Abstract
Background and Aim
Gastric motility dysfunction is most commonly seen in diabetic and idiopathic gastroparesis patients. Recently we reported that impaired nitrergic relaxation and a reduced NO (nitric oxide) bioavailability were responsible for gastric motility dysfunction in diabetic female rats. One of the main factors involved in the inactivation of the nitrergic system is oxidative stress commonly seen in diabetic patients. Hyperlipidemia may also be one of the detrimental causes for impaired gastric motility associated with diabetes. In the current study, we investigated whether apolipoprotein E knockout mice (ApoE-KO), an oxidative stress animal model with a hyperlipidemia burden, also displays an impaired nitrergic system. To test this, nitrergic relaxation (AUC/mg tissue) was measured at 2 Hz through electric field stimulation using gastric pyloric strips prepared from C57BL WT or ApoE-KO female mice. Protein expression was determined by Western blots.
Results
Nitrergic relaxation was reduced in gastric strips from ApoE-KO versus WT mice. Protein levels of nNOS (neuronal nitric oxide synthase), GCH-1 (GTP cyclohydrolase 1), Nrf2 (nuclear factor E-2 related factor 2) and GCSc (glutamate-cysteine ligase catalytic) were also reduced in ApoE-KO compared to controls, with no significant change in GCSm (glutamate-cysteine ligase modifier) and HO-1 (heme oxygenase 1). The activities of DHFR (dihydrofolate reductase) and antioxidant enzymes were also reduced in ApoE-KO mice.
Conclusions
This novel study is the first to reveal that a deficiency in ApoE impairs gastric motility functions, and that hyperlipidemia and the suppression of selective antioxidants may be an underlying mechanism for this pathological change.
Keywords: Apolipoprotein E, Nitric oxide, Gastroparesis, Nitrergic relaxation, nNOS dimerization, Nrf2, Phase II enzymes, BH4
Introduction
Apolipoprotein E (ApoE) is a class of apolipoprotein found among the chylomicron and intermediate-density lipoproteins that bind to a specific receptor on liver and peripheral cells. It is essential for the normal catabolism of triglyceride-rich lipoprotein constituents [1]. ApoE transports lipoproteins, fat-soluble vitamins, and cholesterol into the lymph system and then into the blood. It is synthesized principally in the liver, but has also been found in other tissues such as the brain, kidneys, and spleen. ApoE lipoproteins are ligands for interaction with low density lipoprotein receptors in tissues [2]. Defects in ApoE result in type III hyperlipoproteinemia (HLP III), in which increased plasma cholesterol and triglycerides are the result of impaired clearance of VLDL and LDL remnants leading to increased lipid storage in the body. This may eventually increase oxidative stress and lead to diabetes [3–7]. Lack of ApoE also leads to decreased antioxidant and NO levels [8, 9].
Gastroparesis is a clinical condition which is associated with abnormal gastric motility and predominantly affects young women [10–16]. We have recently reported that BH4 availability is reduced in diabetic females and leads to delayed gastric emptying which has been restored by supplementation of BH4 [12, 13, 17]. Elevated oxidative stress is one of the detrimental factors for diabetes induced complications. Apart from BH4, Nrf2 (nuclear factor erythroid 2-related factor) is another vital element which is an anti-oxidant element and is involved in defense mechanisms. Nrf2 is a transcription factor that protects the cells from oxidative stress by activating the phase II antioxidant enzymes [18–21].
It has been reported that exaggerated postprandial hypertriglyceridemia is common in diabetic patients [22]. Along with LDL protein ApoE also plays an important role in the efficient production and clearance of lipoproteins. ApoE acts as a ligand for the interaction of low density lipoprotein receptors in the tissues [2, 7]. So deficiency of ApoE results in the impairment of LDLR interaction with the tissues thus leading to increased plasma cholesterol and triglycerides along with oxidative stress. Previous studies have demonstrated that ApoE serves as a direct antioxidant and protects cells from oxy radical damage [5, 7, 8, 23, 24]. There have been no studies demonstrating the role of ApoE in diabetic gastroparesis. Therefore, in this present study we have used ApoE-KO mice to demonstrate whether deficiency of ApoE is involved in the pathogenesis of diabetic gastroparesis through impairment of the nitrergic system.
