Summary
Objectives
Recent large cholera outbreaks highlight the need for improved understanding of the pathogenesis and epidemiology of cholera. The incubation period of cholera has important implications for clinical and public health decision-making, yet statements of the incubation period of cholera are often imprecise. Here we characterize the distribution of cholera’s incubation period.
Methods
We conducted a systematic review of the literature for statements of the incubation period of cholera and data that might aid in its estimation. We extracted individual-level data, parametrically estimated the distribution of toxigenic cholera’s incubation period, and evaluated evidence for differences between strains.
Results
The incubation period did not differ by a clinically significant margin between strains (except O1 El Tor Ogawa). We estimate the median incubation period of toxigenic cholera to be 1.4 days (95% CI, 1.3–1.6). Five percent of cholera cases will develop symptoms by 0.5 days (95% CI 0.4–0.5), and 95% by 4.4 days (95% CI 3.9–5.0) after infection.
Conclusions
We recommend that cholera investigations use a recall period of at least five days to capture relevant exposures; significantly longer than recent risk factor studies from the Haitian epidemic. This characterization of cholera’s incubation period can help improve clinical and public health practice and advance epidemiologic research.
Keywords: Cholera, Incubation period, Vibrio cholerae
Introduction
Cholera has been studied since before the time of John Snow, yet many important aspects of its transmission and clinical course are not fully understood. One aspect, the incubation period, plays a significant role in cholera surveillance, prevention, and control. The incubation period of an infectious disease is the time between infection and the development of symptoms. The full range of variability in incubation periods across infected individuals shapes epidemic dynamics and informs public health decision-making. Simple summary measures, such as the average or range of incubation periods, don’t describe this variability and may be of limited usefulness in clinical and public health practice.
Vibrio cholerae causes an estimated 3 to 5 million cases of cholera and 100,000 to 120,000 deaths each year.1 Cholera is transmitted by ingestion of V. cholerae bacteria, resulting from either direct transmission via fecal contamination of food, water, or fomites, without entry into a larger aquatic ecosystem, or through ingestion of seafood or water from aquatic reservoirs.2 Whether an individual develops clinical cholera, and the time to the development of symptoms, depends upon mode of transmission, the quantity of bacteria ingested, and host factors.3,4 Though the majority of cases are thought to be asymptomatic, cholera infection can cause diarrhea and vomiting, and in severe cases extreme dehydration, metabolic acidosis, and death.4 Case fatality rates from cholera can be maintained below 1% with appropriate case management; however, without appropriate treatment case fatality rates can exceed 20%.4
V. cholerae are typically classified into one of over 200 serogroups by their somatic O antigen.4 Only two serogroups, O1 and O139, are known causes large outbreaks in humans. V. cholerae O1 can be divided on the basis of phenotypic or biochemical differences into the classical and El Tor biotypes. Within each biotype, isolates are classified into serotypes by their antigenic form, with Inaba and Ogawa being the most important. Classical cholera is generally thought to be more pathogenic and virulent than El Tor, whereas El Tor may survive better in the environment and human hosts.5
Knowledge of the incubation period has practical value in clinical practice, public health, and epidemiological studies. Historically, health officials and politicians worldwide debated the duration of cholera’s incubation period in discussions of global cholera control including when deciding on the length of quarantine imposed on sailors and ships.6 More recently, estimates of the incubation period have been used to define the end of epidemics and to identify etiologically relevant time periods to assess disease-associated risk factors.7,8 Over the past decade computational models of cholera transmission and control have played an increasingly important role in shaping our understanding of transmission dynamics and guiding policy. Cholera transmission models often utilize an assumed incubation period distribution.9
Statements about the incubation period of cholera are often imprecise, unsourced, or based on limited information. Statements of the incubation period such as “a few hours to a few days” or “five days” are common, and it is often unclear if they refer to the mean incubation period, its maximum, the full range or some other interval. In this paper, we perform a systematic review of the literature on cholera’s incubation period and estimate its full distribution in a pooled analysis of published data.
