Abstract
The law of natural selection can be used to understand cancer development at the level of species as well as at the level of cells and tissues. Through this Perspective I seek to explain: 1) Why the lack of sufficient selective pressure to prevent cancers in old age helps explain the exponential increase in cancer incidence in the elderly. 2) Why the evolution of long-lived animals necessitated the acquisition of potent tumor suppressive mechanisms. 3) How the requirement to prevent inappropriate somatic cell expansion and cancer has constrained developmental and tissue architectural modalities. 4) How the evolution of well-adapted stem cells with complex niche requirements has conferred resistance to oncogenic mutations, as phenotype-altering genetic change is almost always disadvantageous within a well-adapted cell population. 5) How the impairment of stem cell fitness, as occurs in old age, can promote selection for adaptive mutations and cancer initiation. 6) Why differential maintenance of stem cell fitness may explain how different vertebrate species with enormous differences in lifespan and body size similarly avoid cancer through reproductive years.
Cancer and Natural Selection
why the paucity of selective pressure to prevent cancers in old age helps explain the exponential increase in cancer incidence in the elderly.
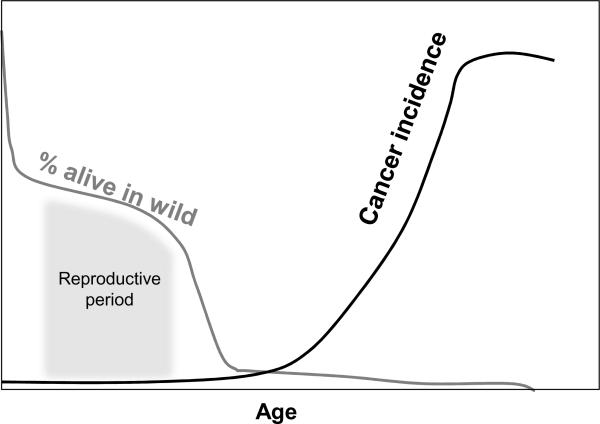
Natural selection is relatively blind to the elderly, which during most of our evolutionary history were relatively rare (1). The chances of a human older than 40 contributing to the gene pool of future generations were low, as an earlier demise due to disease, starvation, predators or other causes was more likely. The decline in the soma with age has been postulated to reflect the lack of selection for somatic maintenance beyond the age when the animal contributes to population fitness by passing its genes on to future generations (1–3). The same reasoning applies to cancer, and as such there is little selective pressure to limit cancer in old animals. Thus, an estimated curve for cancer rates at any given age overlaps little with survival percentages at that age (Fig 1). While these curves for the typical vertebrate animal in the “wild” suggest that cancer does not substantially limit survival past breeding age, they do not suggest that cancer has been unimportant in vertebrate evolution. Indeed, the evolution of long-lived and large animals, such as vertebrates, necessarily entailed the acquisition of potent tumor suppressive mechanisms. While there may have been some advantage for better suppression of cancer in the elderly (old males can often still breed, “grandmothers” serving as care-givers, etc.), enhanced tumor suppression probably comes with a cost, which, if it required increased energy allocation in youth, may have been disfavored. Thus, evolution has in effect weighed the costs and benefits of somatic cell maintenance and tumor suppression, favoring a strategy that maximizes reproductive success.
Figure 1. Natural selection can explain cancer incidence at the organismal level.
There is minimal selection against cancer beyond the age where most animals would already be dead by other causes. The curves depicted should not be viewed as quantitative, but as “ballpark” estimates. For example, for some species, the loss of individuals before sexual maturity is much greater.
Evolved Tumor Suppressive Mechanisms
how the acquisition of tumor suppressive mechanisms facilitated the evolution of large and long-lived organisms.
The evolution of long-lived multicellular animals required acquisition of potent tumor suppressive mechanisms. I consider these different tumor suppressive mechanisms as either intrinsic (primarily cell autonomous) or integral (related to tissue architecture/organization and organism-wide systems). Finally, I will argue for another important mechanism of tumor suppression based on the resistance of fit stem cell populations to phenotype-altering heritable changes.
