Abstract
Purpose
Prenatal smoking is a preventable risk factor for poor perinatal outcomes and is more prevalent in pregnant smokers of low socioeconomic status (SES). We describe the intervention model and factors associated with quitting from the Pittsburgh STOP Program, an evidence-informed dissemination intervention for low-SES pregnant smokers.
Setting
STOP is delivered in community health care clinics serving economically disadvantaged women.
Participants
Participants were 856 pregnant women who were current smokers (93%) and recent quitters (7%). Most were white (59%) or black (35%), single (74%), young (mean age = 25), and experiencing an unplanned pregnancy (84%); 90% were insured by Medicaid/uninsured.
Methods
An evidence-informed intervention for community pregnant women was delivered individually in a single-group pre-post evaluation design. Measures were demographics, participation and retention, smoking status, satisfaction, and cost. Analyses included descriptive statistics and logistic regression.
Results
Participants attended an average of 4.7 sessions. Dropout rate after the first session was 5%. Over 11% of smokers quit; 48% of preenrollment spontaneous quitters remained abstinent. Factors significantly associated with quitting included race, mother’s age, nicotine dependence, and number of sessions attended.
Limitations
STOP is a community program with self-selected participants and no control group.
Conclusion
Low-income pregnant smokers will engage in an evidence-informed cessation program tailored for this group, with quit rates that compare to controlled research results.
Keywords: Pregnant Women, Smoking Cessation, Low Socioeconomic Status, Risk Factors, Prevention Research
PURPOSE
Introduction
There is clear evidence that smoking rates during pregnancy have declined in the United States. All U.S. states surveyed achieved the Healthy People 20101 objective that 30% of smokers would stop smoking during pregnancy.2 The U.S. smoking rate during pregnancy declined from 19.5% in 19893 to 13.2% by 2006.4 Many women quit smoking before becoming pregnant, although the exact rate is not known,5 and another 23% quit spontaneously in early pregnancy.6
Despite this success, subgroups of the U.S. population remain at risk; pregnancy smoking rates vary by socioeconomic status (SES) and race/ethnicity. In general, lower socioeconomic groups show higher rates of prenatal smoking. Pregnant smokers tend to be younger, less well educated, more likely to be insured by Medicaid, and to have lower occupational achievement.2 Rates of spontaneous quitting during pregnancy are lower for low-income women (11%–15%)7,8 and prenatal relapse rates are high (23%–35%).9–11
Ethnic groups vary in their rates of smoking during pregnancy. Rates are higher than the national average among Native American (20.0%)12 and non-Hispanic white women (18.1%).4 Non-Hispanic black women (10.6%),4 Hispanic women (2.8%),4 and Asian women (2.2%)13 smoke less than other racial/ethnic groups during pregnancy. Thus, despite decline over the past two decades in rates of smoking during pregnancy, subgroups of the population are at increased risk of continuing to smoke during pregnancy and, thereby, for smoking-related birth complications.
Evidence-based smoking cessation services for pregnant women are a critical component of comprehensive health care services in general and in medically underserved communities specifically. The Public Health Service (PHS) has published and updated a tobacco control Clinical Practice Guideline14–16 recommending that pregnant smokers be advised to quit and offered psychosocial cessation interventions with specific treatment components to improve effectiveness.16 The guideline acknowledges that individuals of low SES “bear a disproportionate burden from tobacco” and that reducing this disparity “is an important part of improving the overall health of the American public.”16(p151) A recent Cochrane review of the effects of cessation interventions for pregnant women on smoking behavior and perinatal health outcomes summarized the results of 72 controlled trials. The review concluded that interventions are effective at helping approximately 6% of pregnant women stop smoking, measured by self-report plus biological confirmation, and that such interventions reduced the incidence of adverse birth outcomes.17
As with other evidence-based results,18,19 it has been challenging to disseminate efficacious tobacco control interventions into general community settings. A recent article called for a critical evaluation of programs to reduce prematurity and low birth weight among Medicaid recipients as a key factor in improving the status of this population.20 The provision of evidence-based cessation services for pregnant smokers is clearly one component of such a program.
