Abstract
The Rh(III) catalyzed oxidative coupling of alkenes with arenes provides a greener alternative to the classical Heck reaction for the synthesis of arene-functionalized alkenes. The present mechanistic study gives insights for the rational development of this key transformation. The catalyst resting states and the rate law of the reaction have been identified. The reaction rate is solely dependent on catalyst and alkene concentrations and the rate determining step is the migratory insertion of alkene into a Rh–C(aryl) bond.
Catalytic dehydrogenative cross-coupling reactions offer the desirable possibility of forming a new C–C bond directly from two simple C–H bonds which, alongside the atom economy advantage, is fundamentally fascinating because a catalytic system allows the coupling of two stable centers.1 In this regard, the oxidative version of the Heck cross-coupling, also known as the Fujiwara-Moritani reaction,2 is an interesting approach providing access to aryl-functionalized alkenes directly from two C–H bonds without the need for prefunctionalized partners (Scheme 1). This method is also of particular interest for the prospective production of styrene directly from benzene and ethylene.3
Scheme 1.

The Oxidative Heck or Fujiwara-Moritani reaction.
A number of substrates have been oxidatively coupled to alkenes using Pd(II) as a catalyst and various oxidants (Cu(OAc)2, Ag2CO3, benzoquinone, etc.).1a,4 More recently, Rh(III) complexes have proven to be efficient and versatile catalysts for a variety of oxidative cross-couplings5 and especially for the Fujiwara-Moritani reaction,6 even allowing the coupling of simple unactivated alkenes.6n However, this methodology could be made even more useful by broadening the types of directing groups and the range of C-H bonds that can be functionalized, and by identification of more convenient and cost effective stoichiometric oxidants.1a In order to improve these cross-couplings on a rational basis it is crucial to understand their mechanism. While the arylation of different substrates with Rh(III) cationic catalysts have been investigated,7 there are few reports on the mechanism of oxidative reactions,8 none of which include alkenes. In this paper, we report a mechanistic study of the coupling of 2-phenylpyridine with styrene catalyzed by [Cp*RhCl2]2, activated by AgSbF6, and employing a copper carboxylate as an oxidant. We report a kinetic study which identifies the resting states and the turnover-limiting step for this transformation.
We chose 2-phenylpyridine (1) as a substrate because it is known to form stable rhodacycles,9 and styrene derivatives 2 are known to be efficiently coupled by Rh(III).10 Fluorine substitution at the aryl para-position of both reagents provided a convenient 19F NMR handle that allowed careful monitoring of their concentrations during the reaction and the observation of catalyst resting states. Previously, we have reported the coupling of aryl oximes with simple alkenes using a mixture of [Cp*RhCl2]2 and AgSbF6 as the catalyst in THF.6n We therefore tested the coupling of 1 with 2 under the same conditions. Although Cu(OAc)2 gave excellent yields as an oxidant, it exhibited low solubility in THF. This would hamper NMR monitoring and make a kinetic study challenging. To ensure the homogeneity of the reaction solutions, we addressed this problem by using copper heptanoate (4) as the oxidant, a complex fully soluble in THF. Employing 2 equivalents of pyridine over styrene to avoid any overalkylation, we recovered the expected coupling product 3 in 91% yield (Scheme 2). An added benefit was that the solubility of 4 allowed us to reduce the amount of oxidant to 1 equivalent where previously 2.1 equivalents of Cu(OAc)2 were needed.
Scheme 2. Rh(III)-Catalyzed Oxidative Coupling of 2-(4-fluorophenyl)pyridine (1) and 4-fluorostyrene (2)a.

a0.1 mmol of 1, 0.05 mmol of 2, 2.5 μmol of [Cp*RhCl2]2, 0.01 mmol of AgSbF6, 0.05 mmol of Cu(O2C7H13)2 (4), 1 mL THF, T = 50 °C, 12 h.
