Abstract
The levels of circulating oxidized phospholipids (OxPLs) become increased in chronic and acute pathologic conditions such as hyperlipidemia, atherosclerosis, increased intimamedia thickness in the patients with systemic Lupus erythematosus, vascular balloon injury, acute lung injury (ALI), and acute respiratory distress syndrome (ARDS). These pathologies are associated with inflammation and activation of endothelial cells. Depending on the biological context and the specific group of phospholipid oxidation products, OxPL may exhibit both proinflammatory and anti-inflammatory effects. This review will summarize the data showing a dual role of OxPL in modulation of chronic and acute inflammation as well as OxPL effects on pulmonary endothelial permeability. Recent reports show protective effects of OxPL in the models of endotoxin and ventilator-induced ALI and suggest a potential for using OxPL-derived cyclopenthenone-containing compounds with barrier-protective properties for drug design. These compounds may represent a new group of therapeutic agents for the treatment of lung syndromes associated with acute inflammation and lung vascular leak.
Abbreviations: ALI, acute lung injury; cAMP, cyclic adenosine monophosphate; COX-2, cyclooxygenase-2; CS1, connecting segment 1; EC, endothelial cell; eNOS, endothelial nitric oxide synthase; Erk1/2, extracellular signaling kinase 1/2; EGR-1, early growth response factor-1; FAK, focal adhesion kinase; GAS, gamma-interferon activation sequence; GPCR, G-protein-coupled receptor; GPI, glycosylphosphatidylinositol; GTP, guanosine triphosphate; HO-1, heme oxygenase-1; ICAM-1, intercellular adhesion molecule-1, IL-8, interleukin-8; KOdiA-PC, 5-keto-6-octendioic acid ester of 2-lyso-phosphocholine; LBP, LPS binding protein; LDL, low-density lipoprotein; L-NAME, N-nitro-L-arginine-methyl ester; LPS, lipopolysaccharide; MCP1, monocyte chemotactic protein 1; MLC, myosin light chain; MLC, myosin light chain; MM-LDL, minimally modified LDL; mRNA, messenger RNA; NFκB, nuclear factor κB; OxLDL, oxidated LDL; OxPAPC, oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine; OxPL, oxidized phospholipids; PAF, platelet activation factor; PAPC, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine; PAPE, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylethanolamine; PAPS, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylserine; PECPC, 1-palmitoyl-2-(5,6-epoxycyclopentenone)-sn-glycero-3-phsphocholine; PEIPC, 1-palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phsphocholine; PGE2, prostaglandin E2; PGPC, 1-palmitoyl-2-glutaroyl-sn-glycero-phosphocholine; PKA, protein kinase A; PKC, protein kinase C; PLA2, phospholipase A2; POVPC, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphocholine; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; siRNA, small interfering RNA; SREBP, sterol response element binding protein; TF, tissue factor; TLR, toll-like receptor; TNF-α, tumor necrosis factor-α; UPR, unfolded protein response; VCAM-1, vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor; VILI, ventilator-induced lung injury
Increased levels of oxidized phospholipids (OxPL), which result from an enhanced generation of reactive oxygen species (ROS) or decreased antioxidant defense, are involved in certain pathologic conditions such as lung inflammation,1, 2 ventilator-induced lung injury (VILI), atherosclerosis,3, 4 and cell apoptosis.5, 6 Cell membrane phospholipids and phospholipids contained in the circulating lipoproteins are the major source for OxPL. The cell membrane and low-density lipoproteins (LDLs) are enriched in phospholipids that contain polyunsaturated fatty acids, which are highly prone to oxidative modification. The formation of OxPL is initiated either by enzymes, such as lipoxygenase, or by reactive oxygen species (ROS). OxPL has been detected in human atherosclerotic lesions and the aortas of cholesterol-fed and heritable hyperlipidemic rabbits.3, 4, 7
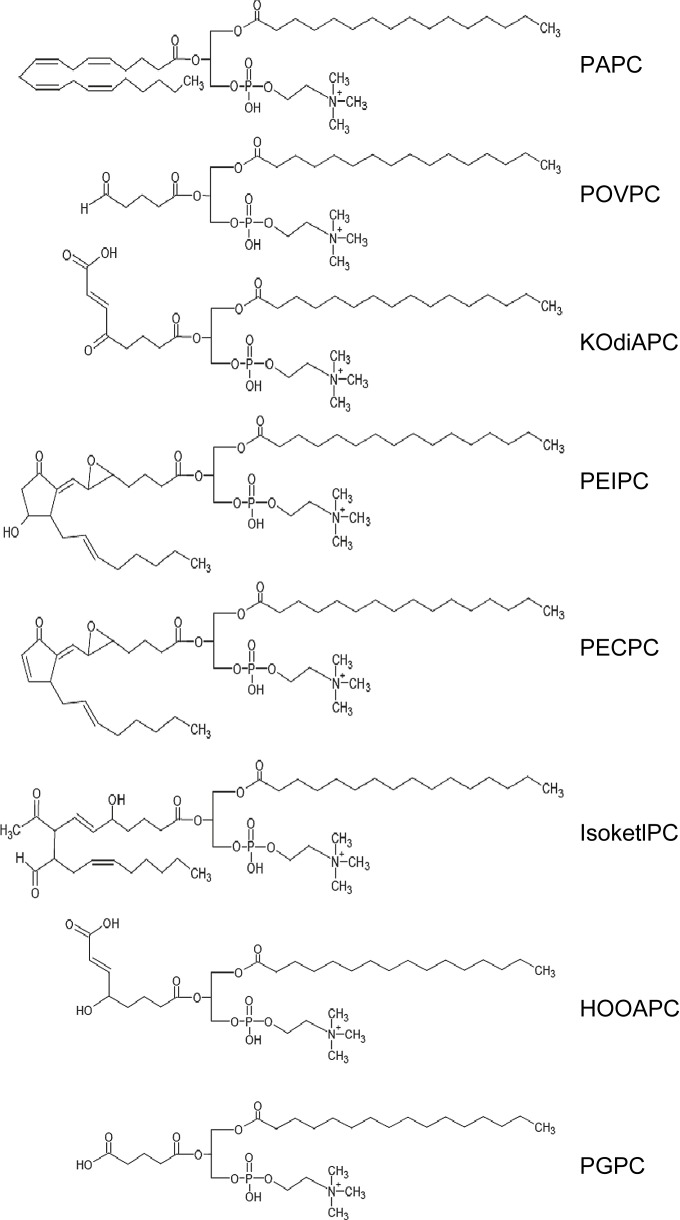
One major phospholipid present in plasma membrane and minimally modified LDL (MM-LDL) is 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC), which generates a heterogeneous group of oxygenated full-length products or compounds during oxidation with truncated oxidized residues present at the sn-2 position. Biologically active “fragmented OxPL,” in which the sn-2 fatty acid residues are oxidatively truncated, include 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphocholine (POVPC), 1-palmitoyl-2-glutaroyl-sn-glycero-phosphocholine (PGPC), and 5-keto-6-octendioic acid eater of 2-lyso-phosphocholine (KOdiA-PC). The other group of OxPLs, such as 1-palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phsphocholine (PEIPC) and 1-palmitoyl-2-(5,6-epoxycyclopentenone)-sn-glycero-3-phsphocholine (PECPC), represents “oxygenated OxPL,” which is generated through the addition of oxygen atoms to the sn-2 fatty acid residues. The chemical structures of these biologically active OxPL are shown in Fig 1 . In addition to phosphatidylcholine, other classes of OxPL that contain different polar heads and fatty acids, such as 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylethanolamine (PAPE) and 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylserine (PAPS), may undergo oxidative modification. This structural diversity accounts for a remarkable variety of biological activities of OxPL, which are described below.