Methods
Our review follows the general approach taken in Lessler et al.10 We highlight key features of this approach and note differences between the two when applicable. Our approach and reporting follow the PRISMA guidelines.11
Search strategy and selection criteria
We searched PubMed, Google Scholar, and ISI Web of Knowledge in January 2011. On PubMed, we searched the terms “incubation”, “period”, “cholera” and “cholerae.” On Google Scholar we searched for “incubation period of cholera”, “incubation period for cholera”, “incubation period of V. cholerae”, “incubation period for V. cholerae”, “incubation period of V. cholerae”, and “incubation period for V. cholera”. We used, “incubation period AND cholera”, and “incubation period AND cholerae” in ISIWeb of Knowledge. We also consulted two medical textbooks and a classic cholera publication.12–14
Two independent reviewers evaluated all abstracts for inclusion in the full text review. Differences were resolved by discussion and consensus. Non-English documents and dead links returned by Google Scholar were excluded from abstract review. Documents were excluded from full-text review if they were definitively about a different disease or reported a non-human study. Otherwise, all articles returned by the searches were included in the full-text review.
Assessment
Documents in the full-text review were classified as containing: (1) an incubation period estimate based on original data or analysis, (2) a cited statement of the incubation period, (3) an unsourced statement of the incubation period, and/or (4) no statement of the incubation period. Relevant citations within each document were similarly reviewed. Documents were further classified based on whether they contained individual-level data appropriate for analysis. Two reviewers independently examined each paper potentially containing original data for figures, tables, or text describing individual-level incubation period data. Discrepancies between reviewers were resolved by discussion and consensus. Two experts reviewed the list of documents containing incubation period data and suggested any documents not identified by the search criteria.
We restricted this review to infections from V. cholerae O1 and O139, which have been identified as the primary causes of cholera epidemics and pandemics. Since serogroup classification was developed in the 1930’s, and given the wide held belief that previous cholera pandemics were caused by V. cholerae O1, we included all references to cholera from previous pandemics (i.e. data from before 1923) as O1 cholera with no assumptions about biotype or serotype. In sensitivity analyses we excluded this data and found no significant differences (see supplement).
Data abstraction and analysis
We took two approaches to characterize the incubation period of cholera; we summarized general statements about the incubation period of cholera, and pooled and reanalyzed individual-level data.
Statement summarization
We extracted all statements of the incubation period of cholera to characterize the consensus in the medical literature (excluding individual observations). We present the range of incubation periods that would be consistent with the majority of statements (i.e. consistent with over 50% of the published estimates), and the modal statement of the central tendency.
Pooled analysis
We included all individual-level observations where an exact value or a range for both the time of exposure and the time of symptom onset was reported or could be deduced. We did not include times between cases within households in endemic settings as it was not possible to place a lower bound on exposure times without strong assumptions. We treated all data as doubly interval censored observations (e.g. “two days later” would be treated as an interval censored observation in the range of 48–72 h).15
The logarithm of incubation periods of acute infectious diseases have been shown to approximately follow a normal distribution.10 Using this assumption, we pooled individual observations and fit the data to a log-normal distribution, characterized by its median and dispersion (the exponential of the standard deviation of the logarithm of the data). When log-normal distributed, approximately two-thirds of incubation periods will fall between median ÷ dispersion and median × dispersion. We fit models to serogroup specific data, and further stratified by biotype and serotype (within each biotype) to investigate differences between strains. We constructed hierarchical models adjusting for study type (i.e. observational or experimental) to assess whether strain specific differences could be explained by study-level factors.
We estimated the median, dispersion, and key percentiles of the incubation period using a Bayesian adaptation (see supplement) of previously published methods.15 To check the robustness of our results, we compared our estimates to those made from alternative parametric and nonparametric models (see supplement). All analyses were performed using the R statistical language (version 2.14.0). All data used in the pooled analyses and a full bibliography are available from the authors on request.
