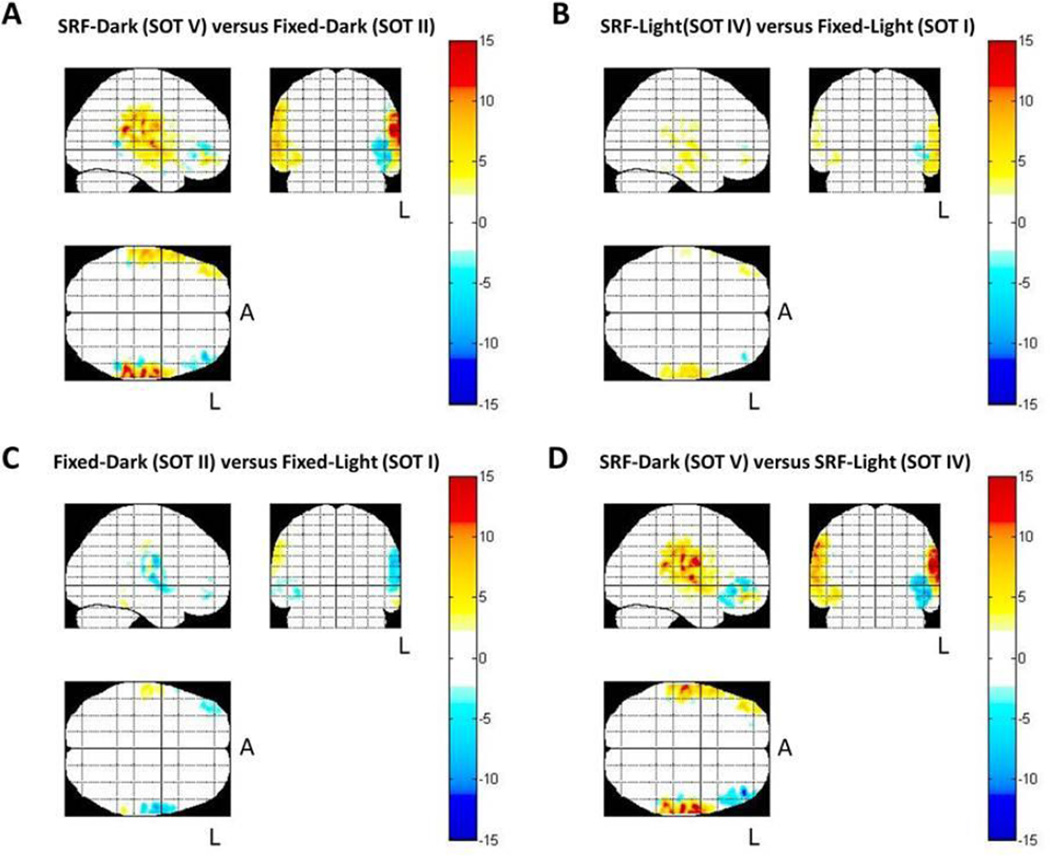
Based on the recorded fNIRS data from all of the source-detector pairs, an image of brain activity can be reconstructed. Figure 3 shows the estimated spatial maps of oxy-hemoglobin based on the fNIRS data and the probe registration for each subject based on the methods described elsewhere (Abdelnour and Huppert, 2011; Abdelnour et al., 2009).
Figure 3.
Estimated spatial maps (T-test) of the oxy-hemoglobin data collected using fNIRS for the change in brain activity between the test and baseline conditions using all source-detector combinations. The color bar represents the results of the t-statistic (T-score). Areas in red indicate regions more activated (increased oxy-hemoglobin) during the comparison. Areas in blue indicate decreased oxyhemoglobin during the test condition in comparison to the baseline condition. Data are displayed as a maximum intensity projection along the sagittal, coronal, and axial directions. The L indicates the left side of the brain and the A represents anterior. Only areas with significant activation (p<0.05; corrected) are shown.