With this model in hand, our first objective was to gain insight into the nature of the catalyst resting state or states and their formation. It has been shown earlier that the C–H activation of phenylpyridine by [Cp*RhCl2]2 leads to cyclometalated Rh complexes that are either neutral when an acetate salt is employed9b or cationic when using AgSbF6.7 In the present case the reaction mixture contained both AgSbF6 and copper carboxylate salts. To distinguish cationic from neutral rhodacycles under operating conditions, we synthesized a series of such complexes with different ligands and compared their 19F NMR chemical shifts (Scheme 3). We observed that the 19F signals appear between −107 and −109 ppm for cationic complexes 7 – 10 and ca. −111 ppm for neutral complexes 5 and 6 in THF-d8.
Scheme 3.

Syntheses of Cationic and Neutral Rhodacycles
We were also able to obtain single crystals useful for X-ray diffraction analysis of complexes 5–7, 9 and 10. The solid-state structures presented the expected piano stool geometry with Rh–C distances slightly longer for cationic complexes, although the differences are small and within experimental error: 5, 2.024(2) Å ≤ 6, 2.030(6) Å < 9, 2.042(4) Å ≤ 7, 2.046(7) Å < 10, 2.065(7) Å (Figure 1).11
Figure 1.
ORTEP views of complexes 5–7, 9 and 10. H atoms and counteranions SbF6− have been omitted for clarity.
We then carried out the stoichiometric cyclometallation of 1 by [Cp*RhCl2]2 and AgSbF6 in the presence of copper carboxylate 4 (4:1:4:4 respectively) in THF. By comparing the 19F and 1H NMR data of the reaction mixture to those of synthesized samples we confirmed the formation of carboxylate and cationic rhodacycles 6 and 7 (Scheme 4).
Scheme 4.

C–H Activation and Cyclometalation of 1
We next utilized 19F NMR to monitor an actual catalytic run with 6 as precatalyst.12 Although 6 was the predominant resting state, part of the rhodium was transformed into a new species characterized by a set of two 19F resonances at δ −108.5 and −110.0 ppm, which we reasoned corresponded to the rhodacycle styrene adduct 11 (Scheme 5). By increasing the temperature the signals of 6 and 11 became broader, fading into the baseline at 65 °C. Upon cooling the signals became sharper again and two rotamers of 11 could be observed at 25 °C (see below and Figure S-2). This fluxional behavior can be attributed to ligand exchange between 6 and 11.
Scheme 5. Resting States during Oxidative Coupling.
a The integration is not fully accurate due to broad NMR signals. b Two rotamers.
Independent synthesis and isolation of 11 from 2 and [Cp*RhCl2]2 by addition of AgSbF6 proved to be difficult. We observed the formation of a dark violet species whose 1H and 19F NMR spectra were in agreement with the structure of 11, but it decomposed rapidly into an undefined mixture of products. However, in-situ observation by NMR of the addition of 2 to rhodacycle 7 in THF-d8 at −10 °C showed the clean formation of 11 as a mixture of the syn and anti rotamers (Scheme 6).
Scheme 6.

Formation of the Rhodacycle Styrene Adduct
By varying the temperature we observed the reversible interconversion of the rotamers due to free rotation of styrene around the Rh–alkene bond (Figure S-3). This result confirmed that 11 was indeed a π-olefin complex and not a new metallacycle formed by styrene insertion into the Rh–C bond. Although we were not able to observe the insertion process, it is safe to assume that it takes place to eventually form 3. This would be in agreement with the work of Jones et al. who showed that the reaction of ethylene or propylene with Ir or Rh metallacycles led to unstable π-alkene complexes similar to 11 with iridium or to insertion products with rhodium.13
From the above experiments we concluded that 6 and 11 are both resting states of the catalyst. These two species interconvert through the exchange of the carboxylate anion for styrene. This process is upstream from the migratory insertion of styrene into the Rh–C bond. Since the ring-expanded metallacycle could not be detected, it is reasonable to assume that the ensuing steps leading to 3 are faster than the alkene insertion, which determines the rate of the catalytic reaction.