Results
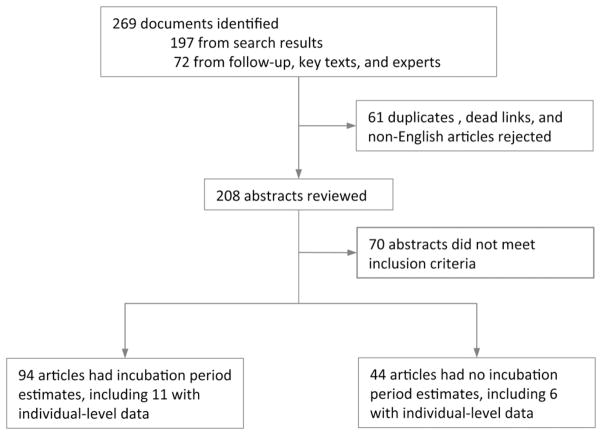
We identified ninety-three documents containing 179 statements of the incubation period of cholera (Fig. 1). Of these, 35% (63/179) came from original studies, 25% (45/179) cited a source, and 40% (71/179) gave no source. Statements of the incubation period pertained to the mean, the range of the mean, the median, the range of the full incubation period, and to non-specific measures of the range or central tendency. The majority of published estimates of cholera’s incubation period are consistent with a duration of 1–5 days with a mode of 3 days. In most cases, no serogroup, serotype or biotype was specified in these statements.
Figure 1.
Flow chart for search process.
Pooled analysis
Seventeen documents with individual-level data met the criteria for inclusion in the pooled analysis (Table 1). From these we extracted data on 323 cholera cases with 37% (120/323) coming from 8 experimental studies,3,16–22 and 63% (203/323) from 9 observational studies.12,23–30 Most observations came from the current seventh pandemic, while 38 came from the second to fifth cholera pandemics, including 25 observations reported by John Snow during an 1854 cholera outbreak in London.12,23,29 Most observations came from V. cholerae O1 infections (294/323), the predominant serogroup circulating globally, and 29 observations were from infections with V. cholerae O139. We found statistical evidence for a significant difference in incubation periods between the O1 and O139 serotypes (see supplement). However, due to the paucity of incubation period observations for O139 infections, we considered both serogroups together in our primary analysis.
Table 1.
Details of papers that included individual-level data for inclusion in the pooled analysis. (Note: that studies that involved more than one strain are shown on separate lines.)
| Source | Location | Study type | N | Population | Dose (CFU) | Infection mechanism | Comments |
|---|---|---|---|---|---|---|---|
| BMJ. Jan 16, 189723 | Malta | Observational | 8 | British soldiers | NA | Natural | Assumed O1 |
| Cash et al. (1974)16 | USA | Experimental | 42 | Healthy male inmates | 106 | Water w/2 g NaHCO3 | O1 Classical Inaba 569B |
| Cash et al. (1974)16 | USA | Experimental | 22 | Healthy male inmates | 105 | Water w/2 g NaHCO3 | O1 Classical Ogawa 395 |
| Cohen et al. (1999)22 | USA | Experimental | 4 | Healthy 18–40 y/o’s | 105/106 | 30 ml NaHCO3 buffer solution | V. cholerae O139 4260B (frozen) |
| Coster et al. (1995)17 | USA | Experimental | 5 | Healthy 18–40 y/o’s | 5 × 106 | Water with buffer | O139 MO10 |
| Dowse G (2004)24 | Bali | Observational | 1 | 33 y/o Australian man | NA | Natural | O1 |
| Eberhart-Phillips et al. (1996)25 | Airplane | Observational | 66 | 9–77 y/o passengers | NA | Natural | O1 El Tor Inaba and Ogawa |
| Goh et al. (1984)26 | Singapore | Observational | 18 | Male construction workers 16–56 y/o | NA | Natural | O1 El Tor Ogawa |
