Abstract
Ubiquitin-like modifications are macromolecular chemistry for which our understanding of the enzymatic mechanisms is lacking. Most E3 ligases in ubiquitin-like modifications do not directly participate in chemistry, but are thought to confer allosteric effects; however the nature of the allosteric effects has been elusive. Recent molecular dynamics simulations suggested that an E3 binding enhances the population of the conformational states of E2~SUMO thioester that favor reactions. In this study, we carried out the first temperature-dependent enzyme kinetic analysis to investigate the role of an E3 on activation entropy and enthalpy. The small ubiquitin-like modifier (SUMO) E3, RanBP2, confers unusually large, favorable activation entropy to lower the activation energy of the reaction. Mutants of RanBP2, designed to alter flexibilities of the E2~SUMO thioester, showed a direct correlation of their favorable entropic effects with their ability to restriction the conformational flexibility of the E2~SUMO thioester. While the more favorable activation entropy is consistent with the previously suggested role of E3 in conformational selection, the large positive entropy suggests a significant role of solvent in catalysis. Indeed, molecular dynamics simulations in explicit water revealed that the more stable E2~SUMO thioester upon E3 binding results in stabilization of a large number of bound water molecules. Liberating such structured water at transition state can result in large favorable activation entropy but unfavorable activation enthalpy. The entropy-driven mechanism of the E3 is consistent with the lack of structural conservation among E3s despite their similar functions. This study also illustrates how proteins that bind both SUMO and E2 can function as E3s and how intrinsically unstructured proteins can enhance macromolecular chemistry in addition to their known advantages in protein-protein interactions.
Keywords: ubiquitin, SUMO, E3 ligase, allosteric effect, solvent effect, intrinsically disordered proteins
The significant role of water in protein structure and function has been well documented by many elegant studies (1–4). Release of ordered water from protein to bulk solvent leads to a small enthalpic penalty due to loss of hydrogen bonds maintained by ordering of water. However, the entropic gain of releasing ordered water far outweighs the enthalpic loss, resulting in as much as 2 kcal/mol in free energy gain for each sequestered water molecule (1). Such entropy gain contributes greatly to protein folding, binding and in entropy driven catalysis, such as in peptide bond synthesis on ribosome (3).
Ubiquitin and ubiquitin-like modifications are among the most important post-translational modifications that regulate functions of target proteins (5, 6). In such modification process, a ubiquitin-like protein (Ubl) is first activated by an E1 enzyme, and then is transferred to E2 by forming a thioester bond with the -SH group of the catalytic Cys residue of E2. In the final step, which usually requires the participation of an E3 ligase, the Ubl is attached to target proteins by the formation of an isopeptide bond with a specific Lys residue on the target protein. The E3s that do not form thioester intermediates with Ubl constitute the largest family. These E3s bind to an E2 site that is distal from the catalytic active site (7, 8), and are thought of as adaptors to bring E2 and substrate proteins together. However, many studies have indicated that E3s are not simply adaptors, although the nature of their allosteric effects remains unknown despite extensive structural analysis of E2–E3 complexes and E2~Ubl thioester intermediate mimetics (7).
One of the outstanding questions in ubiquitin-like modifications is how E3 ligases confer their functions despite their diverse structures ranging from RING-domain-containing large proteins or multi-protein complexes to intrinsically unstructured proteins (9). While the majority of E3s that do not form thioester intermediates with Ubls contain a RING or U-box E2-binding motif, recent studies have identified a class of SUMO-binding motif (SBM, also known as SUMO-interacting motif, SIM)-dependent E3 ligases that contain a conserved SIM instead of a conserved E2-binding motif. Increasing numbers of SIM-dependent E3 ligases have been identified, including Pc2 (10–12), a Kaposi’s sarcoma-associated herpes virus encoded protein (13), and RanBP2 (14, 15), which is the best characterized member of this class. Both X-ray crystallographic studies and solution NMR studies showed that, similar to other E3 ligases, RanBP2 binds to a surface of E2 that is distal from the catalytic and substrate-binding sites, and thus does not directly participate in the chemistry (8, 16). RanBP2 offers an attractive model for investigating the allosteric effect of E3 ligases, and understanding its allosteric effect through binding a remote site of E2 has general implications for other E3 ligases.
In this study, we carried out the first temperature-dependent enzyme kinetic analysis in ubiquitin-like modifications to investigate RanBP2’s allosteric effect on activation entropy and enthalpy. In combination with mutants that were designed to alter the conformational flexibility of the SUMO~E2 thioester, measurements of the enthalpic and entropic components of the activation Gibbs free energy revealed that RanBP2 confers an unusual large favorable activation entropy and that RanBP2’s allosteric effect is conferred through its ability to alter the conformational flexibility of the E2~SUMO thioester. Consistent with previous molecular dynamic simulations, our simulation also suggested that RanBP2 restricts the flexibility of the E2~SUMO thioester. In addition, we further analyzed structured water and found that stabilized E2~SUMO thioester by RanBP2 allows sequestration of water molecules in the E2~SUMO thioester conjugates in the ground state. Releasing ordered water to bulk solvent in the transition state due to conformational changes is consistent with the observation of increased enthalpy cost but the large favorable entropy gain that lowers activation energy of the reaction in the presence of the E3. This novel model of E3 function does not require a specific structure of E3 and offers a unifying theme of the similar allosteric effects of E3s in the absence of a conserved structure.
EXPERIMENTAL PROCEDURES
Plasmids
Expression constructs for SUMO E1 (SAE1/SAE2), Ubc9, wild-type and C52A mutant SUMO-11–97, GST-Sp100241–360, and RanGAP1 were made as previously described (16–19). cDNAs encoding the IR1-M domain (2629–2710), the E3_ΔSIM (or IR1-M_ΔSIM) (2637–2710) domain of RanBP2, E3_G and E3_GG (inserted after residue 2638) followed by His6-tags at the C-termini were amplified by PCR, and inserted between the NdeI and BamHI sites of pET11a+ (Novagen). All constructs were confirmed by DNA sequencing.
Expression and Purification of Recombinant Proteins
Purification of human SUMO-1 (C52A), SUMO E1, Ubc9, and GST-Sp100 used protocols described previously (16–18). The various E3 constructs were expressed in E. Coli BL21(DE3) strain, purified using Ni-NTA column (Qiagen) and dialyzed against phosphate buffered saline (PBS) containing 1 mM DTT. The IR1-M domain of RanBP2 used in NMR analysis was 2D, 15N, and 13C labeled by growing E. coli cells (37°C) in M-9 minimal medium in D2O and supplemented with trace minerals and Basal Medium Eagle vitamins (Gibco) using 15NH4Cl and 13C-glucose as the nitrogen and carbon sources, respectively.
Pull-down Assay
Protein pull-down assays were performed as previously described (17). Microtiter plates (Corning Costar) were coated (4°C, overnight) with 0.5 μg/well of the IR1-M or the IR1-M_ΔSIM domain of RanBP2 in PBS (100 μL/well). Wells were washed twice and blocked (1 h, room temperature) with 150 μL of blocking buffer (2% BSA, 0.1% Tween 20 in PBS). Either 100 ng of purified recombinant Ubc9 or 5 μL in vitro translated 35S-labeled RanGAP1 sample was added with 100 μL blocking buffer followed by incubation (1 h, room temperature). Wells were washed 3 times with 150 μL blocking buffer and then 4 times with 150 μl PBS containing 0.1% Tween 20. Bound Ubc9 and RanGAP1 (or SUMOylated RanGAP1) were extracted with SDS sample buffer and detected by Western blot using anti-Ubc9 antibody or by autoradiography using a phosphor imager (Molecular Dynamics), respectively.
