Abstract
OBJECTIVE
To assess risk factors for methicillin-resistant Staphylococcus aureus (MRSA) acquisition among extended care residents focusing on level of care (residential vs rehabilitation) and room placement with an MRSA-positive resident.
DESIGN
Prospective cohort study.
SETTING
Extended care units at 2 healthcare systems in Maryland.
PARTICIPANTS
Four hundred forty-three residents with no history of MRSA and negative MRSA surveillance cultures of the anterior nares and areas of skin breakdown at enrollment.
METHODS
Follow-up cultures were collected every 4 weeks and/or at discharge for a period of 12 weeks. Study data were collected by a research nurse from the medical staff and the electronic medical records. Cox proportional hazards modeling was used to calculate adjusted hazards ratios (aHRs) and 95% confidence intervals (CIs).
RESULTS
. Residents in rehabilitation care had 4-fold higher risk of MRSA acquisition compared with residents in residential care (hazard ratio [HR], 4. [95% CI, 2.2–8.8]). Being bedbound was significantly associated with MRSA acquisition in both populations (residential care, aHR, 4.3 [95% CI, 1.5–12.2]; rehabilitation care, aHR, 4.8 [95% CI, 1.2–18.7]). Having an MRSA-positive roommate was not significantly associated with acquisition in either population (residential care, aHR, 1.4 [95% CI, 0.5–3.9]; rehabilitation care, aHR, 0.5 [95% CI, 0.1–2.2]); based on concordant spa typing, only 2 of 8 residents who acquired MRSA and had room placement with an MRSA-positive resident acquired their MRSA isolate from their roommate.
CONCLUSION
Residents in rehabilitation care appear at higher risk and have different risk factors for MRSA acquisition compared to those in residential care.
Residence in an extended care facility is increasingly common and has long been identified as an important risk factor for methicillin-resistant Staphylococcus aureus (MRSA) colonization and infection.1–3 The prevalence of MRSA colonization among residents in these facilities is high, and published results suggest it may be between 8% and 28%.1,3,4 Older adults, who constitute a large proportion of residents in extended care facilities, are potentially at increased risk of morbidity and mortality from MRSA infections due to decreased immune function and increased prevalence of underlying co-morbid conditions.5,6
Despite this increased risk, relatively few studies have reported on risk factors for MRSA acquisition in extended care facilities.1,7,8 As MRSA colonization is a strong risk factor for MRSA infection, these data are essential to identify appropriate infection control strategies in extended care facilities. Recently, the Centers for Disease Control and the Prevention Healthcare Infection Control Practices Advisory Committee, the Society for Healthcare Epidemiology of America, and the Association for Professionals in Infection Control and Epidemiology published guidelines for isolation precautions suggested that decisions regarding placing residents on contact precautions should be made on a case-by-case basis and based primarily on the resident’s clinical situation; however, the guideline acknowledges that extended care settings vary greatly and that few data are available on which to make these decisions.9,10 The current guideline recommends a single room, when possible, for those infected or colonized with MRSA. Room placement is also a difficult decision in the extended care setting because single rooms may not be readily available and also because changing rooms is disruptive, particularly in a residential care environment.
The goal of this study was to assess risk factors associated with MRSA acquisition in extended care facilities in a prospective cohort study. We focused on level of care (residential vs rehabilitation) and room placement with an MRSA-positive resident as the primary variables of interest because these are controversial questions for those working in infection control in extended care settings. To our knowledge, this is the first study to compare MRSA acquisition in different levels of care.
METHODS
Study Setting
The study population consisted of extended care residents at 2 healthcare systems located in Maryland. The Veterans Affairs Maryland Health Care System (VAMHCS) has 5 extended care units within 2 facilities. The Baltimore Rehabilitation and Extended Care Center has 2 units with a total of 120 beds providing postacute care, chronic rehabilitation, hospice, and residential care. The Perry Point Veterans Affairs Medical Center has 3 units with a total of 150 beds providing chronic rehabilitation and residential care. The University Specialty Hospital, part of the University of Maryland Medical System, is a 180-bed postacute care hospital that has an 88-bed rehabilitation extended care unit. These extended care facilities covered a spectrum of care from acute rehabilitation (in which the individual is expected to participate in at least 3 hours of therapy per day) to chronic rehabilitation (typically 1 hour of therapy per day), with the expectation of return to the community, and residential care (custodial care is given with therapy offered only as needed), without the expectation of return to community.
