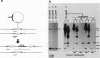
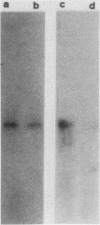
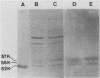
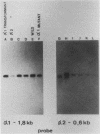
Abstract
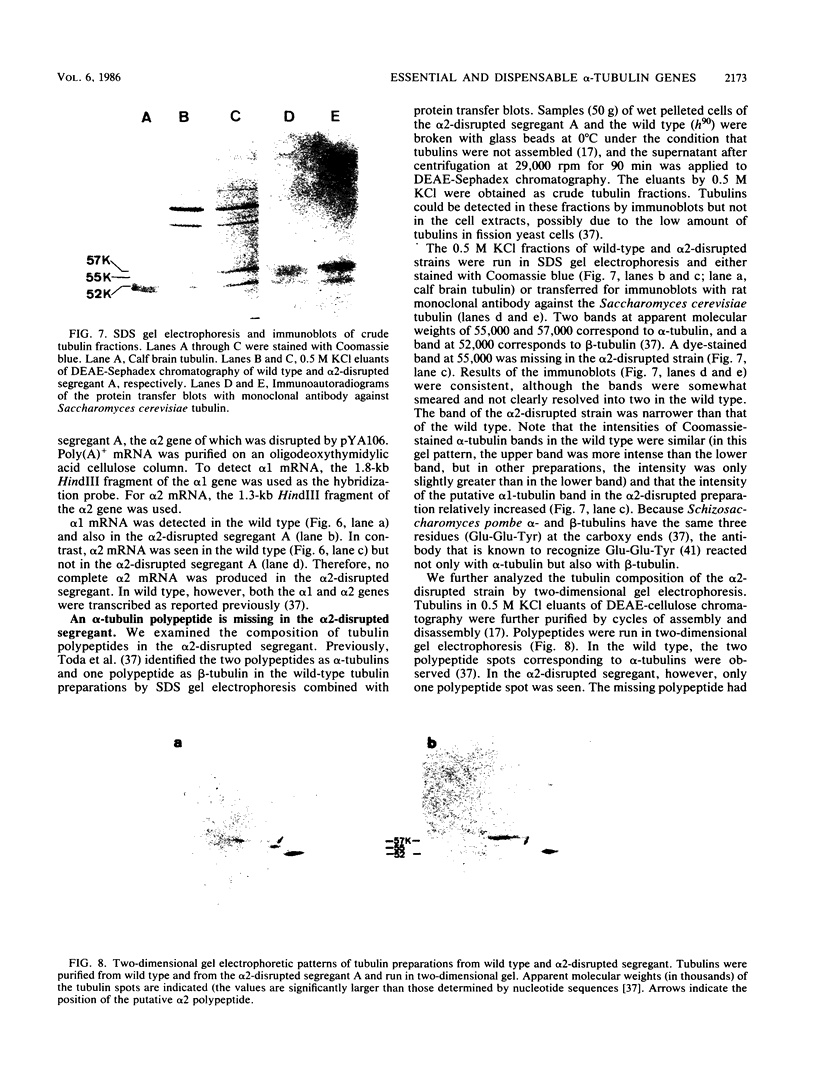
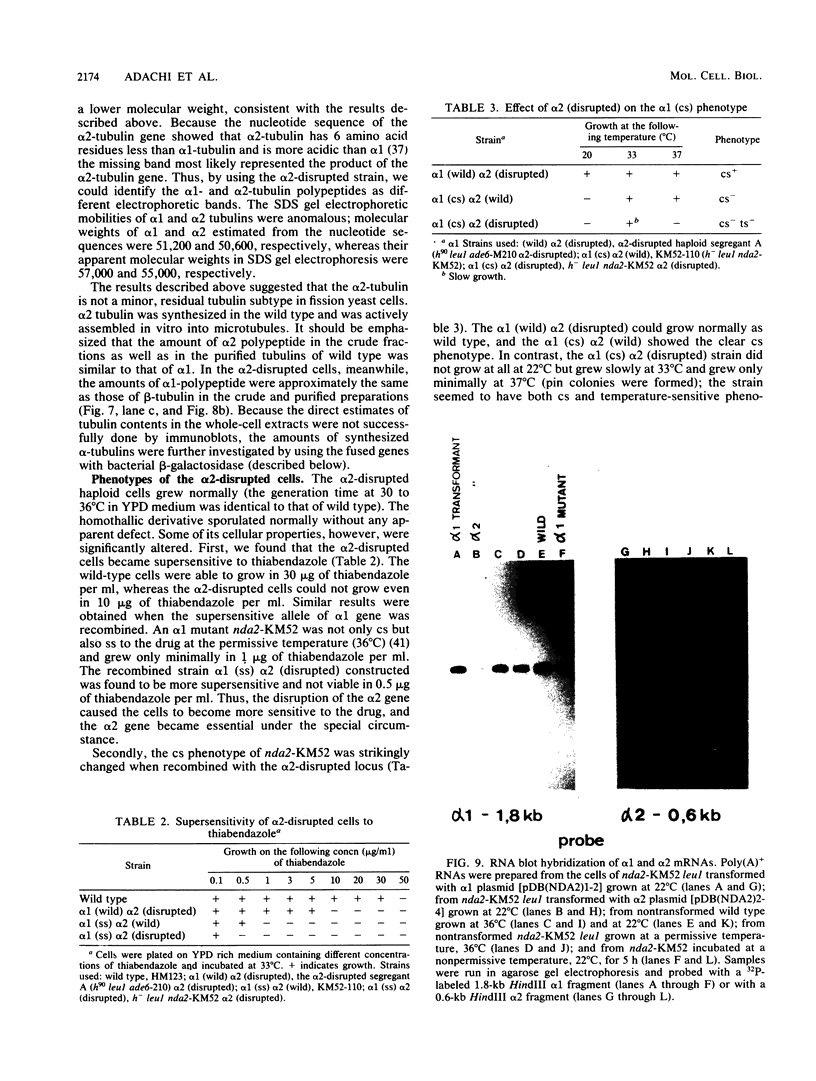
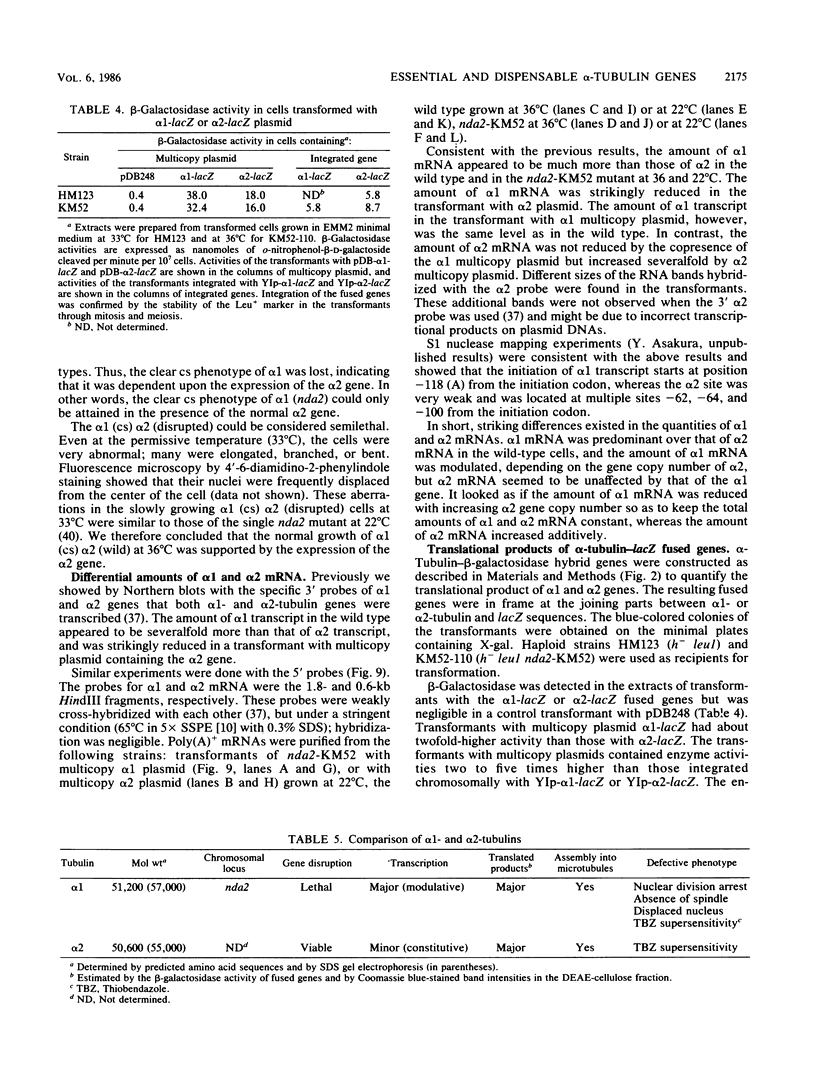
The fission yeast Schizosaccharomyces pombe has two alpha-tubulin genes and one beta-tubulin gene. Gene disruption experiments showed that the alpha 1-tubulin gene (NDA2) is essential whereas the alpha 2 gene is dispensable. The alpha 2-disrupted cells missing alpha 2 transcript and alpha 2 polypeptide could grow and sporulate normally. The alpha 2 gene, however, was expressed in the wild type and the alpha 1 mutant. Alpha 2-Tubulin was distinguished as an electrophoretic band and was assembled into microtubules. The alpha 2-disrupted cells had an increased sensitivity to an antimicrotubule drug thiabendazole, and the alpha 1(cold-sensitive [cs]) alpha 2 (disrupted) cells became not only cs but also temperature sensitive. Northern blot experiments indicated that alpha 2 transcription was minor and constitutive whereas alpha 1 transcription was major and modulated, depending on the gene copy number of the alpha 2 gene. The amounts of alpha 1 and alpha 2 polypeptides estimated by beta-galactosidase activities of the lacZ-fused genes integrated on the chromosome and by intensities of the electrophoretic bands in crude tubulin fractions, however, were comparable, indicating that alpha 2 tubulin is not a minor subtype. We assume that the cells of Schizosaccharomyces pombe have no excess tubulin pool. alpha 1 mutants would then be blocked in the cell cycle because only half the amount of functional alpha-tubulin required for growth can be produced by the alpha 2 gene. On the other hand, the alpha 2-disrupted cells became viable because the synthesis of alpha 1 tubulin was increased by transcriptional or translational modulation or both. The real cause for essential alpha 1 and dispensable alpha 2 genes seems to be in their regulatory sequences instead of the coding sequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach D., Piper M., Nurse P. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol Gen Genet. 1982;187(2):326–329. doi: 10.1007/BF00331138. [DOI] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Havercroft J. C. Is apparent autoregulatory control of tubulin synthesis nontranscriptionally regulated? J Cell Biol. 1983 Sep;97(3):919–924. doi: 10.1083/jcb.97.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Pittenger M. F., Feramisco J. R. Elevation of tubulin levels by microinjection suppresses new tubulin synthesis. Nature. 1983 Oct 20;305(5936):738–740. doi: 10.1038/305738a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Sullivan K. F. Molecular biology and genetics of tubulin. Annu Rev Biochem. 1985;54:331–365. doi: 10.1146/annurev.bi.54.070185.001555. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. The tubulins: from DNA to RNA to protein and back again. Cell. 1983 Sep;34(2):330–332. doi: 10.1016/0092-8674(83)90366-5. [DOI] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y., Toda T., Yanagida M. The NDA3 gene of fission yeast encodes beta-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984 Dec;39(2 Pt 1):349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfayan L., Wensink P. C. alpha-Tubulin genes of Drosophila. Cell. 1981 Apr;24(1):97–106. doi: 10.1016/0092-8674(81)90505-5. [DOI] [PubMed] [Google Scholar]
- Kemphues K. J., Kaufman T. C., Raff R. A., Raff E. C. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982 Dec;31(3 Pt 2):655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V. Purification of yeast tubulin by self-assembly in vitro. Biochemistry. 1981 Jun 9;20(12):3629–3633. doi: 10.1021/bi00515a050. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Havercroft J. C., Chow L. T., Cleveland D. W. Four unique genes required for beta tubulin expression in vertebrates. Cell. 1983 Mar;32(3):713–724. doi: 10.1016/0092-8674(83)90057-0. [DOI] [PubMed] [Google Scholar]