Materials and Methods
Experimental Mice
Adult female ApoE-KO mice (12 weeks old) were procured from Jackson Laboratories (Sacramento, CA) and maintained in the institutional animal care facility. The protocols for using these mice were approved by the Institutional Animal Care and Use Committees at the Meharry Medical College, Nashville, Tennessee. Tissue samples collected from animals were snap frozen in liquid nitrogen and stored at −80°C.
Organ Bath Studies
Electric field stimulation (EFS)-induced NANC relaxation was studied in circular gastric pyloric strips using organ bath (Radnoti Glass Technology, Monrovia, CA, USA). Pyloric strips were used as delayed emptying is increased by abnormal pyloric contractions [12]. Strips from WT and ApoE-KO mice were collected and NANC relaxation is measured through EFS induction. The NO dependence nitrergic relaxations were confirmed by preincubation with NG-nitro-l-arginine-methyl ester (l-NAME, 100 µM). Comparisons between groups were performed by measuring the area under the curve (AUC/mg tissue).
Western Blot Analysis
nNOSα, GCH-1, DHFR, Nrf2, GCSc, GCSm and HO-1 proteins were quantified in gastric antrum homogenates from the two groups using standard western blot analysis, as described in our previous study. Proteins were measured by Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA) and separated by SDS polyacrylamide gel electrophoresis. The membrane was immunoblotted with polyclonal primary antibodies (Zymed Laboratories Inc., CA; Santacruz Biotechnology, CA) and their respective secondary antibodies.
Activity of DHFR and Antioxidant Enzymes (SOD and Catalase) in ApoE-KO Mice
Activity of the enzyme DHFR is measured using a commercially available kit (Sigma Aldrich). CAT activity was assayed by a commercially available kit from Cayman Chemical Company (Ann Arbor, MI, USA) and the data were expressed as nmol/min/mg protein). SOD1 activity in stomach antrum was assayed by a commercially available kit from Cayman Chemical Company.
Statistics
Data were presented as mean ± standard error (SE). Statistical comparisons between groups were determined by Student’s t test or the Tukey test after one-way analysis of variance (ANOVA). A P value of less than 0.05 was considered statistically significant.