Fig 1.
Chemical structures of biologically active oxidized phospholipids. The abbreviations are as follows: KOdiA-PC = 5-keto-6-octendioic acid ester of 2-lyso-phosphocholine; HOOA-PC = 5-hydroxy-8-oxo-6-octenoyl- phosphocholine; PGPC = 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphocholine.
The ability of OxPL to induce monocyte adhesion or expression of interleukin-8 (IL-8) is demonstrated both for fragmented and oxygenated OxPL.8, 9 In other biological systems, however, fragmented and oxygenated OxPLs exert different, or even opposite, effects. For example, sn-2-oxygenated, but not sn-2-fragmented phospholipids, exhibited barrier-protective properties in pulmonary endothelial cells (ECs). 10 Consistent with barrier-protective effects, sn-2-oxygenated, but not sn-2-fragmented, phospholipids activated Rac and Cdc42 small guanosine triphosphate (GTP)ase involved in signaling to the endothelial cells in the cytoskeleton. Furthermore, even molecules that contain a similar sn-2-fragmented residue exhibit different activities. POVPC and PGPC contain ω-aldehyde and ω-carboxyl groups, respectively. POVPC increases monocyte but not neutrophil binding to ECs.3 In addition, POVPC strongly inhibits the lipopolysaccharide-mediated induction of neutrophil binding and expression of E-selectin protein and messenger RNA (mRNA).3 In contrast, PGPC induces both monocyte and neutrophil binding as well as the expression of the E-selectin and the vascular cell adhesion molecule 1 (VCAM-1).11
Receptors For OxPLS
Several lines of evidence reveal that both receptor-mediated and receptor-independent pathways are involved in OxPL-mediated cell activation. However, because of a broad spectrum of effects and diversity in chemical structures, it is reasonable to postulate that OxPL may interact with multiple receptors. Available data suggest several different families of receptors are activated by OxPL.
G-protein-coupled receptors (GPCRs)
It has previously been shown that OxPL may act by binding to a GPCR. Parhami et al12 demonstrated that the treatment of aortic ECs with MM-LDL resulted in a saturable dose-dependent increase in cyclic adenosine monophosphate (cAMP) levels. The cAMP increase in response to MM-LDL treatment was caused by the stimulation of Gs complexes and inhibition of Gi complexes. Subsequent studies revealed involvement of different GPCR in the OxPL-mediated signaling pathway. During nonenzymatic deacylation and enzymatic hydrolysis, oxidized fatty acyl chains can be released from the sn-2 position of OxPL. Lipoprotein-associated phospholipase A2, which is also known as platelet-activation factor (PAF)-acetylhydrolase, catalyzes the conversion of OxPL into lysophospholipids, which can bind and activate various GPCRs. For example, lysophosphatidylcholine activates GPR4 and G2A receptors,13, 14, 15 whereas lysophosphatidic acid stimulates LPA1–LPA4 receptors.16, 17 Furthermore, some OxPLs have a similar structure to PAF and are considered as potential PAF receptor agonists. The major PAF-like lipid in oxidated LDL (OxLDL) is 1-O-hexadecyl-2-(butanoyl or butenoyl)-sn-glycero-3-phosphocholine.18 The activation of the PAF receptor by the OxPL resulted in IL-8 expression and monocyte binding to ECs.19, 20 However, some OxPL effects cannot be reproduced by the activation of the PAF receptor. As a well-recognized edemagenic agent, PAF does not mimic the barrier-protective effects of oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC)21 as well as induction of vascular endothelial growth factor (VEGF) by OxPAPC,22 which suggests the involvement of receptor mechanisms rather than the PAF receptor. More recently, a Gs-coupled GPCR, prostaglandin E2 (PGE2) has been implicated in the activation of beta-1 integrin and the stimulation of monocyte binding to ECs induced by OxPAPC and PEIPC.23 Butaprost, which is the specific agonist of PGE2, mimicked the effect of OxPAPC on the regulation of tumor necrosis factor-alpha (TNF- α) and IL-10 in monocyte-derived cells.23 Furthermore, EP2 antagonist AH6809 blocked the activation of EP2 by OxPAPC in HEK293 cells and blocked the IL-10 response to PEIPC in monocytic THP-1 cells.23
Peroxisome proliferator-activated receptors (PPARs)
A line of evidence shows that PPAR γ, which is a nuclear receptor protein that plays an essential role in the regulation of genes, was activated by a fragmented alkyl phospholipid in OxLDL called hexadecyl azelaoyl phosphatidylcholine.24 In addition to PPARγ, PPARα may be also activated by OxPL or its components POVPC and PGPC. Furthermore, by using transient transfection assays, Delerive et al25 demonstrated that OxLDL but not LDL activated PPARα dose dependently in ECs without affecting PPARα protein expression. Inhibitory analysis showed that phospholipase A2 but not lipoxygenases or cyclooxygenases was required for this activation.25
Scavenger receptors
CD36, which is an 88-kD transmembrane glycoprotein expressed on monocytes/macrophages, platelets, and microvascular endothelium, has been implicated as a putative receptor for OxLDL.26, 27, 28 More recently, the acyl chains of OxPL that protrude from the plasma membrane were defined as a key recognition site for macrophage binding to the cell surface. Two conserved, positively charged amino acids in CD36 (lysines at positions of 164 and 166) are critical for OxPL and OxLDL binding to CD36.29 In contrast to the implication of CD36 in OxLDL recognition by macrophage, work by Walton et al30 demonstrated that IL-8 induction by OxPAPC is independent of CD36 and mediated by toll-like receptor 4 (TLR4) and a 37-kDa glycosylphosphatidylinositol-anchored protein.