Intrinsic tumor suppression
Cells of multicellular organisms, even those with body plans as simple as sponges (4), have evolved mechanisms to maintain appropriate numbers of cells within tissues. Both cell division and survival are regulated by various social cues, including growth factor stimulation, attachment to a basement membrane, contact with other cells, and adequate blood supply. Importantly, there is often substantial redundancy in these control systems, as well as feedback mechanisms that penalize cells that disobey these social cues. A cell that inappropriately enters the cell cycle in the absence of appropriate signals will typically be committed to die or senesce. This tight control of cell numbers is necessary for proper functioning of the tissue. It is also clear that mechanisms that eliminate cells from the replicative pool in response to inappropriate signals are powerful intrinsic tumor suppressive mechanisms (5). Indeed, many oncogenic mutations affect signaling pathways that promote apoptosis, cell senescence or other means to prevent clonal expansion. In addition, DNA damage and other cellular stresses trip similar pathways in order to limit the risk of propagating damaged cells. The evolution of multicellular animals (metazoans) demanded the acquisition of new tumor suppressor gene functions that limit inappropriate cell expansion, and it is not surprising that the origin of metazoans coincides with the emergence of some oncogene and tumor suppressor founder genes (6).
While the requirement for telomere maintenance is certainly not limited to multicellular organisms, progressive telomere shortening in somatic cells would be expected to limit tumor development, particularly if initiated in non-stem cells that lack telomerase activity (7). Finally, effective DNA repair and epigenetic code maintenance certainly also contribute to intrinsic tumor suppression by limiting somatically heritable diversity, although evidence that that maintenance of genetic and epigenetic codes has been significantly improved during the evolution of multicellularity is lacking.
Integral tumor suppression
A highly underappreciated but profound concept advanced by a number of investigators, starting with Cairns, is that the evolution of multicellular organisms has been constrained by the requirement to avoid cancer (or any somatic cell evolution that disrupts normal tissue function) (8–16). The constraints put on tissue development and organization are more critical for organisms with larger bodies and longer lives, which require tissue renewal during adulthood. In particular, the hierarchical organization of tissues, with few stem cells and progressive differentiation of most cells, has certainly contributed to effective tumor suppression. Moreover, stem cells tend to divide infrequently, with rapid proliferation delegated to short-lived progenitor cells derived from these stem cells. The compartmentalization of stem cells, such as colonic stem cells in the crypts at the base of each villus (protected from the nasty lumenal contents), would be expected to further limit somatic cell evolution. The relatively small size of each population of stem cells should serve to impede clonal evolution, given the limited target size for advantageous mutations (9–11, 14). On the other hand, small population sizes are more favorable for mutation fixation, even for disadvantageous mutations (such as those conferring genetic instability), which could be fixed by genetic drift. While most self-renewing epithelial tissues appear to have adopted the strategy of compartmentalizing stem cells, the hematopoietic system (for unknown reasons) instead evolved a single large intermixing population of stem cells.
So both by strategic location (reducing carcinogen exposure) and by reducing stem cell numbers (small effective population size), vertebrates evolved intestines with low cancer rates, despite a dizzying number of cell divisions required daily to maintain this organ. The hierarchical organization of tissues, with a few stem cells maintaining a tissue, is a beautiful evolved strategy, leaving the inherently dangerous cell expansion to short-term progenitors, who will soon be destined to terminal differentiation (and, in the case of intestinal cells, a one-way trip out the anus). Thus, potentially oncogenic mutations get “flushed” (pun intended) (10, 17). There were probably other possible solutions to constructing a good food absorbing organ, but among the evolutionarily possible solutions vertebrates needed one that was compatible with a sufficiently low tumor rate to accommodate larger body sizes and longer life spans.