Data from research trials and epidemiological surveys have identified factors associated with quitting in pregnant women. Black women may be less likely than others to quit spontaneously very early in pregnancy (e.g., Ockene et al.21), although this finding is not consistent in the literature (e.g., LeClere and Wilson,6 Solomon and Quinn22). Those more likely to quit smoking later in pregnancy but by the time of delivery were nonwhite and younger women, those who were in their first pregnancy, those who were less addicted to nicotine, those who had commercial insurance (i.e., not Medicaid), those who entered treatment earlier in pregnancy, those who smoked fewer cigarettes per day prior to pregnancy, those who perceived a risk to themselves and the fetus from smoking, those who experienced lower life stress including job strain, those who had higher social support and no husband or partner who smoked, and those who had a lack of exposure to physical/sexual violence and lower depression and anxiety scores.23,24 Little information is available, however, on factors associated with smoking cessation in low-SES pregnant women treated in clinical community programs.
Objective
Smoking rates during pregnancy in Pennsylvania are higher than the national average (16.6%).25 Given that Pittsburgh was reported in a national survey26 to have the highest rate of smoking during pregnancy of any large U.S. city (24%) and that the rate of prenatal smoking among low-income pregnant women in the city approaches 50%,27 we developed and delivered the Pittsburgh STOP Program, an evidence-informed community program that disseminates the expertise derived from tobacco control research to high risk pregnant smokers in the community. The objective of this report is to describe the STOP participant sample, intervention model, and factors associated with quitting.
METHODS
Program Design/Description
Developed in 2000 as a collaboration between a women’s specialty hospital and behavioral specialists at a large psychiatric facility, STOP works primarily with pregnant smokers from zip codes representing the most economically disadvantaged neighborhoods in the area. The mission of the Pittsburgh STOP Program is to enhance the health of pregnant women and babies in the community through the delivery of an evidence-informed, individualized, and motivational intervention among underserved pregnant smokers. Intervention was funded primarily by state master tobacco settlement funds via the Pennsylvania Department of Health. Specific goals of STOP were to develop and maintain community referral sources, to deliver interventional strategies deemed effective in tobacco control research, to assist current smokers (CSs) in quitting smoking or cutting down on daily cigarette use and recent spontaneous quitters to maintain abstinence, and to maintain excellent program participation. A single-group pre-post evaluation design was employed. STOP was approved by the University of Pittsburgh Medical Center Total Quality Council and, for manuscript preparation, by the Institutional Review Board of the University of Pittsburgh.
Sample
We report on the sample characteristics and factors associated with quitting for 856 pregnant women enrolled in STOP between November 2000 and September 2008. Participants were referred to STOP via several methods: (1) direct referrals from obstetrical providers in the hospital clinic and satellite offices (the major referral source) and from other community clinics, drug and alcohol rehab centers, school-based programs for pregnant teens, and health plan pregnancy case management programs; and (2) self-referrals from flyers publicizing STOP in recruitment sites. Recruitment materials emphasized that a woman did not need to be sure she was ready to quit to enroll. CSs and recent spontaneous quitters were recruited, because relapse before delivery is prevalent among the latter group.10,11 Any pregnant smoker was eligible to participate and all interested participants received the intervention.
Measures
Women completed paper and pencil measures of demographic information and nicotine dependence (using the Fagerstrom Test of Nicotine Dependence,28 on which scores range from 0 to 10) at enrollment and, for nicotine dependence, at the last visit before delivery (LBD). Program satisfaction was assessed postpartum using a 10-item paper and pencil questionnaire. Rates of referral, enrollment, and session attendance were recorded to evaluate participation and retention.
Smoking status was assessed using a combination of self-report and expired air carbon monoxide (CO) measurement, conservatively using an 8–parts per million (ppm) cutpoint, at the low end of the CO cutpoint range recommended by the SRNT Subcommittee on Biochemical Verification.29 Smoking status at entry was categorized as CS or recent quitter (RQ). RQs self-reported having quit since becoming pregnant and achieved a CO score of ≤8 ppm. Dropouts were those who enrolled but attended only one session; all others were completers. Final quit status was categorized as quit (self-report at the LBD of zero cigarettes in the past week and a CO of ≤8 ppm) or smoking (self-report of any cigarettes and/or CO >8 ppm at LBD). Timing of the LBD varied among completers (see Results section).
Finally, cost of program delivery was determined by summing the total dollars received from contract and grant funding sources from the beginning of the program in 2000 through September 2008, minus costs associated with evaluation. Cost for two handheld CO monitors (~$1000 each; CareFusion MicroCO, model MC02; www.carefusion.com) was included as a programmatic cost, because the monitors served as both an intervention component and an evaluation tool. Costs per participant and per quitter were determined by dividing the total programmatic cost by the number of participants who enrolled in the program and by the number of participants who were not smoking at the LBD.