Non-receptor-mediated effects of OxPL
In addition to receptor-mediated mechanisms, some effects of OxPL are probably not mediated by cell membrane receptors. For example, the sterol response element binding protein (SREBP) seems to be activated as a consequence of cholesterol depletion by OxPAPC in ECs through a nonreceptor mechanism.31 During oxidation, esterified fatty acids in phospholipids are converted into a series of highly electrophilic ketoaldehyde isomers, which are ready to adduct to proteins by direct chemical modification. A study by Brame et al32 provides a new insight into the mechanism by which OxPL influences the function of cardiac K+ channel, and it suggests a generalized cellular mechanism for the alteration of the membrane function as a consequence of oxidative stress induced by OxPL. By using N-biotin labeled OxPAPC, Gugiu et al33 demonstrated the binding of OxPAPC to cytoskeleton-associated protein 4. This type II reversibly palmitoylated transmembrane protein regulates the tissue plasminogen activator on the surface of smooth muscle cells.
OxPL-Induced Signaling
The complexity of OxPL-induced effects on endothelial function is illustrated by a spectrum of cell response to OxPL, such as cytoskeletal remodeling, barrier function, inflammation, procoagulant activity, redox reaction, sterol metabolism, unfolded protein response (UPR), and angiogenesis. A microarray study showed that OxPAPC regulated the expression of over 700 genes in human aortic ECs.34 In this part, we will discuss mechanisms activated by OxPL and summarize the effects of OxPL on the vascular barrier.
Regulation of inflammatory pathways by OxPL
The accumulation of OxPL at the sites of chronic vascular inflammation such as atherosclerotic lesions and its role in propagation of this process is well recognized. The initial study by Henriksen et al35 formed the basis of the hypothesis that oxidation of LDL might be an important step in the atherogenesis process. Subsequent studies demonstrated the roles of OxPL in the activation of monocyte extravasation and adhesion, induction of inflammatory cytokines production, and increased thrombogenesis, as well as the effects on coagulation and redox balance.
Extravasation and activation of monocytes
A characteristic feature of atherosclerosis is the accumulation of lipids in the lesion area. Numerous studies have clearly demonstrated that OxPL activates monocyte binding to the vascular EC; the initial event triggers vascular inflammation and atherosclerosis plaque formation and induces synthesis of monocyte-specific chemoattractants such as monocyte chemotactic protein 1 (MCP1) and monocyte/EC adhesion proteins.8 Connecting segment 1 (CS1) fibronectin is an endothelial membrane protein that binds monocyte integrin α4β1, which causes the firm adhesion of monocyte with ECs.11 OxPAPC promotes the surface deposition of the CS-1 fibronectin, which is mediated by increased R-Ras activity and decreased H-Ras activity. In this model, OxPAPC increased cAMP levels, which are responsible for R-Ras activation. In turn, R-Ras activated PI3 K, which results in the activation of α5β1 integrin on the apical surface.36 A candidate receptor that mediates this pathway is prostaglandin receptor EP2,23 which belongs to the GPCR family. EP2 agonists increased the activation of beta 1 integrin. A line of evidence suggests lipoxygenase pathway as an alternative mechanism that regulates OxPL-induced monocyte adhesion. The treatment of ECs with a lipoxygenase inhibitor, but not cyclooxygenase, decreased MM-LDL and POVPC-induced monocyte binding to ECs.37 Furthermore, the addition of 12(S)- hydroperoxy tetraenoic eicosatetraenoic acid reversed the inhibitory effects of a lipoxygenase inhibitor on monocyte binding. Importantly, the mechanism of OxPL-induced monocyte binding to ECs is different from that induced by other inflammatory factors, such as lipopolysaccharide (LPS), TNFα, or IL-1. OxPL does not upregulate intercellular adhesion molecule-1 (ICAM-1), VCAM-1, and E-selectin expression, but it upregulates MCP1, CS1, and P-selectin, which leads to selective monocyte, but not neutrophil, adhesion to the vascular endothelium.
Upregulation of proinflammatory cytokines
Evidence indicates that OxPL increases the expression of several chemokines, including MCP-1, IL-8, IL-6, macrophage inflammatory protein-1 alpha (MIP-1 α), MIP-1 β, and growth-related protein α, which are described well in previous reviews.38, 39, 40 These cytokines play important roles in the initiation and development of chronic inflammation. IL-8 transcription induced by OxPAPC lasts at least 18 h, with the peak of mRNA expression at approximately 6 h following OxPAPC treatment. In contrast, TNFα-induced IL-8 transcription reaches its peak at 1 h and remains increased during 4 h following treatment.41 Unlike classic inflammatory mediators such as LPS and TNFα, the induction of these inflammatory mediator genes by OxPL is achieved through different transcription mechanisms. IL-8 transcription is induced by TNFα mainly through the nuclear factor κB (NFκB) pathway. The activation of IL-8 transcription by OxPL, however, is independent of the NFκB pathway and may be mediated by various pathways that include the c-Src/JAK2/STAT3 pathway,41 PPARγ pathway,8 eNOS/SREBP,42 and UPR.22, 34 Src kinases participate in growth factor/cytokine signal transduction pathways that mediate proliferation, survival, differentiation, and apoptosis. OxPAPC activates Src kinase and its downstream effector STAT3, which regulates IL-8 transcription through the binding to a gamma-interferon activation sequence element in the IL-8 promoter.43 Moreover, the activation of Src kinase by OxPAPC is independent of cAMP/protein kinase A (PKA) pathway used by OxPAPC in activating β1 integrin for monocyte binding mentioned above. Evidence indicates that OxPAPC activates SREBP in human artificial episomal chromosome after 1 h treatment, and the activation persisted for 8 h.31 More investigation provided evidence for the role of endothelial nitric oxide synthase (eNOS) in the activation of SREBP by OxPAPC. OxPAPC treatment of ECs induced a dose- and time-dependent activation of eNOS, and the NOS inhibitor N-nitro-L-arginine-methyl ester significantly inhibited SREBP activation and IL-8 synthesis by OxPAPC.42 Recent findings have shown a role for a UPR signaling cascade in OxPAPC-induced induction inflammatory cytokines. An immunohistochemical analysis of human atherosclerotic lesions indicated the activation of UPR in the areas that contain OxPL.34 The small interfering RNA (siRNA)-mediated knockdown of ATF4 and XBP1, which are the 2 branches of UPR, downregulated both the basal and the OxPAPC-stimulated expression of IL-8, IL-6, MCP-1, and CXC motif ligand 3 in the human artery ECs.34 Inhibition of UPR by pharmacologic inhibitors or knockdown of cochaperone HTJ-1 decreased VEGF mRNA induction by OxPL.22 The expression levels of VEGF in OxPL-treated cells strongly correlated with the induction of ATF4 target genes ATF3 and TRB3. A chromatin immunoprecipitation assay demonstrated that OxPL stimulated the binding of ATF4 to a regulatory site in the VEGF gene.