Vertebrates have evolved effective immune systems, and even invertebrates possess innate immune systems. In addition to limiting pathogen infections, these systems can target malignant cells for destruction, thus contributing to tumor suppression. Tissue organization may also contribute to the “peer pressure” exerted on malignant cells, whereby a normal microenvironment can suppress the malignant phenotype (18, 19). In addition, a growing tumor alters its microenvironment, and thus the adaptive landscape. As described by Gatenby and Gillies (20), alterations in the microenvironment create new barriers to continued cancer evolution, and these barriers are typically effective, preventing further tumor development. Such barriers include oxygen and nutrient limitation that require the acquisition of mutations that promote glycolysis and/or increase the blood supply. These barriers could account for the ubiquitous observation of benign but largely innocuous growths in tissues in the elderly (15): most oncogenically initiated clones are dead ends. Thus, the reason that most of us survive to our Golden Years without developing cancer is because evolved tumor suppressive mechanisms are so good at impeding tumor development.
Robust Fitness and Tumor Suppression
how the evolution of well-adapted stem cell pools has conferred resistance to oncogenic mutations.
Cancer progression occurs by a process of somatic cell evolution, whereby a single normal cell gives rise to a highly complex tumor, consisting of populations of cells harboring many genetic and epigenetic alterations. This evolutionary process is driven by two major forces: diversification of heritable types through acquisition of genetic and epigenetic changes, and selection for cells that harbor mutations increasing their fitness. For this discussion it is important to distinguish organismal fitness (a measure of the ability of individuals to pass their genotype to subsequent generations) from somatic cell fitness (a measure of the ability of stem/progenitor cells to pass their epigenotype/genotype to subsequent generations of cells).
From an evolutionary perspective, initiating oncogenic mutations will typically need to provide a positive fitness gain in order to be fixed and trigger clonal expansion (21). We have argued that this fitness gain is relative to other cells in a tissue, and for the initial stages of tumorigenesis these other cells are the normal cells competing for the same niches as the oncogenically-initiated cells (22, 23). Therefore, understanding the initial stages of tumor evolution requires careful consideration of the change in cell fitness conferred by an oncogenic mutation relative to the fitness of other cells in the same progenitor cell population.
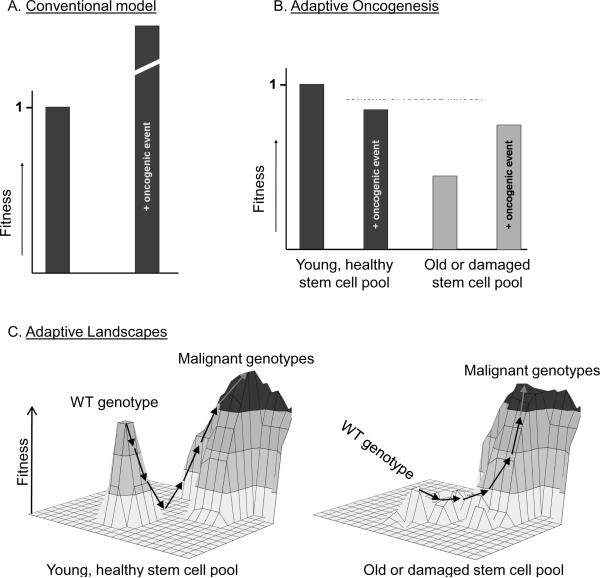
The predominant paradigm is that oncogenic events generally improve cellular fitness (Fig. 2A), leading to clonal selection (24). Thus, the occurrence of oncogenic mutations is believed to limit cancer initiation and progression. Instead, I will argue that long-lived multicellular organisms have evolved highly fit stem cell populations, not only as a means of efficiently maintaining a tissue, but also because a high degree of fitness of a cell population will oppose somatic cell evolution (22, 23). Like animal populations well adapted to their environments, stabilizing selection should limit fixation of changes that improve fitness in a highly fit population of stem cells (Fig 2B). Highly effective competition in a young, healthy stem cell population should serve to maintain the status quo, preventing somatic cell evolution. Effective competition will also facilitate the elimination of the occasional damaged cell from the stem cell pool, helping to maintain tissue fitness.