| Hornick et al. (1971)3 | USA | Experimental | 1 | Healthy volunteers | 108 | Water w/2 g NaHCO3 | O1 classical Inaba 569B |
| Hornick et al. (1971)3 | USA | Experimental | 1 | NA | 106 | Water w/2 g NaHCO3 | O1 classical Ogawa 395 |
| Levine (1980)18 | USA | Experimental | 7 | University students and community adults | NA | Water w/2 g NaHCO3 | O1 El Tor Inaba P27459 and N16961 |
| Levine et al. (1979)19 | USA | Experimental | 3 | University students and community adults | 106 | Water w/2 g NaHCO3 | O1 classical Inaba 569B |
| Levine et al. (1979)19 | USA | Experimental | 13 | University students and community adults | 106 | Water w/2 g NaHCO3 | O1 classical Ogawa 395 |
| Morris (1995)20 | USA | Experimental | 20 | Healthy adults 19–35 y/o | NA | Water w/2 g NaHCO3 | O139 Bengal A11837 |
| Quain (1883)29 | UK | Observational | 5 | NA | NA | Natural | Assumed O1 |
| Sack et al. (1998)21 | USA | Experimental | 2 | Health volunteers | 105 | Water w/2 g NaHCO3 | O1 El Tor Inaba N16961 (frozen) |
| Schiraldi et al. (1974)27 | Italy | Observational | 54 | Hospitalized patients | NA | Natural | O1 El Tor Ogawa, some patients had impaired gastric function |
| Snow (1854)12 | UK | Observational | 25 | NA | NA | Natural | Assumed O1 |
| Sutton (1974)28 | Airplane | Observational | 23 | Passengers on flight | NA | Natural | O1 El Tor |
| Taylor et al. (1993)30 | USA | Observational | 3 | Females 29–36 y/o | NA | Natural | O1 El Tor Ogawa |
We estimate the median incubation period of toxigenic cholera to be 1.4 days (95% Credible Interval (CI), 1.3–1.6) with a dispersion of 1.98 (95% CI 1.87–2.11). Five percent of cholera cases will develop symptoms by 0.5 days (95% CI 0.4–0.5), and 95% by 4.4 days (95% CI 3.9–5.0) after infection.
Serogroup O1
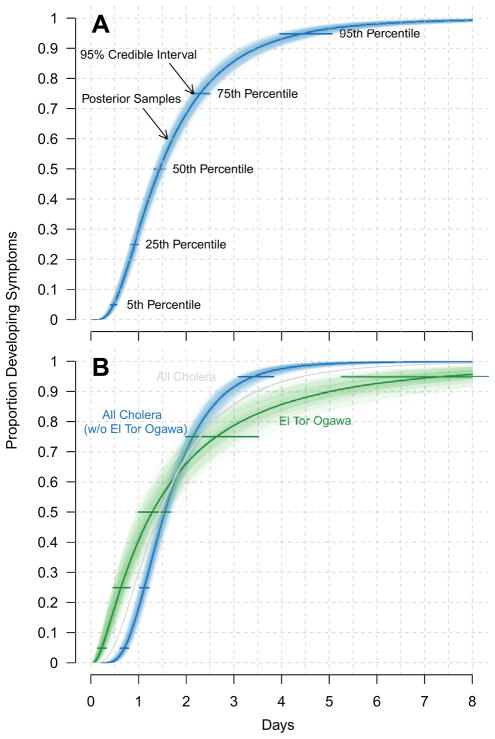
O1-specific incubation period data came from 91 observations from 5 human challenge studies,3,16,18,19,21 and from 203 observations from 9 outbreak investigations,12,23–30 including two outbreaks on international flights.25,28 Based on this data, we estimate the median incubation period for O1 cholera to be 1.5 days (95% CI 1.4–1.7) with a dispersion of 2.04 (95% CI 1.92–2.19) (Fig. 2A, Table 2). Five percent of O1 cases will develop symptoms by 0.5 days (95% CI 0.4–0.5), and 95% by 4.9 days (95% CI 4.3–5.7) after infection. In a sensitivity analysis, we excluded data reported before serogroup definitions existed (38 observations in 3 studies12,23,29) and found that our estimates of 5th, 25th, 50th, 75th and 95th percentiles of the incubation period did not vary by a clinically significant margin (less than a third of a day for each, see supplement).
Figure 2.
Estimated cumulative distribution of incubation period for (A) all toxigenic cholera (O1 and O139), and (B) all toxigenic cholera without O1 El Tor Ogawa (blue), O1 El Tor Ogawa (green), and, for reference, the fit from (A) in grey. Horizontal bars show the 95% credible intervals and samples from the posterior distribution (illustrating the uncertainty in the estimates) are shown as transparent lines.
Table 2.
