Abstract
Purpose
This post hoc analysis of the Medical Therapy of Prostatic Symptoms (MTOPS) trial examined the effect of finasteride alone compared to placebo on clinical progression of benign prostatic hyperplasia (BPH) in men with baseline prostate volume (PV) <30 mL and ≥30 mL.
Materials and Methods
Men were randomized to placebo (n=737), doxazosin alone (4 to 8 mg) (n=756), finasteride alone (5 mg) (n=768), or doxazosin plus finasteride (n=786) (average duration of follow-up was 4.5 yrs); ~50% of patients had a baseline PV ≥30 mL. The present analysis was based on the finasteride alone and placebo arms only and included patients for whom baseline and end of study data were available. We examined the effect of treatment on the cumulative percentage of men who did not experience clinical progression of BPH by study end.
Results
In men with baseline PV ≥30 mL, treatment with finasteride produced a significant (p<0.001) increase relative to placebo in the cumulative percentage of patients who did not experience clinical progression of BPH (finasteride, 88.1%, versus placebo, 77.8%). There was no significant (p=0.441) between-group difference in men with baseline PV <30 mL (91.4% versus 89.1%, respectively).
Conclusions
Long-term treatment with finasteride led to a significant beneficial effect compared to placebo on clinical progression of BPH in LUTS patients with enlarged prostates (baseline PV ≥30 mL). Finasteride had no significant effect, compared to placebo on clinical progression of BPH in LUTS patients with smaller prostates (baseline PV <30 mL).
Keywords: benign prostatic hyperplasia, lower urinary tract symptoms, finasteride, total prostate volume
INTRODUCTION
The Medical Therapy of Prostatic Symptoms (MTOPS) trial is the largest and longest placebo-controlled study to assess the effect of medical (drug) therapy on the clinical progression of benign prostatic hyperplasia (BPH).1,2 In that trial, the risk of clinical progression of BPH was significantly reduced by both finasteride (34% relative risk reduction) and doxazosin (39% relative risk reduction) alone compared to placebo; however, the two drugs combined resulted in a superior placebo-corrected risk reduction (66% relative risk reduction) compared to that for either drug alone.1 Finasteride is an inhibitor of type 2 5α-reductase (5AR), which is the predominant 5AR isozyme in the prostate that catalyzes the reduction of testosterone to the more potent androgen, dihydrotestosterone (DHT). Treatment with finasteride results in a marked decrease in intraprostatic DHT, leading to a reduction in prostate volume (PV), improvement in urinary symptoms, and a reduction in the risk of serious BPH-related outcomes, including acute urinary retention and the need for BPH-related surgery.3,4 The beneficial effects of finasteride are generally believed to be restricted to men with enlarged prostates.4 We assessed the long-term effects of finasteride alone compared to placebo on urinary symptoms and clinical progression of BPH in men enrolled in the MTOPS trial subgrouped by baseline PV. We defined men with a baseline prostate volume of <30 mL as having smaller prostates and those with a volume ≥30 ml as having enlarged prostates.
MATERIALS AND METHODS
Study Design and Patient Selection
The design and major outcomes of the MTOPS Study have been reported previously.1 ,2 Briefly, men at least 50 years of age with an American Urological Association Symptom Index (AUA SI) score of 8 to 30 and a maximum urinary flow rate (Qmax) of 4 to 15 mL/s with a voided volume of at least 125 mL were eligible for the trial. A total of 3047 men were randomized 1:1:1:1 to either placebo, doxazosin (4-8 mg), finasteride (5 mg), or combination therapy with doxazosin and finasteride. The present analysis was based on the finasteride alone and placebo arms only and included patients for whom baseline and end of study data were available. The primary outcome, overall clinical progression of BPH, was defined as the first occurrence of either an increase from baseline of at least 4 points in the AUA SI score (confirmed within four weeks), acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection. Secondary outcomes included change from baseline to end of study in the AUA SI score, Qmax, and post-void residual volume (PVR). PV was measured by transrectal ultrasound (TRUS) in all men at baseline and at the end of Year 5 or at the end-of-study follow-up, whichever came first. The average duration of follow-up was 4.5 years.
