Abstract
All adult mammals examined thus far exhibit sleep bout durations that follow an exponential distribution and wake bout durations that follow a power-law distribution. In altricial rodents such as rats and mice, exponential distributions of sleep bouts are found soon after birth, but the power-law distribution of wake bouts does not emerge until the third postnatal week. Also, both sleep and bouts consolidate across the early postnatal period. It is not known whether similar developmental processes occur in precocial species during the prenatal period. Here we characterize sleep-wake development in a precocial species, the domestic sheep (Ovis aries), from 114–148 days gestational age (DGA). Sleep and wake bout durations exhibited exponential distributions throughout the fetal period with some evidence of an emerging exponential-to-power-law transition for wake bouts toward the end of gestation. Both sleep and wake bouts consolidated in an orderly fashion across development and there was little evidence of circadian variation, even in the oldest subjects. These results indicate that similar patterns of sleep-wake organization are found prenatally in a precocial species as are found postnatally in altricial species. Data from more species are needed to fully realize the benefits of a developmental comparative approach for understanding the forces that have shaped the ontogeny and phylogeny of mammalian sleep.
Keywords: sleep, wake, EMG, fetus
Introduction
Mammals cycle between bouts of sleep and wakefulness throughout the day and night. By examining the statistical distributions of these bouts, previously undetected structure has been revealed. Thus, in all adult mammals studies thus far (i.e., rats and mice: order Rodentia; cats: order Carnivora; and humans: order Primates), sleep bouts follow an exponential distribution [P(t) ~ exp(−t/τ)] with a characteristic time scale τ, whereas wake bouts follow a power-law distribution [P (t) ~ t−α] with a scaling exponent α (Lo, et al., 2002). Similar analyses in newborn rats suggest that the exponential distribution of sleep bouts is a robust finding; in contrast, wake bouts initially follow an exponential distribution before a power-law distribution emerges by the end of the second postnatal week (Blumberg, Seelke, Lowen, & Karlsson, 2005). Similar findings have been reported in infant mice (Blumberg, Coleman, Johnson, & Shaw, 2007).
An improved understanding of the temporal structure of sleep and wake bouts, including their development, can yield useful insights into the neural mechanisms that underlie behavioral state transitions. Importantly, exponential and power-law distributions suggest different kinds of neural interactions and associated connectivities, as well as different vulnerabilities to neural degeneration; for example, destroying terminals from the locus coeruleus selectively prevents the developmental emergence of power-law wake distributions without affecting sleep bout durations (Gall, et al., 2009). Thus, more precise assessment of the temporal structure of sleep and wake bouts allows us to accurately identify those neural systems that contribute to bout consolidation and power-law expression (Blumberg, Coleman et al., 2007; Gall et al., 2009). Such precision is useful for understanding sleep-wake changes across normal development and in response to pathological conditions (Blumberg, Karlsson, & Seelke, 2007).
It may be that the statistical distributions of sleep and wakefulness reported thus far represent universal features of sleep-wake organization and development in mammals. However, before drawing such a conclusion, it is important to sample from a wider array of species. Here, using archival data, we report on the development of sleep-wake dynamics during the day and night in fetal domestic sheep (Ovis aries) from 115 days gestational age (DGA) to DGA 145. The domestic sheep is a member of the order Artiodactyla and, unlike the other species examined thus far, their offspring are highly precocial. As a consequence, much of the sleep-wake development observed postnatally in altricial rodents (e.g., rats) may occur prenatally in sheep.
Although fetal sheep have been the subject of numerous investigations of behavioral state development (Anderson, et al., 1998; Robertson, et al., 1996; Robinson, Wong, Robertson, Nathanielsz, & Smotherman, 1995; Ruckebusch, 1972; Ruckebusch, Gaujoux, & Eghbali, 1977; Shinozuka & Nathanielsz, 1998; Szeto & Hinman, 1985), most studies have focused on the development of state-dependent electroencephalographic (EEG) activity across the prenatal period. Such analyses, although useful for describing developmental changes in cortical activity, do not provide insight into sleep and wakefulness before the developmental emergence of state-dependent EEG activity (e.g., delta waves). In infant rats, for example, bouts of high nuchal muscle tone (indicative of wakefulness) and low muscle tone or atonia (indicative of sleep) are tightly coupled with behavior (e.g., high-amplitude movements, myoclonic twitches, eye movements) and are governed by neural circuits similar to those associated with sleep and wakefulness in adults (Karlsson & Blumberg, 2005; Karlsson, Gall, Mohns, Seelke, & Blumberg, 2005; Seelke, Karlsson, Gall, & Blumberg, 2005). Then, when delta activity is first detected at postnatal day 11, it merges seamlessly with periods of quiet sleep as defined using electromyogram (EMG) activity alone (Seelke & Blumberg, 2008). Thus, EMG activity is not only a proxy for sleep and wakefulness, it is the foundation upon which the other components of sleep and wakefulness are built (Blumberg & Seelke, 2010).