Intervention
Treatment was delivered individually and in person in community health clinics where participants received prenatal services. A small subset of sessions (9%) was delivered by telephone with Medicaid-insured women referred by a local health plan who lived outside the Pittsburgh city limits. Collaboration of program staff with referral sources—clinic receptionists, medical assistants, nurses, midwives, nurse practitioners, physicians, and health plan maternity case managers—was robust and included feedback to providers, informational sessions, and frequent individual informal contact. These collaborative efforts helped STOP be viewed as a core component of health care provision in the clinic settings and enhanced referral flow.
Treatment components included all the evidence-based methods recommended by the PHS guideline for general smoking cessation.15,16 Counselors attended to smokers’ motivations for quitting with strategies that were personally relevant, were repeated at every visit, and addressed health risks of smoking and rewards of and barriers to quitting. Both practical (problem-solving and skills training) counseling and social support were included, as was cessation pharmacotherapy when recommended by participants’ physicians. STOP counselors employed motivational interviewing skills and strategies to frame interactions with participants as opportunities to clarify ambivalence about quitting. They provided collaborative assistance with goal setting in the context of respect for the smoker’s point of view. Two additional components enhanced attendance and motivation: (1) small baby gifts (<5 each) that participants earned by attending sessions, and (2) measurement of CO at all visits with a handheld monitor; CO scores were tracked from visit to visit as an intervention component. The CO measurement was initially included as an evaluation measure to confirm self-report of smoking status. Participant feedback in the first year of the program convinced us that CO monitoring was perceived as a highly useful motivational tool for participants, who, once they learned via program education the importance of CO to their fetuses’ health, were eager to complete CO check-ins as part of the intervention.
The goal for the initial contact was to establish rapport, confirm enrollment, and begin collaborative goal setting, engaging participants in a reflective manner and making use of open-ended questions. STOP counselors explained the program, gathered information about the level of motivation to quit and confidence to do so, and determined the participant’s goal—to quit smoking entirely or to cut down. The latter was seen as an appropriate treatment goal because of the evidence for fetal harm reduction when pregnant smokers reduce daily cigarette use even when they do not quit.30 Counselors helped participants describe smoking triggers and develop initial strategies for addressing these. A handout, written at a fourth-grade reading level,31 explained the effects of circulating CO on adult and fetal health. Counselors described the use of the CO monitor and took a first reading. In the second session, counselors explored progress toward cessation or harm reduction goals and engaged participants in a collaborative discussion of challenges, barriers, and strategies. Attention to continued rapport building and tracking of CO scores continued in this and all additional sessions.
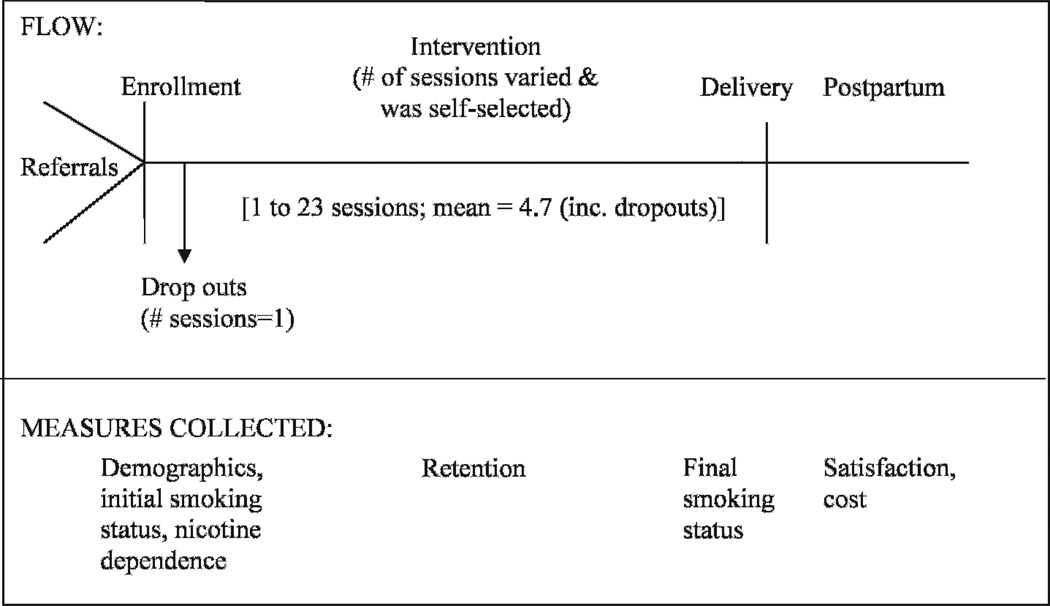
Sessions three and beyond involved assisting participants in reaffirming and meeting their individual goals and in considering coping strategies for dealing with stressful situations and ongoing smoking triggers. The PHS guideline15,16 states that four to eight sessions are most effective for the general population, but no definitive evidence exists for the optimal number in pregnancy. In the spirit of collaborative treatment planning, participants self-selected the number of treatment sessions they thought they needed, although at least four were encouraged. Number of sessions also was affected by when in pregnancy participants entered STOP. As delivery approached, postpartum relapse prevention information was discussed. A final session occurred postpartum during which relapse prevention and/or additional strategies for continuing to try to quit were discussed and reinforced. See the Figure for a display of intervention flow and associated measures.