Estimates of parameters 5th, 25th, 50th (median), 75th, and 95th percentiles, and dispersion for the incubation period of cholera O1 and O139, and O1 El Tor by serotype. 95% confidence intervals are shown in parentheses.
| Strain |
N (studies)
|
5th percentile | 25th percentile | 50th percentile | 75th percentile | 95th percentile | Dispersion | |
|---|---|---|---|---|---|---|---|---|
| Exper. | Obs. | |||||||
| Combined | 120 (8) | 203 (9) | 0.5 (0.4, 0.5) | 0.9 (0.8, 1.0) | 1.4 (1.3, 1.6) | 2.3 (2.1, 2.5) | 4.4 (3.9, 5.0) | 1.98 (1.87, 2.11) |
| Combined (w/o El Tor Ogawa) | 120 (8) | 128 (6) | 0.7 (0.6, 0.8) | 1.1 (1.0, 1.2) | 1.5 (1.4, 1.7) | 2.1 (2.0, 2.3) | 3.4 (3.1, 3.8) | 1.62 (1.54, 1.71) |
| O139 | 29 (3) | 0 (0) | 0.7 (0.6, 0.9) | 1.0 (0.9, 1.2) | 1.3 (1.1, 1.5) | 1.6 (1.4, 2.0) | 2.3 (1.9, 3.1) | 1.42 (1.31, 1.54) |
| O1 | 91 (51) | 203 (9) | 0.5 (0.4, 0.5) | 0.9 (0.8, 1.0) | 1.5 (1.4, 1.7) | 2.4 (2.2, 2.7) | 4.9 (4.3, 5.7) | 2.04 (1.92, 2.19) |
| Inaba (El Tor) | 9 (2) | 23 (1) | 0.7 (0.5, 1.0) | 1.1 (0.9, 1.4) | 1.5 (1.2, 1.8) | 2.0 (1.6, 2.4) | 3.0 (2.3, 4.1) | 1.52 (1.37, 1.81) |
| Ogawa (El Tor) | 0 (0) | 75 (3) | 0.2 (0.1, 0.3) | 0.6 (0.5, 0.8) | 1.3 1.0, 1.6) | 2.6 (2.0, 3.5) | 7.4 (5.2, 11.5) | 2.89 (2.46, 3.62) |
Serotype and biotype
Within the O1 serogroup, we found no significant differences in the incubation period distributions of El Tor and classical biotypes after adjusting for study type. Next, following convention, we compared serotypes within each biotype. All 75 observations for El Tor Ogawa came from three observational studies,18,21,25 and our estimate of its distribution was significantly different than other serotypes. We estimate the median incubation period of O1 El Tor Ogawa to be 1.3 (95% CI 1.0–1.6) days with a dispersion of 2.89 (95% CI 2.46–3.62). Five percent of El Tor Ogawa cases will develop symptoms by 0.2 (95% CI 0.1–0.3) days, with 95% developing symptoms by 7.4 days (95% CI 5.2–11.5) days after infection.
Our estimates suggest larger variability in the incubation period with O1 El Tor Ogawa than other toxigenic strains, with some cases expected to become symptomatic over a week after infection. We re-estimated the incubation period for toxigenic cholera with this influential data removed and found the median incubation period is 1.5 (95% CI 1.4–1.7) days with a dispersion of 1.62 (95% CI 1.54–1.71). Five percent of cases will develop symptoms by 0.7 (95% CI 0.6–0.8) days, with 95% developing symptoms by 3.4 (95% CI 3.1–3.9) days after infection (Fig. 2B, Table 2).
Serogroup O139
Incubation period data for infections with V. cholerae O139 came from 29 observations from 3 human challenge experiments. 17,20,22 From this data we estimate the median incubation period for O139 cholera to be 1.3 days (95% CI 1.1–1.5) with a dispersion of 1.42 (95% CI 1.31–1.54) (Fig. 2A, Table 2). Five percent of O139 cases will develop symptoms by 0.7 days (95% CI 0.6–0.9), with 95% developing symptoms by 2.3 days (95% CI 1.9–3.1) after infection.
Discussion
We estimated the full distribution of cholera’s incubation period by combining 323 individual observations from the past 150 years, providing more detail than any estimate we found in the published literature. The consensus within the literature captures the central tendencies of cholera’s incubation period but fails to reflect its variability. Based on our reanalysis of published data, we expect that the range of incubation periods consistent with the majority of statements in the literature (1–5 days) would exclude 33% (95% CI 0.29–0.38) of cholera incubation periods.