Therefore, the analyses used here in fetal sheep focus on the development of sleep and wake bouts as defined using EMG activity alone. Similar to what has been seen postnatally in altricial rodents, we predict that sleep and wake bouts will consolidate across the fetal period and that sleep bouts will always follow an exponential distribution, even at the earliest gestational ages. In contrast, we predict that wake bouts will follow an exponential distribution at the earliest fetal ages and transition to a power-law distribution toward the end of gestation.
Methods
Subjects
Archival data from 17 sheep fetuses from timed matings of Rambouillet-Colombia (Ovis aries) ewes were used in this study. Ewes and fetuses were cared for according to guidelines established by the New York State College of Veterinary Medicine and the National Institutes of Health (1986). The protocol for this study was approved by the Cornell Institutional Animal Care and Use Committee. All facilities were approved by the American Association for the Accreditation of Laboratory Animal Care. Data from theses fetuses have been published previously (Anderson et al., 1998; Robertson et al., 1996; Robinson et al., 1995).
Surgery and Procedure
Fetuses were instrumented on 113–116 DGA using procedures described previously (Robertson et al., 1996; Robinson et al., 1995). Briefly, ewes were given a ketamine (1000 mg) and glycopyrollate (0.12 mg) preanesthetic. Surgery was conducted with the ewe under 2.0% halothane anesthesia, which also anesthetizes the fetus (Towell, Figueroa, Markowitz, Elias, & Nathanielsz, 1987). The fetus was exteriorized through a midline laparotomy and an incision through the uterine wall. Polyvinyl catheters were placed in a fetal carotid artery and jugular vein and filled with heparinized saline. Fetal blood gases, blood pressure, and heart rate were continuously monitored. Fetal forelimb flexor and extensor muscles (brachialis, triceps) and hindlimb flexor, extensor (tibialis anterior, gastrocnemius) and nuchal muscles were instrumented with pairs of EMG electrodes, implanted 3–5 mm apart. Electrodes were inserted for electrooculogram (EOG) and electrocorticogram monitoring and an indifferent electrode was implanted into the nuchal muscle. All catheters and wires passed through two sites in the ewe’s lateral abdominal wall. A topical antiseptic spray (Blu-kote; Morris, NJ) was applied to all incision sites after closure of the uterus and maternal abdomen.
For the first 2 days after surgery, each ewe was treated with antibiotic and oral analgesic. Maternal and fetal vascular catheters were continuously infused with sterile heparinized saline (20 units/ml of 0.9 g/liter saline) at a rate of 0.5 ml/hr. Maternal and fetal arterial blood samples (0.5 ml) were obtained daily and oxygen saturation, hemoglobin, pH, p02, and pC02 were measured with a blood gas analyzer to monitor the condition of the ewe and fetus. At the completion of the study, ewes and fetuses were euthanized with an overdose of sodium pentobarbital.
Data acquisition began 2 days after the fetus was instrumented and continued until the end of gestation. EMG signals were processed online using custom-written software. The signals were band-pass filtered (3 to 20 Hz), full-wave rectified, lowpass filtered at 10 Hz, and digitized at 32 Hz with 8-bit resolution. The digitized data were integrated over successive 1-s intervals and the resulting time series was stored for subsequent analysis.
Data analysis
Previously described methods (Karlsson & Blumberg, 2002; Karlsson et al., 2005; Karlsson, Kreider, & Blumberg, 2004; Seelke & Blumberg, 2008) developed to derive sleep-wake bout data from EMG signals in infant rats were adapted for the fetal sheep. Nuchal muscle EMG data, integrated and full-wave rectified were imported into NeuroScore (Data Sciences International, St. Paul, MN).