Enhanced thrombogenesis
The tissue factor (TF) is the cell-surface receptor for factor VIIa, which plays important role in thrombus formation. TF is expressed by subendothelial cells that are normally not exposed to circulating blood. ECs do not express TF until they are exposed to inflammatory molecules. OxPAPC induces phosphorylation of extracellular signaling kinase1/2 (Erk1/2) and the expression of early growth response factor-1 (EGR-1). Both of these signals are required for enhanced TF expression. A study by Bochkov et al44 shows that OxPAPC induces the expression of TF by ECs. A methyl ethyl ketone-specific inhibitor PD98059 suppressed Erk1/2 activation as well as EGR-1 and TF increase, which indicates a protein kinase C (PKC)/Erk/EGR-1 pathway as 1 pathway that contributes to the TF induction by OxPAPC. In addition, OxPAPC-induced an increase of cytosolic Ca++ activates calcineurin, which led to the nuclear translocation and DNA binding of nuclear factor activated T cells. Thus, the thrombogenic switch induced by OxPAPC in endothelium promotes the general procoagulant state characteristics of atherosclerosis and inflammation.
Increased oxidative stress
The excessive generation of ROS contributes to lung inflammation and injury. Several observations suggest that OxPL may induce the generation of excessive ROS in ECs. Short-chain polar lipid derivatives cause glutathione depletion in ECs.45 OxPAPC-induced nicotinamide adenine dinucleotide phosphate oxidase activation is apparently a source of increased superoxide production that leads to glutathione depletion.46 Other pathways used by OxPAPC to generate ROS include uncoupled eNOS and increased mitochondrial metabolism.47 In parallel with an increase in oxidative stress, OxPAPC may induce the transcription of antioxidant enzymes. OKL 38 is a newly recognized antioxidant gene, which can be induced by superoxide production in response to OxPAPC.48 The stimulation of OKL 38 expression by OxPAPC is mediated through transcription factor nuclear factor E2-related factor.
Anti-inflammatory effects of OxPL
OxPL also exhibits anti-inflammatory and protective effects in the context of sepsis and acute injury. The anti-inflammatory effects of OxPL include (1) inhibition of “sterile” acute lung injury (ALI) induced by viral and bacterial derived inflammatory mediators49, 50; (2) inhibition of “aseptic” ALI induced by injurious mechanical ventilation,51 and therefore the use of PEIPC and PECPC-like stabilized compounds may show beneficial effects in other “aseptic” lung injury models such as ischemia/reperfusion; (3) inhibition of lung vascular leak and inflammation in the secondary ALI induced by acute necrotizing pancreatitis52; and (4) inhibition of dendritic cell maturation.53 These anti-inflammatory effects are mediated by enhanced endothelial barrier function, induction of signaling pathways that lead to upregulation of anti-inflammatory genes, inhibition of proinflammatory gene expression, and prevention of the interaction of proinflammatory bacterial products with host cells. The signaling pathways that mediate the anti-inflammatory effects of OxPL may overlap with some signaling pathways that mediate the proinflammatory effects. In the following sections, we will discuss these OxPL functions in more detail.
Role of OxPL in endothelial barrier function
The vascular endothelial barrier between circulating blood and interstitial fluid is dynamically regulated by a counterbalance of barrier-protective and barrier-disruptive bioactive molecules present in the circulation. Maintaining the balance is critical for preventing the transmigration of inflammatory cells from the blood vessels to the interstitial tissue. Our previous studies have shown that the treatment of pulmonary ECs with OxPAPC (5–20 μg/mL) enhanced the basal EC monolayer barrier properties in a dose-dependent manner, which lasted over 12 h.10 OxPAPC pretreatment attenuated EC permeability induced by thrombin, LPS, or high-magnitude cyclic stretch,10, 51, 54 and it accelerated the recovery of compromised EC barrier function.10 An analysis of structure–function relationships showed that oxidation products of arachidonic acid-, but not linoleic acid-containing phospholipids, as well as sn-2-oxygenated, but not sn-2-fragmented phospholipids, exhibited barrier-protective properties in pulmonary ECs.10 Of note, nonoxidized phospholipids did not affect basal or agonist-induced permeability in ECs. The OxPAPC protective effects were verified in the rodent models of LPS-induced lung injury and VILI and in the model of pulmonary ECs exposed to high-magnitude cyclic stretch and thrombin stimulation.51
Signaling mechanisms that underlie the EC barrier-protective effects of OxPAPC involve small GTPases (Rho, Rac, and Cdc42), which play pivotal roles in regulating cytoskeletal, focal adhesions, and adhesion junctions. Rac and Cdc42 signaling promotes EC barrier-protective functions. OxPAPC-induced activation of Rac and Cdc42 in pulmonary ECs leads to the enhancement of peripheral actin cytoskeleton and the formation of microspike-like actin structures at the cell–cell interface.10 The coexpression of constitutively active Rac and Cdc42 reproduced the unique actin rearrangement observed in the OxPAPC-stimulated EC monolayer.10 An analysis of upstream mechanisms showed the involvement of PKA, Src, and PKC activities in the OxPAPC-induced Rac/Cdc42 activities.21, 55 The molecular mechanisms of the barrier-protective effects of PKA may be caused by its ability to attenuate the endothelial myosin light chain (MLC) kinase activity that leads to a decreased basal level of MLC phosphorylation; PAK-mediated inhibition of Rho activity; and phosphorylation of the actin-binding proteins filamin, adductin, dematin, as well as the focal adhesion proteins paxillin and focal adhesion kinase (FAK). These proteins lead to the disappearance of stress fiber and F-actin in the membrane ruffles. Our studies also indicated that OxPAPC induced the phosphorylation of cofillin,55 which promotes peripheral actin polymerization. In addition, OxPAPC induced the phosphorylation and membrane translocation of focal adhesion protein paxillin and FAK. A recent study demonstrated a potential role of paxillin in the feedback mechanism of Rac regulation by focal adhesions in the OxPAPC-stimulated ECs.56 The expression of paxillin with a mutated PAK1 attenuated OxPAPC-induced PAK1 activation and an EC barrier-protective response.56 The use of an siRNA approach provided evidence that the Rac/Cdc42-specific guanine nucleotide exchange factors Tiam1 and βPIX are involved in the OxPAPC-induced Rac activation and EC barrier protection in response to OxPAPC treatment.57