Figure 2. Contrasting the Conventional Model for cancer initiation with Adaptive Oncogenesis.
A. For the Conventional Model, oncogenic events are generally advantageous, and thus cancer initiation is limited by the incidence of these events. B. For the Adaptive Oncogenesis Model, phenotype-altering epigenetic or genetic changes will rarely be advantageous within a population of healthy, well-adapted stem and progenitor cells. But following aging or damage-induced reductions in stem/progenitor pool fitness, oncogenic events that improve fitness should provide selective advantages. C. Proposed adaptive landscapes of stem cell populations within a healthy young individual or within an aged or carcinogen-exposed individual. The X and Y axes represent the potential genetic and epigenetic diversity. The Z axis represents fitness. Small arrows indicate mutational or epigenetic changes that could lead to cancer, which come with a high cost for cellular fitness for a young healthy stem cell population, but much less of a cost within a damaged stem cell pool.
So why do we develop cancer? It is well known that age is the greatest risk factor for cancer in mammals (7, 24), with the incidence of most human cancers rising exponentially with age [see (11, 15) for evolutionary perspectives on childhood cancers]. In stem and progenitor cell pools damaged by aging or other insults, cellular fitness will be reduced and the adaptive landscape dramatically altered (Fig 2B and 2C). We have proposed that age-related or carcinogen-induced reductions in the fitness of stem cell pools, due to accumulation of genomic damage as well as to alterations in the microenvironment, should promote selection for adaptive mutations and epigenetic events (Fig 3A). This Adaptive Oncogenesis hypothesis is supported by studies using mouse models for the hematopoietic system (25–29).
Figure 3. Maintenance of fit somatic stem cells limits tumor development.

A. When mutations and epigenetic changes (heritable damage) accumulate to the point where the buffering capacity of the stem cell pool is exhausted (denoted by vertical dotted line), the fitness of the stem cell population will begin to decline. Declining fitness will then increase selective pressure for adaptive oncogenic mutations that in turn promote cancer initiation. B. Proposed model for how vertebrates with large differences in somatic cell numbers (103–107 fold) and lifespans (10–100 fold) similarly avoid cancer through reproductive years.
Still, just as evolution of species is driven by mutation and selection (and genetic drift), this model does not negate the importance of genetic and epigenetic diversity, which increase with age and following carcinogenic exposure, in providing fuel for selection. Moreover, it is important to emphasize that the decline of stem cell fitness with age or other insults will not just reflect cell intrinsic damage, but will also involve microenvironmental alterations (indirectly reducing the fitness of stem cells) that could promote oncogenic adaptation (30, 31). Indeed, Bagby and colleagues have proposed an adaptive model to explain the association of bone marrow failure syndromes (such as fanconi anemia) with leukemia: increased selection for oncogenic mutations that are adaptive within defective/poorly fit hematopoietic progenitor cells, resulting from both cell-intrinsic and microenvironmental effects (30). Studies using mouse models of fanconi anemia provide experimental support for this model (32–34). Thus, in addition to reducing the intrinsic fitness of stem cells, heritable damage associated with aging or other insults will further change the fitness landscape by altering niches, creating new opportunities for adaptation (new fitness peaks that may facilitate transitions to malignancy; Fig 2C).
There are obvious parallels between this model of “status quo” maintenance of stem cell pools and evolutionary ideas on species stasis, a critical component of the Punctuated Equilibrium Theory advanced by Eldredge and Gould (35). As Eldredge wrote in Reinventing Darwin, “species, and the ecosystems that their component organisms staff, are tenacious. They `work' perfectly well and, once entrenched, are unlikely either to change or to be displaced by newly evolved taxa -unless and until extinction knocks ecosystems off their tracks” (36). And as Wright noted in 1931, gene frequencies “tend to remain constant in the absence of disturbing forces” (37). I would argue that the same holds true for stem cells, with similar pressures preventing the fixation of oncogenic mutations, at least until substantial change happens (such as irradiation, which could mirror a catastrophic event such as occurred at the K–T boundary).