Results
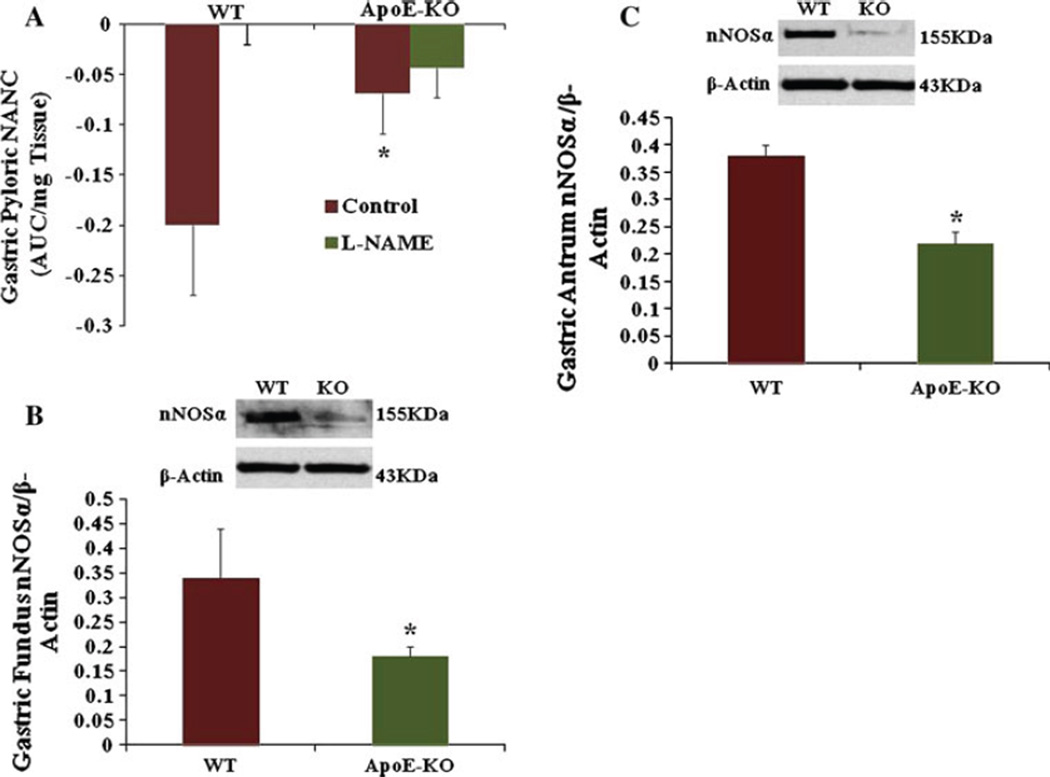
Gastric Pyloric Nitrergic Relaxation Is Impaired in Female ApoE-KO Compared to WT Mice
Figure 1a depicts the gastric nitrergic relaxation following EFS (2 Hz) in WT and ApoE-KO mice. The elevated levels of oxidative stress and hyperlipidemia in female ApoE-KO significantly impaired gastric nitrergic relaxation compared to WT mice (0.069 ± 0.04 vs −0.2 ± 0.07, P < 0.05).
Fig. 1.
a Nitrergic relaxation in WT and apolipoprotein E knockout mice (ApoE-KO) gastric muscular pyloric tissues in vivo. Nitrergic relaxation was measured in WT and ApoE-KO pyloric gastric tissue. Nitrergic relaxation is reduced in ApoE-KO mice when compared to WT mice. b Expression of nNOSα in WT and ApoE-KO female mice gastric muscular tissue. Representative immunoblot and densitometric analysis data for nNOSα protein in female mice gastric fundus muscular tissue. There was a significant decrease in nNOSα in female ApoE-KO mice compared to WT mice. c Representative immunoblot and densitometric analysis data for nNOSα protein in female mice gastric antrum muscular tissue. A significant decrease was seen in nNOSα in female ApoE-KO mice compared to WT mice. Values are mean ± SE (N = 4). Statistical significance was determined by Tukey test after one-way ANOVA. *P < 0.05 compared with control group
Oxidative Stress and Hyperlipidemia in ApoE-KO Decreases Gastric nNOSα Protein Expression
According to Fig. 1b the protein level of nNOSα, the only functional isoform of nNOS in gastric fundus tissue, was significantly (P < 0.05) decreased (0.18 ± 0.02 vs 0.34 ± 0.1) in ApoE-KO mice when compared to WT. Similarly, Fig. 1c shows the reduced expression of nNOSα protein in gastric antrum in ApoE-KO mice when compared to WT (0.22 ± 0.04 vs 0.38 ± 0.02, P < 0.05). Therefore, all our subsequent experiments were carried out in gastric antrum muscular tissue.
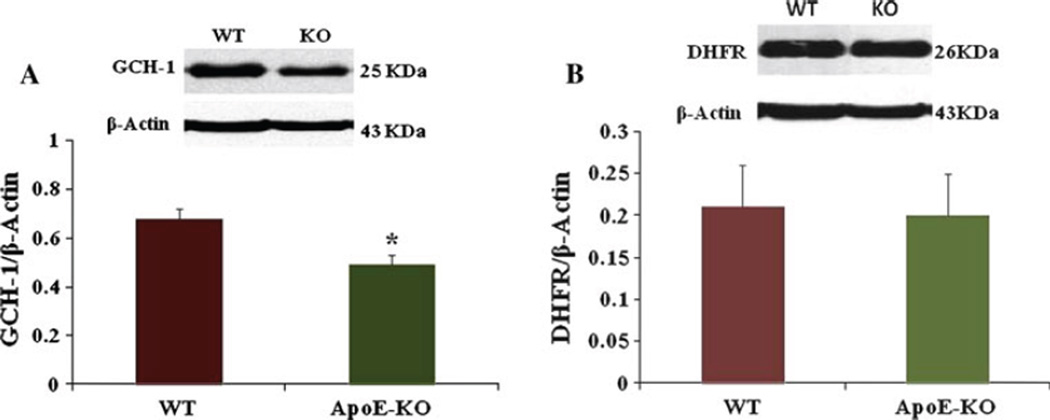
Reduced Expression of Gastric Antrum GCH-1 in ApoE-KO Mice
Figure 2a shows a significant decrease in GCH-1 protein expression in female ApoE-KO mice gastric antrum compared to WT mice (0.49 ± 0.04 vs 0.68 ± 0.04, P < 0.05).