We find evidence for differences between one strain, O1 El Tor Ogawa, and all other toxigenic cholera strains. One explanation for these differences is that all data for O1 El Tor Ogawa come from observational studies; however, these differences persist when restricting data on other strains to observational studies. Since serotypes are based on antigen differences, it is plausible that the incubation periods between antigen-defined strains would be different. The combined estimate of cholera’s incubation period (Table 2, Fig. 2A) is an appropriate and conservative estimate for use in most settings; however, when O1 El Tor Ogawa is the main circulating strain, the O1 El Tor Ogawa-specific estimate (Table 2, Fig. 2B) may be preferred.
Over the past two decades, a variant of O1 El Tor, which produces classical cholera toxin (the toxin produced by the classical biotype strains), has nearly displaced prototypical El Tor strains worldwide,5 and is responsible for the current Haitian epidemic.31 Although the cholera toxin allele for all strains in this analysis were not characterized, it is unlikely that any incubation period observations were a result of this, seemingly more virulent, hybrid strain. Other virulence factors, many of which are encoded on mobile genetic elements within the V. cholerae genome, may also modify the incubation period. More investigations are needed to understand the properties of emerging strains, such as the incubation period. Given the diverse use of incubation periods and uncertainty related to variant O1 El Tor strains, expert judgment based on contemporary observations should be used in deciding the appropriate distribution to use.
Outbreak investigation guidelines from the WHO for foodborne diseases suggest limiting the recall period for potential exposures to the length of the incubation period from symptom onset.8 Current guidance by the WHO and US Centers for Disease Control,1,32 state that the incubation period of cholera ranges between 2 h and 5 days. However, some risk factor studies conducted in the recent Haitian epidemic used exposure recall periods of as short as 3 days,33,34 which we estimate will miss 21% (95% CI 0.14–0.21) of O1 El Tor Ogawa exposures. This could cause investigators to miss important risk factors or sources of exposure. We recommend that cholera investigations use a recall period of at least 5 days, and up to 7 days in the case of El Tor Ogawa.
There are a number of limitations on inferences from this pooled data. Differences in symptom onset definitions between studies can introduce variability into measured incubation periods, though all papers that included a definition of symptom onset defined it as onset of diarrhea after known/suspected ingestion of V. cholerae. The incubation period may also vary by dose. Hornick et al. showed a dose–response relationship between the inoculum dose and the incubation period, with lower doses leading to longer incubation periods.3 Most experimental studies included in this analysis administered 106 colony-forming units along with a buffer solution, but the infectious dose from the observational studies is unknown. For a short period of time after V. cholerae exits a human host, it is thought to exist in a hyperinfectious state.35 This state reduces the infectious dose required to produce illness, and could plausibly lead to different incubation periods when compared to infections from non-hyperinfectious vibrios. Subjects in the experimental studies in this analysis were not exposed to hyperinfectious vibrios however those exposed under natural conditions (i.e. the observational studies), may have been. When analyzed separately, the incubation periods fit to data from observational and experimental studies have similar distributions, but with more variability in the distribution fit to the observational data (see supplement). These differences presumably arise from the heterogeneity in dose and exposure pathway between the two types of settings.
Previous exposure to cholera provides some protection against clinical cholera and may modify the incubation period. While data on previous exposures were unavailable for the observational studies reviewed, the experimental studies reviewed were exclusively performed on North American volunteers, most with no previous exposure to cholera. There is evidence that host genetics and nutritional status may play a role in the development of cholera symtoms.4 Hence, extrapolation from one population to another may be suspect.
Our estimates characterize the variability in the incubation period of cholera and reveal differences between one strain, O1 El Tor Ogawa, and the others. As cholera continues to persist throughout South Asia, Africa, and the Caribbean, these estimates can help improve epidemiologic and clinical investigations, and enhance computational models aimed at understanding transmission dynamics and intervention effects. These estimates allow us to move past simple statements of the incubation period towards incorporation of individual variability and uncertainty in decision-making.
Supplementary Material
Acknowledgments
Role of the funding source
The study funders had no role in the design, conduct or analysis of this study.