Of Mice and Men
why alterations in programs of stem cell fitness maintenance may best explain differential tumor suppression for different vertebrates.
Let's step back and consider the issue of cancer rates in mice and humans. It is striking that humans manage largely to avoid cancer until after 40 years of age, despite >1000-fold greater cellularity and >20-fold longer lifespans than mice (which die of cancer at similar percentages in their short 2–4 year maximal lifespans). What mechanism(s) best explains species-specific differences in cancer occurrence? Notably, humans and mice appear to have similar numbers of tumor suppressor genes, which possess apparently similar functions (9). It is also not clear that humans have evolved a superior immune system relative to mice, or that humans have more effective intrinsic tumor suppressive mechanisms or architectural/hierarchical constraints that limit somatic cell evolution. Perhaps most surprisingly, given the prevalent view that the acquisition of oncogenic mutations limits cancer development, mouse and human cells do not appear to differ appreciably in mutation rates (9, 38). It is notable that human primary cells do appear harder to transform than primary mouse cells, in that there are differences in the numbers of oncogenic hits required for cell transformation in vitro (39). But evidence that this relative resistance to in vitro transformation extends to physiological contexts is lacking (9). Thus, none of these tumor suppressor functions have been shown to be substantially more robust in humans than mice.
Importantly, cellular fitness, which declines in both mice and humans roughly in the latter halves of their potential life spans, does inversely correlate with cancer incidence in both species (Fig 3A). While a larger animal with more stem cells per tissue presents a bigger target size for oncogenic mutations, the ability of a fit stem cell pool to impede somatic cell evolution via stabilizing selection should be relatively size-independent (Fig 3B). Moreover, larger stem cell population size should better limit mutation fixation by drift and buffer against fitness reductions (with fit stem cells replacing damaged ones). Basically, natural selection ensured maintenance of somatic fitness for as long as it was beneficial for species propagation: in addition to ensuring adequate tissue function and prevention of many age-associated diseases, maintaining fit stem cells should greatly limit cancer development through reproductive years.
If we revisit Fig 1, we can picture grossly similarly shaped curves for humans and mice, but with a different scale for age. Natural selection should “tune” somatic cell fitness maintenance towards optimization of organismal fitness. There is an energetic cost for increased somatic maintenance (1), and this investment, which should delay the onset of aging-associated decline and diseases (including cancer), would only provide a selective advantage to an organism if the change increased the probability of passing genes to future generations. As a side-note, it is interesting to consider whether the sustained maintenance of fit somatic stem cells in the “immortal” hydra (40) contributes to the apparent absence of either aging or malignant growths in this simple metazoan.
So what are the implications of this model of tumor suppression by maintenance of stem cell fitness? For one, an increase in body size or life-span may not require selection of new tumor suppressor mechanisms, but simply a shift in energy allocation towards more prolonged somatic tissue maintenance. In this regard, it is interesting that closely related species or even members of the same species can exhibit different rates of aging and maximum life-spans (41, 42), often dependent on predation rates, suggesting that alterations of somatic maintenance programs can maximize the effective allocation of energy as an investment towards lifespans appropriate for optimal reproduction. And delayed cancer development should result from improved/prolonged somatic maintenance: the same tuning can provide for both delayed aging and delayed cancer.
The fitness model can perhaps also explain the “paradox of the blue whale” or “Peto's paradox” (9, 43): how could such a large animal so effectively avoid cancer (Fig 3B)? Similarly, elephants and tortoises exhibit very long lives with apparently low cancer rates (15). But if we consider tumor suppression as dependent on maintenance of somatic tissue, then the same evolutionary alterations that facilitated longer lives and bigger bodies for these species (justified by longer periods of reproductive success) would at the same time serve to limit cancer. Thus, a key open question, both for cancer and aging biology, is the identity of the mechanism(s) that are altered during evolution to modulate programs of somatic cell maintenance.