Fig. 2.
Expression of GCH-1, dihydrofolate reductase (DHFR) in WT and apolipoprotein E knockout (ApoE-KO) female mice gastric antrum muscular tissue. a Representative immunoblot and densitometric analysis data for GCH-1 protein in female mice gastric antrum. A significant decrease was seen in GCH-1 in female ApoE-KO mice compared to WT mice (0.49 ± 0.04 vs 0.68 ± 0.04). Values are mean ± SE (N = 4). Statistical significance was determined by Tukey test after one-way ANOVA. *P < 0.05 compared with control group. b Representative immunoblot and densitometric analysis data for DHFR protein in female mice gastric antrum. Values are mean ± SE (N = 4)
Expression of DHFR Protein in WT and ApoE-KO Female Mice Gastric Tissue
Figure 2b shows no change in DHFR expression in ApoE-KO mice compared to WT mice (0.20 ± 0.05 vs 0.21 ± 0.05).
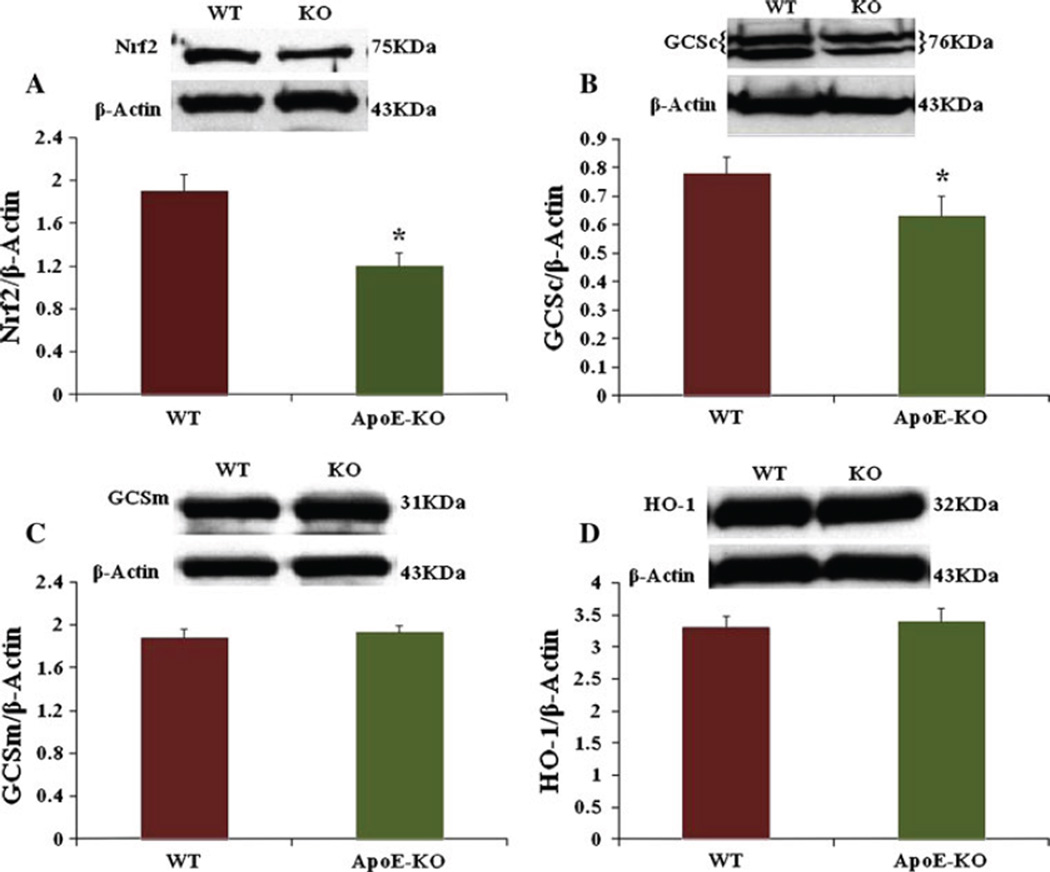
Reduced Expression of Gastric Antrum Nrf2 Protein in ApoE-KO Mice
Figure 3a demonstrates the expression of gastric antrum Nrf2 in ApoE-KO mice. The protein level of gastric antrum Nrf2 is reduced significantly in ApoE-KO mice (0.26 ± 0.13 vs 0.32 ± 0.016, P < 0.05) as a result of high oxidative stress when compared to WT mice. We next examined if a reduction observed in Nrf2 protein alters the phase II enzyme levels in ApoE-KO gastric antrum muscular tissue.