Infection control practices during the study period were similar at the VAMHCS and the University Specialty Hospital and remained the same for the duration of the study. They consisted of MRSA surveillance cultures performed on admission and the use of modified contact precautions for patients colonized with MRSA. Modified contact precautions consisted of a tiered approach to room placement for MRSA-positive residents, with preference given to single rooms, placement with another MRSA-positive resident, and finally, placement with an MRSA-negative resident at low risk for MRSA infection. Gowns and gloves were used during contact with body substances. Alcohol-based hand rinses were available for hand hygiene in both systems. The University of Maryland Baltimore Institutional Review Board and the VAMHCS Research and Development Committee approved this study.
Study Population and Design
This was a prospective cohort study of extended care facility residents without MRSA colonization at the start of the study who were followed over a series of 12-week study cycles and watched for the acquisition of MRSA colonization. The study was conducted from March 2005 to September 2008. Two 12-week cycles (at University Specialty Hospital) or 3 12-week cycles (at all others) were conducted on each unit approximately 1 year apart. The study population included residents who had (1) no history of MRSA colonization, (2) at least 1 anterior nares sample and skin breakdown (if present) that was negative for MRSA by culture and polymerase chain reaction (PCR) at enrollment, (3) a length of stay of more than 7 days, and (4) at least 1 follow-up set of cultures to detect acquisition. Every 4 weeks during the study cycle and before planned discharges, we collected culture samples from the anterior nares and the largest area of skin breakdown.
Laboratory Methods
Although the study units routinely used surveillance cultures for infection control purposes, all surveillance culture specimens in this study were collected by a research nurse using a rayon-tipped swab with Amies transport medium (BactiSwab, Remel). The swab was premoistened with transport gel before obtaining the nares specimen. All swab specimens were streaked for isolation onto tryptic soy agar containing 5% sheep blood agar (Remel). Plates were incubated at 37°C for 48 hours. Isolates were identified as S. aureus on the basis of catalase and coagulase production (Pastorex, Bio-Rad Laboratories). S. aureus isolates were plated on oxacillin agar (6 μg/mL) screening plates and incubated at 37°C. Colonies on the oxacillin agar plates were classified as oxacillin resistant (ie MRSA). Isolates that were classified as S. aureus and did not grow on oxacillin agar were considered methicillin sensitive. Enrollment swab specimens that were negative by culture for S. aureus underwent real-time PCR for the detection of MRSA using the BD GeneOhm MRSA assay (Becton Dickinson) according to the manufacturer’s instructions. All follow-up cultures were analyzed using standard microbiologic testing, as described above.
Molecular Typing
Chromosomal DNA was extracted from cells after growth in an overnight culture of tryptic soy broth at 37°C using methods described elsewhere.7 Each MRSA isolate was also characterized by DNA amplification and DNA sequencing of the protein A (spa) gene hypervariable region.11 The spa repeats were defined based on comparison to the sequences in a public database.12 We defined MRSA isolates as concordant spa types if the spa type was identical.
Variable Definitions
MRSA acquisition was defined as present if culture of a specimen from the anterior nares or an area of skin breakdown (if present) was positive for MRSA. The date that the positive culture was taken was used as the date of acquisition. Colonization with methicillin-susceptible S. aureus (MSSA) was defined as present if at least 1 specimen from the anterior nares obtained during the study cycle grew MSSA on culture. During the study cycle, a research nurse collected data from the medical staff and the electronic medical records and entered the following variables into a relational database (Microsoft Access): demographic characteristics (age, race, and sex); length of stay; level of care (residential, hospice, respite, acute, or chronic rehabilitation); presence of skin breakdown (pressure ulcer, surgical wound, or placement of a percutaneous foreign device); level of mobility, by measuring whether a patient was able to attend therapy and other activities with little assistance or whether he had his meals in his room and required assistance with most activities; and level of dependency, as measured by activities of daily living. Level of care was dichotomized as residential or rehabilitation care on the basis of whether a return to independent living in the community was expected. Any antibiotic use in the 30 days before and during the study cycle was also recorded. In addition, room placement was also recorded as a single room, a non-single room with at least 1 roommate with MRSA colonization, or a nonsingle room with no roommates with MRSA colonization. The Charlson Comorbidity Index was used as a measure of aggregate comorbidity and calculated using International Classification of Diseases, Ninth Edition (ICD-9) codes.13 To assure the quality of the data, we performed logic checks on the database and monitored source documentation for key variables in 5% of enrolled residents.