- Minami S. A., Collis P. S., Young E. E., Weeks D. P. Tubulin induction in C. reinhardii: requirement for tubulin mRNA synthesis. Cell. 1981 Apr;24(1):89–95. doi: 10.1016/0092-8674(81)90504-3. [DOI] [PubMed] [Google Scholar]
- Mischke D., Pardue M. L. Organization and expression of alpha-tubulin genes in Drosophila melanogaster. One member of the alpha-tubulin multigene family is transcribed in both oogenesis and later embryonic development. J Mol Biol. 1982 Apr 15;156(3):449–466. doi: 10.1016/0022-2836(82)90260-1. [DOI] [PubMed] [Google Scholar]
- Neff N. F., Thomas J. H., Grisafi P., Botstein D. Isolation of the beta-tubulin gene from yeast and demonstration of its essential function in vivo. Cell. 1983 May;33(1):211–219. doi: 10.1016/0092-8674(83)90350-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H., O'Farrell P. Z. Two-dimensional polyacrylamide gel electrophoretic fractionation. Methods Cell Biol. 1977;16:407–420. doi: 10.1016/s0091-679x(08)60116-8. [DOI] [PubMed] [Google Scholar]
- Rose M., Botstein D. Construction and use of gene fusions to lacZ (beta-galactosidase) that are expressed in yeast. Methods Enzymol. 1983;101:167–180. doi: 10.1016/0076-6879(83)01012-5. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Russell P. R., Hall B. D. Structure of the Schizosaccharomyces pombe cytochrome c gene. Mol Cell Biol. 1982 Feb;2(2):106–116. doi: 10.1128/mcb.2.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rykowski M. C., Wallis J. W., Choe J., Grunstein M. Histone H2B subtypes are dispensable during the yeast cell cycle. Cell. 1981 Aug;25(2):477–487. doi: 10.1016/0092-8674(81)90066-0. [DOI] [PubMed] [Google Scholar]