ASA’s work on this project was funded by the Johns Hopkins Sommer Scholars Program. JL and DATC were funded by a grant from the Bill and Melinda Gates Foundation (the Vaccine Modeling Initiative, 705580-3). DATC’s work was also supported by a grant from the NIH (NIGMS, U54 GM088491-01). JL is the recipient of a Research Scholar Development Award from the NIH (NIAID, K22 AI092150-01). DATC holds a Career Award at the Scientific Interface from the Burroughs Welcome Fund. KER was funded by the Division of Intramural Research Programs at the National Institute of Mental Health. The authors thank J.G. Morris and David Sack for useful comments.
Appendix A. Supplementary material
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jinf.2012.11.013.
Footnotes
Contributors
All authors were involved in conception of this review and drafting of the manuscript. ASA and KER conducted the systematic literature review. ASA and JL analyzed the data.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributor Information
Andrew S. Azman, Email: aazman@jhsph.edu.
Kara E. Rudolph, Email: krudolph@jhsph.edu.
Derek A.T. Cummings, Email: dcumming@jhsph.edu.
References
- 1.World Health Organization. Cholera. World Health Organization; 2012. [accessed 09.02.12]. http://www.who.int/mediacentre/factsheets/fs107/en/ [Google Scholar]
- 2.Morris JG. Cholera–modern pandemic disease of ancient lineage. Emerg Infect Dis. 2011;17:2099–104. doi: 10.3201/eid1711.111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hornick RB, Music SI, Wenzel RP, Cash R, Libonati JP, Snyder MJ, et al. The broad street pump revisited: response of volunteers to ingested cholera vibrios. Bull N Y Acad Med. 1971;47:1181–91. [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson EJ, Harris JB, Morris JG, Calderwood SB, Camilli A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol. 2009;7:693–702. doi: 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safa A, Sultana J, Dac Cam P, Mwansa JC, Kong RYC. Vibrio cholerae O1 hybrid El Tor strains, Asia and Africa. Emerg Infect Dis. 2008;14:987–8. doi: 10.3201/eid1406.080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong HE. Discussion on cholera. Br Med J. 1893;2:364–71. [Google Scholar]
- 7.Bopp CA, Ries AA, Wells JG. Laboratory methods for the diagnosis of epidemic dysentery and cholera. National Center for Infectious Diseases (US) Centers for Disease Control and Prevention & World Health Organization Regional Office for Africa; 1999. [Google Scholar]
- 8.World Health Organization. Foodborne disease outbreaks: guidelines for investigation and control. World Health Organization; 2008. [Google Scholar]
- 9.Chao DL, Halloran ME, Longini IM. Vaccination strategies for epidemic cholera in Haiti with implications for the developing world. Proc Natl Acad Sci. 2011;108:7081–5. doi: 10.1073/pnas.1102149108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DAT. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J Altman DG PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. PloS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snow J. On themode of communication of cholera. 2. 1854. [Google Scholar]
- 13.Pediatrics AAO. Cholera (Vibrio cholerae) In: Pickering L, editor. Red book: 2009 Report of the Committee on Infectious Diseases. 28. Elk Grove Village, IL: 2009. pp. 727–9. [Google Scholar]
- 14.Cohen J, Opal S, Powderly W. Infectious diseases. 3. Mosby: Elsevier Ltd; 2010. [Google Scholar]
- 15.Reich NG, Lessler J, Cummings DAT, Brookmeyer R. Estimating incubation period distributions with coarse data. Stat Med. 2009;28:2769–84. doi: 10.1002/sim.3659. [DOI] [PubMed] [Google Scholar]
- 16.Cash RA, Music SI, Libonati JP, Snyder MJ, Wenzel RP, Hornick RB. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J Infect Dis. 1974;129:45–52. doi: 10.1093/infdis/129.1.45. [DOI] [PubMed] [Google Scholar]
- 17.Coster TS, Killeen KP, Waldor MK, Beattie DT, Spriggs DR, Kenner JR, et al. Safety, immunogenicity, and efficacy of live attenuated Vibrio cholerae O139 vaccine prototype. Lancet. 1995;345:949–52. doi: 10.1016/s0140-6736(95)90698-3. [DOI] [PubMed] [Google Scholar]
