Abstract
Background: Elevated level of plasma homocysteine has been related to various diseases. Patients with hyperhomocysteinemia can develop hepatic steatosis and fibrosis. We hypothesized that oxidative stress induced by homocysteine might play an important role in pathogenesis of liver injury. Also, the cellular response designed to combat oxidative stress is primarily controlled by the transcription factor Nrf2, a principal inducer of anti-oxidant and phase II-related genes. Methods: HepG2 cells were treated with homocysteine in different time periods. Glutathione content was measured by flowcytometry. Using electrophoretic mobility shift assay (EMSA) and Western-blotting, anti-oxidant response element (ARE)-binding activity of Nrf2 for heme ocygenase-1 (HO-1) was demonstrated. Real time RT-PCR and Western-blotting were performed to evaluate whether homocysteine was able to induce mRNA and protein expression of HO-1. Results: The role of Nrf2 in cellular response to homocysteine is substantiated by the following observations in HepG2 cells exposed to homocysteine (i) Western-blotting revealed that Nrf2 is strongly stabilized and became detectable in nuclear extracts. (ii) EMSA demonstrated increased binding of Nrf2 to oligomers containing HO-1 promoter-specific ARE-binding site. (iii) Real time RT-PCR and Western-blotting revealed increased mRNA and protein expression of inducible gene HO-1 after treatment with homocysteine. Conclusion: Data presented in the current study provide direct evidence that the immediate cellular response to oxidative stress provoked by homocysteine is orchestrated mainly by the Nrf2-ARE pathway. Therefore, induction of Nrf2-ARE-dependent expression of HO-1 could be a therapeutic option for hepatic cells damage induced by homocysteine.
Key Words: Heme oxygenase-1, HepG2 cells, Oxidative stress
INTRODUCTION
Homocysteine is a thiol-containing amino acid, which produces at the cross road of methionine metabolism. Liver is the tissue where methionine is metabolized. Elevated plasma homocysteine or hyperhomocysteinemia has been implicated as a risk factor for atherosclerosis, cerebrovascular, peripheral vascular diseases and hepatic dysfunction [1, 2].
The enzymes 5, 10 methylenetetrahydrofolate reductase and cystathionine β-synthase play critical roles in regulating plasma homocysteine. Patients with hyperhomocysteinemia due to deficiency in these enzymes can develop hepatic steatosis and fibrosis [3]. Consistent with these findings, homozygous cystathionine β-synthase -deficient mice having hyperhomocysteinemia also develop hepatic steatosis [4, 5]. It is also shown that in chronic alcoholism, hyperhomocysteinemia due to insult in methionine metabolism can contribute to the development of alcoholic liver disease, that is characterized by fatty liver, steatohepatitis, fibrosis, cirrhosis and potentially hepatocellular carcinoma [6, 7].
Little is known about the cellular and biochemical mechanisms of homocysteine-induced hepato-toxicity, but it has been confirmed that oxidative stress plays an important role in different types of liver injury [8, 9]. To counteract damage induced by oxidative stress, cells have developed an adoptive defense mechanism that leads to rapid and efficient induction of detoxifying and anti-oxidative stress enzymes. Among them, induction of a family of anti-oxidant detoxification enzymes is a key element in maintaining cellular redox homeostasis and reducing oxidative damage. One of these enzymes is heme oxygenase 1 (HO-1). Increased homocysteine levels may affect the expression of intracellular defensive genes such as HO-1, which is induced in response to stressful conditions [10]. The HO-1 expression contributes to protection against oxidative liver damage and is a potential therapeutic target for hepato-protection [11]. Data indicate that anti-oxidant gene responsible for encoding this enzyme is coordinately regulated through consensus cis-acting element called anti-oxidant response element (ARE) in its 5'-flanking promoter region [12, 13].