During the comparisons where subjects went from fixed-dark (SOT II) to SRF-dark (SOT V) (Figure 3A) and from SRF-light (SOT IV) to SRF-dark (SOT V) (Figure 3D), bilateral activation in the temporal-parietal regions (superior temporal gyrus, STG, and supramarginal gyrus, SMG) was observed. It can be seen that these conditions caused similar bilateral temporal-parietal activations as well as slight activation of the right PFC (prefrontal cortex) and deactivations of the left PFC. Only statistically changed regions (<0.05; corrected for effective degrees-of-freedom) are shown for each comparison. The brain images showed a slight hemispheric dominance between conditions. The temporal-parietal activation was more prominent in the left hemisphere during both of these conditions.
During the comparisons when subjects went from fixed-light (SOT I) to SRF-light (SOT IV) (Figure 3B) there were left cortical activations in the temporal-parietal area as well as small activations/deactivations in the right/left PFC, respectively. When subjects went from fixed-light (SOT I) to fixed-dark (SOT II) (Figure 3C) there were overall deactivations (decrease in oxy-hemoglobin) primarily in the right prefrontal cortex and left temporo-parietal area and small activations in the right temporo-parietal area.
Table 2 summarizes the activation areas observed in each comparison and provides the significance of the left and right temporal regions of interest (ROI) for oxy- and deoxy-hemoglobin. The center of activation in Talairach space for the group-level inferences and mean response amplitude of the area was defined from a k-means cluster-based analysis (threshold set to p < 0.05). Regions of the superior temporal gyrus covering approximately Brodmann areas 38, 22, and 21 were identified for all four comparisons. Because of the limited spatial resolution of fNIRS and the uses of an atlas-based analysis in this study, caution should be exhibited in interpreting the precise location of these regions. Deoxy-hemoglobin images demonstrated changes centered at the same location as the oxy-hemoglobin results. Deoxy-hemoglobin responses (not shown) were much smaller than the corresponding oxyhemoglobin changes.
Table 2.
