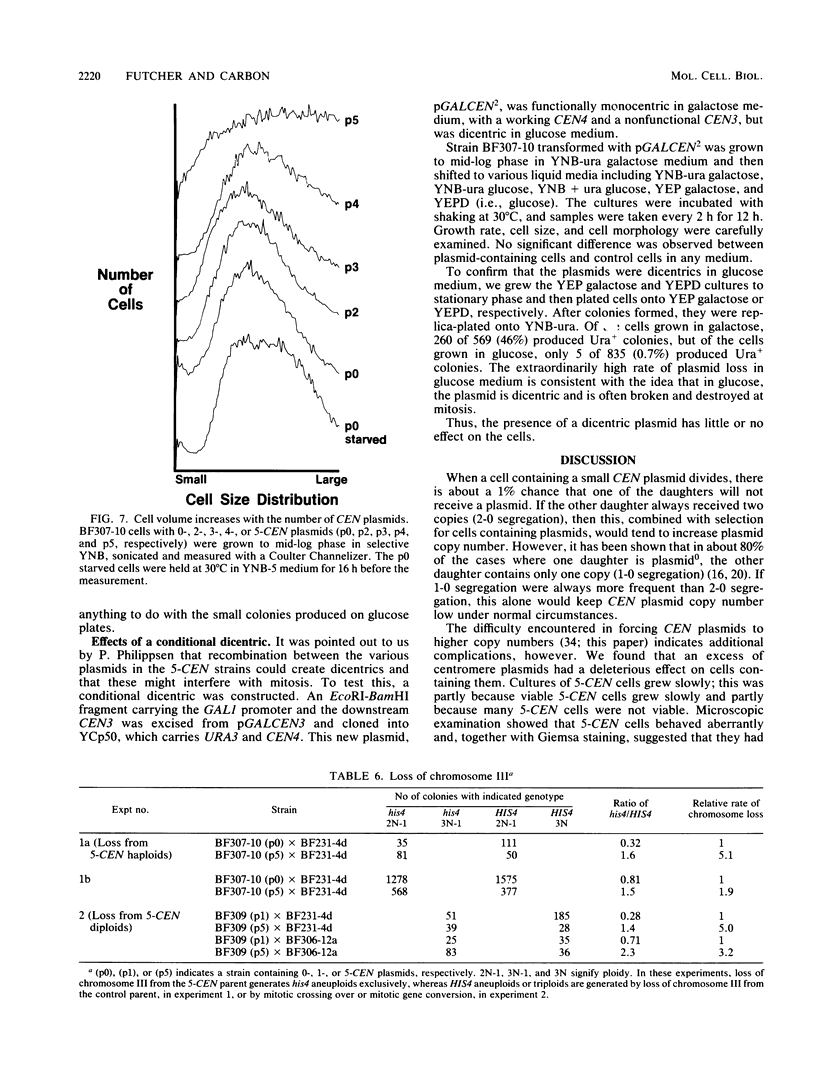
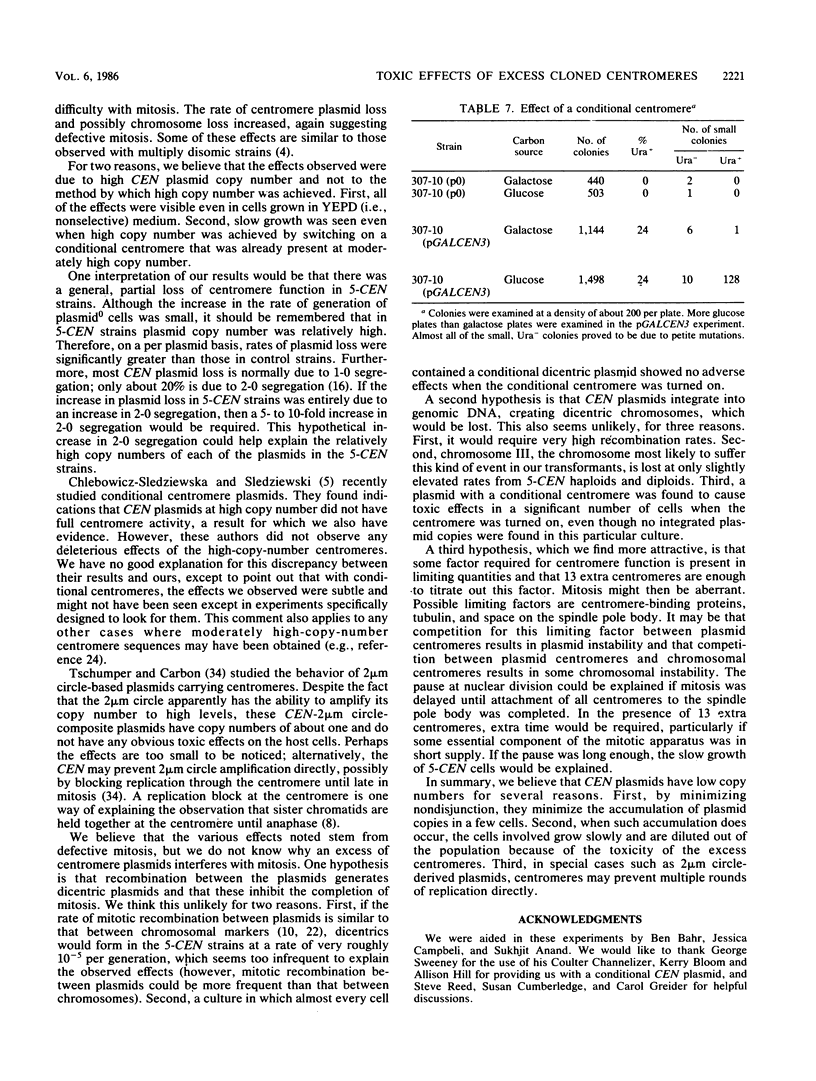
Abstract
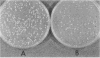
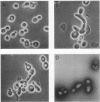
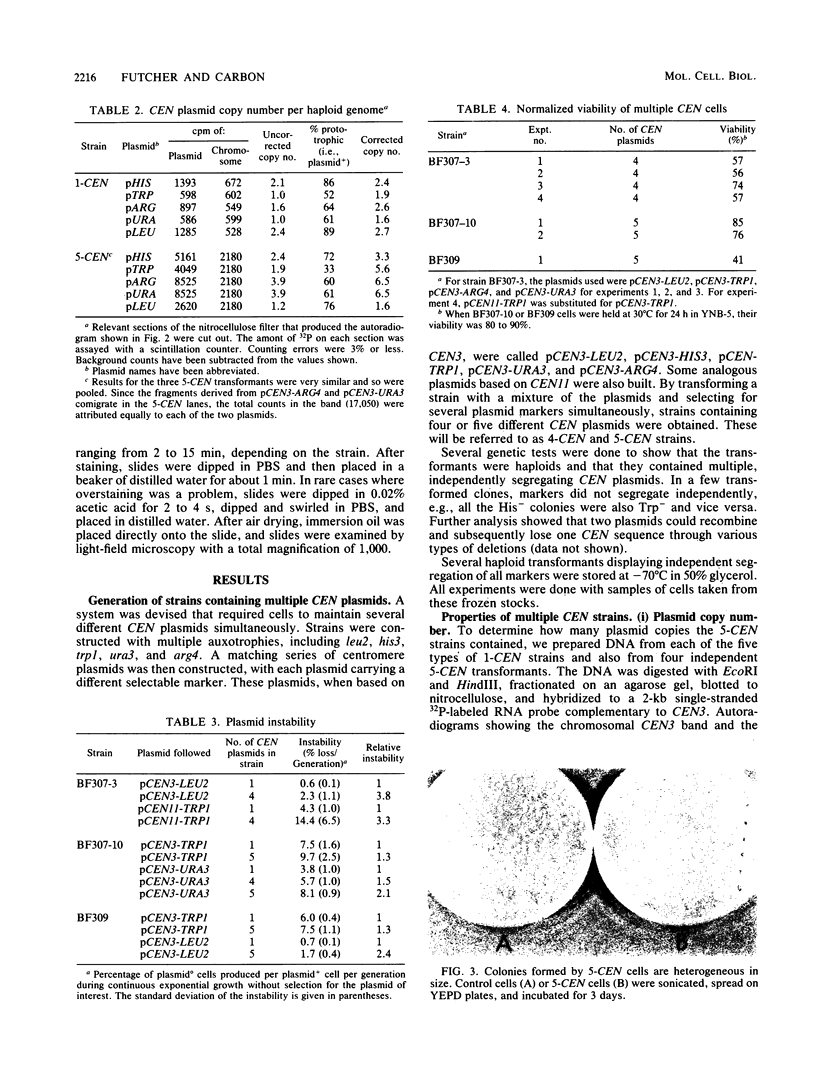
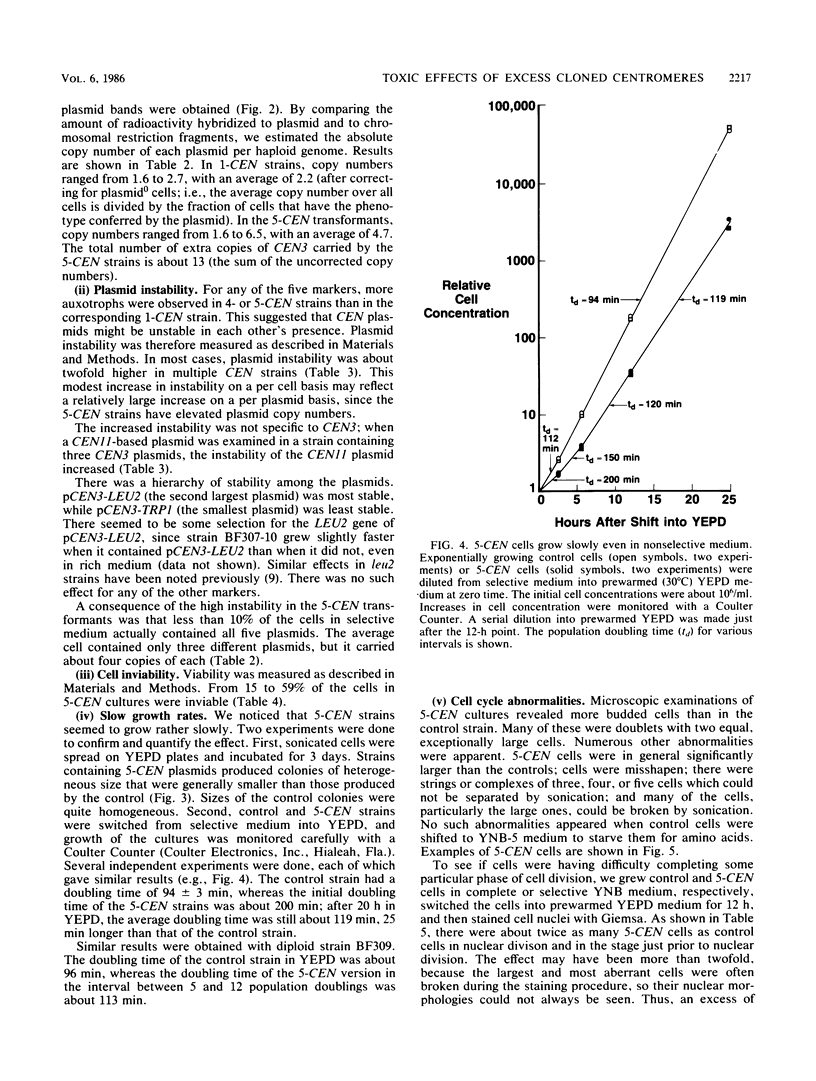
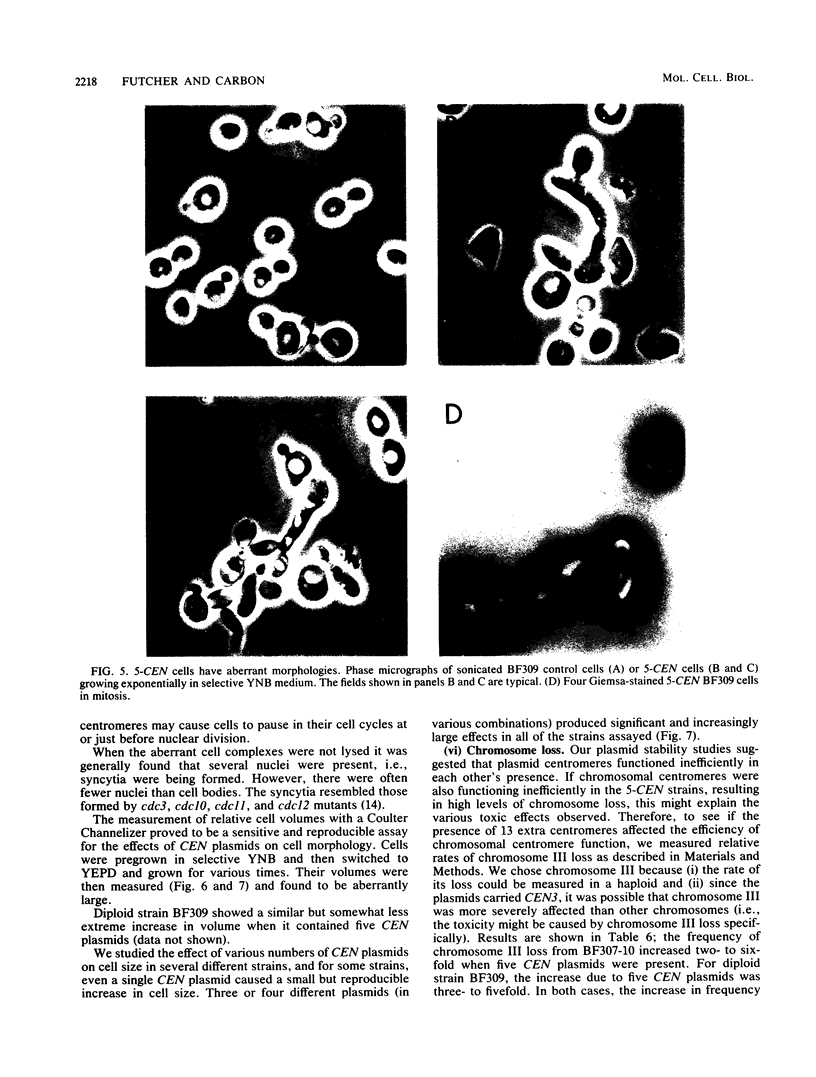
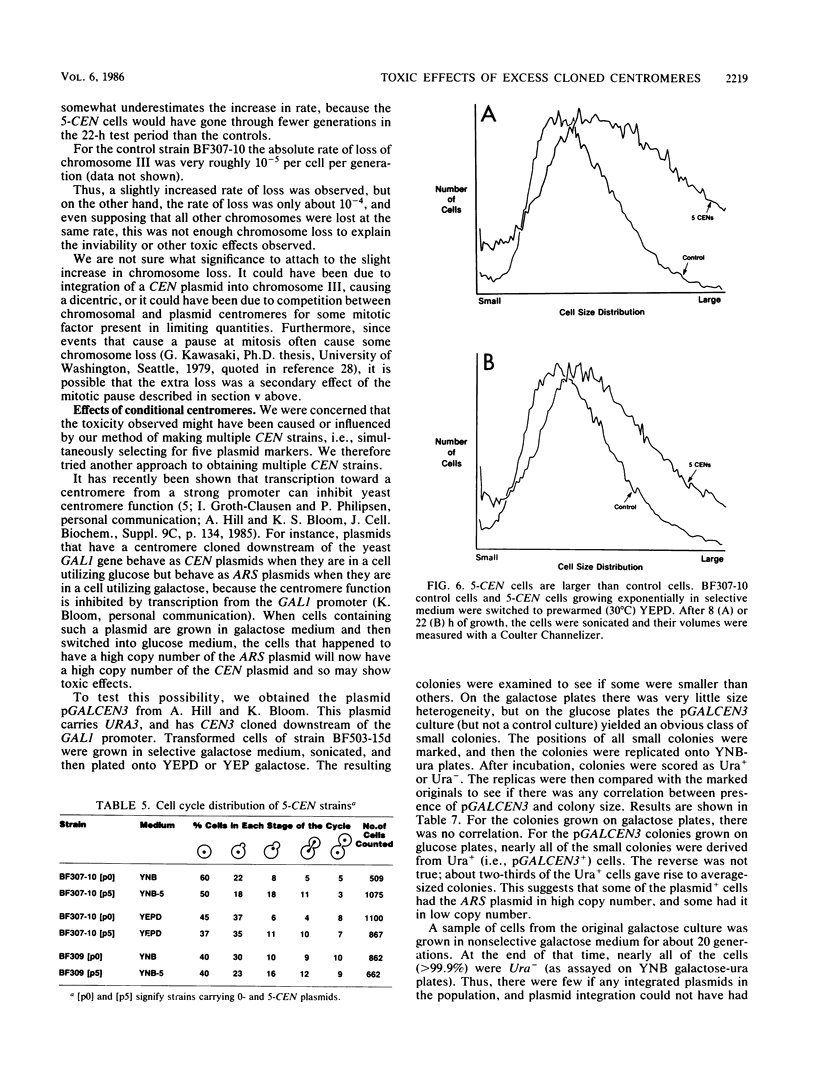
Plasmids carrying a Saccharomyces cerevisiae centromere have a copy number of one or two, whereas other yeast plasmids have high copy numbers. The number of CEN plasmids per yeast cell was made artificially high by transforming cells simultaneously with several different CEN plasmids carrying different, independently selectable markers. Some host cells carried five different CEN plasmids and an average total of 13 extra copies of CEN3. Several effects were noted. The copy number of each plasmid was unexpectedly high. The plasmids were mutually unstable. Cultures contained many dead cells. The viable host cells grew more slowly than control cells, even in nonselective medium. There was a pause in the cell cycle at or just before mitosis. We conclude that an excess of centromeres is toxic and that the copy number of centromere plasmids is low partly because of selection against cells