Figure.
Intervention Flow and Associated Measures
Analysis
Descriptive statistics were calculated overall and separately by final smoking status. Dropouts were conservatively presumed to have continued smoking and were included in quit analyses. Logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs; for the ORs) for the outcome of final smoking status; an OR >1.0 would correspond to greater odds of continuing to smoke. Both unadjusted and adjusted results were obtained from corresponding logistic models. Because the predictor variables in the model were carefully chosen based on theoretical considerations, all predictor variables were included in the multivariable model regardless of statistical significance. In a sensitivity analysis (not shown here), a multivariable model was formulated using only those that were at least marginally significant alone; results were very similar to those shown here for the significant variables that remained in the multivariable model. To assess the fit of the model and any potential problems with collinearity, we conducted the Hosmer and Lemeshow (H-L) test of fit, examined deviance residuals, and calculated variance inflation factors (VIFs).
Descriptive summaries also were calculated to summarize responses to satisfaction questions. Cost analyses were completed as described above.
RESULTS
Sample Characteristics
Participants’ ages ranged from 13 to 46, with a mean of 24.6 years (SD = 6.34). CSs comprised 93% of the sample at entry. Fifty-nine percent reported being white, 37% were African-American, and 5% other (Asian and Hispanic each <1%; mixed 4%); 74% were single; 90% were insured by Medicaid or were uninsured; and 84% were experiencing an unplanned pregnancy. Mean gestational age at entry was 19.9 weeks (SD = 7.24); about half (54%) were in their second trimester and the rest were evenly divided between the first and third trimesters (24% and 22%, respectively). For those with more than one visit (i.e., who were not dropouts), the mean length of time between first visit and LBD was 101 days (median = 97; SD = 57). Mean length of time between LBD and delivery was 44 days (median = 22; SD = 55). Mean nicotine dependence score was 3.4 (SD = 2.6; minimally to moderately dependent).
Referrals and Retention
A total of 3379 referrals resulted in 856 participants (25% of those referred). Referrals grew steadily from 245/y in 2000 to 561 in 2008. Only 42 of 856 participants (5%) dropped out. The mean number of sessions per participant (including dropouts) was 4.7 (SD = 2.92).
Factors Associated With Quitting
Overall, 119 (13.9%) participants quit or remained quit, and 737 were smoking at the last session before delivery. Of the 797 smoking at entry, 91 (11.4%) quit and 28 (47.5%) of RQs stayed smoke free. An additional 27.3% of entering CSs who did not quit by the last visit cut down on the number of cigarettes smoked per day. See Tables 1 and 2 for descriptions of participant characteristics and final smoking status.
Table 1.
Demographic Categories by Final Smoking Status*
| Final Smoking Status | ||
|---|---|---|
|
|
||
| Discrete Variables | Quit, No. (%) | Smoking, No. (%) |
| Race | ||
| White | 59 (11.8) | 442 (88.2) |
| AA/other | 62 (17.5) | 292 (82.5) |
| Marital status | ||
| Single | 95 (15.1) | 535 (84.9) |
| Married/other | 25 (11.3) | 197 (88.7) |
| Gestational age at entry | ||
| 0–3 mo | 32 (15.6) | 173 (84.4) |
| 3–6 mo | 62 (13.5) | 398 (86.5) |
| 6–9 mo | 26 (14.1) | 158 (85.9) |
| Entry status smoking | ||
| Smoking | 91 (11.4) | 706 (88.6) |
| Recent quit | 28 (47.5) | 31 (52.5) |
AA indicates African-American.
Table 2.