Kinetic Analysis
Recombinant SUMO-1 C52A mutant was used instead of wild-type SUMO-1 to avoid complications from SUMO-1/SUMO-1 disulfide-linked dimer. The mutation does not alter conjugation activities (19). In vitro SUMOylation of Sp100 was carried out by incubating reaction mixture (4.2 μL) containing 0.78 μM E1, 0.35 μM Ubc9, 22.7 μM SUMO-1(C52A), 3.4 mM ATP, 2.6 μM of each wild-type and mutant IR1-M domains of RanBP2 (E3, E3_G, E3_GG, and E3_linker) or assay buffer (5 mM MgCl2, 0.1% Tween 20, 20 mM Hepes pH 7.5, 50 mM NaCl) for non-E3 assays, and SUMOylation was initiated by the addition of various GST-Sp100 concentrations (2–9 μM) at different temperatures. Prior to adding GST-Sp100, mixtures were incubated (10 min, room temperature) for saturation of RanBP2 SUMOylation. A different set of temperatures was used for each E3 condition. For wild-type E3 and E3_GG insertion, the reactions were incubated at 25, 28, 31, and 37°C for 3 min. For E3_linker insertion and no E3 (NE) assays, the temperatures used were 31, 37, 40, and 43°C for 10 min. Reactions were then quenched with 9 μL of 360 mM DTT loading buffer and components were separated on SDS PAGE gel followed by Western Blot. All experiments were performed in triplicate with the exception of E3_linker in duplicate. The use of different temperatures was due to the vastly different reaction rates of the different E3 variants. The reactions in the presence of E3 and E3_GG were very fast; thus in order to ensure that product formation is linearly dependent on time over reasonable reaction time for proper estimation of initial rates, they were carried out at lower temperatures. Reactions in the absence of E3 or with E3_linker occurred at much slower rates, and thus higher temperatures and longer reaction time were necessary to obtain detectable product formation.
Western Blot analysis
Gels were transferred to a PVDF-FL membrane in TOWBIN buffer (containing 192 mM Glycine and 25 mM Tris base). The membrane was washed twice with PBS-0.1% Tween 20 buffer and then incubated with blocking buffer overnight. Free SUMO and SUMOylated products were detected with a mouse anti-SUMO-1 antibody (Abgent, San Diego, CA), donkey anti-mouse secondary antibody and imaged and quantitated with an Odyssey scan (Li-Cor Biosciences, Nebraska).
MD simulations
MD simulations were carried out in explicit water (TIP3P) (20) with the NAMD program (21). The simulations were carried out with Charmm27 force field (22), isothermal-isobaric (NPT) ensemble, periodic boundaries and particle-mesh Ewald (full) electrostatics calculations (23, 24). Conjugate gradient minimization was performed for 1000 steps until convergence of force to 0.1kcal/mol-Å. Then, the whole systems were heated to 310 K in steps of 1 K for every 5 MD steps. Both systems were equilibrated for 10 ns. The trajectory files were save every 2.5 ps. MD simulations were carried out for 125 ns for the complex without E3 and 150 ns for the complex with E3. MD simulations were prepared by VMD1.8 (25) and carried out by NAMD2.6 using a local HPC computer cluster. The trajectory analyses were carried out by VMD1.8. Only the last 100 ns simulations were used for the calculations. The thioester reaction site was defined by residues K524(RanGAP1), C93(Ubc9) and G97(SUMO). Water molecules, which were within 5.0 Å from these residues and appeared at the same position longer than 10 ns, are listed in Table 2. The boundary of the pocket at the SUMO1 - UBC9 interface was defined by residues Q29, D30, S31, I34, Y51, R54, Q55, and R63 of SUMO1 and K48, K49, T51, A106, T108, K110, Q111 and N121 of UBC9. Water molecules, which were within 5.0 Å from these residues and appeared at the same position longer than 10 ns, are listed in Table 3.
Table 2.
A list of water molecules within 5 Å of residues involved in the chemistry, K524 (RanGap1), C93 (UBC9) and G97 (SUMO1), and their residence time. The water molecules in upper box are specifically located at the interface between RanGap1 and UBC9.
| Without E3 | With E3 | ||
|---|---|---|---|
|
| |||
| Atm_index | Time (ns) | Atm_index | Time (ns) |
| 44573 | 27.9 | ||
| 30239 | 24.5 | ||
|
| |||
| 16740 | 17.7 | 35090 | 42.8 |
| 26157 | 15.8 | 62360 | 35.9 |
| 34425 | 13.8 | 43952 | 17.7 |
| 39090 | 13.0 | 46241 | 13.4 |
| 17643 | 11.1 | 47168 | 12.2 |
| 27312 | 11.0 | 15878 | 11.6 |
Table 3.
A list of water molecules that are trapped in a pocket at the interface of UBC9 and SUMO1 for more than 10 ns and their residence time. The definition of SUMO-Ubc9 interface is given in Methods.