- Schedl T., Burland T. G., Gull K., Dove W. F. Cell cycle regulation of tubulin RNA level, tubulin protein synthesis, and assembly of microtubules in Physarum. J Cell Biol. 1984 Jul;99(1 Pt 1):155–165. doi: 10.1083/jcb.99.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Haber J. E., Botstein D. Lethal disruption of the yeast actin gene by integrative DNA transformation. Science. 1982 Jul 23;217(4557):371–373. doi: 10.1126/science.7046050. [DOI] [PubMed] [Google Scholar]
- Silflow C. D., Rosenbaum J. L. Multiple alpha- and beta-tubulin genes in Chlamydomonas and regulation of tubulin mRNA levels after deflagellation. Cell. 1981 Apr;24(1):81–88. doi: 10.1016/0092-8674(81)90503-1. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Adachi Y., Hiraoka Y., Yanagida M. Identification of the pleiotropic cell division cycle gene NDA2 as one of two different alpha-tubulin genes in Schizosaccharomyces pombe. Cell. 1984 May;37(1):233–242. doi: 10.1016/0092-8674(84)90319-2. [DOI] [PubMed] [Google Scholar]
- Toda T., Umesono K., Hirata A., Yanagida M. Cold-sensitive nuclear division arrest mutants of the fission yeast Schizosaccharomyces pombe. J Mol Biol. 1983 Aug 5;168(2):251–270. doi: 10.1016/s0022-2836(83)80017-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Toda T., Hayashi S., Yanagida M. Cell division cycle genes nda2 and nda3 of the fission yeast Schizosaccharomyces pombe control microtubular organization and sensitivity to anti-mitotic benzimidazole compounds. J Mol Biol. 1983 Aug 5;168(2):271–284. doi: 10.1016/s0022-2836(83)80018-7. [DOI] [PubMed] [Google Scholar]
- Wehland J., Schröder H. C., Weber K. Amino acid sequence requirements in the epitope recognized by the alpha-tubulin-specific rat monoclonal antibody YL 1/2. EMBO J. 1984 Jun;3(6):1295–1300. doi: 10.1002/j.1460-2075.1984.tb01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M. Genetic analysis of resistant mutants to antimitotic benzimidazole compounds in Schizosaccharomyces pombe. Mol Gen Genet. 1980;180(1):231–234. doi: 10.1007/BF00267375. [DOI] [PubMed] [Google Scholar]