Summaries of Age, Addiction Level, and Participation by Quit Smoking Status*
| Final Smoking Status | ||||
|---|---|---|---|---|
| Quit | Smoking | |||
| Continuous Variables | Mean (Median) | SD (IQR) | Mean (Median) | SD (IQR) |
| Mother’s age | 22.7 (21.0) | 6.46 (18.0, 26.0) | 24.9 (24.0) | 6.27 (20.0, 29.0) |
| Degree of addiction | 2.51 (2.0) | 2.51 (0.0, 4.0) | 3.55 (3.0) | 2.58 (1.0, 5.0) |
| No. of sessions | 5.92 (6.0) | 2.63 (4.0, 7.0) | 4.48 (4.0) | 2.93 (3.0, 6.0) |
IQR indicates interquartile range.
We modeled demographic and program factors associated with quitting for the combined group of CSs and RQs. For the single-variable associations (i.e., unadjusted p values), with the exception of marital status and gestational age at entry into the program, demographic factors were significantly related to prenatal cessation (with the 95% CI excluding 1.0). As shown in Tables 1 and 3, white women were more likely to continue smoking (OR = 1.59), as were those who were older (OR = 1.81 for a 10-year difference in age), completed fewer treatment sessions (OR = .86 for a one-session difference) and were more nicotine dependent (OR = 1.18 for 1-point increase). After fitting the multivariable logistic model, age (OR = 1.5), attending fewer sessions (OR = .8), and being more nicotine dependent (OR = 1.19) all still showed a strong association with final smoking status (as evidenced by a 95% CI that excluded 1.0). None of the VIFs were above 2.0 (where 5–10 is generally considered potentially problematic in terms of collinearity), and the H-L test was highly nonsignificant (p = .30, where nonsignificant results reflect acceptable model fit). Further, only a small percentage of the deviance residuals were outside the usual range of ±2.0.
Table 3.
Logistic Modeling for Factors Associated With Continuing to Smoke*
| Single-Variable Model | Multivariable Model | ||||
|---|---|---|---|---|---|
| Variable | Exposure Level | Odds Ratio | 95% CI | Odds Ratio | 95% CI |
| Race | White | 1.59 | 1.08, 2.35 | 1.34 | 0.85, 2.09 |
| Marital status | Married/other | 1.36 | 0.86, 2.23 | 0.97 | 0.58, 1.67 |
| Gestational age at entry | 3–6 mo | 1.21 | 0.76, 1.90 | 1.19 | 0.71, 1.96 |
| 6–9 mo | 1.17 | 0.67, 2.07 | 0.89 | 0.46, 1.71 | |
| Age | 10-y increase | 1.81 | 1.29, 2.58 | 1.54 | 1.06, 2.27 |
| Degree of addiction | 1-point increase | 1.18 | 1.08, 1.29 | 1.19 | 1.09, 1.31 |
| No. of sessions | 1 additional session | 0.86 | 0.81, 0.91 | 0.84 | 0.78, 0.90 |
CI indicates confidence interval.
Satisfaction
More than 90% of participants who answered a question about the quality of service they received were “very satisfied,” including both quitters (95%) and nonquitters (91%); slightly more quitters (88%) than nonquitters (73%) answered the question. Almost all respondents (98.5%) said STOP services helped them deal more effectively with quitting or cutting down, and 95.5% said they would refer a friend to the program. The following were rated as the single most helpful treatment components: interventionist support (40.8%); CO monitoring (30.5%); education about the effects of smoking (14.8%); improved health (5.1%); incentives (3.5%); problem-solving assistance (2.1%); and other (3.3%).
Cost
The total cost for delivering the STOP Program over the 8 years reported here was $527,631. Cost per enrolled participant was $616.91 and per quitter was $4433.87.
DISCUSSION
Results of the Pittsburgh STOP Program show that a quality tobacco control intervention, informed by the research literature, can be delivered with low-income pregnant women in a community setting and that outcomes on a par with those described in the research literature are possible. STOP results compare favorably with those described in the recent Cochrane review of cessation interventions during pregnancy.17 Over 11% of smokers quit as a result of STOP Program intervention, compared to 6% overall from the randomized controlled trials described in the Cochrane review. In addition, a substantial percentage (28%) of those who didn’t quit were able to cut down, an improved result compared to that described in the review that showed no significant evidence of reduced smoking in non-quitters. In our sample, African-American and other nonwhite women were more likely to quit, as were younger women, women with lower nicotine dependence, and women who attended more treatment sessions. Programmatically, we showed that with a strong focus on development of program relationships with referral sources, very high levels of referrals can be established and maintained over time. In addition, despite Cochrane findings that withdrawals from intervention trials were common,17 we maintained very high participation rates (95%) in a low-income sample.