| Without E3 | With E3 | ||
|---|---|---|---|
|
| |||
| Atm_index | Time (ns) | Atm_index | Time (ns) |
| 17007 | 46.1 | 21407 | 81.5 |
| 48039 | 31 | 38807 | 81.4 |
| 35412 | 28.5 | 17408 | 56.9 |
| 24117 | 19.4 | 40091 | 46.7 |
| 28446 | 17.4 | 50282 | 45.2 |
| 38376 | 17.4 | 41810 | 35.3 |
| 19854 | 17.1 | 35387 | 28.3 |
| 44481 | 15.7 | 32966 | 23.9 |
| 36753 | 14.3 | 60995 | 22.7 |
| 6411 | 13.3 | 29270 | 22.4 |
| 13491 | 13.3 | 50009 | 21.6 |
| 45138 | 12.8 | 42896 | 21.4 |
| 23046 | 11.7 | 51074 | 17.8 |
| 29730 | 11.5 | 8669 | 17.4 |
| 54999 | 11.2 | 54833 | 16.3 |
| 18270 | 11.1 | 32972 | 15.1 |
| 15273 | 11 | 50132 | 14.3 |
| 52158 | 10.7 | 26162 | 13.9 |
| 8346 | 10.4 | 24584 | 13.4 |
| 49590 | 10.1 | 39071 | 13 |
| 26519 | 12.8 | ||
| 63854 | 12.5 | ||
| 48206 | 12.4 | ||
| 48239 | 12.1 | ||
| 25697 | 11.8 | ||
| 61010 | 11.5 | ||
| 32090 | 11.2 | ||
| 21989 | 11.2 | ||
| 22433 | 11.2 | ||
| 53285 | 11.2 | ||
| 33860 | 11.1 | ||
| 26720 | 11 | ||
| 11285 | 11 | ||
| 50135 | 11 | ||
| 12053 | 10.9 | ||
| 14066 | 10.8 | ||
| 38627 | 10.7 | ||
| 25148 | 10.6 | ||
| 37670 | 10.5 | ||
| 43919 | 10.5 | ||
| 28493 | 10.5 | ||
| 29126 | 10.1 | ||
| 30086 | 10.1 | ||
| 57053 | 10 | ||
RESULTS
Design of E3 variants to examine the requirements for ligase function
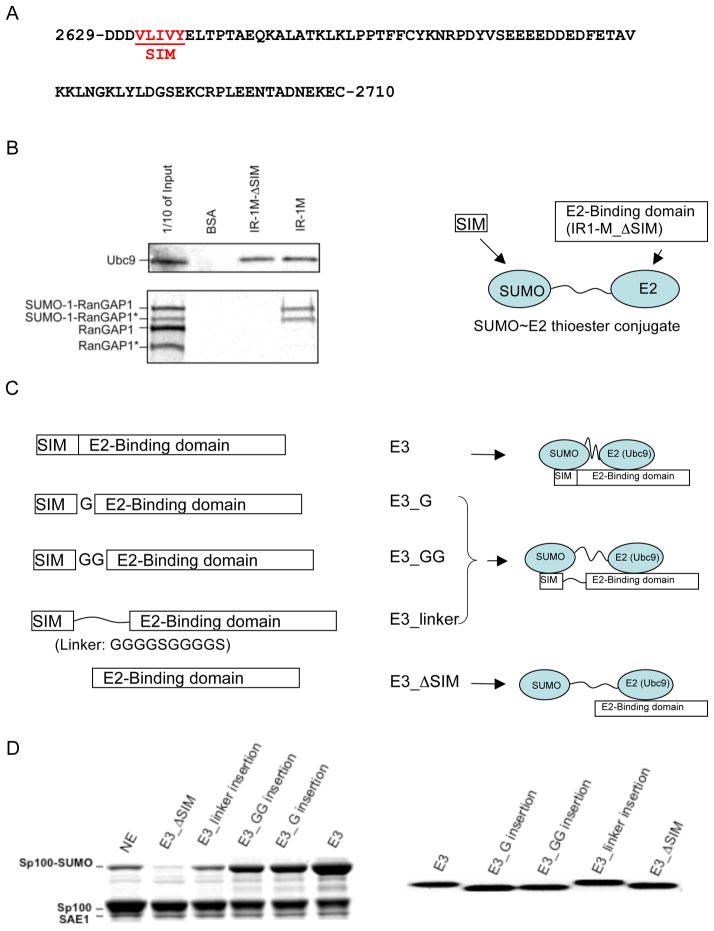
Within RanBP2, the IR1-M (internal repeat 1 and M) domain, which contains a SIM and an E2 (Ubc9)-binding segment, confers the E3 ligase activity for SUMOylation (. 1A) (14). IR1-M is highly flexible in solution and has the characteristic sequence of a disordered protein, but adopts a β-strand upon binding SUMO and forms two α-helices upon binding the E2 (8, 16). Previous structural studies suggested that the SUMO and Ubc9-binding segments of IR1-M do not overlap. To confirm the independence of the SUMO-binding segment from the E2-binding region, the SIM was deleted from IR1-M; the resulting construct was referred to as IR1-M_ΔSIM (residues 2637–2710, Fig. 1B) or E3_ΔSIM (Fig. 1C). While pull-down assays showed that the affinity of IR1-M_ΔSIM for binding Ubc9 was similar to that of IR1-M, IR1-M_ΔSIM failed to bind the SUMO-conjugated protein RanGAP1 (Fig. 1B) (17, 26), confirming that the SUMO- and the E2-binding activities are independent of each other.
Figure 1.
Independence of the SUMO and Ubc9-binding sites in the IR1-M domain of RanBP2. (A) The IR1-M domain of RanBP2 is rich in Pro, polar and charged residues, similar to other disordered proteins. The SUMO-binding motif (SIM) is underlined and indicated in red. (B) The SUMO- and E2-binding regions in E3 are independent of each other. Pull-down experiments demonstrate that the SIM is required for binding SUMO (shown with SUMOylated RanGAP1). However, SIM deletion does not affect the interaction with Ubc9. Bound Ubc9 was detected by Western blot. RanGAP1 was labeled with 35S by in vitro transcription/translation reaction using rabbit reticulocyte extracts in the presence of 35S-methionine and was detected by autoradiography. Both RanGAP1 and SUMOylated RanGAP1 were produced during the translation process because rabbit reticulocyte extracts contained all the necessary components for SUMOylation of RanGAP1 (26). Asterisks indicate truncated version of RanGAP1 due to the presence of an ATG start codon in the middle of RanGAP1 cDNA. (C) Schematic diagrams of the E3 variants that contained linker insertions between the SIM and Ubc9-binding regions. Their expected effects on the SUMO~E2 thioester are shown to the right. (D) Comparison of ligase activity of the different E3 variants. The assay mixture (7 μL) containing 0.78 μM E1, 0.35 μM E2, 2.6 μM E3, 4 mM ATP, 22.7 μM SUMO, and 8.4 μM of Sp100 was incubated (37°C, 14 min) and quenched with freshly prepared DTT loading buffer (360 mM, 8.5 μL). The panel on the right shows Coomassie staining of the E3 variants to demonstrate that similar amounts were used in the activity assay.
IR1-M is a prototype of the SIM-dependent SUMO ligases. To identify factors required for the E3 ligase activity in addition to SUMO- and Ubc9-binding ability, we created mutations in IR1-M by introducing flexible linkers of various lengths between the E2 and SUMO-binding segments. Three constructs were generated, in which the linkers consisted of one or two Gly residues (G and GG), or 10 residues of the sequence GGGGSGGGGS (Fig. 1C). The linker insertion constructs contained the same SUMO and E2-binding segments. We hypothesized that if the E3 were to induce a specific structure of the SUMO~E2 thioester th7at is required for SUMO transfer, the G and GG mutants were likely to have different orientation effects on the SUMO and E2-binding segments due to the different number of chemical bonds introduced between the two segments. In addition, variable linker lengths allow the evaluation of how spacing between the SUMO- and Ubc9-binding segments affects E3 ligase activity.
E3_ΔSIM did not display any E3 activity under any conditions (Fig. 1D), consistent with previous finding that both the SUMO- and E2-binding sites are required for E3 ligase activity (8, 16). However, the longer the linker between SIM and E2-binding segments, the lower the E3 activity (Fig. 1D). Activity levels of E3 variants were: wild-type IR1-M (E3) > E3_G ≈ E3_GG > E3_linker > E3_ΔSIM≈ no E3, and these results were independent of Sp100 concentrations and reaction temperatures. Furthermore, because E3_ΔSIM and E3 have the same binding affinity for Ubc9, that E3_ΔSIM showed a complete loss of ligase function indicates that the E3’s allosteric effect is not on the structure of E2, but on the E2~SUMO thioester. E3_G and E3_GG had similar activities, which correlated with their similar linker length but not their structural effects on SUMO and Ubc9 in the thioester conjugate due to the different number of bonds introduced into the linker. These observations suggest that the allosteric effect of E3 is unlikely to be on the structure of the E2~SUMO thioester. Instead, the E3 activities of the mutants were directly correlated to the linker lengths: the longer the linker, the lower the activity.