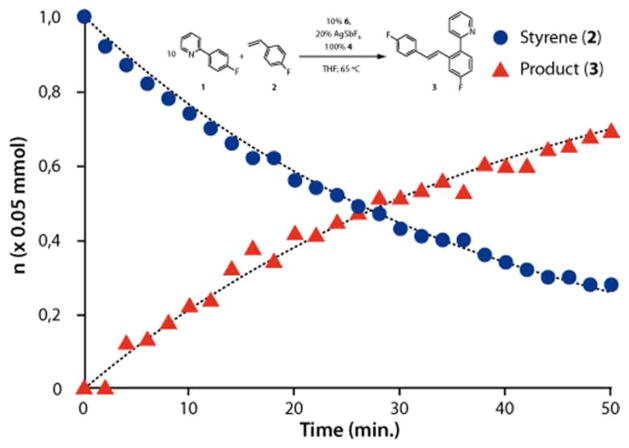
This qualitative conclusion was tested by carrying out a kinetic study designed to establish the rate law of the catalytic transformation. We monitored the progress of the reaction by 19F NMR at 65 °C using 6 as precatalyst under pseudo first order kinetic conditions with excess of 1 relative to 2. Using a 10-fold excess of 1 the reaction followed pseudo-first order kinetics with k = 0.0269 min−1 for the consumption of 2 and a slightly lower rate for product 3 formation with k = 0.024 min−1 (Figure 2). The rate of consumption of 2 is the same (within experimental error) when the original catalyst, 5% of [Cp*RhCl2]2, is used instead of 6, confirming that the same active species are generated in both cases.
Figure 2.
Plot of the concentrations of substrate 2 and product 3 versus time obtained by 19F NMR. Conditions: 0.5 mmol of 1, 0.05 mmol of 2, 5 μmol of 6, 0.01 mmol of AgSbF6, 0,05 mmol of 4, 0.7 mL THF, 0.3 mL THF-d8, 1 μL C6F6 (as internal standard), T = 65 °C. Black dashed lines are simulations [2]t = [2]0e−kt (k = 0.0269 min−1) and [3]t = [2]0(1 – e−kt) (k = 0.024 min−1).
The order of each reactant was then determined by measuring the reaction rate in the presence of variable excess concentrations of 1, catalyst 6, AgSbF6 and Cu(O2C7H13)2 (4) (Table S-1).
The kinetic analysis showed zero-order dependence on copper carboxylate 4 and 4-fluorophenyl-pyridine (1) and first order dependence on catalyst 6 (see SI). Interestingly, in early experiments the silver salt exhibited apparently bimodal behavior. Initially, the starting material consumption followed first-order kinetics with a similar rate for all silver concentrations (7.5 – 30 mol% with regard to 2) indicating zero-order dependence on [Ag]. However, for AgSbF6 concentrations lower than 20 mol % the behavior changed and styrene consumption deviated from first-order after 12 min (Figure S-8). We believe this is due to inadvertent catalyst decomposition, and so in subsequent reactions the silver concentration was kept at 20 mol%. First order behavior was reproducibly observed in these runs.
Monitoring the reactions under operating catalytic conditions established that the concentration of 6 and 11 hardly varied during the course of the reaction.
 |
(1) |
Assuming a steady state situation for 11, its concentration can be expressed according to eq 4, which upon substitution in the rate law (eq 2) furnishes eq 5; this expresses the rate as a function of the concentrations of reactants 6 and 2.
| (2) |
| (3) |
| (4) |
| (5) |
With eq 5 we obtained excellent fits with kobs = 2.72 min−1 for the styrene 2 consumption in the catalytic runs with different amounts of catalyst 6, showing that only catalyst and styrene concentrations are kinetically relevant.