Fig. 3.
Expression of Nrf2, GCSc, GCSm and HO-1 in WT and apolipoprotein E knockout (ApoE-KO) female mice gastric antrum muscular tissue. Representative immunoblot and densitometric analysis data for Nrf2 protein in female mice gastric antrum. a A significant decrease in Nrf2 in female ApoE-KO mice compared to WT mice. Values are mean ± SE (N = 4). Statistical significance was determined by Tukey test after one-way ANOVA. *P < 0.05 compared with control group. b Representative immunoblot and densitometric analysis data for GCSc protein in female mice gastric antrum. c Representative immunoblot and densitometric analysis data for GCSm protein in female mice gastric antrum. d Representative immunoblot and densitometric analysis data for HO-1 protein in female mice gastric antrum. Values are mean ± SE (N = 4)
Expression of Gastric Antrum GCSc, GCSm, HO-1 Proteins in ApoE-KO Mice
The protein levels of GCSc in WT and ApoE-KO mice gastric antrum are shown in Fig. 3b. The protein level of GCSc is significantly (P < 0.05) reduced in ApoE-KO mice (0.63 ± 0.02 vs 0.78 ± 0.06) when compared to WT. However, Fig. 3c and d shows no change in GCSm and HO-1 protein expression in ApoE-KO mice compared to WT mice.
Activity of DHFR and Antioxidant Enzymes (CAT and SOD1) in ApoE-KO Female Mice
Table 1 depicts gastric DHFR enzyme activity which was significantly (P < 0.05) reduced in ApoE-KO mice when compared to WT mice (0.006 ± 0.004 vs 0.2 ± 0.004). It also shows a reduction in SOD and CAT activity in ApoE-KO mice compared to WT mice [(6.07 ± 0.77 vs 2.97 ± 0.72, P < 0.05); (6.79 ± 0.57 vs 5.09 ± 0.48, P < 0.05), respectively].
Table 1.
Activity of DHFR, antioxidant enzymes (SOD and catalase) in ApoE-KO mice gastric antrum muscular tissue. This table depicts a marked decrease of DHFR activity in ApoE-KO mice
| Activity | WT | ApoE-KO |
|---|---|---|
| DHFR activity (micro mole/min/mg protein) | 0.2 ± 0.004 | 0.006 ± 0.004* |
| SOD activity (U/mg protein) | 6.07 ± 0.77 | 2.97 ± 0.72* |
| Catalase activity (nmol/min/mg protein) | 6.79 ± 0.57 | 5.09 ± 0.48* |
DHFR dihydrofolate reductase, ApoE-KO apolipoprotein E knockout Note the significant reduction of SOD and CAT in ApoE-KO mice compared to WT mice. Values are mean ± SE (N = 4). Statistical significance was determined by Tukey test after one-way ANOVA.