These results suggest a dynamic allosteric effect—the ability of E3 to restrict the flexibility of the E2~SUMO thioester conjugates. Recent NMR studies of various ubiquitin~E2 thioesters revealed that ubiquitin and E2 in their thioester conjugate or its mimetics have significant relative flexibility (27–30). The lack of a stable structure of the Ubl~E2 thioester is consistent with a previous molecular dynamic simulations of the E2~SUMO thioester (31), although the thioester is not sufficiently stable to be characterized by structural methods (32). The flexibility of the thioester conjugate should be restricted by binding IR1-M. Thus, the linker-length-dependent E3 activities suggest that the ligase activity is directly linked to their abilities to restrict the flexibility of E2~SUMO thioester conjugate.
Entropy driven mechanism of E3
To gain insights into how the flexibility of E2~SUMO thioester is related to the effect of IR1-M, the entropy and enthalpy components of activation energy of SUMO transfer from E2 to a substrate, in the presence and absence of the E3, were measured by temperature-dependent enzyme kinetic analysis. Such analysis provides the necessary insights into how an E3 lowers the activation energy. Activation enthalpy and entropy can be extracted using the Eyring–Polanyi equation:
where k is the reaction rate constant, R is the gas constant, T is the absolute temperature, h is the Planck constant and kB is the Boltzmann constant. Therefore, the enthalpy of activation, ΔH≠, can be extracted from the slope of the Eyring plots, ln(k/T) versus 1/T, and the activation entropy, ΔS≠, can be extracted from the intercepts.
Activation entropy and enthalpy had not been measured previously for ubiquitin-like modifications, and thus an innovative approach had to be designed. Although the single turnover reaction of transferring SUMO from the E2~SUMO thioester to target proteins gives information on the microscopic rate constants (8), it is far too quick (20–60 s) to handle the large number of reactions required to assay for multiple temperatures at multiple substrate concentrations, in the absence and presence of wild-type and mutant E3, at the same time to eliminate systematic errors. Instead, we chose the measurements of the net transfer rate constants (k = kcat/Km) of SUMO from E2 to substrates using the overall conjugation reactions carried out under limiting E2 concentrations (19, 33–38). The rate constant also directly reflects the E3-dependent step when the step is rate-limiting of the overall conjugations. In addition, the product formation rate was controlled by the low E2 concentrations, and thus the initial rates were measured over 3 to 10 min to eliminate measurement errors arising from insufficient temperature equilibration or slight time variations in sample handling.
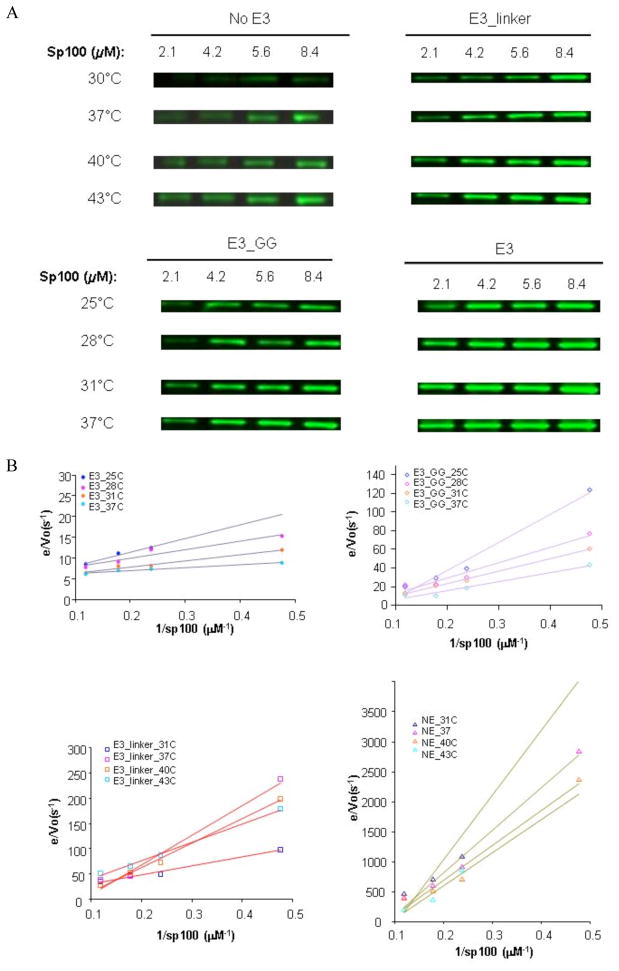
The net transfer rate constants (k = kcat/Km) of SUMO from E2 to substrates were measured using the plot of [E]/Vo versus the inverse of substrate concentration (here 1/[GST-Sp100]), under the conditions that transfer of SUMO from E2 to substrate is rate-limiting in this multi-enzyme, multi-step modification, as described previously (36). These experiments used Sp100, a well-established SUMO substrate whose SUMOylation is stimulated by RanBP2 (14, 16). Different temperatures and reaction times were chosen for the various E3 variants because they have significantly different reaction rates. Because the E3_G and E3_GG mutants had comparable effects (Fig. 1D), E3_GG was chosen to represent the two in temperature-dependent kinetic experiments. In addition, reactions in the absence of E3 are representative of reactions in the presence of E3_ΔSIM, because they have identical effects on SUMOylation rates at various substrate concentrations and reaction temperatures (Fig. 1) (8). For wild-type E3 and E3_GG, the reactions were fast (3 min) and were performed at 25–37°C. For the E3_linker insertion and no E3 (NE) assays, higher temperatures (31–43°C) and a longer reaction time (10 min) were used, because the reactions occurred at much slower rates. Conjugation reactions were performed over durations in which product formation was proportional to time and represented initial rates Vo. Each kinetic experiment involved 64 reactions (4 substrate concentrations for 4 E3 variants at 4 different temperatures) that were carried out at the same time to minimize systematic errors (representative data shown in Fig. 2A). The measurements were repeated three times to obtain uncertainty of the measurements. A couple of points (out of 64) were dropped in each repeat due to band intensities at low substrate concentrations that were too low to measure accurately. Plots of [E]/Vo versus 1/[GST-Sp100] were linear, indicating that the conditions were appropriate for Michaelis-Menton kinetics (Fig. 2B).
Figure 2.
Temperature dependent enzyme kinetic measurements of the wild-type and mutant E3. (A) Examples of Western blots used to detect the Sp100~SUMO product. The differences in temperatures and reaction times between the E3 variants were necessary to ensure that Sp100~SUMO can be accurately detected. (B) Double reciprocal plots of data shown in (A). The assay mixture (4.2 μL) contained 0.78 μM E1, 0.35 μM E2, 2.6 μM E3, 4 mM ATP, 22.7 μM SUMO, and four Sp100 concentrations: 2.1, 4.2, 5.6, and 8.4 μM. E3 variants used: no E3 (NE), E3_linker, E3_GG insertion, and wild-type E3. Temperatures: 31–43°C, 10 min for NE and E3_linker assays; 25–37°C, 3 min for E3_GG insertion and wild-type E3 assays. Assays were quenched with freshly prepared 360 mM DTT loading buffer (8.5 μL), separated by SDS-PAGE and detected by Western blots using an anti-SUMO-1 antibody.