Brain activation in regions of interest identified from statistical cluster analysis based on oxy-hemoglobin signals. For each condition, the Talairach coordinate, the ROI mean, and mean/standard deviation of the effect size of oxy-hemoglobin changes over the region-of-interest (ROI) is provided.
| ROI Location | ||||||
| BA | X | Y | Z | ROI Mean | Standard Deviation | |
| SRF Dark (SOT V) versus | BA40 (L) | −58 | 3 | −10 | 4.13 | 1.40 |
| Fixed-Dark (SOT II) | BA48 (R) | 53 | 22 | −2 | 3.95 | 0.91 |
| SRF-Light (SOT IV) versus | BA40 (L) | −58 | 4 | −11 | 1.46 | 0.56 |
| Fixed-Light (SOT I) | BA48 (R) | 53 | 23 | −2 | 1.00 | 0.28 |
| Fixed-Dark (SOT II) versus | BA40 (L) | −58 | 2 | 10 | −0.76 | 0.70 |
| Fixed-Light (SOT I) | BA48 (R) | 53 | 23 | −2 | −0.36 | 0.49 |
| SRF-Dark (SOT V) versus | BA40 (L) | −58 | 4 | −9 | 3.23 | 1.45 |
| SRF-Light (SOT IV) | BA48 (R) | 53 | 23 | −1 | 3.76 | 0.93 |
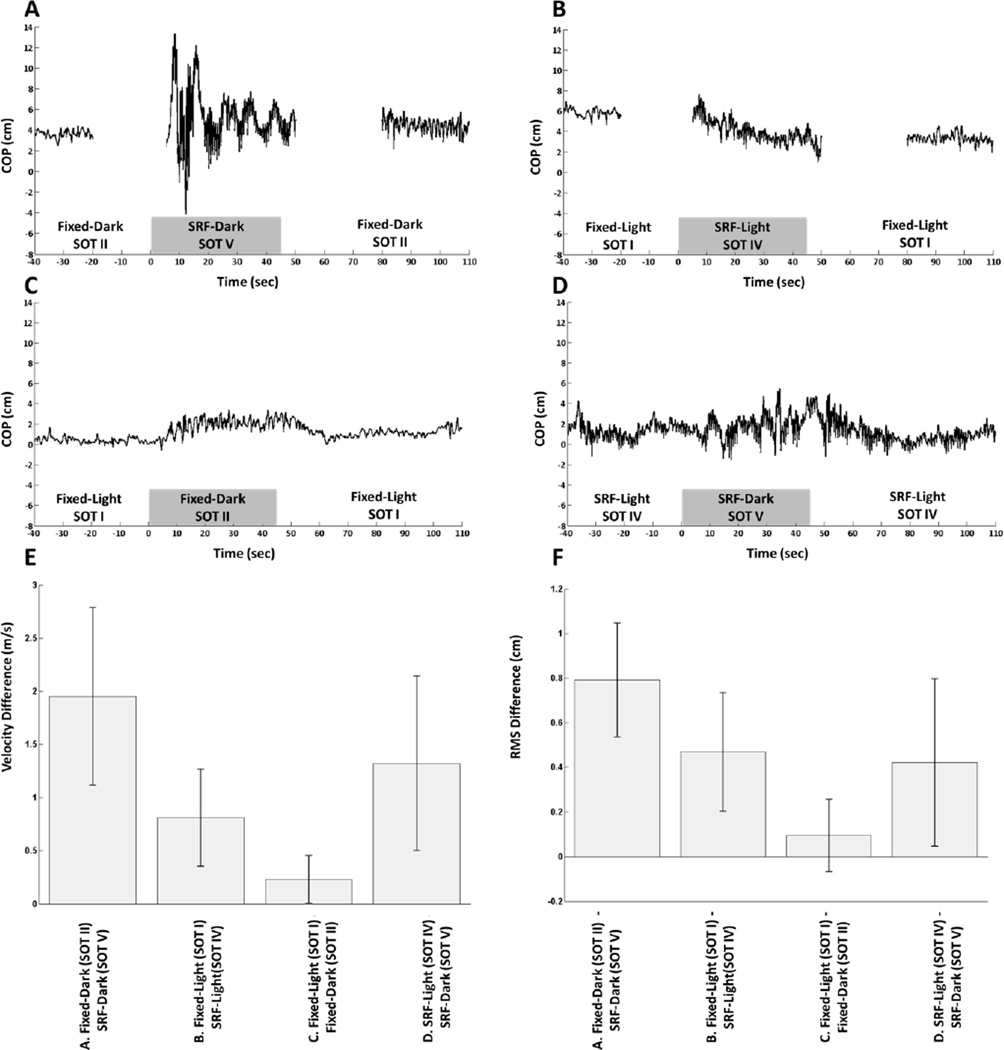
Figure 4 shows the corresponding body-sway data for one representative subject (figure 4A-D) along with the group-level average velocity (figure 4E) and average root-mean-squared (RMS) displacements (Figure 4F) for each of the four test conditions. The gaps in data in figure 4A and B are due to the limitations of the Equitest system as it normally does not transition from fixed to SRF as this is not done clinically. We observed that the degradation of proprioception occurring in the midst of degraded visual feedback (fixed-dark to SRF-dark; Figure 4A) caused the largest change in postural sway. The changes in postural sway were similar for subjects who lost proprioception but retained visual information (fixed-light to SRF-light; Figure 4B) and when subjects lost vision in the absence of proprioceptive information (SRF-light to SRF-dark; Figure 4D). Loss of vision in the presence of proprioceptive information (fixed-light to fixed-dark; Figure 4C) produced the smallest changes in sway. This is supported by both the velocity difference and RMS difference. The means tests show that the velocity differences differed significantly from zero for all condition pairs. Single-sample T-tests for each condition show that for all the SOT combinations except the comparison of fixed-light (SOT I) to fixed-dark (SOT II), the RMS differences between the non-baseline condition and baseline conditions differ significantly from zero.
Figure 4.