Transcription factor Nrf2, a member of the cap'n collar family of transcription factors, is present in the cytoplasm attaching to an actin-binding protein named Klech-like ECH-associated protein [14]. After activation, it dissociates from Klech-like ECH-associated protein 1 and translocates into the nucleus, where it activates transcription of several detoxification and anti-oxidant genes [15]. It has been demonstrated that the Nrf2-dependent adaptive response provides a pivotal defense mechanism against oxidative stress [16]. However, a direct link between homocysteine-mediated HO-1 induction and activation of Nrf2 expression via stimulation of ARE-binding activity remains to be examined. The current study was designed to investigate whether homocysteine induces the expression of HO-1 in HepG2 cells, and whether this induction is mediated by ARE present within its promoter.
MATERIALS AND METHODS
Cell culture, treatment and cell viability assay. The HepG2 cells (National Cell Bank, Pasteur Institute of Iran) were grown in DMEM medium containing 10% heat inactivated fetal calf serum and 100 U/ml penicillin G sodium, 100 µg/ml streptomycin and L-glutamine in humidified atmosphere containing 5% CO2 at 37ºC. For RNA extraction and glutathione detection, the cells were seeded at a density of 5 × 105 per well in 6-well culture plates. For isolation of whole cell and nuclear protein extract, cells were treated in 25-cm2 flasks. D,L-homocysteine (Sigma Chemical Co, USA) was used at a final concentration of 50 µM corresponding to 25 µM L-homocysteine. The culture medium was replaced with fresh media and the different treatments were initiated. The untreated cells were used as control. The cells were treated with homocysteine for 15 min, 30 min, 45 min, 3 h, 6 h and 9 h. For cell viability assay, HepG2 cells (1 × 106 cells/ml) were incubated in control or test medium supplemented with 5% fetal calf serum at 37°C for 18 h. Cell viability was assessed by counting cells on a hemocytometer after suspension in PBS (pH 7) containing 0.5% Trypan blue stain. The data shows the means ± S.D. from three independent experiments.
Flow cytometry. Cellular glutathione (GSH) levels were measured using the thioltracker Violet GSH detection reagent (Invitrogen/Molecular Probes, Eugene, OR, USA) according to the manufacturer's protocol. Cells were seeded in a 6-well plate, incubated overnight and treated the following day. After designated treatment times, the cells were washed with PBS and incubated in Dulbecco's PBS containing 10 µM (final concentration) thioltracker Violet at 37ºC for 30 min. Afterwards, cells were analyzed on a flow cytometer (Partec, Germany). Analysis of cellular GSH content was restricted to PI negative (intact) cells.
RNA extraction and real time quantitative PCR. Total RNA was extracted from HepG2 cells using RNeasy cell mini kit (Qiagen GmbH, Hiden, Germany) according to the manufacturer's instruction. RNA samples with A260/A280 ratios above 1.8 were stored at -70°C for further analysis. Total RNA (3 µg) was subjected to reverse transcription using QuantiTect Revese Transcription Kit (Fermentase, Vilnius, Lithuania). Real time quantitative PCR was done using SYBR Premix Taq (Takara, Japan) and primer for HO-1 (QT00092645, QIAGEN GmbH, Hiden, Germany). For data normalization, the transcript for the constitutive gene product β-actin (QT01680476, QIAGEN GmbH, Hiden, Germany) was used. Each cycle consisted of denaturation at 95ºC for 5s and combined annealing and extension at 60ºC for 30 s. Each sample type was run in duplicate and each experiment was performed four times. The expression levels of the gene were quantified using REST 2009 Software using the real time PCR data.