Ten 5-s segments of either uninterrupted high muscle tone or atonia periods were measured for each fetus, averaged, and the midpoint between high tone and atonia periods was determined. The mean midpoint value across all fetuses was 10.1 μV; therefore, the midpoint of 10 μV was adopted for all subsequent analysis. Each 5-s segment during a recording period comprised 5 EMG measurements (i.e. a 1 Hz sampling rate) and a bout of sleep (or wake) was defined as a 5-s segment in which muscle tone was below (or above) the 10 μV midpoint for at least 4 of the 5 measurements. Using automatic analysis tools in Neuroscore, the duration of each period of high nuchal muscle tone (indicative of wakefulness) and nuchal atonia (indicative of sleep) was measured in 5-s epochs for each subject across 24-h of continuous recording.
Five age groups were used for these analyses: DGA 114–118 (hereafter referred to as DGA 116, n = 7), DGA 122–124 (hereafter referred to as DGA 123, n = 10), DGA 130–132 (hereafter referred to as DGA 131, n = 10), DGA 138–140 (hereafter referred to as DGA 139, n = 10), and DGA 144–148 (hereafter referred to as DGA 146, n = 7). Each fetus contributed data to at least 2, and up to 4, age groups.
Data were imported into JMP 6.0 (SAS, Cary, NC) and DeltaGraph 5.6.3 (SPSS, Chicago, IL) for statistical analysis. Survivor distributions were produced for each fetus and from pooled data at each age. Data were plotted using semi-log and log-log coordinates and regression analyses were performed to calculate r2 values and, therefore, the degree of fit of the data to exponential and power-law distributions, respectively (Blumberg et al., 2005).
Analysis of variance (ANOVA) was used to test for differences across age. Because each subject was tested at more than 1 age, we adjusted the degrees of freedom in the ANOVAs to reflect the total number of subjects and to compensate for the lack of independence. Paired t tests were used to test for within-age differences. For all tests, the Type 1 error rate (alpha) was set at .05. All means are presented with their standard errors.
Results
Figure 1A depicts a recording from a fetus at DGA 116 and Figure 1B depicts a recording from the same fetus at DGA 146. Black bars below the EMG traces indicate periods of nuchal muscle atonia (indicative of sleep) and red bars represent periods of high nuchal muscle tone (indicative of wakefulness). The developmental change from fragmented to consolidated sleep between the two prenatal ages is clearly visible. As a measure of REM-sleep processes, we analyzed electrooculogram (EOG) bursts (Pace-Schott & Hobson, 2002). Figure 1C depicts a representative EOG burst occurring after the onset of atonia and terminating before the onset of high muscle tone. A similar pattern of activity has been demonstrated in infant rats (Seelke et al., 2005).
Figure 1.
Representative nuchal EMG recordings from (A) a DGA 115 subject and (B) the same fetus at 145 DGA. Black bars denote bouts of sleep and red bars denote bouts of wake. Note the more fragmented sleep-wake pattern at DGA 115. (C) Representative EMG recording for a different fetus at DGA 145 with the associated electooculogram (EOG) recording.
Sleep and wake bouts consolidate while total sleep duration remains unchanged
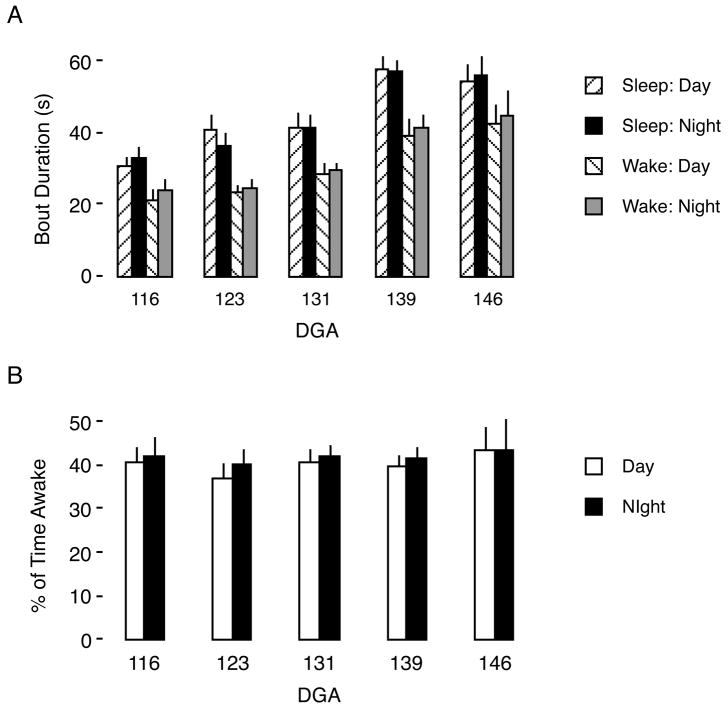
As shown in Figure 2, neither mean sleep and wake bout durations nor percentage of time awake exhibited circadian variation at any age tested. However, as shown in Figure 2A, mean sleep bout duration increased significantly with age (F4,16 = 7.74, P < 0.002), as did mean wake bout duration (F4,16 = 6.72, P < 0.003). In contrast, as depicted in Figure 2B, the percentage of time asleep was maintained within a narrow range of values (59–66%) across all ages examined (F4,16 = 0.5, NS).