Statistical Analysis
We first performed an intention-to-treat (ITT) analysis which included all randomized men with evaluable PV data. Mean (median for PVR) change from baseline to study end in AUA SI score, Qmax, PVR, and PV as well as the cumulative percentage (95% CI) of patients who did not have clinical progression of BPH by study end was examined in the placebo and finasteride treatment groups in men with baseline PV <30 mL and compared with those with PV ≥30 mL. We also performed a per-protocol analysis to assess the consistency of the results of our ITT analysis for AUA SI, Qmax, PVR, and PV. The per protocol analysis excluded men who had either been treated with open-label medical therapy for BPH or who had undergone invasive therapy for BPH prior to the end-of-study TRUS measurement. We used a one-way analysis of variance to compare finasteride with placebo with respect to the change from baseline for AUA SI score, Qmax, and PV separately for men with baseline PV <30 ml and those with baseline PV ≥30 ml. For PVR, the Wilcoxon rank sum test was used to compare treatment arms with respect to change from baseline. The log-rank test was used to compare the treatment arms with respect to time to clinical progression of BPH.
RESULTS
Baseline and end of study PV measurements were available for a total of 1140 men from the finasteride and placebo treatment groups (Table 1). Among these men, baseline PV ranged from 8.8 mL to 181.0 mL, with 49% having a baseline PV <30 mL. Within each PV subgroup (baseline PV <30 mL and ≥30 mL), men randomized to either placebo or finasteride were generally similar with respect to baseline demographic and clinical characteristics (Table 1). Men with baseline PV ≥30 mL were slightly older and had higher mean PVR and PSA levels compared to men with baseline PV <30 mL.
Table 1. Baseline and disease characteristics, presented by baseline prostate volume (ITT patient population).
| Parameter | Baseline Prostate Volume <30 mL | Baseline Prostate Volume ≥30 mL | ||
|---|---|---|---|---|
|
|
||||
| Placebo N=276 |