Statistical Analysis
Because the study was performed in 12-week cycles approximately 1 year apart, residents could participate in more than 1 study cycle. We used the last eligible study cycle per resident as the unit of analysis. Descriptive statistics, including the Student t test, the Wilcoxon test, and the χ2 test, were used to compare differences between groups. Kaplan-Meier plots were created using data from the last study cycle per resident, and both the log-rank and the Wilcoxon tests were used to test for equality of survival functions. Cox proportional hazards modeling was used to calculate unadjusted and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs). Variables included in the initial multivariable statistical model were significantly associated with MRSA acquisition at the P < .10 level in the descriptive analyses. Variables not significantly associated (α = 0.05) with MRSA acquisition were then removed from the models. Each of the removed variables was then reinserted into the model to assess whether its presence altered the regression coefficient by 20% or more. If so, this confounding variable was included in the final model. The resulting multivariable model was considered the final model. Additionally, because of the specific interest and potential policy implications regarding room placement with an MRSA-positive resident, we forced this variable into the final model.
RESULTS
Among 1,444 resident N study cycles during the study period, 617 (43%) were excluded because there was a previous history of MRSA colonization or infection. Of the 827 resident N study cycles without previous MRSA history, others were excluded because the resident refused to participate (50 study cycles from 50 residents), because the resident was MRSA positive at study enrollment (41 study cycles from 41 residents), and because the resident had a length of stay less than 6 days (40 study cycles from 40 residents). One hundred forty-four residents were lost to follow-up before undergoing at least 1 follow-up anterior nares surveillance culture. When we compared residents lost to follow-up (n = 144) to those who were not lost to follow-up (n = 443), those not lost to follow-up were more likely to be in residential care (65% vs 49%); however, the groups were similar with respect to admission to a VA facility (84% vs 80%), antibiotic use (39% vs 32%), and being bedbound (10% vs 12%). The final resulting sample at risk for acquisition was 443 residents with 537 cycles. Twenty-two residents had 3 study cycles, 49 had 2 study cycles, and 371 had 1 study cycle. We used the last eligible study cycle per resident for analysis.
Approximately 64% (n = 286) of participants were in residential care with the remaining 36% (n = 157) in rehabilitation care. Almost all residential care residents and 46% of rehabilitation care residents were from VA-affiliated facilities. Table 1 displays characteristics of the participants and a comparison between residential and rehabilitation participants. Residents of residential care facilities were significantly older and more likely to be male, required greater than moderate assistance with activities of daily living, and had an MSSA-positive culture during the study period. Residents of rehabilitation facilities were significantly more likely to be African American, to have received antibiotics, to have skin break-down, and to have limited mobility, including being bedbound.
TABLE 1.