Protective effects of OxPLs against LPS-induced inflammation
LPS, which is the outer membrane component of gram-negative bacteria, is known as an endotoxin that induces a variety of proinflammatory genes leading to septic shock and death. Several studies have been performed to elucidate the molecular mechanism that underlies LPS-induced inflammation. LPS binds with the serum-binding protein called LPS binding protein and is delivered to the phosphatidylinositol glycan-linked cell surface protein CD14. The complex then interacts with TLR4 and its accessory protein MD2, and it recruits adaptor proteins MyD88 and Mal to the cytoplasmic domain of TLR4.58, 59, 60, 61 The downstream signaling pathways of TLR4 include NF-κB, AP-1 and Egr-1 transcription factors.62
During inflammatory insult, cells produce a plethora of ROS, which modify phospholipids peroxidatively that lead to the generation of OxPL. Thus, OxPL may represent a negative feedback mechanism that induces excessive inflammation. OxLDL and OxPL inhibit activation of NF-κB induced by LPS and CpG DNA.49, 63 OxLDL inhibited LPS-induced IL-12 protein expression and cyclooxygenase-2 (COX-2) expression and activity in macrophages64 as well as an induction of E-selectin protein expression in ECs induced by LPS.65 Lysophosphatidylcholine inhibited the LPS induction of TF in monocytes.66 Bochkov et al65 revealed the mechanism of an OxPAPC inhibitory effect on LPS-induced inflammation using in vitro and in vivo models. The inhibitory effect of OxPAPC was specific for LPS but not for other inflammatory agonists such as IL-1 and TNFα; OxPAPC inhibited LPS signaling by blocking the binding of LPS to LBP and CD14. A later study by Walton et al67 proposed an alternative mechanism via an OxPAPC-induced disruption caveolae and inhibition of the assembly of the LPS signaling complex in ECs. In addition to blocking an inflammatory signaling cascade, our studies suggested that independent of the inhibitory mechanisms mentioned above, OxPAPC may also attenuate LPS-induced and VILI via the direct enhancement of vascular endothelial barrier function.50, 54
Anti-inflammatory mechanisms activated by OxPL
The anti-inflammatory effects of increased cAMP intracellular concentrations have been previously described. Increased cAMP levels inhibit E-selectin and VCAM-1 expression in ECs,68 inhibit oxidative burst in neutrophils, suppress p38 MAP kinase,69 and upregulate suppressor of cytokine signaling 3.70 Heme oxygenase-1 (HO-1) is an enzyme that mediates the catabolism of heme into carbon monoxide in human endothelial and smooth muscle cells.71 The anti-inflammatory effects of HO-1 are mediated by the generation of carbon monoxide, which inhibits the expression of IL-1β, TNFα, and macrophage inflammatory protein 1β.72, 73 Moreover, HO-1 induces the expression of the anti-inflammatory IL-10, which in turn upregulates the expression of HO-1. COX2 is another enzyme that is involved in OxPL-mediated anti-inflammatory signaling and the resolution of inflammation. The expression of COX-2 is induced by OxPL in a cAMP-response-element-binding-protein-dependent and PPARγ-dependent manner.74 OxPL may also activate eNOS.42 Increased NO production has been associated with many anti-inflammatory activities including the downregulation of adhesion molecules, suppression of chemokine production, and inhibition of leukocyte extravasation. In summary, the above findings indicate that OxPL induces several anti-inflammatory signaling molecules and transcription factors and decreases the expression of inflammatory cytokines in pathologic conditions associated with acute injury or local inflammation.
The generation of OxPL is a general feature of lung injury and accompanying activation of ROS production induced by different pathogen infections. A study by Imai et al75 shows the accumulation of oxidized phosphorylcholine products in human and animal lungs infected with the severe acute respiratory syndrome virus, H5N1 avian influenza virus, and anthrax, as well as in a mouse model of acid-induced lung injury. This study also suggests the involvement of OxPL in the development of ALI. OxPL increases in that study were monitored by tissue staining with a monoclonal EO6 antibody. The most potent antigens for this antibody are products of aldol condensation of fragmented products of PAPC oxidation, such as P(POVPC)VPC, diLysoPC-C9, and diOVPC, as well as Schiff bases that form covalent bonds between protein lysine residues and aldehyde groups of fragmented oxidized phospholipids such as POVPC-bovine serum albumin.76 As discussed above, fragmented PL oxidation products induce endothelial barrier disruption (see Table I 77).10 Thus, EO6 antibodies do not discriminate between barrier-protective and barrier-disruptive OxPL. The generation of EO6-detectable OxPL in bronchioalveolar lavage and alveolar macrophages may represent the accumulation of fragmented PAPC oxidation products, aldol condensates, and Schiff bases rather than increased levels of PEIPC or PECPC. The OxPAPC doses used for intratracheal instillation (20 μg/g body weight) that affected lung elastance parameter were 5–10 times higher compared with the doses used in other studies that showed protective effects in the model of LPS-induced and CpG DNA-induced ALI.49 It also seems that in animal models of LPS-induced lung injury and VILI, as well as lung dysfunction associated with acute necrotizing pancreatitis, higher OxPAPC doses up to 40 mg/kg may be well tolerated if administered intravenously.49, 50, 51, 52 These doses promote vascular endothelial barrier function. In turn, the pathologic effects of high doses of intratracheal OxPAPC may be caused by the direct effects of OxPAPC on alveolar epithelial cells. Because the precise effects of OxPAPC on alveolar epithelial permeability and other physiologic responses have not been yet tested, it is possible that OxPAPC at reported doses may cause barrier-disruptive effects on epithelial cells. These possibilities need more testing.
Table I.