Finasteride N=281 |
Placebo N=288 |
Finasteride N=295 |
|
| Mean ± SD Age (yrs) | 60.3 ± 7.1 | 60.7 ± 7.0 | 64.1 ± 6.9 | 63.9 ± 7.1 |
| Mean ± SD AUA SI | 16.2 ± 5.7 | 17.2 ± 5.8 | 16.8 ± 6.0 | 17.1 ± 5.8 |
| Mean ± SD Qmax (mL/sec) | 10.5 ± 2.8 | 10.6 ± 2.5 | 10.4 ± 2.8 | 10.5 ± 2.7 |
| Median (range) PVR (mL) | 38.0 (0-399) | 36.0 (0-411) | 52.0 (0-535) | 40.0 (0-552) |
| Mean ± SD PV (mL) | 21.8 ± 5.1 | 22.3 ± 4.9 | 47.2 ± 17.4 | 50.4 ± 21.1 |
| PV range (mL) | 8.8-29.9 | 9.3-29.9 | 30.0-181.0 | 30.0-170.3 |
| Mean ± SD PSA (ng/mL) | 1.3 ± 1.1 | 1.3 ± 1.2 | 3.1 ± 2.3 | 3.3 ± 2.3 |
AUA SI=AUA Symptom Index score; Qmax=maximum urinary flow rate; PVR=post-void residual volume; PV=prostate volume; PSA=prostate-specific antigen
Change in AUA SI Score
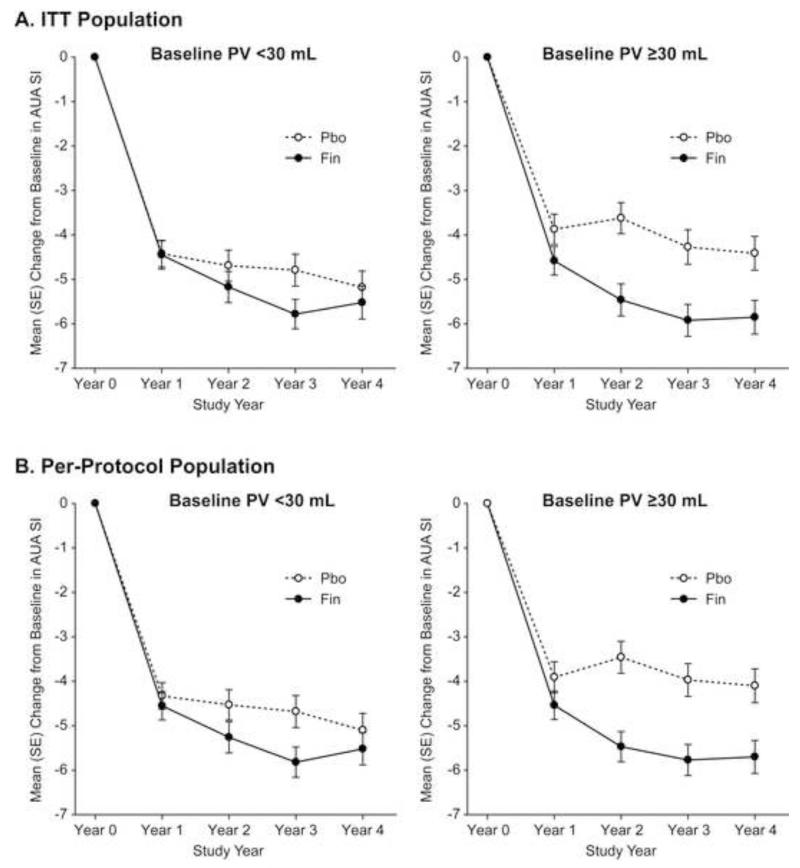
In the ITT analysis, treatment with finasteride led to a significant (p=0.045) mean decrease from baseline to study end in the AUA SI score relative to placebo in men with baseline PV ≥30 mL (mean decrease of −5.69 versus −4.65 in finasteride and placebo groups, respectively), whereas there was no significant between-group difference in the mean change from baseline in AUA SI score in men with baseline PV <30 mL (mean decrease of −6.01 versus −5.06 in finasteride and placebo groups, respectively) (Table 2; Figure 1A). The AUA SI score results for the per-protocol analysis were generally consistent with those for the ITT analysis (Table 2; Figure 1B), with significant between-group differences achieved for both PV cohorts. The largest improvement with finasteride observed in the per-protocol analysis occurred at Year 2 in the baseline PV ≥30 mL cohort, with a mean between-group difference of approximately 2 AUA SI score units (Figure 1B); this improvement appeared to be generally sustained in Years 3 and 4.
Table 2. Effect of finasteride versus placebo on change from baseline in urinary symptoms and prostate volume at study end in patients with baseline prostate volume <30 mL and those with baseline prostate volume ≥30 mL (ITT and per-protocol population).