Figure 2.
Mean sleep and wake bout durations and percentage of time awake across development in fetal sheep. (A) Mean sleep and wake bout duration increase significantly with age. At each age, sleep bout duration is longer than wake bout duration during both the day and night. (B) Mean percentage of time awake during the day and night. Means are presented with their standard error.
Sleep and wake bout durations follow an exponential distribution
Survivor distributions of sleep and wake bouts, recorded during the day and night at 3 DGA groups, are presented in Figure 3. Survival data that follow an exponential distribution fall on a straight line on a semi-log plot and those that follow a power-law distribution fall on a straight line on a log–log plot.
Figure 3.
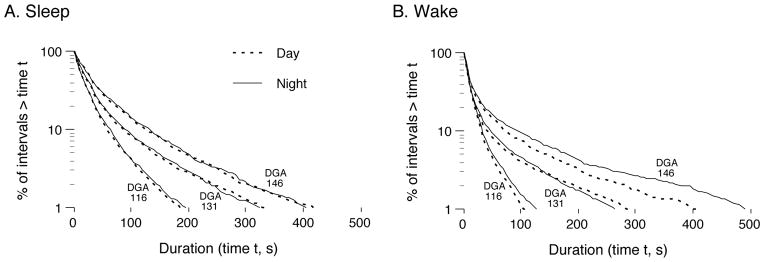
Log-survivor plots of pooled sleep (A) and wake (B) bout durations for fetal sheep at DGA 116, 131, and 146 during the day (dotted lines) and night (solid lines). The plots were constructed using semi-log coordinates; in such plots, data that follow an exponential distribution fall along a straight line.
For sleep bout durations (Figure 3A), the data for all age groups fall on a straight line on the semi-log plot. As shown in Figure 4A, paired t tests revealed that an exponential distribution provided a significantly better fit to the sleep data than did a power-law distribution at each of the 5 ages examined [DGA 116 (t=4.2, df = 9, P < 0.05); DGA 123 (t = 10.1, df = 16, P < 0.05); DGA 131 (t = 11,5, df = 17, P < 0.05); DGA 139 (t = 10,6, df = 17, P < 0.05); DGA 146 (t = 3.0, df = 9, P < 0.05)].
Figure 4.
Best-fit (r2) values of log-survivor plot data at 5 ages in fetal sheep. For each individual, the degree of fit of the data to exponential and power-law distributions was determined, yielding a value of r2 that was then averaged across subjects at each age. (A) Values of r2 for sleep bout durations showing that the data are significantly better fit to an exponential distribution. (B) Values of r2 for wake bout durations showing that the data are significantly better fit to an exponential distribution at all ages except DGA 116 and 146. Means are presented with their standard error. * significant within-age difference.
For wake bout durations (Figure 3B), the data for most, but not all, age groups fall on a straight line on the semi-log plot. As depicted in Figure 4B, an exponential distribution provided a significantly better fit to the wake bout duration data at DGA 123 (t = 5.25, df = 15.0, P < 0.05), DGA 131 (t =5.5, df = 17.7, P < 0.05) and DGA 139 (t = 1.8, df = 17.9, P < 0.05). Between DGA 139 and DGA 146, the graphs of the two distributions intersect; however, at DGA 146 the mean r2 values are not significantly different (t = 0.9, df = 9.5).