Characteristics of Methicillin-Resistant Staphylococcus aureus (MRSA)–Negative Residents of Extended Care Facilities, Stratified by Level of Care
| Variable | Residential (n = 286) | Rehabilitation (n = 157) | P value* |
|---|---|---|---|
| Age, years, mean ± SD | 76 ± 11 | 57 ± 16 | <.01 |
| Male sex | 273 (95) | 123 (78) | <.01 |
| Black race | 114 (40) | 81 (52) | .02 |
| No. of study cycles | <.01 | ||
| 1 | 218 (76) | 153 (97) | |
| 2 | 46 (16) | 4 (3) | |
| 3 | 22 (8) | 0 (0) | |
| Residents in a Veterans Affairs facility | 282 (99) | 72 (46) | <.01 |
| Prestudy length of stay ≤6 months | 173 (60) | 154 (98) | <.01 |
| Antibiotic therapy during study cycle | 61 (21) | 82 (52) | <.01 |
| Skin breakdown | |||
| Decubitus ulcers | 45 (16) | 42 (27) | .01 |
| Invasive device | 40 (14) | 76 (48) | <.01 |
| Surgical incision | 12 (4) | 63 (40) | <.01 |
| Required more than moderate assistance for ADL | 109 (38) | 33 (21) | <.01 |
| Mobility | |||
| Limited mobility | 77 (27) | 59 (36) | .02 |
| Bedbound | 24 (8) | 28 (18) | <.01 |
| Tested MSSA positive during study cycle | 84 (29) | 30 (19) | .02 |
| Room placement with MRSA-positive resident | 59 (21) | 28 (18) | .48 |
| Charlson Comorbidity Index, median (IQR) | 2.0 (1.0–4.0) | 2.0 (0.0–3.0) | <.01 |
NOTE. Data are no. (%) of patients, unless indicated otherwise. Study cycles are 12-week study periods that were repeated approximately 1 year apart on each unit. Two residents had 1 study cycle in rehabilitation care followed by 1 or more cycles in residential care during the study. These residents appear only in the residential care column. IQR, interquartile range; MSSA, methicillin susceptible Staphylococcus aureus; SD, standard deviation; ADL, activities of daily living.
P values are from the Student t test or the Kruskall-Wallis test for continuous variables and the χ2 test for categorical variables.
Thirty-six residents (8%) acquired MRSA colonization; however, the incidence among rehabilitation residents (17 residents [11%]) was approximately twice the incidence among residents of residential facilities (17 residents [6%]). Survival analyses utilizing Kaplan-Meier plots and comparing acquisition by level of care further confirmed the differences in incidence over time (Figure 1). The risk of acquisition was 4 times higher among residents in rehabilitation care, compared with the risk among those in residential care (HR, 4.4 [95% CI, 2.2–8.8]). Based on these results and the comparisons in Table 1, further analyses were stratified by these 2 levels of care.
Figure 1.
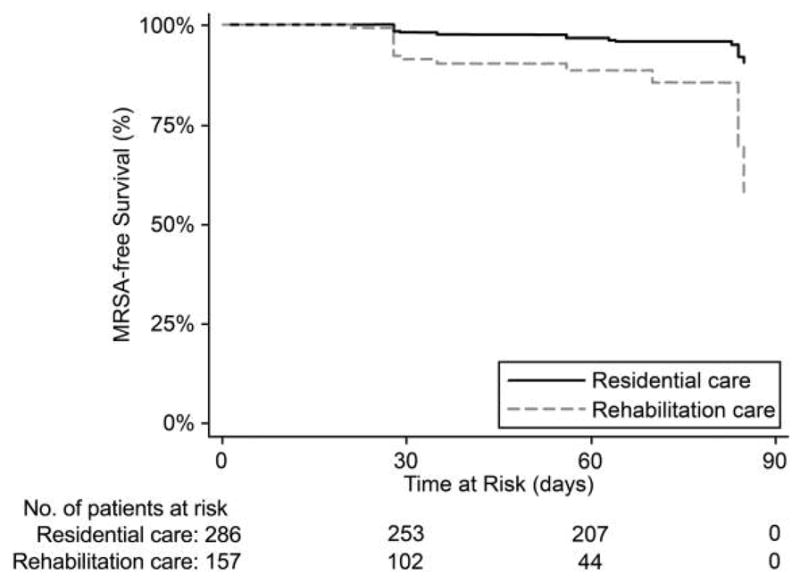
Acquisition-free survival in methicillin-resistant Staphylococcus aureus (MRSA)–negative residents of extended care facilities stratified by level of care (n = 443). Log-rank test for equality of survival functions, P < .01; Wilcoxon (Breslow) test for equality of survival functions, P < .01; hazard ratio (rehabilitation vs residential), 4.40 (95% confidence interval, 2.21–8.75).