carrying multiple centromere plasmids. The toxicity may be caused by competition between the centromeres for some factor present in limiting quantities, e.g., centromere-binding proteins, microtubules, or space on the spindle pole body.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K. S., Fitzgerald-Hayes M., Carbon J. Structural analysis and sequence organization of yeast centromeres. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1175–1185. doi: 10.1101/sqb.1983.047.01.133. [DOI] [PubMed] [Google Scholar]
- Campbell D., Doctor J. S., Feuersanger J. H., Doolittle M. M. Differential mitotic stability of yeast disomes derived from triploid meiosis. Genetics. 1981 Jun;98(2):239–255. doi: 10.1093/genetics/98.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowicz-Sledziewska E., Sledziewski A. Z. Construction of multicopy yeast plasmids with regulated centromere function. Gene. 1985;39(1):25–31. doi: 10.1016/0378-1119(85)90103-9. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Miklos G. L. Localization and quantification of circular DNA in yeast. Eur J Biochem. 1974 Jan 16;41(2):359–365. doi: 10.1111/j.1432-1033.1974.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature. 1980 Oct 9;287(5782):504–509. doi: 10.1038/287504a0. [DOI] [PubMed] [Google Scholar]
- Erhart E., Hollenberg C. P. The presence of a defective LEU2 gene on 2 mu DNA recombinant plasmids of Saccharomyces cerevisiae is responsible for curing and high copy number. J Bacteriol. 1983 Nov;156(2):625–635. doi: 10.1128/jb.156.2.625-635.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S. Evidence that spontaneous mitotic recombination occurs at the two-strand stage. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4436–4440. doi: 10.1073/pnas.75.9.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald-Hayes M., Buhler J. M., Cooper T. G., Carbon J. Isolation and subcloning analysis of functional centromere DNA (CEN11) from Saccharomyces cerevisiae chromosome XI. Mol Cell Biol. 1982 Jan;2(1):82–87. doi: 10.1128/mcb.2.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald-Hayes M., Clarke L., Carbon J. Nucleotide sequence comparisons and functional analysis of yeast centromere DNAs. Cell. 1982 May;29(1):235–244. doi: 10.1016/0092-8674(82)90108-8. [DOI] [PubMed] [Google Scholar]