Western-blot analysis. For nuclear extraction preparation, HepG2 cells were washed twice with PBS (pH 7.4). Cells were then harvested in 1 ml of PBS and centrifuged at 2,800 ×g at 4ºC for 10 min. The cell pellet was resuspended in 250 µl of cold buffer A consisting of 10 mM HEPES (pH 7.9),1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol and protease inhibitor cocktail (Roche, Mannheim, Germany). The pellet was then incubated on ice for 15 min to allow cells to swell. Then, 10% Nonidet P-40 was added and the tube was vortex-mixed for 10 s. The homogenate was then centrifuged at ,800 ×g at 4ºC for 10 min. The resulting nuclear pellet was resuspended in 30 µl of cold buffer B consisting of 5 mM HEPES (pH 7.9), 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol , 26% glycerol and protease inhibitor. The pellet was then incubated on ice for 15 min and vortex-mixed for 10-15 s every two min. The nuclear extract was finally centrifuged at 14800 ×g at 4ºC for 5 min. The protein concentration was measured by Bradford's method. For preparation of the whole cell extract, HepG2 cells not treated (control) or treated with homocysteine (as indicated previously) were washed in PBS, resuspended in 200 µl of radioimmuno precipitation assay buffer (150 mM sodium chloride, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris pH 8) and protease inhibitor cocktail. Afterward, it was incubated on ice for 10 min. The cells were then centrifuged at 9300 ×g for 10 min and the supernatant was taken as the whole cell extract. The amount of protein was measured with Bradford's method. Sample proteins from whole cell lysates (30 µg) and nuclear extracts (40 µg) were loaded onto 12% sodium dodecyl sulfate polyacrylamide gel for HO-1 and 10% polyacrylamide gel for Nrf2. They separated by electrophoresis, transferred to a nitrocellulose membrane and blocked. Immunoblot analysis was carried out using anti-HO-1 (1/1000) polyclonal antibody (Abcam Co., UK), anti-Nrf2 (1/500) polyclonal antibody (Abcam Co., UK). Anti-β-actin (1/3000) polyclonal antibody and anti-lamin B (1/2000) polyclonal antibody (Abcam Co., UK) were used to confirm the equal loading of proteins prepared from HepG2 cells after different treatments. The second antibody was goat polyclonal secondary antibody to rabbit IgG and was horseradish peroxidase conjugate. Visualization was accomplished by the enhanced chemiluminescence (ECL plus Western-blotting detection system, GE Healthcare). The intensity of bands was calculated with image J software (version 1.46a).
Electrophoretic mobility shift assay (EMSA). Synthetic complementary oligonucleotides (Sigma, USA) were 3'-biotinylated using the biotin 3'-end DNA labeling Kit (Pierce, USA) according to the manufacturer's instructions. After labeling, complementary strands were mixed together in an equimolar ratio and allowed to anneal at 37ºC for 2 h to form double-stranded probe. The sequences of the oligonucleotides, 5'-GATCTTTTATGCTGTGTCATGGTTT-3' and its complementary strand 5'-AAACCATGACACAGC ATAAAAGATC-3' were used as the probe. Binding reactions were carried out at room temperature for 20 min in the presence of 50 ng/µl poly (dI-dc), 0.05% Nonidet P-40, 5 mM MgCl2, 10m M EDTA and 2.5% glycerol in 1× binding buffer (Lightshift chemilu-minescent EMSA Kit, Pierce) using 20 fmol biotin end-labeled target DNA and 10 µg of nuclear extract (as described previously). In antibody super shift assays, 1 µl of 1µg/µl Nrf2 antibody (Santa Cruz, sc-722X) was added to the reaction mixture prior to the addition of the probe. A 200-fold excess of unlabeled oligonucleotide (competitor) was added where necessary. Assays were loaded onto native 5% polyacrylamide gels pre-electrophoresed in 0.5× Tris borate/EDTA for 60 min and electrophoresed at 100 V before being transferred onto a positively charged nylon membrane in 0.5× Tris borate/EDTA at 100 V for 30 min. The membrane was UV cross-linked and the biotin end-labeled probe was detected with streptavidin-horseradish peroxidase using a luminol enhancer solution (Lightshift chemiluminescent EMSA Kit, Pierce, USA) according to the manufacturer's instruction.
Statistical analysis. Data analysis was done using SPSS version 16. Data are presented as the means ± S.E. (standard error). Significance of differences between control and treated groups was determined by one-way analysis of variance (ANOVA). Least significant difference post-hoc analysis was performed for comparing the groups. For all analyses, P values less than 0.05 were considered significant.