The double-reciprocal plots fit well to a line (Fig. 2B) based on R-values, and the slopes of the double reciprocal plots, corresponding to Km/kcat ratios, were well determined. The Km/kcat ratios correspond to the net transfer rate constant of SUMO from E2 to substrates. We found that kcat/Km were 1.7±0.6 ×102 (M−1s−1) and 1.1±0.4 ×105 (M−1s−1) in the absence and presence of IR1-M, respectively (Table 1). These results are very consistent with published values that were independently determined using single turnover reactions (transferring SUMO from E2~SUMO thioester to substrate). Recent measurements of k2/Kd, which is similar to kcat/Km, in the absence and presence of IR1-M were 2.3±0.3×102 (M−1s−1) and 9.4±0.9×104 (M−1s−1), respectively, for a substrate (MEF2p) that has similar modification efficiency as Sp100 (39). Thus, our results have been independently validated.
Table 1.
Summary of measured rates, activation entropy and enthalpy of the reactions as well as the calculated activation free energy in the absence of E3 and in the presence of wild-type and different E3 mutants with insertions.
| k (M−1 s−1)* | ΔH≠ (kcal/mol) | TΔS≠ (kcal/mol)* | ΔG≠ (kcal)* | |
|---|---|---|---|---|
| E3 | (1.1 +/− 0.4) × 105 | 24.0 +/− 2.4 | 12.9 +/− 2.2 | 11.0 +/− 3.3 |
| E3_GG insertion | (1.2 +/− 0.5) × 104 | 14.7 +/− 2.7 | 2.2 +/− 2.4 | 12.4 +/− 3.6 |
| E3_linker insertion | (1.8 +/− 0.6) × 103 | 12.0 +/− 1.1 | −1.4 +/− 0.9 | 13.6 +/− 1.4 |
| No E3 activity | (1.7 +/− 0.6) × 102 | 10.7 +/− 0.3 | −4.3 +/− 0.3 | 15.0 +/− 0.4 |
Values correspond to those measured at 37°C.
Eyring analysis was carried out to extract the activation entropy and enthalpy for reactions with and without E3 (Fig. 3, Table 1). The activation entropy, TΔS≠, without E3 showed a negative value (−4.3 kcal/mol), which suggests that the transition state is more orderly than the ground state of reactants in the absence of E3. Interestingly, reactions in the presence of wild-type E3 showed a large favorable activation entropy TΔS≠ (12.9 kcal/mol) that is the driving force for lowered activation Gibbs free energy to accelerate the reaction. The entropic advantage achieved by the E3 was reduced when flexible linkers were inserted between the SUMO and E2-binding sites: the longer the linker, the smaller the entropic advantage achieved by the E3 (Table 1). In contrast, reactions carried out with E3 had higher (unfavorable) activation enthalpy, ΔH≠, than reactions without E3. ΔH≠ was lower when the E3 contained linker insertions, and the longer the flexible linker, the lower the ΔH≠, with ΔH≠ being the lowest for reactions without E3 or with E3_ΔSIM (Table 1).
Figure 3.
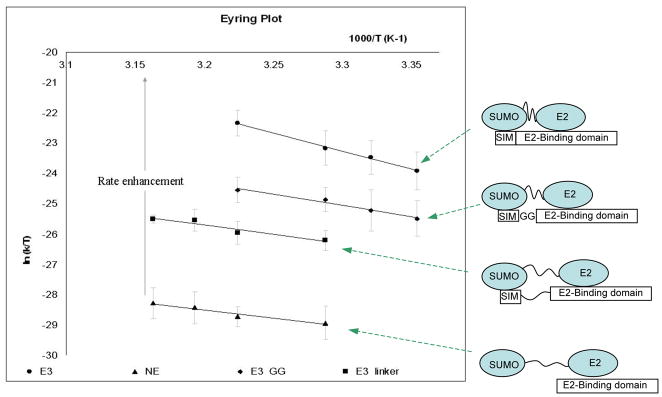
Eyring plots of kinetic experiments. Slopes of the plot were used to calculate the activation enthalpy, and the intercepts were used to calculate the activation entropy. Included are schematic drawings of each E3 variant and their expected effects on the E2~SUMO thioester conjugate.
The effect of E3 on the stability of SUMO~E2 thioester and its interaction with water
To gain insight into the origin of the unfavorable change of activation enthalpy and favorable change of activation entropy, we carried out molecular dynamics (MD) simulations. It was not feasible to investigate the SUMO~Ubc9 thioester conjugate experimentally, because it converts to Ubc9-SUMO isopeptide bond within an hour (32). In addition, thioester mimics using either ester bond or disulfide bond had low solubility, likely due to the non-covalent interaction between SUMO and the N-terminal region of Ubc9 (40), resulting in the formation of large aggregates of the thioester mimic at concentrations required for structural studies.
Similarly to a recent report, the structure of RanBP2 in complex with SUMOylated RanGAP1 and Ubc9 were used to deduce the structure of E2~SUMO thioester in complex with the E3 (8, 31). Two MD simulations were designed to mimic the thioester conjugate: one with the E3 and the other one with the E3 deleted. In order to mimic SUMO~Ubc9 thioester, the isopeptide bond between SUMO1 (G97) and RanGAP1 (K524) was broken, and a thioester bond between SUMO1 and UBC9 was constructed without significant structural alteration, because the two atoms to be bonded are already in close proximity (~3.5 Å). The simulation was carried out for 125 ns for the complex without E3 and for 150 ns for the complex with E3.
As found in the recent report, analysis of the structures in MD simulations confirmed that SUMO1-Ubc9 thioester explores a much smaller conformation space in the present of E3 than in the absence of E3 (Fig. 4A and 4B). The relative flexibility of SUMO and the E2 in their thioester conjugate is consistent with experimental observations of ubiquitin~E2 thioester conjugates (27–30), and is expected due to the lack of extensive contacts between SUMO and Ubc9 that are needed for a stable structure. The flexibility observed for the E2~SUMO thioester conjugate in MD simulation is not as extensive as that observed by NMR and small angle X-ray scattering studies of ubiquitin~E2 thioester mimetics (27), likely due to the short simulation time. The more favorable activation entropy in the presence of the E3 than in the absence of the E3 is agreeable with the recently reported MD studies suggesting that the E3 binding may select the population that favors the reaction and substrate binding. In addition, if the transition state remains the same with and without the presence of E3, the more ordered thioester reactive center in the ground state in the presence of E3 than in the absence of E3 would reduce the entropic penalty for the formation of transition state. However, this model does not fully account for the large positive activation entropy observed above.
Figure 4.
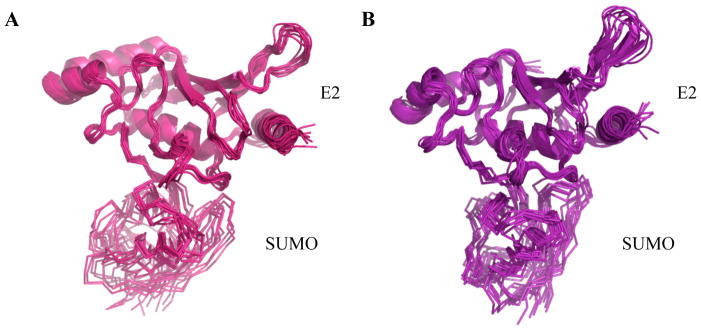
Superposition of the structures of the E2~SUMO thioester conjugate from the last 50 ns of simulations in the absence (A) and presence (B) of the E3. A structure from every 5 ns was taken for the superposition. The superposition minimized the root-mean-square-deviation of the E2 only; thus fluctuation in the SUMO structures reflects variations in the relative positioning between the two proteins in the thioester conjugate.