The Cochrane report recommends that to avoid “victim blaming and compounding issues of social disadvantage closely associated with smoking,”17(p20) attention in best practices programs needs to be paid to consumer concerns and to appropriately supporting those from economically disadvantaged populations. In that vein, STOP satisfaction results demonstrate that our low-income participants were very satisfied with the treatment they received. Nearly half reported that interventionist support was the key factor promoting their success. The finding that CO monitoring was rated by one-third of participants as the most helpful treatment component is of interest, because use of CO monitoring as a motivational tool was an innovation in this program. Of note, the STOP Program was awarded a Science and Service Award by the Substance Abuse and Mental Health Administration in 2008, recognizing “community-based organizations and coalitions for exemplary implementation of evidence-based services.”32(p1)
There are a number of implications that derive from our findings, as well as concerns about sustainability of community evidence-informed programs such as the Pittsburgh STOP Program. Our results demonstrate that it is possible for a community program to achieve results comparable to randomized controlled interventions for smoking during pregnancy, even with a very disadvantaged subgroup of the population. The potential effectiveness of including ongoing CO monitoring as a treatment component needs more study.
The implications for improvements in care for this population are great. It is clear that there is a rapid and significant return on investment for prenatal cessation programs when comparing the cost to deliver such programs with the reduction in costs associated with diverting low birth weight and other negative birth outcomes related to smoking during pregnancy.17 For example, a typical neonatal intensive care unit stay for a preterm infant is $51,600, and average first-year medical costs are 10 times greater for preterm ($32,325) than for term infants ($3325).33 A recent systematic review of smoking cessation and relapse prevention programs for pregnant women found that the few studies including formal cost-benefit analysis found a favorable benefit-cost ratio of up to 3:1, comparing dollars invested in treatment with later health care costs.34
However, the practical aspects of funding interventions such as STOP are disheartening. High-quality programs are relatively expensive to deliver. A significant concern is that third-party reimbursement for crucial cessation services does not, at the current levels, support the provision of effective best practices interventions. Most states in the United States, including Pennsylvania, provide Medicaid coverage for smoking cessation intervention in health care. At the current rate of state Medicaid reimbursement,35 STOP Program services provided over the 8-year period described here, had they been billed for, would have brought in approximately $192,000. This amount is only slightly more than one-third of actual program costs. The cost in staff time to attend to participants who otherwise might drop out is substantial, yet unreimbursable. Time for following up with missed appointments, for participants whose phones are often disconnected, and for high-level data collection and analysis accrue program costs but are not supported in traditional fee-for-service reimbursement models. These delivery cost versus reimbursement discrepancies occur at the same time that allocation of state master tobacco settlement monies for tobacco control programs is diminishing rapidly across the United States.36 In fact, by 2009, no state was funding cessation programs at the level recommended by the Centers for Disease Control.37
Attention needs to be paid in this post–Healthy People 2010 era to methods by which reimbursement policies can be brought in line with the level of support needed for effective implementation of demonstrated best practices interventions such as described here. It is possible that new health care reform regulations requiring insurers to provide more coverage for smoking cessation services may help to ameliorate the funding decrease from state master tobacco settlement monies.
Limitations
STOP is a community program with no control group. Participants self-selected the number of sessions to complete and three-quarters enrolled after the first trimester. Because of the differential rate of responding to satisfaction questions among quitters and nonquitters (the latter category including dropouts), it is possible that we overestimated satisfaction with the program. Although ours is a real-life sample, these limitations decrease our ability to understand potential results from a program with a more homogeneous sample and standardized structure.
Conclusion
Low-income pregnant smokers can be retained in an evidence-informed cessation program tailored for this group. Approximately 11% of pregnant smokers quit and about half of those who quit spontaneously in early pregnancy before enrolling remained smoke free through delivery. Quitting was significantly associated with several demographic characteristics of the mother, as well as nicotine dependence and degree of involvement with the treatment program. Concerns about the potential for sustainability of funding for best practices tobacco control interventions for low-income pregnant smokers are substantial, despite the clear and rapid return on investment that such services provide.
SO WHAT? Implications for Health Promotion Practitioners and Researchers.
What is already known on this topic?
Smoking during pregnancy is a significant risk factor for poor neonatal outcomes. Prenatal smoking cessation efforts in the U.S. have been very successful, but low income women continue to be at significant risk for smoking during pregnancy. Efficacious research interventions for smoking during pregnancy have not been widely disseminated into general community healthcare practice.