We identified several potential risk factors using unadjusted Cox proportional hazards models stratified by level of care (residential vs rehabilitation) (Table 2). Among participants in residential care, receiving antibiotics (HR, 4.4 [95% CI, 1.7–11.1]), being bedbound (HR, 4.9 [95% CI, 1.8–14.0), and requiring more than moderate assistance with activities of daily living (HR, 2.7 [95% CI, 1.0–6.9]) were significantly associated with MRSA acquisition. In multivariable models, receiving antibiotics (adjusted HR [aHR], 3.8 [95% CI, 1.4–9.9]) and being bedbound (aHR, 4.3 [95% CI, 1.5–12.2]) were independently and significantly associated with MRSA acquisition among participants in residential care (Table 3).
TABLE 2.
Risk of Methicillin-Resistant Staphylococcus aureus (MRSA) Acquisition for MRSA-Negative Residents of Extended Care Facilities, Stratified by Level of Care (n = 443)
| Variable | Residential hazard ratio (95% CI) (n = 286) |
Rehabilitation hazard ratio (95% CI) (n = 157) |
|---|---|---|
| Age >65 years | 0.48 (0.17–1.36) | 0.93 (0.33–2.63) |
| Male sex | NAa | 1.11 (0.31–3.90) |
| Black race | 1.54 (0.61–3.88) | 0.89 (0.35–2.24) |
| Prestudy length of stay <6 months | 2.20 (0.82–5.90) | NAa |
| Antibiotic therapy during study cycle | 4.40 (1.74–11.1) | 0.80 (0.31–2.03) |
| Skin breakdown | ||
| Pressure ulcers | 1.69 (0.56–5.14) | 1.96 (0.77–4.98) |
| Invasive device | 1.38 (0.40–4.77) | 1.08 (0.41–2.86) |
| Surgical incision | NAa | 1.63 (0.64–4.10) |
| Required more than moderate assistance for ADL | 2.66 (1.03–6.87) | 1.68 (0.59–4.73) |
| Mobility | ||
| Limited mobility | 0.58 (0.17–2.01) | 1.56 (0.62–3.92) |
| Bedbound | 4.89 (1.78–14.0) | 2.41 (0.85–6.87) |
| Tested MSSA positive during study cycle | 0.61 (0.20–1.84) | 0.43 (0.10–1.84) |
| Room placement with MRSA-positive resident | 1.84 (0.69–4.92) | 0.55 (0.13–2.38) |
| Charlson Comorbidity Index >2 | 1.33 (0.53–3.36) | 0.61 (0.20–1.85) |
Indicates that a hazard ratio could not be calculated because there were no acquisitions in 1 or both of the comparison groups. ADL, activities of daily living.
TABLE 3.
Adjusted Hazard Ratios (aHRs) for Methicillin-Resistant Staphylococcus aureus (MRSA) Acquisition in MRSA-Negative Residents of Extended Care Facilities, Using Cox Proportional Hazards Models
| Variable | aHR (95% CI) |
|---|---|
| Residential (n = 286) | |
| Antibiotic therapy during study cycle | 3.75 (1.43–9.88) |
| Bedbound | 4.28 (1.50–12.16) |
| Room placement with MRSA-positive resident | 1.42 (0.51–3.93) |
| Rehabilitation (n = 157) | |
| Limited mobility | 2.59 (0.80–8.45) |
| Bedbound | 4.81 (1.24–18.68) |
| Room placement with MRSA-positive resident | 0.47 (0.10–2.16) |
Among participants in rehabilitation care, no variables were significantly associated with acquisition in bivariable analyses, although being bedbound (HR, 2.4 [95% CI, 0.9–6.9]) and having pressure ulcers (HR, 2.0 [95% CI, 0.8–5.0]) were associated with an increased risk. In multivariable models, only being bedbound was significantly associated with acquisition (aHR, 4.8 [95% CI, 1.2–18.7]).