Biologic effect of selected products of oxidized phospholipids
| OxPLs | Potential Receptors | Bioactivities |
|
|---|---|---|---|
| Proinflammatory Effects | Anti-Inflammatory Effects | ||
| POVPC | PPARγ, PAF receptor CD36 | Inhibits LPS-induced E-selectin expression and neutrophil adhesion3 | |
| PGPC | PPARα, VEGFR2 | ||
| PEIPC | EP2, TLR4, PPARα | Enhances EC barrier function10 | |
| PECPC | PPARα | Increases MCP-1 and IL-1 synthesis9 | Enhances EC barrier function10 |
| Lyso-PC | GPR4, G2A | Upregulates VCAM-1, ICAM-1, and P-selectin expression77 | |
| KOdiAPC | CD36 | Inhibits LPS induction of E-selectin and IL-867 | |
Consistent with the notion about the deleterious effects of high local OxPAPC doses on lung elastance in vivo, the high OxPAPC concentrations also caused endothelial barrier dysfunction in vitro.10 The quantitative and qualitative analysis of OxPL generated in ALI of tissue samples using a mass spectrometry approach still remains to be performed. These important studies will improve the characterization of the composition of endogenous OxPL generated in the injured lung as well as the understanding of their role in the pathogenesis of ALI. Many published studies support the notion that cellular responses to OxPL are critically dependent on local OxPL concentrations.10 Thus, OxPL may play a dual role in the progression of ALI. During the acute phase, a high concentration of OxPL may exert a barrier dysfunction effect, whereas decreased OxPL concentrations at the later phase of injury restore the vascular barrier and contribute to the resolution of lung inflammation and injury. Additional studies are required to test this hypothesis.
Conclusion
OxPL generated in various pathologic conditions exhibits a broad range of biologic activities, including proinflammatory and anti-inflammatory effects as well as the regulation of lung permeability. Unlike classic inflammatory factors, OxPL triggers unique mechanisms that lead to the activation of inflammatory signaling pathways, stimulation of inflammatory transcription factors, expression of inflammatory cytokines, and generation of excessive ROS. However, OxPLs are potent inhibitors of acute inflammation mediated by the TLR family. A specific group of cyclopenthenone-containing OxPLs that include PEIPC and PECPC induces potent barrier-protective responses in the vascular ECs and inhibits hyperpermeability induced by edemagenic agonists, which are mediators of inflammation and pathologic mechanical strain. Thus, increased OxPL levels observed in the course of acute inflammation, such as bacterial infection or acute tissue injury, may represent a negative feedback mechanism that leads to the downregulation of cellular inflammatory cascades. Additional screening and characterization of OxPL compounds with barrier-protective and anti-inflammatory properties and synthesis of bioactive OxPL derivates with stabilized structure may be an exciting area of research that could potentially lead to a new group of pharmacologic molecules beneficial for the treatment of VILI, acute respiratory distress syndrome, lung inflammation, and other diseases associated with increased vascular leakage.
Chicago, Ill
Footnotes
Supported by grants HL76259 and PO1 HL58064 from the National Institutes of Health National Heart, Lung, and Blood Institute (to K.G.B.).
References
- 1.Yoshimi N., Ikura Y., Sugama Y. Oxidized phosphatidylcholine in alveolar macrophages in idiopathic interstitial pneumonias. Lung. 2005;183:109–121. doi: 10.1007/s00408-004-2525-0. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T., Henson P.M., Murphy R.C. Occurrence of oxidized metabolites of arachidonic acid esterified to phospholipids in murine lung tissue. Anal Biochem. 1998;262(15):23–32. doi: 10.1006/abio.1998.2749. [DOI] [PubMed] [Google Scholar]
- 3.Subbanagounder G., Leitinger N., Schwenke D.C. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler Thromb Vasc Biol. 2000;20:2248–2254. doi: 10.1161/01.atv.20.10.2248. [DOI] [PubMed] [Google Scholar]
- 4.Watson A.D., Leitinger N., Navab M. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;23:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 5.Huber J., Vales A., Mitulovic G. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 6.Chang M.K., Binder C.J., Miller Y.I. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiltunen T.P., Gough P.J., Greaves D.R., Gordon S., Yla-Herttuala S. Rabbit atherosclerotic lesions express scavenger receptor AIII mRNA, a naturally occurring splice variant that encodes a non-functional, dominant negative form of the macrophage scavenger receptor. Atherosclerosis. 2001;154:415–419. doi: 10.1016/s0021-9150(00)00519-0. [DOI] [PubMed] [Google Scholar]
- 8.Lee H., Shi W., Tontonoz P. Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ Res. 2000;87:516–521. doi: 10.1161/01.res.87.6.516. [DOI] [PubMed] [Google Scholar]
- 9.Subbanagounder G., Wong J.W., Lee H. Epoxyisoprostane and epoxycyclopentenone phospholipids regulate monocyte chemotactic protein-1 and interleukin-8 synthesis. Formation of these oxidized phospholipids in response to interleukin-1beta. J Biol Chem. 2002;277:7271–7281. doi: 10.1074/jbc.M107602200. [DOI] [PubMed] [Google Scholar]
- 10.Birukov K.G., Bochkov V.N., Birukova A.A. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95:892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- 11.Leitinger N., Tyner T.R., Oslund L. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci U S A. 1999;96:12010–12015. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parhami F., Fang Z.T., Yang B., Fogelman A.M., Berliner J.A. Stimulation of Gs and inhibition of Gi protein functions by minimally oxidized LDL. Arterioscler Thromb Vasc Biol. 1995;15:2019–2024. doi: 10.1161/01.atv.15.11.2019. [DOI] [PubMed] [Google Scholar]
- 13.Kabarowski J.H., Zhu K., Le L.Q., Witte O.N., Xu Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293:702–705. doi: 10.1126/science.1061781. [DOI] [PubMed] [Google Scholar]
- 14.Lum H., Qiao J., Walter R.J. Inflammatory stress increases receptor for lysophosphatidylcholine in human microvascular endothelial cells. Am J Physiol. 2003;285:H1786–H1789. doi: 10.1152/ajpheart.00359.2003. [DOI] [PubMed] [Google Scholar]
- 15.Zhu K., Baudhuin L.M., Hong G. Sphingosylphosphorylcholine and lysophosphatidylcholine are ligands for the G protein-coupled receptor GPR4. J Biol Chem. 2001;276:41325–41335. doi: 10.1074/jbc.M008057200. [DOI] [PubMed] [Google Scholar]