- Futcher A. B., Cox B. S. Copy number and the stability of 2-micron circle-based artificial plasmids of Saccharomyces cerevisiae. J Bacteriol. 1984 Jan;157(1):283–290. doi: 10.1128/jb.157.1.283-290.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971 Dec;69(2):265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- Hicks J. B., Hinnen A., Fink G. R. Properties of yeast transformation. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1305–1313. doi: 10.1101/sqb.1979.043.01.149. [DOI] [PubMed] [Google Scholar]
- Hieter P., Mann C., Snyder M., Davis R. W. Mitotic stability of yeast chromosomes: a colony color assay that measures nondisjunction and chromosome loss. Cell. 1985 Feb;40(2):381–392. doi: 10.1016/0092-8674(85)90152-7. [DOI] [PubMed] [Google Scholar]
- Hieter P., Pridmore D., Hegemann J. H., Thomas M., Davis R. W., Philippsen P. Functional selection and analysis of yeast centromeric DNA. Cell. 1985 Oct;42(3):913–921. doi: 10.1016/0092-8674(85)90287-9. [DOI] [PubMed] [Google Scholar]
- Hyman B. C., Cramer J. H., Rownd R. H. Properties of a Saccharomyces cerevisiae mtDNA segment conferring high-frequency yeast transformation. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1578–1582. doi: 10.1073/pnas.79.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D., Kent J. C., Hartwell L. H. Genetic analysis of the mitotic transmission of minichromosomes. Cell. 1985 Feb;40(2):393–403. doi: 10.1016/0092-8674(85)90153-9. [DOI] [PubMed] [Google Scholar]