The large positive activation entropy, which indicates that the ground state is more orderly than the transition state, suggests ordered water is released upon formation of transition state. Thus we carried out detailed analysis of residence time of water molecules and locations of structured water along the MD trajectory. The more stable E2~SUMO thioester in the presence of the E3 results in more stably bound water at the thioester reaction site as well as at the SUMO-E2 interface. Table 2 shows the residence time of water molecules that stayed within 5 Å of residues involved in the chemistry, K524 (RanGap1), C93(UBC9) and G97(SUMO1), for longer than 10 ns during the simulation. The E3-bound structure not only has more water molecules that stayed for more than 10 ns near the thioester bond, but also with longer residence time. The waters are found to form hydrogen bonds with polar sidechains and backbone. Snapshots of the MD trajectory illustrate the positions of some of these bound waters in the vicinity of the thioester bond in the presence of the E3 (Fig. 5A) and in the absence of the E3 (Fig. 5B). The long-residence water molecules did not always stay in a particular hydrogen-bonding pattern, but stayed in the same area. Conformational changes upon the formation of the transition state likely cause the release of water bound at ground state. Similar mechanism has been implicated for other enzyme catalytic mechanisms such as ribosomal catalysis of peptide synthesis (41–45). Two structured water molecules were also found at the interface between RanGAP1 and Ubc9 (E2), which may contribute to the slightly enhanced affinity (approximately 7 fold) of the substrate for E2 in E3 stimulated reactions (8). These water molecules were not present in the crystal structure of SUMOylated RanGAP1 in complex with Ubc9 and RanBP2, possibly due to the lack of the thioester bond and experimental conditions.
Figure 5.
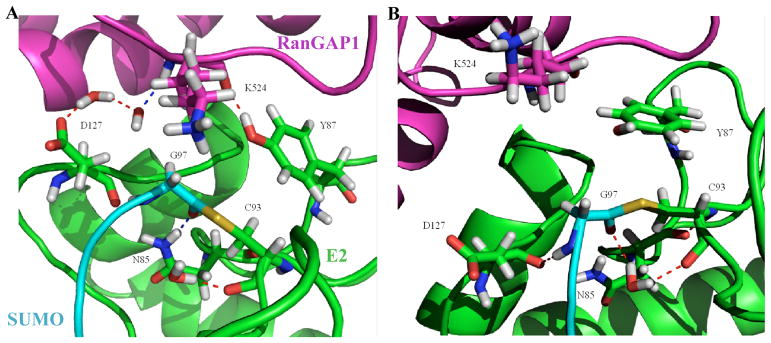
Molecular dynamics simulations to obtain insights into the entropy driven mechanism. Snapshots of the molecular dynamics trajectory to show the water molecules in the vicinity of the thioester bond that stayed for more than 10 ns in the presence (A) and absence (B) of the E3. Sidechains of key residues are shown: N85, Y87, C93, and D127 of the E2 (green), which are important in catalysis, the conjugation residue G97 of SUMO (cyan), and the modification residue K524 of RanGAP1 (magenta).
E3 may also contribute to an overall entropic force to the reaction through water molecules that are stabilized at the interface between SUMO1 and UBC9 (Table 3 and Fig. 6). Along the MD trajectory, more than twice the number of water molecules (as many as 44) stayed in a “water pocket” at their interfaces for more than 10 ns in the presence of the E3 than in the absence of the E3, apparently due to the more stable structure of the thioester conjugate in the complex with the E3. Several water molecules in this location were observed in the crystal structure of SUMOylated RanGAP1 in complex with Ubc9 and RanBP2 (8). These “trapped” water molecules are expected to be lost upon the completion of the reaction when SUMO-1 dissociates from E2, and contribute to the overall entropic force of the E3 stimulated reaction.
Figure 6.
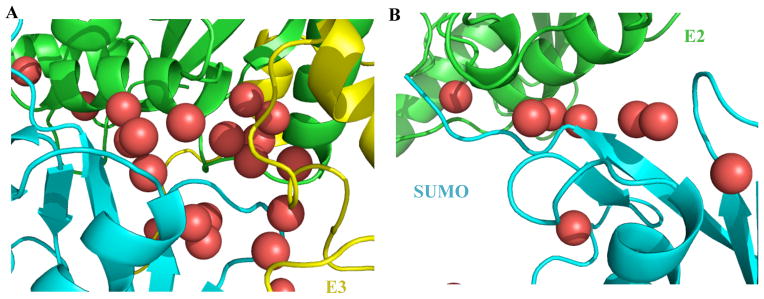
Snapshots of the molecular dynamics trajectory showing the water molecules at the interface between SUMO and Ubc9 in the presence (A) and absence (B) of the E3. Water molecules are shown as red balls, and SUMO, Ubc9 and the E3 are shown with the same color codes as their labels.
DISCUSSION
In this study, we have measured activation entropy and enthalpy for ubiquitin-like modifications for the first time, and found that the E3, RanBP2, confers a large entropic effect to lower the activation energy, thereby accelerating the reactions. Molecular dynamic simulations suggest that solvent effect is likely a major contribution to the entropy driven mechanism. The more stable structure of the E2~SUMO thioester conjugate in the presence of E3 than in the absence of E3 traps more stably bound water. Liberating such structured water to bulk water at the transition state can reduce order and result in favorable activation entropy (1). Such model is also consistent with the observation of less favorable activation enthalpy in the presence of E3 than in the absence of E3, which may reflect the breakage of hydrogen bonds of ordered water. The present study does not exclude the contributions of other factors to the favorable activation entropy, but suggests a novel concept in E3 catalysis that is consistent with all experimental and simulation studies.
The effect of the E3 on activation entropy and enthalpy suggests that the E3 unlikely confers a structural allosteric effect to improve the interaction of the E2 with the transition state, like many well studied enzymes, because the activation enthalpy, which reflects direct interactions through hydrogen bonding, hydrophobic and ionic interactions, is more unfavorable in the presence than in the absence of the E3. In addition, similar activity of E3_G and E3_GG suggests that the determinants of the E3 ligase activity do not lie in its ability to confer a structural effect on the E2~SUMO thioester. In stead, the linker-length dependent activities of the E3 variants are consistent with a dynamic effect. Taken together, these data suggest that the ability of RanBP2 to activate SUMOylation depends on its ability to restrict the flexibility of E2~SUMO thioester conjugates.
The novel concept proposed here offers a unifying theme for the allosteric effect of E3. Enthalpic effects are generally evident from X-ray and NMR structures, which include hydrogen bonds and hydrophic and charge interactions. Entropic effects, on the other hand, arise from a diverse combination of factors due to solvent and energetics of conformational ensembles, as discussed above. Entropic effects explain the poor correlation of structure and affinity of E2-E3 complexes with the activities of E3. Previous studies have also indicated that the E2-bound ubiquitin plays an important role in E3-stimulated ubiquitination reactions (46, 47). Even polycations or Zn2+ ion can stimulate ubiquitination reactions (48, 49), and they can reduce the flexibility of the E2~Ubl thioester by charge interactions. The E3 ligase activity of many ubiquitin E3 ligases depends on its affinity for both E2 and target proteins (6). By bringing the reactants (the ubiquitin~E2 thioester and a target protein) together, there is a significantly reduced loss of rotational and translational entropies of the substrates upon forming the transition state, contributing to favorable activation entropy. Overall, the entropy-driven mechanism, particularly the solvent effect due to the stablilized E2~SUMO thioester by E3 binding, does not require a conserved structure of E3, and may be a general theme of E3’s allosteric effect.