Discussion
Here we demonstrate that the sleep-wake dynamics of fetal sheep follow a developmental trajectory comparable to that previously described postnatally in rats and mice (Blumberg, Coleman et al., 2007; Blumberg et al., 2005; Karlsson et al., 2004). First, average sleep and wake bout durations increased over gestation even as total sleep time remained roughly constant. Second, in agreement with data from infant and adult rats and mice and adult humans and cats (Blumberg, Coleman et al., 2007; Blumberg et al., 2005; Lo et al., 2002; Lo, et al., 2004), sleep bouts at all ages were best characterized by an exponential distribution. This statistical feature of sleep bout structure persisted despite substantial age-related increases in sleep bout durations. Finally, we found no evidence of circadian sleep-wake rhythmicity during the fetal period in these sheep; in contrast, rats exhibit detectible circadian differences in sleep and wake bout durations soon after birth and a clear nocturnal pattern by the beginning of the third postnatal week (Gall, Todd, Ray, Coleman, & Blumberg, 2008).
In contrast with sleep bout distributions, adult mammals studied thus far exhibit wake bout durations that follow a power-law distribution (Lo et al., 2002; Lo et al., 2004). This organization develops postnatally in rats and mice during the third postnatal week (Blumberg et al., 2005). In fetal sheep, the wake bouts were better characterized by an exponential distribution at all ages, except for the oldest group where we observed a non-significant difference between exponential and power-law distributions. Recordings of neonatal and adult sheep are needed to determine if and when power-law wake behavior emerges in this species.
The present results appear inconsistent with previously published reports on the development of behavioral state in fetal sheep (Ruckebusch, 1972; Ruckebusch et al., 1977; Szeto & Hinman, 1985). Early studies reported that organized behavioral states emerge between DGA 115–120 and that the nuchal EMG lags behind the development of organized EEG activity (Szeto & Hinman, 1985). As shown here, however, measures of EMG activity using 5-s epochs revealed highly organized behavioral states in the youngest fetuses from which recordings were made. It may be that the shorter epochs (5 s as opposed to 1 min) used here contributed to our differing results, as 1-min epochs filter out relatively high-frequency data and can thus obscure behavioral state organization at the youngest ages when cycling between sleep and wake is more rapid.
Fetal sheep have previously been reported to sleep approximately 80% of the time (Ruckebusch, 1972), with a decrease in the percentage of time asleep just before and after parturition (Ruckebusch et al., 1977). In contrast, based on the nuchal EMG scoring method used here, sleep was estimated to occupy approximately 60% of the time throughout the prenatal period. Again, differences in scoring methodologies, especially epoch size, likely contributed to these differing estimates of sleep time.
Detailed assessments of the temporal organization of sleep and wake bouts provide a more accurate picture of typical and atypical development (Blumberg, Karlsson et al., 2007). They also provide a sound basis for making meaningful comparisons across species. Although interest in the evolution of sleep has been and continues to be strong (Capellini, Barton, McNamara, Preston, & Nunn, 2008; Lesku, Roth, Amlaner, & Lima, 2006; Siegel, 2005), there has been consistently less interest in the role that developmental processes may play in constraining and biasing the evolution of sleep. However, because evolutionary transformations arise through modifications of developmental processes (Blumberg, 2009; West-Eberhard, 2003), detailed developmental analyses of sleep-wake rhythms across a diversity of species will be needed to gain a complete picture of the evolution – and also the functions – of sleep.
Acknowledgments
Preparation of this article was supported by Icelandic Centre for Research grant 070801051 awarded to K.Æ.K. and NIH grants MH40701 and MH66424 awarded to M.S.B. We thank Matthias Schwab and Peter Nathanielsz for helpful comments.