- 16.Tomura H., Mogi C., Sato K., Okajima F. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: a novel type of multi-functional receptors. Cell Signal. 2005;17:1466–1476. doi: 10.1016/j.cellsig.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Anliker B., Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol. 2004;15:457–465. doi: 10.1016/j.semcdb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Marathe G.K., Davies S.S., Harrison K.A. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J Biol Chem. 1999;274:28395–28404. doi: 10.1074/jbc.274.40.28395. [DOI] [PubMed] [Google Scholar]
- 19.Mao Y.J., Wang L., Wang W.J. Effect of ginkgolide B on the function of rat aorta smooth cells and U937 cells stimulated by oxLDL. Yao Xue Xue Bao. 2006;41:36–40. [PubMed] [Google Scholar]
- 20.Beaudeux J.L., Said T., Ninio E. Activation of PAF receptor by oxidised LDL in human monocytes stimulates chemokine releases but not urokinase-type plasminogen activator expression. Clin Chim Acta. 2004;344:163–171. doi: 10.1016/j.cccn.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Birukova A.A., Chatchavalvanich S., Oskolkova O., Bochkov V.N., Birukov K.G. Signaling pathways involved in OxPAPC-induced pulmonary endothelial barrier protection. Microvasc Res. 2007;73:173–181. doi: 10.1016/j.mvr.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oskolkova O.V., Afonyushkin T., Leitner A. ATF4-dependent transcription is a key mechanism in VEGF up-regulation by oxidized phospholipids: critical role of oxidized sn-2 residues in activation of unfolded protein response. Blood. 2008;112:330–339. doi: 10.1182/blood-2007-09-112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R., Mouillesseaux K.P., Montoya D. Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circ Res. 2006;98:642–650. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- 24.Davies S.S., Pontsler A.V., Marathe G.K. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. J Biol Chem. 2001;276:16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 25.Delerive P., Furman C., Teissier E., Fruchart J., Duriez P., Staels B. Oxidized phospholipids activate PPARalpha in a phospholipase A2-dependent manner. FEBS Lett. 2000;471:34–38. doi: 10.1016/s0014-5793(00)01364-8. [DOI] [PubMed] [Google Scholar]
- 26.Podrez E.A., Poliakov E., Shen Z. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 27.Boullier A., Gillotte K.L., Horkko S. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J Biol Chem. 2000;275:9163–9169. doi: 10.1074/jbc.275.13.9163. [DOI] [PubMed] [Google Scholar]
- 28.Gillotte-Taylor K., Boullier A., Witztum J.L., Steinberg D., Quehenberger O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J Lipid Res. 2001;42:1474–1482. [PubMed] [Google Scholar]
- 29.Kar N.S., Ashraf M.Z., Valiyaveettil M., Podrez E.A. Mapping and characterization of the binding site for specific oxidized phospholipids and oxidized low density lipoprotein of scavenger receptor CD36. J Biol Chem. 2008;283:8765–8771. doi: 10.1074/jbc.M709195200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walton K.A., Hsieh X., Gharavi N. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8. A role for Toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. J Biol Chem. 2003;278:29661–29666. doi: 10.1074/jbc.M300738200. [DOI] [PubMed] [Google Scholar]
- 31.Yeh M., Cole A.L., Choi J. Role for sterol regulatory element-binding protein in activation of endothelial cells by phospholipid oxidation products. Circ Res. 2004;95:780–788. doi: 10.1161/01.RES.0000146030.53089.18. [DOI] [PubMed] [Google Scholar]
- 32.Brame C.J., Boutaud O., Davies S.S. Modification of proteins by isoketal-containing oxidized phospholipids. J Biol Chem. 2004;279:13447–13451. doi: 10.1074/jbc.M313349200. [DOI] [PubMed] [Google Scholar]
- 33.Gugiu B.G., Mouillesseaux K., Duong V. Protein targets of oxidized phospholipids in endothelial cells. J Lipid Res. 2008;49:510–520. doi: 10.1194/jlr.M700264-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Gargalovic P.S., Gharavi N.M., Clark M.J. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 35.Henriksen T., Mahoney E.M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981;78:6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole A.L., Subbanagounder G., Mukhopadhyay S., Berliner J.A., Vora D.K. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler Thromb Vasc Biol. 2003;23:1384–1390. doi: 10.1161/01.ATV.0000081215.45714.71. [DOI] [PubMed] [Google Scholar]
- 37.Huber J., Furnkranz A., Bochkov V.N. Specific monocyte adhesion to endothelial cells induced by oxidized phospholipids involves activation of cPLA2 and lipoxygenase. J Lipid Res. 2006;47:1054–1062. doi: 10.1194/jlr.M500555-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Bochkov V.N. Inflammatory profile of oxidized phospholipids. Thromb Haemost. 2007;97:348–354. [PubMed] [Google Scholar]
- 39.Bochkov V.N., Leitinger N. Anti-inflammatory properties of lipid oxidation products. J Mol Med. 2003;81:613–626. doi: 10.1007/s00109-003-0467-2. [DOI] [PubMed] [Google Scholar]
- 40.Birukov K.G. Oxidized lipids: the two faces of vascular inflammation. Curr Atheroscler Rep. 2006;8:223–231. doi: 10.1007/s11883-006-0077-x. [DOI] [PubMed] [Google Scholar]
- 41.Yeh M., Gharavi N.M., Choi J. Oxidized phospholipids increase interleukin 8 (IL-8) synthesis by activation of the c-src/signal transducers and activators of transcription (STAT)3 pathway. J Biol Chem. 2004;279:30175–30181. doi: 10.1074/jbc.M312198200. [DOI] [PubMed] [Google Scholar]
- 42.Gharavi N.M., Baker N.A., Mouillesseaux K.P. Role of endothelial nitric oxide synthase in the regulation of SREBP activation by oxidized phospholipids. Circ Res. 2006;98:768–776. doi: 10.1161/01.RES.0000215343.89308.93. [DOI] [PubMed] [Google Scholar]
- 43.Gharavi N.M., Alva J.A., Mouillesseaux K.P. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. J Biol Chem. 2007;282:31460–31468. doi: 10.1074/jbc.M704267200. [DOI] [PubMed] [Google Scholar]
- 44.Bochkov V.N., Mechtcheriakova D., Lucerna M. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood. 2002;99:199–206. doi: 10.1182/blood.v99.1.199. [DOI] [PubMed] [Google Scholar]
- 45.Therond P., Abella A., Laurent D. In vitro study of the cytotoxicity of isolated oxidized lipid low-density lipoproteins fractions in human endothelial cells: relationship with the glutathione status and cell morphology. Free Radic Biol Med. 2000;28:585–596. doi: 10.1016/s0891-5849(99)00265-8. [DOI] [PubMed] [Google Scholar]