Representative anterior-posterior center of pressure (COP) data from a single subject collected from the Equitest™ system (A-D). When subjects went from SOT II to SOT V and from SOT I and SOT IV, the COP data were collected in three separate files (indicated by a gap in the data) since the clinical Equitest™ system does not support a smooth transition between fixed to sway-referenced floor (SRF). FNIRS data were recorded continuously. Velocity difference (E) and RMS difference (F) were calculated across all subjects. Error bars represent one standard deviation.
Discussion
i. Brain Imaging of Multi-sensory Vestibular Processing
In this study, functional near-infrared spectroscopy (fNIRS) was used during upright posturography testing to quantify brain activation in cortical regions during conditions of the sensory organization test (SOT). The primary finding was increased activation in the temporo-parietal regions, in particular the area around the superior temporal gyrus, when subjects relied primarily on vestibular information when both vision and proprioceptive feedback were degraded. This finding is consistent with previous studies investigating the vestibular cortical network using functional MRI or PET imaging during artificial vestibular stimulation (caloric irrigation or galvanic stimulations (Dieterich et al., 2003; Fasold et al., 2002; Stephan et al., 2005)). These studies reported activations in the parietal-insular vestibular cortex (PIVC), visual temporal sylvian area (VTS), superior temporal gyrus (STG), supramarginal gyrus (SMG), and inferior parietal lobe (IPL) (Dieterich and Brandt, 2008). Furthermore, Karim et al. showed that similar regions of the temporal-parietal cortex were involved in a simulated skiing task (Karim et al., 2011). The importance of the supramarginal gyrus (SMG) has also been demonstrated by trans-cranial magnetic stimulation and fMRI to play a role in proprioception and resolution of conflicting sensory information (Tsakiris et al., 2008; Tsakiris et al., 2010). The observation of SMG involvement during the SOT conditions is consistent with this previous observation and extends this finding to lower body and vestibular sensory systems as well. This is consistent with previous work showing the activation of the regions around the temporal parietal junction during vestibulo-ocular (Dieterich et al., 2003; Fasold et al., 2002) and during vestibulo-collic reflex activity (Schlindwein et al., 2008).
The increased activation we observed during various combinations of accurate sensory feedback provides additional support for theories of sensory reweighting described by Brandt/Dieterich et al (Brandt et al., 1998). In this study, we found that the temporo-parietal (STG and SMG) regions were activated the most during the conditions forcing vestibular reliance (fixed-dark to SRF-dark and SRF-light to SRF-dark), which occurred when both visual and proprioceptive information had been degraded. The STG and SMG were activated strongly because there was a necessary reweighting toward the most accurate sensory system, the vestibular system, during these conditions. This suggests that these regions are involved in reweighting the visual, vestibular, and proprioceptive sensory inputs. Previous studies have found that visual-vestibular interactions affect the activation/deactivation of these regions (Brandt et al., 1998; Dieterich et al., 2003) and others have implicated these regions’ (especially SMG) importance in processing proprioceptive information (Tsakiris et al., 2008; Tsakiris et al., 2010). Tsakiris et al. specifically found that the supramarginal gyrus is activated during sensorimotor conflicts (Tsakiris et al., 2010).
In both of the conditions that degraded visual and proprioceptive information, we also observed activations in the right prefrontal cortex (PFC) region as well as deactivations in the left PFC, which is involved in motor planning and executive function. We believe these frontal regions are involved with the attentional demand aspect of standing balance. Previous studies of active balancing have found that the STG and SMG as well as frontal regions such as the PFC and dorsolateral prefrontal cortex (DLPFC) are activated (Mihara et al., 2008; Ouchi et al., 1999). Frontal activations are thought to be involved with the allocation of attention, which is required for postural stability (Ouchi et al., 1999). It is unclear what these frontal deactivations mean, but are likely involved in allocation of attentional resources as well. This may be a mechanism for reducing conflicting/irrelevant information from the main task.