References
- Anderson CM, Mandell AJ, Selz KA, Terry LM, Wong CH, Robinson SR, et al. The develoment of nuchal atonia associated with active (REM) sleep in fetal sheep: presence of recurrent fractal organization. Brain Research. 1998;787:351–357. doi: 10.1016/s0006-8993(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Blumberg MS. Freaks of nature: What anomalies tell us about development and evolution. New York: Oxford University Press; 2009. [Google Scholar]
- Blumberg MS, Coleman CM, Johnson ED, Shaw C. Developmental divergence of sleep-wake patterns in orexin knockout and wild-type mice. European Journal of Neuroscience. 2007;25:512–518. doi: 10.1111/j.1460-9568.2006.05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Karlsson KA, Seelke AM. Sleep, development, and human health. Sleep. 2007;30:549–550. doi: 10.1093/sleep/30.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AM. The form and function of infant sleep: From muscle to neocortex. In: Blumberg MS, Freeman JH, Robinson SR, editors. The Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 391–423. [Google Scholar]
- Blumberg MS, Seelke AM, Lowen SB, Karlsson KA. Dynamics of sleep-wake cyclicity in developing rats. Proceedings of the National Academy of Sciences. 2005;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capellini I, Barton RA, McNamara P, Preston BT, Nunn CL. Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution. 2008;62:1764–1776. doi: 10.1111/j.1558-5646.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall AJ, Joshi B, Best J, Florang VR, Doorn JA, Blumberg MS. Developmental emergence of power-law wake behavior depends upon the functional integrity of the locus coeruleus. Sleep. 2009;32:920–926. doi: 10.1093/sleep/32.7.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall AJ, Todd WD, Ray B, Coleman CM, Blumberg MS. The development of day-night differences in sleep and wakefulness in Norway rats and the effect of bilateral enucleation. Journal of Biological Rhythms. 2008;23:232–241. doi: 10.1177/0748730408316954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. The union of the state: myoclonic twitching is coupled with nuchal muscle atonia in infant rats. Behavioral Neuroscience. 2002;116:912–917. doi: 10.1037//0735-7044.116.5.912. [DOI] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. Active medullary control of atonia in week-old rats. Neuroscience. 2005;130:275–283. doi: 10.1016/j.neuroscience.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Gall AJ, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biology. 2005;3:891–901. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Kreider JC, Blumberg MS. Hypothalamic contributions to sleep-wake cycle development. Neuroscience. 2004;123:575–582. doi: 10.1016/j.neuroscience.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Lesku JA, Roth TC, 2nd, Amlaner CJ, Lima SL. A phylogenetic analysis of sleep architecture in mammals: the integration of anatomy, physiology, and ecology. American Naturalist. 2006;168:441–453. doi: 10.1086/506973. [DOI] [PubMed] [Google Scholar]
- Lo CC, Amaral LAN, Havlin S, Ivanov PC, Penzel T, Peter JH, et al. Dynamics of sleep-wake transitions during sleep. Europhysics Letters. 2002;57:625–631. [Google Scholar]
- Lo CC, Chou T, Penzel T, Scammell TE, Strecker RE, Stanley HE, et al. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proceedings of the National Academy of Sciences. 2004;101:17545–17548. doi: 10.1073/pnas.0408242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nature Reviews Neuroscience. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Robertson SS, Johnson SL, Bacher LF, Wood JR, Wong CH, Robinson SR, et al. Contractile activity of the uterus prior to labor alters the temporal organization of spontaneous motor activity in the fetal sheep. Developmental Psychobiology. 1996;29:667–683. doi: 10.1002/(SICI)1098-2302(199612)29:8<667::AID-DEV3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Wong CH, Robertson SS, Nathanielsz PW, Smotherman WP. Behavioral responses of the chronically instrumented sheep fetus to chemosensory stimuli presented in utero. Behavioral Neuroscience. 1995;109:551–562. [PubMed] [Google Scholar]
- Ruckebusch Y. Development of sleep and wakefulness in the foetal lamb. Electroencephalography and Clinincal Neurophysiology. 1972;32:119–128. doi: 10.1016/0013-4694(72)90134-4. [DOI] [PubMed] [Google Scholar]
- Ruckebusch Y, Gaujoux M, Eghbali B. Sleep cycles and kinesis in the foetal lamb. Electroencephalography and Clinical Neurophysiology. 1977;42:226–237. doi: 10.1016/0013-4694(77)90029-3. [DOI] [PubMed] [Google Scholar]
- Seelke AMH, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep. 2008;31:691–699. doi: 10.1093/sleep/31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AMH, Karlsson KÆ, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements, and the development of active and quiet sleep. European Journal of Neuroscience. 2005;22:911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozuka N, Nathanielsz PW. Electrocortical activity in fetal sheep in the last seven days of gestation. Journal of Physiology. 1998;513:273–281. doi: 10.1111/j.1469-7793.1998.273by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH, Hinman DJ. Prenatal development of sleep-wake patterns in sheep. Sleep. 1985;8:347–355. doi: 10.1093/sleep/8.4.347. [DOI] [PubMed] [Google Scholar]
- Towell ME, Figueroa J, Markowitz S, Elias B, Nathanielsz P. The effect of mild hypoxemia maintained for twenty-four hours on maternal and fetal glucose, lactate, cortisol, and arginine vasopressin in pregnant sheep at 122 to 139 days’ gestation. American Journal of Obstetrics and Gynecology. 1987;157:1550–1557. doi: 10.1016/s0002-9378(87)80261-2. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. Oxford: Oxford University Press; 2003. [Google Scholar]