| Parameter | Baseline Prostate Volume <30 mL | Baseline Prostate Volume ≥30 mL | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Finasteride | Placebo | Finasteride | |||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | |
| Intention-to-treat population | ||||||||
| Change from baseline in AUA SI p-value for comparing finasteride vs placebo |
276 | −5.06 ± 5.78 | 281 | −6.01 ± 5.78 | 288 | −4.65 ± 6.17 | 295 | −5.69 ± 6.40 |
| p= 0.054 | p= 0.045 | |||||||
| Change from baseline in Qmax (mL/sec) p-value for comparing finasteride vs placebo |
273 | 3.06 ± 5.96 | 277 | 3.67 ± 6.97 | 282 | 1.78 ± 5.83 | 290 | 3.31 ± 5.74 |
| p= 0.268 | p= 0.002 | |||||||
| Change from baseline in PVR (mL)1 p-value for comparing finasteride vs placebo |
271 | −3.0 (−275, 371) | 280 | −1.5 (−221, 262) |
282 | −0.50 (−458, 641) | 289 | −3.0 (−503, 411) |
| p= 0.992 | p= 0.192 | |||||||
| Change from baseline in PV (mL) p-value for comparing finasteride vs placebo |
276 | 7.19 ± 16.80 | 281 | 0.28 ± 7.98 | 288 | 9.38 ± 17.00 | 295 | −5.79 ± 18.35 |
| p<0.001 | p<0.001 | |||||||
| Per-protocol population | ||||||||
| Change from baseline in AUA SI p-value for comparing finasteride vs placebo |
266 | −4.80 ± 5.62 | 276 | −5.99 ± 5.73 | 261 | −4.16 ± 5.92 | 287 | −5.52 ± 6.13 |
| p= 0.015 | p= 0.008 | |||||||
| Change from baseline in Qmax (mL/sec) p-value for comparing finasteride vs placebo |
263 | 2.88 ± 5.89 | 272 | 3.75 ± 6.92 | 257 | 1.52 ± 5.62 | 284 | 3.19 ± 5.69 |
| p= 0.119 | p<0.001 | |||||||
| Change from baseline in PVR (mL)1 p-value for comparing finasteride vs placebo |
261 | −3.0 (−275, 371) | 275 | 0 (−221, 262) | 256 | 0 (−421, 641) | 283 | −3.0 (−503, 411) |
| p=0.888 | p=0.055 | |||||||
| Change from baseline in PV (mL) p-value for comparing finasteride vs placebo |
266 | 7.35 ± 16.99 | 276 | 0.32± 8.02 | 261 | 11.38 ± 15.00 | 287 | −5.25 ± 17.63 |
| p<0.001 | p<0.001 | |||||||
Median (range) change from baseline shown for PVR
AUA SI=AUA Symptom Index score; Qmax=maximum urinary flow rate; PVR=post-void residual volume; PV= prostate volume
Figure 1.
Mean (SE) change from baseline in AUA Symptom Index score (AUA SI) over time in men with baseline prostate volume (PV) <30 mL versus ≥30 mL for (A) the intention-to-treat (ITT) population and (B) the per-protocol population (excluded patients who had been crossed over to open-label medical therapy or who had invasive therapy prior to the end-of-study transrectal ultrasound measurement) in the Medical Therapy of Prostatic Symptoms (MTOPS) trial.
Change in Qmax
In the ITT analysis, treatment with finasteride led to a significant (p=0.002) mean increase from baseline to study end in Qmax relative to placebo in men with baseline PV ≥30 mL (mean increase of 3.31 mL/sec and 1.78 mL/sec in finasteride and placebo groups, respectively), whereas there was no significant between-group difference in the mean change from baseline in Qmax in men with baseline PV <30 mL (mean increase of 3.67 mL/sec and 3.06 mL/sec in finasteride and placebo groups, respectively) (Table 2). The Qmax results for the per-protocol analysis were generally consistent with those for the ITT analysis (Table 2).
Change in PVR
In the ITT analysis, treatment with finasteride and placebo led to median reductions from baseline to study end in PVR of −3.0 mL and −0.5 mL, respectively, in men with baseline PV ≥30 mL and −1.5 mL versus −3.0 mL, respectively, in men with baseline PV <30 mL, although, the between-group differences were not statistically significant in either of the two baseline PV cohorts (Table 2). The PVR results for the per-protocol analysis were generally consistent with those for the ITT analysis (Table 2).
Change in PV
In the ITT analysis, treatment with finasteride led to a significantly (p<0.001) greater improvement from baseline to study end in PV in men with baseline PV ≥30 mL (mean decrease in PV of −5.79 mL with finasteride compared to a mean increase of 9.38 mL with placebo) and in men with baseline PV <30 mL (mean increase in PV of 0.28 mL with finasteride versus a mean increase of 7.19 mL with placebo) (Table 2). The results from the per-protocol analysis were consistent with those for the ITT analysis (Table 2).