In the condition when only proprioception was degraded (fixed-light to SRF-light), a much smaller activation, compared to activations during conditions that degraded visual and proprioceptive information, in the left superior temporal gyrus was observed. There were also small activations in the right PFC and small deactivations in the left PFC. These patterns are very similar to those seen in conditions where both the visual and proprioceptive inputs were degraded but less so. This condition only degraded proprioceptive inputs and not visual input, thus the subjects were less reliant on just one system. Unlike the other three comparisons, the comparison where only vision was degraded (fixed-light to fixed-dark) caused a decrease in the signal in right frontal and left temporo-parietal area. Loss of visual information is likely a more common scenario in everyday life compared to loss of proprioception or loss of proprioception and visual information. This may account for the deactivation of right frontal and left temporo-parietal areas rather than activation.
ii. Postural Measurements and Brain Imaging
Despite different changes in brain activation, postural sway data showed that the comparison between fixed-light (SOT I) to SRF-light (SOT II) and the comparison that went from SRF-light (SOT IV) to SRF-dark (SOT V) were similar in root mean square (RMS) and velocity difference. In the comparison when only vision was degraded (fixed-light to fixed-dark), a decrease in oxy-hemoglobin in the right PFC and left temporo-parietal area was observed as well as small activations in the right temporo-parietal area. This condition elicited the smallest changes in the posturography data (in terms of RMS and velocity difference). Thus, we found that the magnitudes of the changes in brain signal do not directly correlate with the behavioral (balance) measurements.
In the posturography data, the loss of proprioceptive information in comparison A (fixed-dark to SRF-dark) had a larger effect on body sway compared to comparison D (SRF-light to SRF-dark) even though the test condition in both trials was SRF-dark (SOT V). In the fNIRS brain imaging data, the activation in STG was comparable for these two comparisons on the left side, but larger in comparison D for the left temporal region. Thus, the level of brain activation was not directly correlated to the change in body-sway parameters. The change in body-sway for comparison C (fixed-light to fixed-dark) was not significantly different from that of comparison D (SRF-light to SRF-dark) where both comparisons involve the loss of the visual input. However, the brain response recorded by fNIRS was much larger for comparison D. In comparison C, both vestibular and proprioceptive information are available whereas in comparison D, only vestibular information remains.
iii. Limitations of this study
One of the limitations of fNIRS is the need to place fiber optics over the region of interest in the brain. In order to achieve good signals, these sensors must make firm contact with the scalp in order to measure from the underlying brain. Due to hair, the increased thickness of the skull, and overlying dural sinuses, regions of the brain such as the occipital cortex are more difficult to measure fNIRS signals. Thus, in this current study, we limited our exploration to the temporo-parietal and frontal regions, which had been suggested by previous PET and fMRI studies. The occipital regions (including the visual cortex) would be expected to play a role in sorting sensory information particularly related to the two visual-sway referencing conditions (SOT III and VI), which were not examined in this study partly due to technical limitations and to limit subject fatigue. Because no fNIRS sensors were placed over the occipital areas, our current study also cannot make statements about the roles of these areas in the conditions that were tested and may be investigated in future work. In addition, the limited depth of fNIRS measurements also limits our study to the outer cortex and thus deeper areas involved in vestibular function cannot be observed.
Conclusion
Our results show that the area around the superior temporal gyrus and supramarginal gyrus are preferentially activated during reliance on vestibular information in the control of balance. This is consistent with earlier evidence of the role of these areas in vestibular processing based on caloric, galvanic, and optokinetic stimulation. Furthermore, since fNIRS systems are relatively portable compared to other brain imaging systems like PET or fMRI, an advantage of fNIRS is the ability to be used in studies where subjects are ambulatory. With the exception of 18F-deoxyglucose (FDG) studies, fNIRS has a clear advantage for the study of ambulatory and balance studies compared to PET and fMRI. Although we believe that this is the first study to measure brain activation using fNIRS during the SOT dynamic posturography task, fNIRS has been previously used to study the cortical activations during active balancing (Karim et al., 2011; Mihara et al., 2008), walking (Huppert et al., 2012; Miyai et al., 2001; Suzuki et al., 2008), and during exposure to high g-forces (Benni et al., 2003; Ryoo et al., 2002; Ryoo et al., 2004). Thus, fNIRS provides a complimentary neuroimaging tool to conventional PET and MR imaging. Within the last few years, several commercial wireless fNIRS devices have been introduced, which could allow an even further range of studies to be performed in the future.