Conclusion
It has become increasingly clear that understanding cancer, both at tissue and organismal levels, demands an evolutionary perspective. The evolution of larger bodies, longer lives, and adult tissue maintenance necessitated the acquisition of potent tumor suppressive mechanisms, which operate at levels of individual cells, tissue organization and the whole body to limit malignant growth. I have proposed that one potent mechanism to limit somatic cell evolution (and thus cancer) is the maintenance of highly fit stem cell populations. I further propose that alterations in programs of somatic cell maintenance could account for the vastly different rates (age of onset, with consideration of body size) of cancer in different animals. Different vertebrate species appear to have evolved a common strategy: avoidance of cancer and other consequences of aging by maintaining stem cell fitness to the extent that maximizes organismal fitness.
Acknowledgements
These studies were supported by grants from the National Institutes of Health (R21-CA137262) and the Leukemia Lymphoma Society. I thank Chris Hittinger, Mark Johnston, Carlo Maley, Andrii Rozhok and Andriy Marusyk for their critical comments, and Gayle DeGregori for artwork in Figure 3B.
References
- 1.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–47. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Medawar P. An unsolved problem of biology. H.K.Lewis; London: 1952. [Google Scholar]
- 3.Williams GC. Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 4.Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier ME, Mitros T, et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 2010;466:720–6. doi: 10.1038/nature09201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–15. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 6.Domazet-Loso T, Tautz D. Phylostratigraphic tracking of cancer genes suggests a link to the emergence of multicellularity in metazoa. BMC Biol. 2010;8:66. doi: 10.1186/1741-7007-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DePinho RA. The age of cancer. Nature. 2000;408:248–54. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 8.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 9.Leroi AM, Koufopanou V, Burt A. Cancer selection. Nat Rev Cancer. 2003;3:226–31. doi: 10.1038/nrc1016. [DOI] [PubMed] [Google Scholar]
- 10.Frank SA, Nowak MA. Problems of somatic mutation and cancer. Bioessays. 2004;26:291–9. doi: 10.1002/bies.20000. [DOI] [PubMed] [Google Scholar]
- 11.Crespi B, Summers K. Evolutionary biology of cancer. Trends Ecol Evol. 2005;20:545–52. doi: 10.1016/j.tree.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Galis F, Metz JA. Anti-cancer selection as a source of developmental and evolutionary constraints. Bioessays. 2003;25:1035–9. doi: 10.1002/bies.10366. [DOI] [PubMed] [Google Scholar]
- 13.Kavanagh KD. Perspective: embedded molecular switches, anticancer selection, and effects on ontogenetic rates: a hypothesis of developmental constraint on morphogenesis and evolution. Evolution. 2003;57:939–48. doi: 10.1111/j.0014-3820.2003.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 14.Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924–35. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- 15.Greaves M. Darwinian medicine: a case for cancer. Nat Rev Cancer. 2007;7:213–21. doi: 10.1038/nrc2071. [DOI] [PubMed] [Google Scholar]
- 16.Gatenby RA, Gillies RJ, Brown JS. Evolutionary dynamics of cancer prevention. Nat Rev Cancer. 2010;10:526–7. doi: 10.1038/nrc2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepper JW, Sprouffske K, Maley CC. Animal cell differentiation patterns suppress somatic evolution. PLoS Comput Biol. 2007;3:e250. doi: 10.1371/journal.pcbi.0030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–46. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci USA. 1975;72:3585–9. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 21.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–8. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 22.Marusyk A, DeGregori J. Declining cellular fitness with age promotes cancer initiation by selecting for adaptive oncogenic mutations. Biochim Biophys Acta. 2008;1785:1–11. doi: 10.1016/j.bbcan.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleenor CJ, Marusyk A, DeGregori J. Ionizing radiation and hematopoietic malignancies: altering the adaptive landscape. Cell Cycle. 2010;9:3005–11. doi: 10.4161/cc.9.15.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg RA. The Biology of Cancer. Garland Science; New York: 2007. Chapter 11. [Google Scholar]