Room placement with an MRSA-positive roommate was not significantly associated with acquisition in either population (residential care aHR, 1.4 [95% CI, 0.5–3.9]; rehabilitation care aHR, 0.5 [95% CI, 0.1–2.2]). Overall there were 36 residents who acquired MRSA colonization. Patients in 8 of 109 resident study cycles (7%) who had an MRSA-positive roommate acquired MRSA, in contrast to patients in 28 of 426 resident study cycles (7%) who did not have an MRSA-positive roommate. We performed molecular typing on the MRSA isolates from the 8 roommate pairs. The same spa type was found in 2 of the 8 roommate pairs (data not shown).
DISCUSSION
This was a prospective cohort study of MRSA-negative extended-care facility residents to identify and quantify risk factors for MRSA acquisition. Our data suggest that residents in rehabilitation care are at a 4-fold higher risk of MRSA acquisition than residents in residential care. This implies that characteristics of rehabilitation patients or the types of care they receive may increase their risk of MRSA acquisition. Some risk factors for MRSA acquisition were the same; others differed by level of care. Being bedbound was most strongly associated with MRSA acquisition in both populations, suggesting that healthcare worker contact is the primary mode of transmission, and thus, compliance with hand hygiene should be emphasized at all times. In contrast, antibiotic use was only a risk factor for residents in residential care. Having an MRSA-positive roommate was not significantly associated with acquisition in either population and a minority of roommate pairs had concordant spa types.
Despite increased attention to and the recent guidelines focused specifically on infection control in the long-term care facilities, few studies have prospectively assessed risk factors for MRSA acquisition in this setting, and none, to our knowledge, have compared MRSA acquisition in rehabilitation and residential levels of care. This latter point is relevant because although they potentially represent vastly different subpopulations, residential care and rehabilitation care residents often occupy the same rooms or facilities and as result are subject to the same infection control practices. The limited existing evidence is similar to our results.1,4,7 Bradley et al1 observed that 10% of at-risk residents acquired MRSA over a 1-year period in a 120-bed VA-affiliated residential care facility. Although this study did not identify risk factors specific for MRSA acquisition, there was a trend of increased colonization among residents with lower functional status. Our results suggest that isolation guidelines may need to be stratified by level of care and that intensified isolation precautions, similar to those in acute care settings, may be necessary in rehabilitation care. Rehabilitation care is inherently different from residential or custodial care. It is more time limited and goal oriented and is not the resident’s “home” environment. Thus, a more intensive type of isolation precautions may be justified.14
Despite that, during approximately 20% of the resident study cycles, residents shared a room with an MRSA-positive roommate; we did not detect an association between MRSA acquisition and having an MRSA-positive roommate. Furthermore, molecular typing of the isolates from the 8 residents who acquired MRSA and who had an MRSA-positive roommate identified only 25% concordance on MRSA spa type, suggesting that the acquisition rarely occurred as a result of transmission between roommates. Bradley et al1 also used molecular typing and concluded that 26% of acquisitions in their study resulted from transmission from an MRSA-positive roommate. A similar calculation from our data would conclude that 6% of residents (2 of 36) likely acquired MRSA from a roommate. The difference is most likely because of room placement policies and an increase in the number of available single rooms. Thus, despite the limited number of roommate acquisition pairs, we believe these data from this study support the current tiered room placement policies.
This study was limited by the low number of acquisition events that occurred during the study cycles, which may have been a result of the rigorous methods used to determine that an acquisition represented a transmission event and not a previously undetected colonization and the low acquisition rates in this setting. As a result, we had limited statistical power to identify and quantify potential risk factors. In addition, our study population in residential care consisted largely of VA-affiliated extended care facilities, which may limit the generalizability of our results. Finally, the strengths of the study were its prospective data collection methods, its definition of acquisition, and the use of sequence typing to further refine our risk factor analysis.
In summary, this study demonstrates a more than 4-fold higher incidence of MRSA acquisition in rehabilitation care than in residential care. We found that room placement with an MRSA-positive resident was not a significant risk factor for acquisition and that when acquisition-associated roommate pairs were examined, most of these acquisitions were not from the roommate. These data provide support for current room placement guidelines and suggests that rehabilitation care residents may be at increased risk of acquisition and transmission than residential care. Future studies should focus on further refining the type of isolation precautions needed in each setting.