Highlights.
Functional near-infrared spectroscopy (fNIRS) measures functional activation.
FNIRS was used in subjects during computerized dynamic posturography.
Degrading vision and proprioception caused bilateral temporo-parietal activation.
Bilateral activation may be due to forced reliance on vestibular control.
FNIRS can investigate the cortical role during vestibular and balance tasks.
Acknowledgements
This work was funded by NIH National Institute on Aging (NIH-P30AG024827).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- Abdelnour AF, Huppert T. Real-time imaging of human brain function by near-infrared spectroscopy using an adaptive general linear model. Neuroimage. 2009;46:133–143. doi: 10.1016/j.neuroimage.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour AF, Huppert TJ. A random-effects model for group-level analysis of diffuse optical brain imaging. Biomedical Optics Expres. 2011;2:1–25. doi: 10.1364/BOE.2.000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour F, Genovese C, Huppert T. Hierarchical Bayesian regularization of reconstructions for diffuse optical tomography using multiple priors. Biomedical optics express. 2010;1:1084–1103. doi: 10.1364/BOE.1.001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour F, Schmidt B, Huppert TJ. Topographic localization of brain activation in diffuse optical imaging using spherical wavelets. Phys Med Biol. 2009;54:6383–6413. doi: 10.1088/0031-9155/54/20/023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barin K. Dynamic Posturography: Analysis of Error in Force Plate Measurement of Postural Sway. IEEE Engineering in Medicine and Biology Magazine. 1992;11:52–56. [Google Scholar]
- Benni PB, Li JK, Chen B, Cammarota J, Amory DW. NIRS monitoring of pilots subjected to +Gz acceleration and G-induced loss of consciousness (G-LOC) Advances in experimental medicine and biology. 2003;530:371–379. doi: 10.1007/978-1-4615-0075-9_34. [DOI] [PubMed] [Google Scholar]
- Boas DA, Dale AM, Franceschini MA. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage. 2004;23(Suppl 1):S275–S288. doi: 10.1016/j.neuroimage.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Bonan IV, Colle FM, Guichard JP, Vicaut E, Eisenfisz M, Tran Ba Huy P, Yelnik AP. Reliance on visual information after stroke. Part I: Balance on dynamic posturography. Archives of physical medicine and rehabilitation. 2004a;85:268–273. doi: 10.1016/j.apmr.2003.06.017. [DOI] [PubMed] [Google Scholar]
- Bonan IV, Yelnik AP, Colle FM, Michaud C, Normand E, Panigot B, Roth P, Guichard JP, Vicaut E. Reliance on visual information after stroke. Part II: Effectiveness of a balance rehabilitation program with visual cue deprivation after stroke: a randomized controlled trial. Archives of physical medicine and rehabilitation. 2004b;85:274–278. doi: 10.1016/j.apmr.2003.06.016. [DOI] [PubMed] [Google Scholar]
- Brandt T, Bartenstein P, Janek A, Dieterich M. Reciprocal inhibitory visual-vestibular interaction. Visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain : a journal of neurology. 1998;121(Pt 9):1749–1758. doi: 10.1093/brain/121.9.1749. [DOI] [PubMed] [Google Scholar]
- Chen H, Yao D, Liu Z. A comparison of Gamma and Gaussian dynamic convolution models of the fMRI BOLD response. Magn Reson Imaging. 2005;23:83–88. doi: 10.1016/j.mri.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Custo A, Wells WM, 3rd, Barnett AH, Hillman EM, Boas DA. Effective scattering coefficient of the cerebral spinal fluid in adult head models for diffuse optical imaging. Appl Opt. 2006;45:4747–4755. doi: 10.1364/ao.45.004747. [DOI] [PubMed] [Google Scholar]
- Dehghani H, Eames ME, Yalavarthy PK, Davis SC, Srinivasan S, Carpenter CM, Pogue BW, Paulsen KD. Near infrared optical tomography using NIRFAST: Algorithm for numerical model and image reconstruction. Commun Numer Methods Eng. 2008;25:711–732. doi: 10.1002/cnm.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, Brandt T. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex. 2003;13:994–1007. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Brandt T. Functional brain imaging of peripheral and central vestibular disorders. Brain : a journal of neurology. 2008;131:2538–2552. doi: 10.1093/brain/awn042. [DOI] [PubMed] [Google Scholar]
- Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, Wenzel R. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage. 2002;17:1384–1393. doi: 10.1006/nimg.2002.1241. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Statistical parametric mapping : the analysis of functional brain images. London: Academic; 2007. [Google Scholar]
- Furman JM, Whitney SL. Central causes of dizziness. Physical therapy. 2000;80:179–187. [PubMed] [Google Scholar]
- Holmes C, Hoge R, Collins L, Woods R, Toga A, Evans A. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Huppert T, Hoge RD, Dale AM, Franceschini MA, Boas DA. A Quantitative Spatial Comparison of Diffuse Optical Imaging with BOLD- and ASL-Based fMRI. J Biomed Opt. 2006a;11:064018. doi: 10.1117/1.2400910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert T, Schmidt B, Beluk N, Furman J, Sparto P. Measurement of brain activation during an upright stepping reaction task using functional near-infrared spectroscopy. Human Brain Mapping. 2012 doi: 10.1002/hbm.22106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt. 2009;48:D280–D298. doi: 10.1364/ao.48.00d280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, Boas DA. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage. 2006b;29:368–382. doi: 10.1016/j.neuroimage.2005.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikai T, Kamikubo T, Takehara I, Nishi M, Miyano S. Dynamic postural control in patients with hemiparesis. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2003;82:463–469. quiz 470-462, 484. [PubMed] [Google Scholar]
- Jackson RT, Epstein CM, De l'Aune WR. Abnormalities in posturography and estimations of visual vertical and horizontal in multiple sclerosis. The American journal of otology. 1995;16:88–93. [PubMed] [Google Scholar]
- Karim H, Schmidt B, Dart D, Beluk N, Huppert T. Functional near-infrared spectroscopy (fNIRS) of brain function during active balancing using a video game system. Gait & posture. 2011 doi: 10.1016/j.gaitpost.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fougere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage. 2010;50:1589–1598. doi: 10.1016/j.neuroimage.2009.12.060. [DOI] [PubMed] [Google Scholar]
- Lee D, Lishman J. Visual proprioceptive control of stance. Journal of Human Movement Studies. 1975;1:87–95. [Google Scholar]
- Magnusson M, Enbom H, Johansson R, Wiklund J. Significance of pressor input from the human feet in lateral postural control. The effect of hypothermia on galvanically induced body-sway. Acta oto-laryngologica. 1990;110:321–327. doi: 10.3109/00016489009107450. [DOI] [PubMed] [Google Scholar]
- Mahboobin A, Loughlin PJ, Redfern MS, Sparto PJ. Sensory re-weighting in human postural control during moving-scene perturbations. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2005;167:260–267. doi: 10.1007/s00221-005-0053-7. [DOI] [PubMed] [Google Scholar]
- Mattout J, Phillips C, Penny WD, Rugg MD, Friston KJ. MEG source localization under multiple constraints: an extended Bayesian framework. Neuroimage. 2006;30:753–767. doi: 10.1016/j.neuroimage.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Mergner T, Becker W. A modeling approach to the human spatial orientation system. Annals of the New York Academy of Sciences. 2003;1004:303–315. doi: 10.1196/annals.1303.028. [DOI] [PubMed] [Google Scholar]
- Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Role of the prefrontal cortex in human balance control. Neuroimage. 2008;43:329–336. doi: 10.1016/j.neuroimage.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, Tsunazawa Y, Suzuki T, Yanagida T, Kubota K. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage. 2001;14:1186–1192. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Black FO, Wall C., 3rd Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1982;2:536–544. doi: 10.1523/JNEUROSCI.02-05-00536.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashner LM, Peters JF. Dynamic posturography in the diagnosis and management of dizziness and balance disorders. Neurologic clinics. 1990;8:331–349. [PubMed] [Google Scholar]