P < 0.05 compared with control group
Discussion
The aim of the present study was to demonstrate whether hypercholesteremia and suppression of gastric antioxidants in ApoE-KO mice leads to impairment in nNOS mediated stomach motility function. Recently, we reported an impairment in nitrergic relaxation and reduced expression of nNOSα protein, nNOS dimer which are responsible for the development of gastroparesis in streptozotocin-induced diabetic female rats [12, 13]. We have also reported that suppression of selective antioxidants and hyperlipidemia impairs biopterin and the nitrergic system, thus leading to gastroparesis in LDLR-KO mice model [25]. In this study we tested whether lack of ApoE protein contributes to NO mediated gastric motility dysfunction. For the first time in the GI literature, herein we demonstrated that gastric nitrergic relaxation as well as gastric nNOS alpha expression is reduced in ApoE-KO mice. We also demonstrated that the protein expression/activity of BH4 biosynthesis enzymes of GCH-1, DHFR (enzymes involved in the production of BH4 via de novo and salvage pathways respectively) was reduced in ApoE-KO mice. We also noticed a significant decrease in the expression levels of phase II enzymes as well as antioxidant enzyme activity in ApoE-KO mice stomach muscular tissue. Therefore we suggest that elevated levels of oxidative stress as well as hyperlipidemia observed in diabetic and/or overweight (independent of diabetes) female patients may lead to nitrergic mediated gastric motility dysfunction and thus gastroparesis.
This study is the first comprehensive report of gastric dysmotility in ApoE-KO mice, an animal model of hypercholesteremia. Previous studies have demonstrated that ApoE is a direct antioxidant and protects cells from oxy radical damage [23]. A decreased antioxidant level in ApoE-KO mice appears to correlate with decreased recovery after injury [7]. It has also been reported that there is an increased NO production from human monocyte macrophages in the presence of ApoE [8]. Further, in ApoE-KO mice, vascular relaxation is impaired, vascular superoxide production is increased and NO synthesis is reduced [5]. Loss of NO bioavailability leads to the development of atherosclerosis, thus increasing the risk of cardiovascular disease [24]. However, there have been no studies demonstrating the role of ApoE in diabetic gastroparesis. In the present study, we have first investigated whether apolipoprotein knockout mice (ApoE-KO; displays oxidative stress and high blood cholesterol) display impairment in the gastric nitrergic system. The present study demonstrates that nitrergic relaxation is impaired in ApoE-KO mice along with a reduction in nNOS alpha expression (Fig. 1a, b).
Tetrahydrobiopterin (BH4), an essential co-factor for NOS, is intracellularly produced from GTP via GTP-cyclohydrolase I or, alternatively, from sepiapterin (SP) via the salvage pathway and acts as a redox switch in the oxygenase domain of NOS [24]. Reduced levels of BH4 impair the production of NO and lead to increased superoxide radical production [5]. BH4 deficiency has been associated with diabetic complications including gastroparesis [13]. To investigate the factors involved in reduced BH4 bioavailability we investigate changes in the protein expression of GTPCH (GTP cyclohydrolase) and DHFR (dihydro folate reductase) in both WT and ApoE-KO mice stomach muscular tissues. As shown in Fig. 2, GCH-1 but not DHFR protein levels were reduced in ApoE-KO stomach muscular tissues. Next, we tested the DHFR enzyme activity using an assay kit. As shown in Table 1, the activity of DHFR enzyme is significantly reduced in ApoE-KO mouse gastric muscular tissue. Collectively, these results suggest that hyperlipidemia leads to decreased availability of BH4 and nNOSα uncoupling which results in impaired nitrergic relaxation and thus gastroparesis.