- 46.Rouhanizadeh M., Hwang J., Clempus R.E. Oxidized-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine induces vascular endothelial superoxide production: implication of NADPH oxidase. Free Radic Biol Med. 2005;39:1512–1522. doi: 10.1016/j.freeradbiomed.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landar A., Zmijewski J.W., Dickinson D.A. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2006;290:H1777–H1787. doi: 10.1152/ajpheart.01087.2005. [DOI] [PubMed] [Google Scholar]
- 48.Li R., Chen W., Yanes R., Lee S., Berliner J.A. OKL38 is an oxidative stress response gene stimulated by oxidized phospholipids. J Lipid Res. 2007;48:709–715. doi: 10.1194/jlr.M600501-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Ma Z., Li J., Yang L. Inhibition of LPS- and CpG DNA-induced TNF-alpha response by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2004;286:L808–L816. doi: 10.1152/ajplung.00220.2003. [DOI] [PubMed] [Google Scholar]
- 50.Nonas S., Miller I., Kawkitinarong K. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am J Respir Crit Care Med. 2006;173:1130–1138. doi: 10.1164/rccm.200511-1737OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nonas S., Birukova A.A., Fu P. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care. 2008;12:R27. doi: 10.1186/cc6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L., Wang X.P., Wu K. The therapeutic effect of oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine in rodents with acute necrotizing pancreatitis and its mechanism. Pancreas. 2007;35:e27–e36. doi: 10.1097/mpa.0b013e3181525855. [DOI] [PubMed] [Google Scholar]
- 53.Bluml S., Kirchberger S., Bochkov V.N. Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J Immunol. 2005;175:501–508. doi: 10.4049/jimmunol.175.1.501. [DOI] [PubMed] [Google Scholar]
- 54.Birukova A.A., Fu P., Chatchavalvanich S. Polar head groups are important for barrier-protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007;292:L924–L935. doi: 10.1152/ajplung.00395.2006. [DOI] [PubMed] [Google Scholar]
- 55.Birukov K.G., Leitinger N., Bochkov V.N., Garcia J.G. Signal transduction pathways activated in human pulmonary endothelial cells by OxPAPC, a bioactive component of oxidized lipoproteins. Microvasc Res. 2004;67:18–28. doi: 10.1016/j.mvr.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Birukova A.A., Alekseeva E., Cokic I., Turner C.E., Birukov K.G. Crosstalk between paxillin and Rac is critical for mediation of barrier-protective effects by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2008;295:L593–L602. doi: 10.1152/ajplung.90257.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birukova A.A., Malyukova I., Mikaelyan A., Fu P., Birukov K.G. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. J Cell Physiol. 2007;211:608–617. doi: 10.1002/jcp.20966. [DOI] [PubMed] [Google Scholar]
- 58.Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol. 2002;270:109–120. doi: 10.1007/978-3-642-59430-4_7. [DOI] [PubMed] [Google Scholar]
- 59.Pfeiffer A., Bottcher A., Orso E. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur J Immunol. 2001;31:3153–3164. doi: 10.1002/1521-4141(200111)31:11<3153::aid-immu3153>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 60.Poltorak A., He X., Smirnova I. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 61.Triantafilou M., Miyake K., Golenbock D.T., Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 62.Guha M., Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 63.Hamilton T.A., Ma G.P., Chisolm G.M. Oxidized low density lipoprotein suppresses the expression of tumor necrosis factor-alpha mRNA in stimulated murine peritoneal macrophages. J Immunol. 1990;144:2343–2350. [PubMed] [Google Scholar]
- 64.Eligini S., Brambilla M., Banfi C. Oxidized phospholipids inhibit cyclooxygenase-2 in human macrophages via nuclear factor-kappaB/IkappaB- and ERK2-dependent mechanisms. Cardiovasc Res. 2002;55:406–415. doi: 10.1016/s0008-6363(02)00437-6. [DOI] [PubMed] [Google Scholar]
- 65.Bochkov V.N., Kadl A., Huber J., Gruber F., Binder B.R., Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 66.Engelmann B., Zieseniss S., Brand K. Tissue factor expression of human monocytes is suppressed by lysophosphatidylcholine. Arterioscler Thromb Vasc Biol. 1999;19:47–53. doi: 10.1161/01.atv.19.1.47. [DOI] [PubMed] [Google Scholar]
- 67.Walton K.A., Cole A.L., Yeh M. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler Thromb Vasc Biol. 2003;23:1197–1203. doi: 10.1161/01.ATV.0000079340.80744.B8. [DOI] [PubMed] [Google Scholar]
- 68.Ghersa P., Hooft van Huijsduijnen R., Whelan J., Cambet Y., Pescini R., DeLamarter J.F. Inhibition of E-selectin gene transcription through a cAMP-dependent protein kinase pathway. J Biol Chem. 1994;269:29129–29137. [PubMed] [Google Scholar]
- 69.Rahman A., Anwar K.N., Minhajuddin M. cAMP targeting of p38 MAP kinase inhibits thrombin-induced NF-kappaB activation and ICAM-1 expression in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1017–L1024. doi: 10.1152/ajplung.00072.2004. [DOI] [PubMed] [Google Scholar]
- 70.Fasshauer M., Klein J., Lossner U., Paschke R. Isoproterenol is a positive regulator of the suppressor of cytokine signaling-3 gene expression in 3T3-L1 adipocytes. J Endocrinol. 2002;175:727–733. doi: 10.1677/joe.0.1750727. [DOI] [PubMed] [Google Scholar]
- 71.Ishikawa K., Navab M., Leitinger N., Fogelman A.M., Lusis A.J. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J Clin Invest. 1997;100:1209–1216. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Otterbein L.E., Bach F.H., Alam J. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 73.Otterbein L.E., Zuckerbraun B.S., Haga M. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 74.Pontsler A.V., St Hilaire A., Marathe G.K., Zimmerman G.A., McIntyre T.M. Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor gamma and oxidized alkyl phospholipids from oxidized low density lipoprotein. J Biol Chem. 2002;277:13029–13036. doi: 10.1074/jbc.M109546200. [DOI] [PubMed] [Google Scholar]
- 75.mai Y., Kuba K., Neely G.G. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Friedman P., Horkko S., Steinberg D., Witztum J.L., Dennis E.A. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J Biol Chem. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 77.Kume N., Cybulsky M.I., Gimbrone M.A., Jr. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]