What does this article add?
This article adds to the existing literature by describing a successful translational community program that recruits high risk pregnant smokers, retains them at high rates, and makes use of evidence-informed practices including a motivational approach. Results show outcomes similar to those described in the research literature: 13.9% of participants were smoke-free by delivery and 27.3% reached harm reduction goals by cutting down on daily cigarette use.
What are the implications for health promotion practice or research?
Components of the Pittsburgh STOP Program model will be helpful to health promotion specialists in efforts to provide quality cessation services with high risk pregnant smokers in the community.
Acknowledgments
The authors wish to thank Pittsburgh STOP Program coordinator Patricia Gonzalez, MEd, MS, and interventionist Robin Sutton, BS, for their commitment to STOP participants over the years. Support for program described in this manuscript was provided by the University of Pittsburgh Medical Center, March of Dimes, Tobacco Free Allegheny, Pennsylvania Department of Health, UPMC Health Plan, and the FISA Foundation. Support for data analysis and statistical support was provided by a grant from the Reduce Smoking and Exposure to Tobacco (ReSET) Center at the University of Pittsburgh Graduate School of Public Health and by grant #UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research. This article’s contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Contributor Information
Patricia A. Cluss, Western Psychiatric Institute & Clinic, Department of Psychiatry, School of Medicine.
Michele D. Levine, Western Psychiatric Institute & Clinic, Department of Psychiatry, School of Medicine.
Douglas Landsittel, Center for Research on Health Care Data Center, Division of Internal Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
References
- 1.US Dept of Health and Human Services: Office of Disease Prevention and Health Promotion—Healthy People 2010. NAS Newsl. 2000;15:3. [PubMed] [Google Scholar]
- 2.Suellentrop K, Morrow B, Williams L, et al. Monitoring progress toward achieving maternal and infant Healthy People 2010 objectives—19 states, pregnancy risk assessment monitoring system (PRAMS), 2000–2003. MMWR Surveill Summ. 2006;55:1–11. [PubMed] [Google Scholar]
- 3.Hoyert DL, Freedman MA, Strobino DM, Guyer B. Annual summary of vital statistics: 2000. Pediatrics. 2001;108:1241–1255. doi: 10.1542/peds.108.6.1241. [DOI] [PubMed] [Google Scholar]
- 4.Heron M, Sutton PD, Xu J, et al. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 5.DiClemente CC, Dolan-Mullen P, Windsor RA. The process of pregnancy smoking cessation: implications for interventions. Tob Control. 2000;9:16–21. doi: 10.1136/tc.9.suppl_3.iii16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeClere FB, Wilson JB. Smoking behavior of recent mothers, 18–44 years of age, before and after pregnancy: United States, 1990. Adv Data. 1997;1:11. [PubMed] [Google Scholar]
- 7.Kendrick JS, Zahniser SC, Miller N, et al. Integrating smoking cessation into routine public prenatal care: the smoking cessation in pregnancy project. Am J Public Health. 1995;85:217–222. doi: 10.2105/ajph.85.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Windsor RA, Lowe JB, Perkins LL, et al. Health education for pregnant smokers: its behavioral impact and cost benefit. Am J Public Health. 1993;83:201–206. doi: 10.2105/ajph.83.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McBride CM, Curry SJ, Lando HA, et al. Prevention of relapse in women who quit smoking during pregnancy. Am J Public Health. 1999;89:706–711. doi: 10.2105/ajph.89.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morasco BJ, Dornelas EA, Fischer EH, et al. Spontaneous smoking cessation during pregnancy among ethnic minority women: a preliminary investigation. Addict Behav. 2006;31:203–210. doi: 10.1016/j.addbeh.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Secker-Walker RH, Solomon LJ, Flynn BS, et al. Smoking relapse prevention during pregnancy. A trial of coordinated advice from physicians and individual counseling. Am J Prev Med. 1998;15:25–31. doi: 10.1016/s0749-3797(98)00029-4. [DOI] [PubMed] [Google Scholar]
- 12.Mathews TJ. Division of Vital Statistics. Smoking during pregnancy in the 1990s. Natl Vital Stat Rep. 2001;49:1–15. [PubMed] [Google Scholar]