RESULTS
Effect of homocysteine on cell viability. Exposure of HepG2 cells (1 × 106 cells/ml) to 50 µM homocysteine for up to 18 h had no effect on cell viability (control [live cells: 93 ± 0.7] vs. treatment group with 50 µM homocysteine [live cells: 93 ± 1.0]).
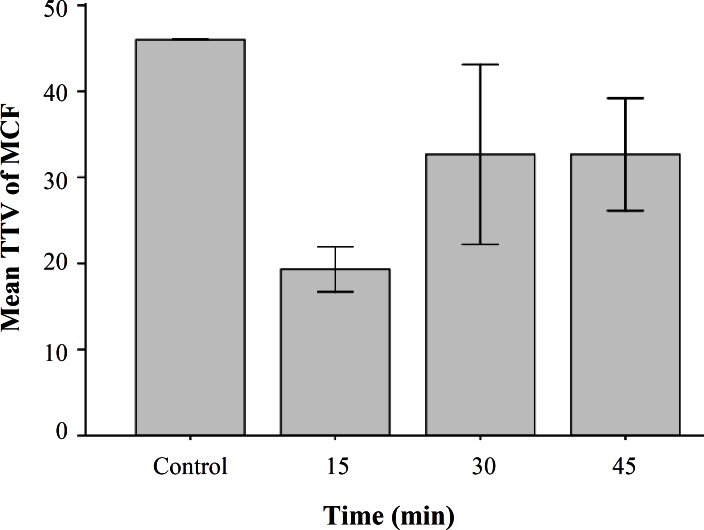
Homocysteine reduced GSH level. To determine if homocysteine cause oxidative stress in HepG2 cells, we evaluated the intercellular levels of the anti-oxidant thiol glutathione using flow cytometry. Incubations with 50 µM D,L-homocysteine resulted in a reduction in thioltracker violet dye fluorescence compared to that seen in untreated cells (Fig. 1). GSH content from 46% in untreated cells significantly decreased to about 19% an 35% within 15 min and 45 min of homocysteine exposure respectively (P<0.001). We did not observe any toxicity using this concentration of homocysteine.
Fig. 1.
Intercellular levels of cellular glutathione using flow cytometry in HepG2 cells exposed to homocysteine. HepG2 cells were treated with D/L homocysteine (50 µM) for the indicated times and harvested for flow cytometric analysis of intracellular glutathione content using mean channel fluorescence (MCF) of thioltracker Violet dye (TTV). Analysis of cellular GSH content was restricted to PI negative (intact) cells. Values are means ± S.E and statistically different from control
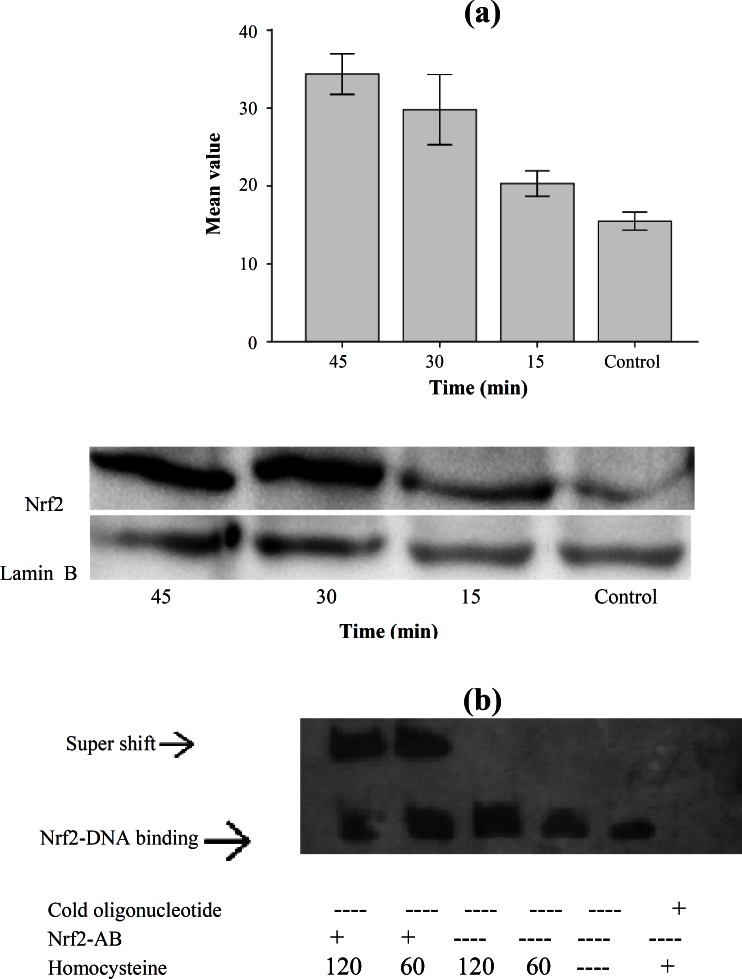
Homocysteine induced Nrf2 protein expression. Since Nrf2 nuclear translocation is a key event in the activation of Nrf2; we examined Nrf2 nuclear accumulation in HepG2 cells treated with homocysteine. The HepG2 cells were incubated with homocysteine (50 µM) and the nuclear cell extracts were electrophoresed. Proteins were transferred and the transferred proteins were visualized and analyzed by Image J software. Western-blot analysis showed that homocysteine led to Nrf2 accumulation in nuclear extract by 1.31, 1.92 and 2.21 folds in HepG2 cells treated with homocysteine after 15, 30 and 45 min in compared with control (Fig. 2A). The statistical analysis also showed that there was a significant difference between Nrf2 nuclear accumulation level in different treatment times (P<0.001).