- Maine G. T., Surosky R. T., Tye B. K. Isolation and characterization of the centromere from chromosome V (CEN5) of Saccharomyces cerevisiae. Mol Cell Biol. 1984 Jan;4(1):86–91. doi: 10.1128/mcb.4.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R. E., Esposito R. E. The RAD52 gene is required for homothallic interconversion of mating types and spontaneous mitotic recombination in yeast. Proc Natl Acad Sci U S A. 1980 Jan;77(1):503–507. doi: 10.1073/pnas.77.1.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Szostak J. W. Construction of artificial chromosomes in yeast. Nature. 1983 Sep 15;305(5931):189–193. doi: 10.1038/305189a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Szostak J. W. Pedigree analysis of plasmid segregation in yeast. Cell. 1983 Oct;34(3):961–970. doi: 10.1016/0092-8674(83)90553-6. [DOI] [PubMed] [Google Scholar]
- Neitz M., Carbon J. Identification and characterization of the centromere from chromosome XIV in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Nov;5(11):2887–2893. doi: 10.1128/mcb.5.11.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzeri L., Philippsen P. Centromeric DNA from chromosome VI in Saccharomyces cerevisiae strains. EMBO J. 1982;1(12):1605–1611. doi: 10.1002/j.1460-2075.1982.tb01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Mann C., Davis R. W. Centromeric DNA from Saccharomyces cerevisiae. J Mol Biol. 1982 Jun 25;158(2):157–190. doi: 10.1016/0022-2836(82)90427-2. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Struhl K., Davis R. W. Isolation and characterisation of a yeast chromosomal replicator. Nature. 1979 Nov 1;282(5734):39–43. doi: 10.1038/282039a0. [DOI] [PubMed] [Google Scholar]
- Storms R. K., McNeil J. B., Khandekar P. S., An G., Parker J., Friesen J. D. Chimeric plasmids for cloning of deoxyribonucleic acid sequences in Saccharomyces cerevisiae. J Bacteriol. 1979 Oct;140(1):73–82. doi: 10.1128/jb.140.1.73-82.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J., Hicks J., Herskowitz I. Control of cell type in yeast by the mating type locus. The alpha 1-alpha 2 hypothesis. J Mol Biol. 1981 Apr 15;147(3):357–372. doi: 10.1016/0022-2836(81)90488-5. [DOI] [PubMed] [Google Scholar]
- Tschumper G., Carbon J. Copy number control by a yeast centromere. Gene. 1983 Aug;23(2):221–232. doi: 10.1016/0378-1119(83)90054-9. [DOI] [PubMed] [Google Scholar]