- Nocera JR, Horvat M, Ray CT. Impaired step up/over in persons with Parkinson's disease. Adapted physical activity quarterly : APAQ. 2010;27:87–95. doi: 10.1123/apaq.27.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi Y, Okada H, Yoshikawa E, Nobezawa S, Futatsubashi M. Brain activation during maintenance of standing postures in humans. Brain : a journal of neurology. 1999;122(Pt 2):329–338. doi: 10.1093/brain/122.2.329. [DOI] [PubMed] [Google Scholar]
- Peterka RJ, Loughlin PJ. Dynamic regulation of sensorimotor integration in human postural control. J Neurophysiol. 2004;91:410–423. doi: 10.1152/jn.00516.2003. [DOI] [PubMed] [Google Scholar]
- Ryoo HC, Hrebien L, Shender BS. Noninvasive monitoring of human consciousness by near-infrared spectroscopy (NIRS) during high +Gz stress. Biomedical sciences instrumentation. 2002;38:1–7. [PubMed] [Google Scholar]
- Ryoo HC, Sun HH, Shender BS, Hrebien L. Consciousness monitoring using nearinfrared spectroscopy (NIRS) during high +Gz exposures. Medical engineering & physics. 2004;26:745–753. doi: 10.1016/j.medengphy.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Schlindwein P, Mueller M, Bauermann T, Brandt T, Stoeter P, Dieterich M. Cortical representation of saccular vestibular stimulation: VEMPs in fMRI. Neuroimage. 2008;39:19–31. doi: 10.1016/j.neuroimage.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Shimada H. Imaging of glucose uptake during walking in elderly adults. Current aging science. 2012;5:51–57. doi: 10.2174/1874609811205010051. [DOI] [PubMed] [Google Scholar]
- Steinbrink J, Villringer A, Kempf F, Haux D, Boden S, Obrig H. Illuminating the BOLD signal: combined fMRI-fNIRS studies. Magn Reson Imaging. 2006;24:495–505. doi: 10.1016/j.mri.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Stephan T, Deutschlander A, Nolte A, Schneider E, Wiesmann M, Brandt T, Dieterich M. Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage. 2005;26:721–732. doi: 10.1016/j.neuroimage.2005.02.049. [DOI] [PubMed] [Google Scholar]
- Suttanon P, Hill KD, Said CM, Logiudice D, Lautenschlager NT, Dodd KJ. Balance and mobility dysfunction and falls risk in older people with mild to moderate Alzheimer disease. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2012;91:12–23. doi: 10.1097/PHM.0b013e31823caeea. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Miyai I, Ono T, Kubota K. Activities in the frontal cortex and gait performance are modulated by preparation. An fNIRS study. Neuroimage. 2008;39:600–607. doi: 10.1016/j.neuroimage.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Toole T, Park S, Hirsch MA, Lehman DA, Maitland CG. The multicomponent nature of equilibrium in persons with parkinsonism: a regression approach. Journal of neural transmission. 1996;103:561–580. doi: 10.1007/BF01273154. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Costantini M, Haggard P. The role of the right temporo-parietal junction in maintaining a coherent sense of one's body. Neuropsychologia. 2008;46:3014–3018. doi: 10.1016/j.neuropsychologia.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Longo MR, Haggard P. Having a body versus moving your body: neural signatures of agency and body-ownership. Neuropsychologia. 2010;48:2740–2749. doi: 10.1016/j.neuropsychologia.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Wang L, Jacques SL, Zheng L. MCML--Monte Carlo modeling of light transport in multi-layered tissues. Comput Methods Programs Biomed. 1995;47:131–146. doi: 10.1016/0169-2607(95)01640-f. [DOI] [PubMed] [Google Scholar]
- Ye JC, Tak S, Jang KE, Jung J, Jang J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. Neuroimage. 2009;44:428–447. doi: 10.1016/j.neuroimage.2008.08.036. [DOI] [PubMed] [Google Scholar]