We next investigated if antioxidant elements like Nrf2 and phase II enzymes are altered in female ApoE-KO mice compared to wild type. Recent work suggests that the Nrf2 (nuclear factor erythroid 2-related factor) is critical for protecting the GI tract against disease by regulating a multifaceted cellular antioxidant defense [19]. Nrf2 is a transcriptional factor that protects the cells from oxidative stress and regulates the expression of phase II genes involved in regulating levels of reactive oxygen species [20]. Our data shows that the expression of Nrf2 and GCSc but not HO1 and GCSm enzymes are significantly reduced in ApoE-KO mice (Fig. 3). This suggests that reduced levels of selective phase II enzymes elevate oxidative stress and thus impair both the nitrergic system and gastric motility functions. Superoxide dismutase is a family of ubiquitous antioxidant enzymes that catalyze the conversion of superoxide anion radicals to hydrogen peroxide (H2O2) and molecular oxygen, whereas, CAT is a ubiquitous antioxidant enzyme that is involved in the conversion of H2O2 to molecular oxygen and water. Our data shows a significant reduction in SOD and catalase in ApoE-KO mice gastric tissue (Table 1).
Previous studies have reported that lack of ApoE contributes to eNOS dysfunction and thus reduced NO synthesis which leads to impaired vascular relaxation and thus leads to the development of atherosclerosis. In ApoE-KO mice, a hypercholesteromic model the superoxide derived from NADPH oxidase reacts with NO to form peroxynitrite which induces oxidative degradation of tetrahydrobiopterin. This leads to eNOS uncoupling and increased production of superoxide thus leading to oxidative stress [8, 9]. It has been well established that lack of ApoE contributes to vascular eNOS dysfunction. Further studies are warranted to investigate the mechanistic role of ApoE in nitrergic mediated gastric motility functions. Nevertheless, this novel study demonstrates that lack of ApoE protein contributes to NO mediated gastric dysmotility.
Acknowledgments
We thank Sutapa Mukhopadhyay (Gangula’s laboratory) for technical assistance. We thank Dr. Diana Marver for review of the manuscript. Financial support for the original research was provided by theNIH-NIDDKR21DKO76704 (PG), P60DK020593 pilot project funds (PG) and fromRCMIG12RR03032 (PG), as start-up funds at Meharry Medical College, Nashville, TN, USA.
Footnotes
Conflict of interest Dr. Gangula has filed a patent application for the use of BH4 in gastroparesis subjects through the University of Texas Medical Branch, Galveston, TX.
Contributor Information
Kalpana Ravella, Department of Physiology, Meharry Medical College, 1005 Dr. D.B. Todd Jr. Blvd, Nashville, TN 37208, USA.
Hong Yang, Department of Physiology, Meharry Medical College, 1005 Dr. D.B. Todd Jr. Blvd, Nashville, TN 37208, USA.
Pandu R. R. Gangula, Email: pgangula@mmc.edu, Department of Physiology, Meharry Medical College, 1005 Dr. D.B. Todd Jr. Blvd, Nashville, TN 37208, USA; Center for Women’s Health Research, Meharry Medical College, Nashville, TN, USA.
References
- 1.Zhang HL, Wu J, Zhu J. The role of apolipoprotein E in Guillain-Barré syndrome and experimental autoimmune neuritis. J Biomed Biotechnol. 2010;357412:1–12. doi: 10.1155/2010/357412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breslow JL, Zannis VI, SanGiacomo TR, Third JL, Tracy T, Glueck CJ. Studies of familial type III hyperlipoproteinemia using as a genetic marker the apoE phenotype E2/2. J Lipid Res. 1982;23:1224–1235. [PubMed] [Google Scholar]
- 3.Feussner G, Feussner V, Hoffmann MM, Lohrmann J, Wieland H, Marz W. Molecular basis of type III hyperlipoproteinemia in Germany. Hum Mutat. 1998;11:417–423. doi: 10.1002/(SICI)1098-1004(1998)11:6<417::AID-HUMU1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Civeira F, Pocovi M, Cenarro A, Casao E, et al. Apo E variants in patients with type III hyperlipoproteinemia. ApoE variants in patients with type III hyperlipoproteinemia. Atherosclerosis. 1996;127:273–282. doi: 10.1016/s0021-9150(96)05969-2. [DOI] [PubMed] [Google Scholar]