In Scheme 7 we depict a mechanism for this transformation that is consistent with our qualitative observations and kinetic results. The mixture of [Cp*RhCl2]2, AgSbF6, pyridine 1 and copper carboxylate 4 gives rise initially to resting state 6 through a C–H activation mechanism of concerted metalation/deprotonation.14 Complex 6 is in equilibrium with 11 through the exchange of styrene. Then, in the turnover-limiting step, the coordinated styrene undergoes migratory insertion to a new metallacycle probably stabilized by a carboxylate ligand similar to that in 6. This new rhodacycle then undergoes β-elimination to release the organic product 3 and a rhodium hydride complex that upon elimination of carboxylic acid is reduced to Rh(I). The latter is then reoxidized by copper to close the catalytic cycle. This Rh(III/I) catalytic cycle involving the sequence C–H activation, alkene insertion, β-elimination and oxidation is in agreement with previous reports on Rh(III) catalysis7–8 and rhodacycle reactivity towards unsaturated molecules9a,13,15 and is similar to that proposed for Pd(II/0).5a,16
Scheme 7.
Proposed Catalytic Cycle
In conclusion, by using a soluble copper carboxylate and fluorine-substituted reactants that allowed monitoring by 19F NMR, we were able to carry out a mechanistic study and propose a catalytic cycle for the oxidative coupling of styrene to phenylpyridine catalyzed by Rh(III). We hope that the understanding provided for this transformation will contribute to the development of other reactions involving the direct coupling of two carbon-hydrogen bonds.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant GM069559 (to J.A.E.), by the Director, Office of Energy Research, Office of Basic Energy Sciences, Chemical Sciences Division, U.S. Department of Energy under Contract DE-AC02-05CH11231 (to R.G.B.), by the Government of Spain Project CTQ2009-11721 and Junta de Andalucía Project FQM6276 (to J.C). M.B. acknowledges the seventh European Community Framework Programme, for a Marie Curie International Outgoing Fellowship that supported this research and Dr. Michael Tauchert for helpful discussion.
Footnotes
Experimental details for the synthesis and characterization; Kinetic experiments; NMR spectra for all compounds; Crystallographic data for 5–7, 9 and 10. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Yeung CS, Dong VM. Chem Rev. 2011;111:1215. doi: 10.1021/cr100280d. [DOI] [PubMed] [Google Scholar]; (b) Liu C, Zhang H, Shi W, Lei A. Chem Rev. 2011;111:1780. doi: 10.1021/cr100379j. [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara Y, Moritani I. Tetrahedron Lett. 1967;12:1119. [Google Scholar]
- 3.Matsumoto T, Periana RA, Taube DJ, Yoshida H. J Catal. 2002;206:272. [Google Scholar]
- 4.Le Bras J, Muzart J. Chem Rev. 2011;111:1170. doi: 10.1021/cr100209d. [DOI] [PubMed] [Google Scholar]
- 5.(a) Song G, Wang F, Li X. Chem Soc Rev. 2012;41:3651. doi: 10.1039/c2cs15281a. [DOI] [PubMed] [Google Scholar]; (b) Patureau FW, Wencel-Delord J, Glorius F. Aldrichimica Acta. 2012;45:31. [Google Scholar]