- 18.Levine MM. Cholera and related diarrheas. Basel, Switzerland: S Karger; 1980. Immunity to cholera as evaluated in volunteers; pp. 195–203. [Google Scholar]
- 19.Levine MM, Nalin DR, Craig JP, Hoover D, Bergquist EJ, Waterman D, et al. Immunity of cholera in man: relative role of antibacterial versus antitoxic immunity. Trans R Soc Trop Med Hyg. 1979;73:3–9. doi: 10.1016/0035-9203(79)90119-6. [DOI] [PubMed] [Google Scholar]
- 20.Morris JG, Losonsky GE, Johnson JA, Tacket CO, Nataro JP, Panigrahi P, et al. Clinical and immunologic characteristics of Vibrio cholerae O139 Bengal infection in North American volunteers. J Infect Dis. 1995;171:903. doi: 10.1093/infdis/171.4.903. [DOI] [PubMed] [Google Scholar]
- 21.Sack DA, Tacket CO, Cohen MB, Sack RB, Losonsky GA, Shimko J, et al. Validation of a volunteer model of cholera with frozen bacteria as the challenge. Infect Immun. 1998;66:1968–72. doi: 10.1128/iai.66.5.1968-1972.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen MB, Giannella RA, Losonsky GA, Lang DR, Parker S, Hawkins JA, et al. Validation and characterization of a human volunteer challenge model for cholera by using frozen bacteria of the new Vibrio cholerae epidemic serotype, O139. Infect Immun. 1999;67:6346–9. doi: 10.1128/iai.67.12.6346-6349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cholera in British ports. Br Med J. 1897 Jan 16;:156–7. [Google Scholar]
- 24.Dowse G. Cholera, imported – Australia (02) 2004 promedmail.org.
- 25.Eberhart-Phillips J, Besser RE, Tormey MP, Koo D, Feikin D, Araneta MR, et al. An outbreak of cholera from food served on an international aircraft. Epidemiol Infect. 1996;116:9–13. doi: 10.1017/s0950268800058891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goh KT, Lam S, Kumarapathy S, Tan JL. A common source food borne outbreak of cholera in Singapore. Int J Epidemiol. 1984;13:210–5. doi: 10.1093/ije/13.2.210. [DOI] [PubMed] [Google Scholar]
- 27.Schiraldi O, Benvestito V, Di Bari C, Moschetta R, Pastore G. Gastric abnormalities in cholera: epidemiological and clinical considerations. Bull World Health Org. 1974;51:349. [PMC free article] [PubMed] [Google Scholar]
- 28.Sutton RG. An outbreak of cholera in Australia due to food served in flight on an international aircraft. J Hyg (Lond) 1974;72:441–51. doi: 10.1017/s0022172400023688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quain SR. A dictionary of medicine. D. Appleton and Co; 1882. [Google Scholar]
- 30.Taylor JL, Tuttle J, Pramukul T, Brien KO, Barrett TJ, Jolbitado B, et al. An outbreak of cholera in Maryland associated with imported commercial frozen fresh coconut milk. J Infect Dis. 1993;167:1330. doi: 10.1093/infdis/167.6.1330. [DOI] [PubMed] [Google Scholar]
- 31.Ceccarelli D, Spagnoletti M, Cappuccinelli P, Burrus V, Colombo MM. Origin of Vibrio cholerae in Haiti. Lancet Infect Dis. 2011;11:262. doi: 10.1016/S1473-3099(11)70078-0. [DOI] [PubMed] [Google Scholar]
- 32.United States Centers for Disease Control. [accessed 09.02.12];Consider cholera: information for healthcare professionals working in the US. http://www.cdc.gov/haiticholera/consider-cholera.htm.
- 33.O’Connor KA, Cartwright E, Loharikar A, Routh J, Gaines J, Fouché M-DB, et al. Risk factors early in the 2010 cholera epidemic, Haiti. Emerg Infect Dis. 2011;17:2136–8. doi: 10.3201/eid1711.110810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunkle SE, Mba-Jonas A, Loharikar A, Fouché B, Peck M, Ayers T, et al. Epidemic cholera in a crowded urban environment, Port-au-Prince, Haiti. Emerg Infect Dis. 2011;17:2143–6. doi: 10.3201/eid1711.110772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merrell DS, Butler SM, Qadri F, Dolganov NA, Alam A, Cohen MB, et al. Host-induced epidemic spread of the cholera bacterium. Nature. 2002;417:642–5. doi: 10.1038/nature00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.