Clinical Progression of BPH
Treatment with finasteride led to a significant (p<0.001) increase relative to placebo in the cumulative percentage of men who did not meet criteria for clinical progression of BPH in men with baseline PV ≥30 mL (finasteride, 88.1%, versus placebo, 77.8%), whereas no significant between-group difference was observed in men with baseline PV <30 mL (91.4% versus 89.1%) (Table 3).
Table 3. Effect of finasteride versus placebo on cumulative percentage at study end of men who did not develop clinical progression of BPH in patients with baseline prostate volume <30 mL and those with baseline prostate volume ≥30 mL.
| Baseline Prostate Volume <30 mL | Baseline Prostate Volume ≥30 mL | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Finasteride | Placebo | Finasteride | |||||
| N | Cumulative percentage (95% CI) |
N | Cumulative percentage (95% CI) |
N | Cumulative percentage (95% CI) |
N | Cumulative percentage (95% CI) |
|
| Men without clinical progression of BPH p-value for comparing finasteride and placebo |
276 | 89.1 (84.8, 92.3) | 280 | 91.4 (87.5, 94.2) | 288 | 77.8 (72.5, 82.1) | 295 | 88.1 (83.9, 91.3) |
| p=0.367 | p<0.001 | |||||||
DISCUSSION
5AR inhibitors are generally recommended for use in patients with symptomatic BPH with enlarged prostates.4 The wide distribution of baseline PV (8.8 – 181.0 mL) for men enrolled in the MTOPS study provided the opportunity to examine the long-term (≥4 years) effects of the 5AR inhibitor finasteride alone compared to placebo on urinary symptoms and clinical progression of BPH in patients with smaller and larger prostates. Although we did not define the volume criterion for an enlarged prostate a priori, our categorization of PV ≥30 mL as enlarged is consistent with currently accepted cutpoints.
We observed in an ITT analysis that treatment with finasteride led to a statistically significant reduction compared to placebo in clinical progression of BPH in LUTS patients with enlarged prostates (baseline PV ≥30 mL), whereas treatment with finasteride did not demonstrate a significant effect relative to placebo on clinical progression of BPH in patients with smaller prostates (baseline PV <30 mL). Additionally, the ITT analysis showed that finasteride produced significant improvement compared to placebo in AUA SI score and Qmax in men with enlarged prostates, but not in men with smaller prostates. In those men who had not either been treated with open-label medical therapy for BPH or had undergone invasive therapy for BPH prior to the end-of-study TRUS measurement, finasteride led to an improvement in AUA SI relative to placebo by Year 2 in the baseline PV ≥30 mL cohort of approximately 2 units, with this improvement being generally sustained through Year 4 of the study (Figure 1B). The larger improvement in PV seen in finasteride-treated patients with baseline PV ≥30 mL (5.79 mL decrease) than in those with baseline PV <30 mL (0.28 mL increase) may have resulted in relieving symptomatic prostatic obstruction, which may have contributed to the added benefits of finasteride on urinary symptoms and clinical progression of BPH in these patients.
Finasteride is approved for the treatment of symptoms of BPH in men with an enlarged prostate based on large, long-term studies that enrolled men over a wide range of PV commonly observed in the clinical setting (<25 mL to ≥40mL).1,3-7 About one-half of the men enrolled in MTOPS had enlarged prostates at baseline as defined by PV ≥30 mL. Whether a similar proportion of men with enlarged prostates as seen in the MTOPS study present to physicians with bothersome symptoms of LUTS associated with BPH is uncertain. Given the significantly lower rate of clinical progression of BPH observed in men with PV ≥30 mL who were treated with finasteride compared to placebo, symptomatic BPH patients with enlarged prostates, and their treating physicians, should consider the benefits observed in this study from treatment with finasteride when determining treatment choice.