Acknowledgments
We thank Deborah Grady, RN, MS, for collection of study specimens and resident data; Alison Lydecker, MPH, for assistance with data analyses and figure creation; Jingkun Zhu, MA, and Coleen Reilly for data management; Gwen Robinson, Mary Lee, and O. Colin Stine, PhD, for their assistance with the performance and interpretation of laboratory testing; and Eli N. Perencevich, MD, MS, for useful suggestions regarding interpretation of the data.
Financial support. This study was funded by a Veterans Affairs Merit Award (M.R.), Centers for Disease Control and Prevention Award R01CI000369 (M.R. and A.D.H), National Institutes of Health awards 1K01AI071015-02 (J.P.F.) and 1K12RR023250-05 (J.K.J.), and University of Maryland General Clinical Research Center award M01RR16500.
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
Presented in part: 19th Annual Scientific Meeting of the Society for Health-care Epidemiology of America, March 19–22, 2009, San Diego, CA.
References
- 1.Bradley SF, Terpenning MS, Ramsey MA, et al. Methicillin-resistant Staphylococcus aureus: colonization and infection in a long-term care facility. Ann Intern Med. 1991;115:417–422. doi: 10.7326/0003-4819-115-6-417. [DOI] [PubMed] [Google Scholar]
- 2.Lucet JC, Grenet K, Armand-Lefevre L, et al. High prevalence of carriage of methicillin-resistant Staphylococcus aureus at hospital admission in elderly patients: implications for infection control strategies. Infect Control Hosp Epidemiol. 2005;26:121–126. doi: 10.1086/502514. [DOI] [PubMed] [Google Scholar]
- 3.Muder RR, Brennen C, Wagener MM, et al. Methicillin-resistant staphylococcal colonization and infection in a long-term care facility. Ann Intern Med. 1991;114:107–112. doi: 10.7326/0003-4819-114-2-1-107. [DOI] [PubMed] [Google Scholar]
- 4.Furuno JP, Hebden JN, Standiford HC, et al. Prevalence of methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii in a long-term acute care facility. Am J Infect Control. 2008;36:468–471. doi: 10.1016/j.ajic.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClelland RS, Fowler VG, Jr, Sanders LL, et al. Staphylococcus aureus bacteremia among elderly vs younger adult patients: comparison of clinical features and mortality. Arch Intern Med. 1999;159:1244–1247. doi: 10.1001/archinte.159.11.1244. [DOI] [PubMed] [Google Scholar]
- 6.Shurland SM, Stine OC, Venezia RA, et al. Colonization sites of USA300 methicillin-resistant Staphylococcus aureus in residents of extended care facilities. Infect Control Hosp Epidemiol. 2009;30:313–318. doi: 10.1086/596114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulhausen PL, Harrell LJ, Weinberger M, Kochersberger GG, Feussner JR. Contrasting methicillin-resistant Staphylococcus aureus colonization in Veterans Affairs and community nursing homes. Am J Med. 1996;100:24–31. doi: 10.1016/s0002-9343(96)90007-8. [DOI] [PubMed] [Google Scholar]
- 8.Trick WE, Weinstein RA, DeMarais PL, et al. Comparison of routine glove use and contact-isolation precautions to prevent transmission of multidrug-resistant bacteria in a long-term care facility. J Am Geriatr Soc. 2004;52:2003–2009. doi: 10.1111/j.1532-5415.2004.52555.x. [DOI] [PubMed] [Google Scholar]
- 9.Siegel JD, Rhinehart E, Jackson M, Chiarello L Health Care Infection Control Practices Advisory Committee. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(suppl 2):65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith PW, Bennett G, Bradley S, et al. SHEA/APIC guideline: infection prevention and control in the long-term care facility. Infect Control Hosp Epidemiol. 2008;29:785–814. doi: 10.1086/592416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harmsen D, Claus H, Witte W, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003;41:5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridom SpaServer. home page, http://www.ridom.de/spaserver.
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14.Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with Contact Precautions: a review of the literature. Am J Infect Control. 2009;37:85–93. doi: 10.1016/j.ajic.2008.04.257. [DOI] [PMC free article] [PubMed] [Google Scholar]