Fig. 2.
Nrf2 protein expression in HepG2 cells exposed to homocysteine. (a) Western-lot of samples from HepG2. Values represent the means ± S.E. of three separate experiments. The bar graph shows the quantization of Nrf2 protein. The nuclear cell extracts were electrophoresed, protein transferred, and blotted with the polyclonal antibody Nrf2, a major band appearing at approximately 68 kDa. Lamin B was used as loading control. Exposure to homocysteine (50 µM) for the indicated time periods causes Nrf2 nuclear accumulation. Quantification of band intensity was performed by Image J (version 1.46a). (b) Gel shift assay using oligomers containing the HO-1 promoter-specific ARE-biding site and nuclear fractions of HepG2 cells that were exposed to homocysteine (50 µM) for the indicated time periods (min). Competitor (200 folds excess) and antibodies were applied as indicated
Homocysteine up-regulates HO-1 expression via activation of Nrf2. Several investigators have defined Nrf2 as a major transcription factor regulating ARE-driven phase 2 gene expression [12, 16]. Therefore, we attempted to determine whether homocysteine could activate Nrf2 in association with HO-1 up-regulation in HepG2 cells. To elucidate the role of Nrf2 in transcriptional activation of ARE, EMSA was performed using oligomers containing HO-1 promoter-specific ARE-binding site. HepG2 cells were exposed to homocysteine (50 µM) for the indicated time periods. Incubation of the nuclear extract from the cells with biotin-labeled HO-1 ARE oligonucleotide resulted in the enhanced HO-1 ARE-binding activity of Nrf2. The nuclear DNA-binding of Nrf2 was increased by homocysteine treatment. The ARE-binding complex appeared at 60 min of homocysteine treatment and was maintained through 120 min. Densitometric evaluation showed that level of nuclear DNA-binding of Nrf2 was increased by 1.8 and 3.3 folds after 60 and 120 min of the treatment. There was a significant difference between binding nuclear protein level at 1 and 2 h with control (P<0.01). Addition of an anti-Nrf2 antibody indicates involvement of the Nrf2 transcription factor at this site (Fig. 2B).