This study also provides further mechanistic insights into the action of E3 ligases that lack a conserved E2-binding motif, but contain a SIM, suggesting that a protein containing a SIM can be potential E3 ligase if it also binds Ubc9. Although only a handful of proteins have been identified as SIM-dependent E3 ligases, this study supports the possibility that many more such E3 ligases exist (11–13, 50).
This study also draws attention to a previously unappreciated function of intrinsically disordered proteins (IDPs)—activating macromolecular chemistry, such as ubiquitin-like modifications. The known prevailing function of IDPs is to bind multiple proteins simultaneously (51). Thus, they frequently mediate signal transduction pathways and function as scaffolds for protein complex formation during transcription and DNA repair. The ability of IR1-M, an IDP, to bind two proteins, SUMO and Ubc9, is required for its E3 ligase activity. This study has shown how IDPs, by binding multiple proteins, can activate macromolecular chemistry instead of merely functioning as scaffold proteins in these events. We hope that this work stimulates interest to carry out further theoretical and experimental studies to investigate the generality of the entropy driven mechanism of E3 ligases in ubiquitin-like modifications.
Acknowledgments
This work was supported by grants from the NIH GM086171 and GM074748 (to Y.C.).
We are grateful to Professor Roman Osman for many helpful discussions, and to Professor Nagarajan Vaidehi for critical reading of the manuscript.
ABBREVIATIONS
- SUMO
Small Ubiquitin-like Modifier
- SIM
SUMO-interacting motif, which is also known as SUMO-binding motif (SBM)
- E1
activating enzyme for ubiquitin-like post-translational modifications
- E2
conjugation enzyme for ubiquitin-like modifications
- E3
ligase for ubiquitin-like modifications
- SAE
SUMO activating enzyme
- RanBP2
Ran binding protein 2
- GST
Glutathione S-transferase
- IR1
internal repeat domain 1 of RanBP2
- PBS
phosphate buffer saline
- DTT
dithiothreitol
- NMR
nuclear magnetic resonance
- SDS PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
- 1.Dunitz JD. The entropic cost of bound water in crystals and biomolecules. Science (New York, NY. 1994;264:670. doi: 10.1126/science.264.5159.670. [DOI] [PubMed] [Google Scholar]
- 2.Privalov PL, Dragan AI, Crane-Robinson C, Breslauer KJ, Remeta DP, Minetti CA. What drives proteins into the major or minor grooves of DNA? Journal of molecular biology. 2007;365:1–9. doi: 10.1016/j.jmb.2006.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beringer M, Rodnina MV. The ribosomal peptidyl transferase. Molecular cell. 2007;26:311–321. doi: 10.1016/j.molcel.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Cozzini P, Fornabaio M, Marabotti A, Abraham DJ, Kellogg GE, Mozzarelli A. Free energy of ligand binding to protein: evaluation of the contribution of water molecules by computational methods. Current medicinal chemistry. 2004;11:3093–3118. doi: 10.2174/0929867043363929. [DOI] [PubMed] [Google Scholar]
- 5.Hershko A, Ciechanover A. The ubiquitin system. Annual review of biochemistry. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 6.Varshavsky A. The ubiquitin system. Trends in biochemical sciences. 1997;22:383–387. doi: 10.1016/s0968-0004(97)01122-5. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman ES, Schulman BA, Zheng N. Structural assembly of cullin-RING ubiquitin ligase complexes. Current opinion in structural biology. 20:714–721. doi: 10.1016/j.sbi.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reverter D, Lima CD. Insights into E3 ligase activity revealed by a SUMO-RanGAP1-Ubc9-Nup358 complex. Nature. 2005;435:687–692. doi: 10.1038/nature03588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annual review of biochemistry. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 10.Yang SH, Sharrocks AD. The SUMO E3 ligase activity of Pc2 is coordinated through a SUMO interaction motif. Molecular and cellular biology. 2010;30:2193–2205. doi: 10.1128/MCB.01510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kagey MH, Melhuish TA, Wotton D. The polycomb protein Pc2 is a SUMO E3. Cell. 2003;113:127–137. doi: 10.1016/s0092-8674(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 12.Kagey MH, Melhuish TA, Powers SE, Wotton D. Multiple activities contribute to Pc2 E3 function. Embo J. 2005;24:108–119. doi: 10.1038/sj.emboj.7600506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang PC, Izumiya Y, Wu CY, Fitzgerald LD, Campbell M, Ellison TJ, Lam KS, Luciw PA, Kung HJ. Kaposi’s sarcoma-associated herpesvirus (KSHV) encodes a SUMO E3 ligase that is SIM-dependent and SUMO-2/3-specific. The Journal of biological chemistry. 285:5266–5273. doi: 10.1074/jbc.M109.088088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The Nucleoporin RanBP2 Has SUMO1 E3 Ligase Activity. Cell. 2002;108:109–120. doi: 10.1016/s0092-8674(01)00633-x. [DOI] [PubMed] [Google Scholar]
- 15.Dawlaty MM, Malureanu L, Jeganathan KB, Kao E, Sustmann C, Tahk S, Shuai K, Grosschedl R, van Deursen JM. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIalpha. Cell. 2008;133:103–115. doi: 10.1016/j.cell.2008.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatham MH, Kim S, Jaffray E, Song J, Chen Y, Hay RT. Unique binding interactions among Ubc9, SUMO and RanBP2 reveal a mechanism for SUMO paralog selection. Nature structural & molecular biology. 2005;12:67–74. doi: 10.1038/nsmb878. [DOI] [PubMed] [Google Scholar]
- 17.Song J, Durrin LK, Wilkinson TA, Krontiris TG, Chen Y. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14373–14378. doi: 10.1073/pnas.0403498101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahyo T, Nishida T, Yasuda H. Involvement of PIAS1 in the sumoylation of tumor suppressor p53. Molecular cell. 2001;8:713–718. doi: 10.1016/s1097-2765(01)00349-5. [DOI] [PubMed] [Google Scholar]
- 19.Tatham MH, Kim S, Yu B, Jaffray E, Song J, Zheng J, Rodriguez MS, Hay RT, Chen Y. Role of an N-terminal site of Ubc9 in SUMO-1, -2, and -3 binding and conjugation. Biochemistry. 2003;42:9959–9969. doi: 10.1021/bi0345283. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen WL, Chandraskhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulationg liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 21.Philips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. CHARMM: A program for macromolecular energy, minimization, and dynamics calculations. J Comput Chem. 1983;4:187–217. [Google Scholar]
- 23.Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. A smooth particle mesh Ewald method. J Chem Phys. 1995;103:8577–8593. [Google Scholar]