- 5.Schreyer SA, Vick C, Lystig TC, Mystkowski P, Leboeuf REC. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab. 2002;282:E207–E214. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- 6.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 7.d’Uscio LV, Baker TA, Mantilla CB, et al. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1017–1022. doi: 10.1161/01.atv.21.6.1017. [DOI] [PubMed] [Google Scholar]
- 8.Laursen JB, Somers M, Kurz S, et al. Endothelial regulation of vasomotion in ApoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 9.Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis—clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501–1504. [PubMed] [Google Scholar]
- 10.Camilleri M. Advances in diabetic gastroparesis. Rev Gastroenterol Disord. 2002;2:47–56. [PubMed] [Google Scholar]
- 11.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G725–G733. doi: 10.1152/ajpgi.00406.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangula PR, Mukhopadhyay S, Ravella K, et al. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298:G692–G699. doi: 10.1152/ajpgi.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 14.Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology. 2000;119:766–773. doi: 10.1053/gast.2000.16509. [DOI] [PubMed] [Google Scholar]
- 15.Klatt P, Pfeiffer S, List BM, et al. Characterization of heme-deficient neuronal nitric-oxide synthase reveals a role for heme in subunit dimerization and binding of the amino acid substrate and tetrahydrobiopterin. J Biol Chem. 1996;271:7336–7342. doi: 10.1074/jbc.271.13.7336. [DOI] [PubMed] [Google Scholar]
- 16.Vasquez-Vivar J, Hogg N, Martasek P, Karoui H, Pritchard KA, Jr, Kalyanaraman B. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J Biol Chem. 1999;274:26736–26742. doi: 10.1074/jbc.274.38.26736. [DOI] [PubMed] [Google Scholar]
- 17.Lee JM, Li J, Johnson DA, et al. Nrf2, a multi-organ protector? FASEB J. 2005;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 18.Aleksunes LM, Manautou JE. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol Pathol. 2007;35:459–473. doi: 10.1080/01926230701311344. [DOI] [PubMed] [Google Scholar]
- 19.Kalthoff S, Ehmer U, Freiberg N, Manns MP, Strassburg CP. Interaction between oxidative stress sensor Nrf2 and xenobiotic-activated aryl hydrocarbon receptor in the regulation of the human phase II detoxifying UDP-glucuronosyltransferase 1A10. J Biol Chem. 2010;285:5993–6002. doi: 10.1074/jbc.M109.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibuya A, Onda K, Kawahara H, et al. Sofalcone, a gastric mucosa protective agent, increases vascular endothelial growth factor via the Nrf2-heme-oxygenase-1 dependent pathway in gastric epithelial cells. Biochem Biophys Res Commun. 2010;398:581–584. doi: 10.1016/j.bbrc.2010.06.124. [DOI] [PubMed] [Google Scholar]
- 21.Xiea W, Xingb D, Zhaob Y, et al. A new tactic to treat postprandial hyperlipidemia in diabetic rats with gastroparesis by improving gastrointestinal transit. Eur J Pharmacol. 2005;510:113–120. doi: 10.1016/j.ejphar.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Gangula PR, Chinnathambi V, Hale AB, Mukhopadhyay S, Channon KM, Ravella K. Impairment of nitrergic system and delayed gastric emptying in low density lipoprotein receptor deficient female mice. Neurogastroenterol Motil. 2011;23:773. doi: 10.1111/j.1365-2982.2011.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colton CA, Czapiga M, Snell-Callanan J, Chernyshev ON, Vitek MP. Apolipoprotein E acts to increase nitric oxide production in macrophages by stimulating arginine transport. Biochim Biophys Acta. 1997;240:391–394. doi: 10.1016/s0925-4439(00)00092-2. [DOI] [PubMed] [Google Scholar]
- 24.Tayeh MA, Marletta MA. Macrophage oxidation of l-arginine to nitric oxide, nitrite, and nitrate: tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989;264:19654–19658. [PubMed] [Google Scholar]
- 25.Lomnitski L, Chapman S, Hochman A, Kohen R, et al. Antioxidant mechanisms in apolipoprotein E deficient mice prior to and following closed head injury. Biochim Biophys Acta. 1999;1453:359–368. doi: 10.1016/s0925-4439(99)00010-1. [DOI] [PubMed] [Google Scholar]