- 25.Bilousova G, Marusyk A, Porter CC, Cardiff RD, DeGregori J. Impaired DNA Replication within Progenitor Cell Pools Promotes Leukemogenesis. PLoS Biology. 2005;3:e401. doi: 10.1371/journal.pbio.0030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marusyk A, Casas-Selves M, Henry CJ, Zaberezhnyy V, Klawitter J, Christians U, et al. Irradiation Alters Selection for Oncogenic Mutations in Hematopoietic Progenitors. Cancer Res. 2009;69:7262–9. doi: 10.1158/0008-5472.CAN-09-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marusyk A, Porter CC, Zaberezhnyy V, DeGregori J. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol. 2010;8:e1000324. doi: 10.1371/journal.pbio.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bondar T, Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell. 2010;6:309–22. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry CJ, Marusyk A, Zaberezhnyy V, Adane B, DeGregori J. Declining lymphoid progenitor fitness promotes aging-associated leukemogenesis. Proc Natl Acad Sci U S A. 2010;107:21713–8. doi: 10.1073/pnas.1005486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagby GC, Fleischman AG. The stem cell fitness landscape and pathways of molecular leukemogenesis. Front Biosci (Schol Ed) 2011;3:487–500. doi: 10.2741/s167. [DOI] [PubMed] [Google Scholar]
- 31.Laconi E, Doratiotto S, Vineis P. The microenvironments of multistage carcinogenesis. Semin Cancer Biol. 2008;18:322–9. doi: 10.1016/j.semcancer.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Sejas DP, Zhang X, Qiu Y, Nattamai KJ, Rani R, et al. TNF-alpha induces leukemic clonal evolution ex vivo in Fanconi anemia group C murine stem cells. J Clin Invest. 2007;117:3283–95. doi: 10.1172/JCI31772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milsom MD, Schiedlmeier B, Bailey J, Kim MO, Li D, Jansen M, et al. Ectopic HOXB4 overcomes the inhibitory effect of tumor necrosis factor-{alpha} on Fanconi anemia hematopoietic stem and progenitor cells. Blood. 2009;113:5111–20. doi: 10.1182/blood-2008-09-180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Le Beau MM, Ciccone S, Yang FC, Freie B, Chen S, et al. Ex vivo culture of Fancc-/-stem/progenitor cells predisposes cells to undergo apoptosis, and surviving stem/progenitor cells display cytogenetic abnormalities and an increased risk of malignancy. Blood. 2005;105:3465–71. doi: 10.1182/blood-2004-06-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gould SJ, Eldredge N. Punctuated equilibria: the tempo and mode of evolution reconsidered. Paleobiology. 1977;3:115–51. [Google Scholar]
- 36.Eldredge N. Reinventing Darwin : the great debate at the high table of evolutionary theory. Wiley; New York: 1995. [Google Scholar]
- 37.Wright S. Evolution in Mendelian Populations. Genetics. 1931;16:97–159. doi: 10.1093/genetics/16.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albertini RJ, Nicklas JA, O'Neill JP, Robison SH. In vivo somatic mutations in humans: measurement and analysis. Annu Rev Genet. 1990;24:305–26. doi: 10.1146/annurev.ge.24.120190.001513. [DOI] [PubMed] [Google Scholar]
- 39.Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer. 2002;2:331–41. doi: 10.1038/nrc795. [DOI] [PubMed] [Google Scholar]
- 40.Bosch TC. Hydra and the evolution of stem cells. Bioessays. 2009;31:478–86. doi: 10.1002/bies.200800183. [DOI] [PubMed] [Google Scholar]
- 41.Selman C, Speakman JR. Theories of Biological Aging: Disposable Soma. [cited 2010 Dec 19];Encyclopedia of Aging. 2002 Available from: http://www.encyclopedia.com. [Google Scholar]
- 42.Austad SN. Retarded senescence in an insular population of Virginia opossums Zoology. 1993;229:695–708. [Google Scholar]
- 43.Caulin AF, Maley CC. Peto's Paradox: evolution's prescription for cancer prevention. Trends Ecol Evol. doi: 10.1016/j.tree.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]