- 24.Darden T, York D, Pedersen LG. Particle mesh Ewald: An N. Log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- 25.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. Journal of molecular graphics. 1996;14:33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 26.Matunis MJ, Wu J, Blobel G. SUMO-1 modification and its role in targeting the Ran GTPase-activating protein, RanGAP1, to the nuclear pore complex. J Cell Biol. 1998;140:499–509. doi: 10.1083/jcb.140.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pruneda JN, Stoll KE, Bolton LJ, Brzovic PS, Klevit RE. Ubiquitin in motion: structural studies of the ubiquitin-conjugating enzyme approximately ubiquitin conjugate. Biochemistry. 2011;50:1624–1633. doi: 10.1021/bi101913m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton KS, Ellison MJ, Shaw GS. Identification of the ubiquitin interfacial residues in a ubiquitin-E2 covalent complex. Journal of biomolecular NMR. 2000;18:319–327. doi: 10.1023/a:1026773008237. [DOI] [PubMed] [Google Scholar]
- 29.Miura T, Klaus W, Gsell B, Miyamoto C, Senn H. Characterization of the binding interface between ubiquitin and class I human ubiquitin-conjugating enzyme 2b by multidimensional heteronuclear NMR spectroscopy in solution. Journal of molecular biology. 1999;290:213–228. doi: 10.1006/jmbi.1999.2859. [DOI] [PubMed] [Google Scholar]
- 30.Serniwka SA, Shaw GS. The structure of the UbcH8-ubiquitin complex shows a unique ubiquitin interaction site. Biochemistry. 2009;48:12169–12179. doi: 10.1021/bi901686j. [DOI] [PubMed] [Google Scholar]
- 31.Tozluoglu M, Karaca E, Nussinov R, Haliloglu T. A mechanistic view of the role of E3 in sumoylation. PLoS computational biology. 6 doi: 10.1371/journal.pcbi.1000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knipscheer P, Flotho A, Klug H, Olsen JV, van Dijk WJ, Fish A, Johnson ES, Mann M, Sixma TK, Pichler A. Ubc9 sumoylation regulates SUMO target discrimination. Mol Cell. 2008;31:371–382. doi: 10.1016/j.molcel.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Bohnsack RN, Haas AL. Conservation in the mechanism of Nedd8 activation by the human AppBp1-Uba3 heterodimer. The Journal of biological chemistry. 2003;278:26823–26830. doi: 10.1074/jbc.M303177200. [DOI] [PubMed] [Google Scholar]
- 34.Haas AL, Bright PM. The resolution and characterization of putative ubiquitin carrier protein isozymes from rabbit reticulocytes. The Journal of biological chemistry. 1988;263:13258–13267. [PubMed] [Google Scholar]
- 35.Siepmann TJ, Bohnsack RN, Tokgoz Z, Baboshina OV, Haas AL. Protein interactions within the N-end rule ubiquitin ligation pathway. The Journal of biological chemistry. 2003;278:9448–9457. doi: 10.1074/jbc.M211240200. [DOI] [PubMed] [Google Scholar]
- 36.Lin D, Tatham MH, Yu B, Kim S, Hay RT, Chen Y. Identification of a substrate recognition site on Ubc9. The Journal of biological chemistry. 2002;277:21740–21748. doi: 10.1074/jbc.M108418200. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Hu W, Cai S, Lee B, Song J, Chen Y. The intrinsic affinity between E2 and the Cys domain of E1 in ubiquitin-like modifications. Mol Cell. 2007;27:228–237. doi: 10.1016/j.molcel.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Lee B, Cai S, Fukui L, Hu W, Chen Y. Conformational transition associated with E1–E2 interaction in small ubiquitin-like modifications. The Journal of biological chemistry. 2009;284:20340–20348. doi: 10.1074/jbc.M109.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohideen F, Capili AD, Bilimoria PM, Yamada T, Bonni A, Lima CD. A molecular basis for phosphorylation-dependent SUMO conjugation by the E2 UBC9. Nature structural & molecular biology. 2009;16:945–952. doi: 10.1038/nsmb.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Q, Jin C, Liao X, Shen Z, Chen DJ, Chen Y. The binding interface between an E2 (UBC9) and a ubiquitin homologue (UBL1) The Journal of biological chemistry. 1999;274:16979–16987. doi: 10.1074/jbc.274.24.16979. [DOI] [PubMed] [Google Scholar]
- 41.Wallin G, Aqvist J. The transition state for peptide bond formation reveals the ribosome as a water trap. Proceedings of the National Academy of Sciences of the United States of America. 107:1888–1893. doi: 10.1073/pnas.0914192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schroeder GK, Wolfenden R. The rate enhancement produced by the ribosome: an improved model. Biochemistry. 2007;46:4037–4044. doi: 10.1021/bi602600p. [DOI] [PubMed] [Google Scholar]
- 43.Sharma PK, Xiang Y, Kato M, Warshel A. What are the roles of substrate-assisted catalysis and proximity effects in peptide bond formation by the ribosome? Biochemistry. 2005;44:11307–11314. doi: 10.1021/bi0509806. [DOI] [PubMed] [Google Scholar]
- 44.Friedman R, Nachliel E, Gutman M. Molecular dynamics of a protein surface: ion-residues interactions. Biophysical journal. 2005;89:768–781. doi: 10.1529/biophysj.105.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sievers A, Beringer M, Rodnina MV, Wolfenden R. The ribosome as an entropy trap. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7897–7901. doi: 10.1073/pnas.0402488101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siepmann TJ, Bohnsack RN, Tokgoz Z, Baboshina OV, Haas AL. Protein interactions within the N-end rule ubiquitin ligation pathway. The Journal of biological chemistry. 2003 doi: 10.1074/jbc.M211240200. [DOI] [PubMed] [Google Scholar]
- 47.Deffenbaugh AE, Scaglione KM, Zhang L, Moore JM, Buranda T, Sklar LA, Skowyra D. Release of ubiquitin-charged Cdc34-S - Ub from the RING domain is essential for ubiquitination of the SCF(Cdc4)-bound substrate Sic1. Cell. 2003;114:611–622. doi: 10.1016/s0092-8674(03)00641-x. [DOI] [PubMed] [Google Scholar]
- 48.Seol JH, Feldman RM, Zachariae W, Shevchenko A, Correll CC, Lyapina S, Chi Y, Galova M, Claypool J, Sandmeyer S, Nasmyth K, Deshaies RJ, Shevchenko A, Deshaies RJ. Cdc53/cullin and the essential Hrt1 RING-H2 subunit of SCF define a ubiquitin ligase module that activates the E2 enzyme Cdc34. Genes & development. 1999;13:1614–1626. doi: 10.1101/gad.13.12.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Z, Li B, Bharadwaj R, Zhu H, Ozkan E, Hakala K, Deisenhofer J, Yu H. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Molecular biology of the cell. 2001;12:3839–3851. doi: 10.1091/mbc.12.12.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang SH, Sharrocks AD. The SUMO E3 ligase activity of Pc2 is coordinated through a SUMO-interaction motif. Molecular and cellular biology. doi: 10.1128/MCB.01510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, Oldfield CJ, Campen AM, Ratliff CM, Hipps KW, Ausio J, Nissen MS, Reeves R, Kang C, Kissinger CR, Bailey RW, Griswold MD, Chiu W, Garner EC, Obradovic Z. Intrinsically disordered protein. J Mol Graph Model. 2001;19:26–59. doi: 10.1016/s1093-3263(00)00138-8. [DOI] [PubMed] [Google Scholar]