There were a number of limitations of our analysis. While the data analysis plan for the MTOPS study prespecified examination of the effect of PV on outcomes, the cutpoints for these subgroups were not prespecified; however, the use of 30 mL or greater to define an enlarged prostate is consistent with current practice.8 In addition, we performed a subgroup analysis, which may be subject to bias.9 There were several strengths of our study, including the large sample size, the wide distribution of baseline PV, and long-term follow-up.
CONCLUSIONS
In this post-hoc analysis of MTOPS trial data, long-term (≥4 years) treatment with finasteride led to a significant beneficial effect compared to placebo on clinical progression of BPH in LUTS patients with enlarged prostates (baseline PV ≥30 mL). Finasteride had no significant effect, compared to placebo on clinical progression of BPH in LUTS patients with smaller prostates (baseline PV <30 mL).
Acknowledgments
The authors wish to acknowledge the contributions of the many investigators, study coordinators, and patients who contributed to the MTOPS trial. MTOPS was supported by the following cooperative agreements from the National Institute of Diabetes, Digestive and Kidney Diseases: U01 DK49977, U01 DK46416, U01 DK41418, U01 DK46429, U01 DK46431, U01 DK46437, U01 DK46437, U01 DK46468, U01 DK46472, U01 DK49880, U01 DK 49912, U01 DK49921, U01 DK49951, U01 DK49954, U01 DK49960, U01 DK49961, U01 DK49963, U01 DK49964, U01 DK49971, U01 DK49980, as well as by the National Center for Minority Health and Health Disparities, NIH. Financial support and drug products for MTOPS were also provided by Merck and Pfizer.
List of Abbreviations
- AUA SI
American Urological Association symptom index
- BPH
benign prostatic hyperplasia
- LUTS
lower urinary tract symptoms
- MTOPS
Medical Therapy of Prostatic Symptoms
- PSA
prostate-specific antigen
- PVR
post-void residual volume
- Qmax
maximum urinary flow rate
- PV
prostate volume
- TRUS
transrectal ultrasound
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Jr., Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. New Engl J Med. 2003;349:2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 2.Bautista OM, Kusek JW, Nyberg LM, McConnell JD, Bain RP, Miller G, et al. Study design of the Medical Therapy of Prostatic Symptoms (MTOPS) trial. Control Clin Trials. 2003;24:224–243. doi: 10.1016/s0197-2456(02)00263-5. [DOI] [PubMed] [Google Scholar]
- 3.Proscar product label. 2010 Mar; http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020180s033lbl.pdf.
- 4.AUA Practice Guidelines Committee AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530–547. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan SA, McConnell JD, Roehrborn CG, Meehan AG, Lee MW, Noble WR, Kusek JW, Nyberg LM, Jr., for the Medical Therapy of Prostatic Symptoms (MTOPS) Research Group Combination therapy with doxazosin and finasteride led to a significantly greater reduction in the risk of clinical progression of BPH compared to either drug alone in LUTS patients with a baseline total prostate volume of ≥25 ml. J Urol. 2006;175:217–220. doi: 10.1016/S0022-5347(05)00041-8. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan SA, Roehrborn CG, McConnell JD, Meehan A, Suryawanshi S, Rotonda JR, Kusek JW, Nyberg LM, Jr., Medical Therapy of Prostatic Symptoms Research Group Long-term treatment with finasteride results in a clinically significant reduction in total prostate volume compared to placebo over the full range of baseline prostate sizes in men enrolled in the MTOPS trial. J Urol. 2008;180:1030–1033. doi: 10.1016/j.juro.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 7.McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T, Guess HA, Lieber MM. Treatment for benign prostatic hyperplasia among community dwelling men: The Olmsted County study of urinary symptoms and health status. J Urol. 1999;162:1301–1306. [PubMed] [Google Scholar]
- 9.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine — reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]