- 13.Martin JA, Kung HC, Mathews TJ, et al. Annual summary of vital statistics: 2006. Pediatrics. 2008;121:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- 14.Fiore MC, Bailey WC, Cohen SJ, et al. Smoking Cessation. Clinical Practice Guideline Number 18. Rockville, Md: US Dept of Health and Human Services; 1996. AHCPR publication 96-0692. [Google Scholar]
- 15.Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, Md: US Dept of Health and Human Services; 2000. [Google Scholar]
- 16.Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, Md: US Dept of Health and Human Services; 2008. [Google Scholar]
- 17.Lumley J, Chamberlain C, Dowswell T, et al. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009:001055. doi: 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McHugh RK, Barlow DH. The dissemination and implementation of evidence-based psychological treatments: a review of current efforts. Am Psychol. 2010;65:73–84. doi: 10.1037/a0018121. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 20.Anum EA, Retchin SM, Strauss JF., III Medicaid and preterm birth and low birth weight: the last two decades. J Womens Health. 2010;19:443–451. doi: 10.1089/jwh.2009.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ockene J, Ma Y, Zapka J, et al. Spontaneous cessation of smoking and alcohol use among low-income pregnant women. Am J Prev Med. 2002;23:150–159. doi: 10.1016/s0749-3797(02)00492-0. [DOI] [PubMed] [Google Scholar]
- 22.Solomon L, Quinn V. Spontaneous quitting: self-initiated smoking cessation in early pregnancy. Nicotine Tob Res. 2004;6:S203–S216. doi: 10.1080/14622200410001669132. [DOI] [PubMed] [Google Scholar]
- 23.Goedhart G, van der Wal MF, Cuijpers P, Bonsel GJ. Psychosocial problems and continued smoking during pregnancy. Addict Behav. 2009;34:403–406. doi: 10.1016/j.addbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Goins KV, Pbert L, Ockene JK. Predictors of smoking cessation in pregnancy and maintenance postpartum in low-income women. Matern Child Health J. 2005;9:393–402. doi: 10.1007/s10995-005-0020-8. [DOI] [PubMed] [Google Scholar]
- 25.Paul IM, Lehman EB, Widome R. Maternal tobacco use and shorter newborn nursery stays. Am J Prev Med. 2009;37:S172–S178. doi: 10.1016/j.amepre.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Annie E. Kids Count: The Right Start: Conditions of Babies and Their Families in America’s Largest Cities. Baltimore, Md: Annie E. Casey Foundation; 1999. Casey Foundation. [Google Scholar]
- 27.Moss DR, Cluss PA, Watt-Morse M, Pike F. Targeting pregnant and parental smokers: long-term outcomes of a practice-based intervention. Nicotine Tob Res. 2009;11:278–285. doi: 10.1093/ntr/ntn035. [DOI] [PubMed] [Google Scholar]
- 28.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 29.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 30.England LJ, Kendrick JS, Wilson HG, et al. Effects of smoking reduction during pregnancy on the birth weight of term infants. Am J Epidemiol. 2001;154:694–701. doi: 10.1093/aje/154.8.694. [DOI] [PubMed] [Google Scholar]
- 31.Flesch R. The Art of Plain Talk. New York, NY: Harper; 1946. [Google Scholar]
- 32.National Implementation Research Network. Implementing evidence-based practices: experiences of the 2007 and 2008 Science and Service Award recipients. Rockville, Md: US Dept of Health and Human Services; 2009. pp. 1–14. [Google Scholar]
- 33.Melnyk BM, Feinstein NF. Reducing hospital expenditures with the COPE (Creating Opportunities for Parent Empowerment) program for parents and premature infants: an analysis of direct healthcare neonatal intensive care unit costs and savings. Nurs Adm Q. 2009;33:32–37. doi: 10.1097/01.NAQ.0000343346.47795.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruger JP, Emmons KM. Economic evaluations of smoking cessation and relapse prevention programs for pregnant women: a systematic review. Value Health. 2008;11:180–190. doi: 10.1111/j.1524-4733.2007.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pennsylvania Dept of Public Welfare. Outpatient fee schedule: Office of Medical Assistance Programs. [Accessed April 3, 2010]; Available at: www.dpw.state.pa.us/PartnersProviders/MedicalAssistance/Schedules/003675750.aspx. [Google Scholar]
- 36.Nelson DE, Reynolds JH, Luke DA, et al. Successfully maintaining program funding during trying times: lessons from tobacco control programs in five states. J Public Health Manag Pract. 2007;13:612–620. doi: 10.1097/01.PHH.0000296138.48929.45. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control. Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR. 2009;58:1127–1132. [PubMed] [Google Scholar]