- 6.(a) Besset T, Kuhl N, Patureau FW, Glorius F. Chem Eur J. 2011;17:7167. doi: 10.1002/chem.201101340. [DOI] [PubMed] [Google Scholar]; (b) Gong TJ, Xiao B, Liu ZJ, Wan J, Xu J, Luo DF, Fu Y, Liu L. Org Lett. 2011;13:3235. doi: 10.1021/ol201140q. [DOI] [PubMed] [Google Scholar]; (c) Li X, Gong X, Zhao M, Song G, Deng J, Li X. Org Lett. 2011;13:5808. doi: 10.1021/ol2023856. [DOI] [PubMed] [Google Scholar]; (d) Park SH, Kim JY, Chang S. Org Lett. 2011;13:2372. doi: 10.1021/ol200600p. [DOI] [PubMed] [Google Scholar]; (e) Patureau FW, Glorius F. J Am Chem Soc. 2010;132:9982. doi: 10.1021/ja103834b. [DOI] [PubMed] [Google Scholar]; (f) Patureau FW, Nimphius C, Glorius F. Org Lett. 2011;13:6346. doi: 10.1021/ol202557w. [DOI] [PubMed] [Google Scholar]; (g) Satoh T, Miura M. Chem Eur J. 2010;16:11212. doi: 10.1002/chem.201001363. [DOI] [PubMed] [Google Scholar]; (h) Ueura K, Satoh T, Miura M. Org Lett. 2007;9:1407. doi: 10.1021/ol070406h. [DOI] [PubMed] [Google Scholar]; (i) Umeda N, Hirano K, Satoh T, Miura M. J Org Chem. 2009;74:7094. doi: 10.1021/jo901485v. [DOI] [PubMed] [Google Scholar]; (j) Wang F, Song G, Du Z, Li X. J Org Chem. 2011;76:2926. doi: 10.1021/jo2002209. [DOI] [PubMed] [Google Scholar]; (k) Wei X, Wang F, Song G, Du Z, Li X. Org Biomol Chem. 2012;10:5521. doi: 10.1039/c2ob25773d. [DOI] [PubMed] [Google Scholar]; (l) Wencel-Delord J, Nimphius C, Patureau FW, Glorius F. Chem Asian J. 2012;7:1208. doi: 10.1002/asia.201101018. [DOI] [PubMed] [Google Scholar]; (m) Zhao P, Wang F, Han K, Li X. Org Lett. 2012;14:3400. doi: 10.1021/ol301371p. [DOI] [PubMed] [Google Scholar]; (n) Tsai AS, Brasse M, Bergman RG, Ellman JA. Org Lett. 2011;13:540. doi: 10.1021/ol102890k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Patureau FW, Besset T, Glorius F. Angew Chem Int Ed. 2011;50:1064. doi: 10.1002/anie.201006222. [DOI] [PubMed] [Google Scholar]
- 7.(a) Li Y, Zhang XS, Li H, Wang WH, Chen K, Li BJ, Shi ZJ. Chem Sci. 2012;3:1634. [Google Scholar]; (b) Tauchert ME, Incarvito CD, Rheingold AL, Bergman RG, Ellman JA. J Am Chem Soc. 2012;134:1482. doi: 10.1021/ja211110h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuart D, Alsabeh P, Kuhn M, Fagnou K. J Am Chem Soc. 2010;132:18326. doi: 10.1021/ja1082624. [DOI] [PubMed] [Google Scholar]
- 9.(a) Li L, Brennessel WW, Jones WD. J Am Chem Soc. 2008;130:12414. doi: 10.1021/ja802415h. [DOI] [PubMed] [Google Scholar]; (b) Li L, Brennessel W, Jones WD. Organometallics. 2009;28:3492. [Google Scholar]
- 10.Umeda N, Hirano K, Satoh T, Miura M. J Org Chem. 2009;74:7094. doi: 10.1021/jo901485v. [DOI] [PubMed] [Google Scholar]
- 11.All complexes 5 to 9 are active precursors for the catalytic reaction, only 10 is inactive due to styrene’s inability to displace the PMe3 ligand.
- 12.The same experiment was conducted using [Cp*RhCl2]2 as a precatalyst but it showed the presence of several unidentified species along with 6 and 11. Starting with 6 allowed cleaner NMR spectra.
- 13.Li L, Jiao Y, Brennessel W, Jones WD. Organometallics. 2010;29:4593. [Google Scholar]
- 14.Lapointe D, Fagnou K. Chem Lett. 2010;39:1118. [Google Scholar]
- 15.Boutadla Y, Davies DL, Al-Duaij O, Fawcett J, Jones RC, Singh K. Dalton Trans. 2010;39:10447. doi: 10.1039/c0dt00280a. [DOI] [PubMed] [Google Scholar]
- 16.Beccalli E, Broggini G, Martinelli M, Sottocornola S. Chem Rev. 2007;107:5318. doi: 10.1021/cr068006f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.