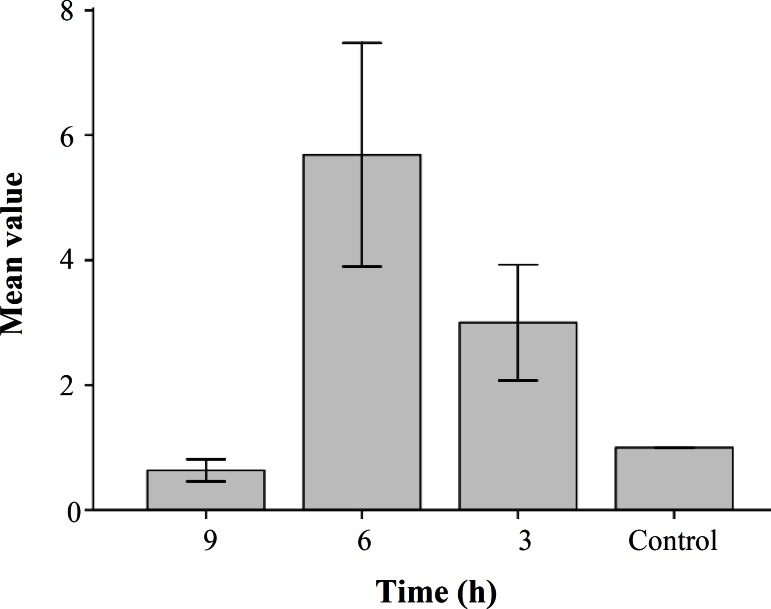
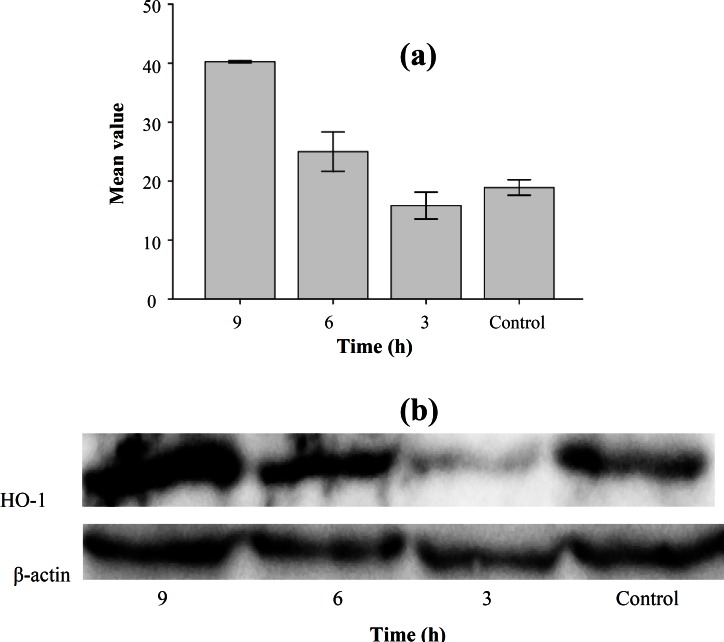
Effect of homocysteine on HO-1 mRNA expression and protein expression. To determine the effect of Nrf2 activation on gene expression, levels of HO-1 gene (one of the Nrf2 target genes) were analyzed. To evaluate HO-1 mRNA expression, cells were treated with homocysteine and quantitative real time RT-PCR analysis was performed. As shown in Figure 3, mRNA expression of HO-1 in HepG2 cells exposed to homocysteine was increased in 3 and 6 h compared with the control and declined to basal level after 9 h. Levels of HO-1 were increased 3 folds ±1.5 and 5.7 folds ±1.82 in 3 and 6 h, respectively. The statistical analysis showed that there was a significant difference between expression level at 6 h and control (P = 0.001). The statistical analysis also showed that there was a significant difference between expression levels in different treatment time (P<0.05). To confirm the result obtained from real time RT-PCR experiment, Western-blot analysis was performed. The HepG2 cells were treated with homocysteine (50 µM) for 3, 6 and 9 h. The cell extracts were electrophoresed, and then the separated proteins were incubated with primary and secondary antibodies. Finally, the transferred proteins were visualized. The results showed that the levels of HO-1 protein started to decline 3 h after exposure to homocysteine and then increased by 1.32 and 2.1 folds in HepG2 cells treated with homocysteine for 6 and 9 h, respectively (Fig. 4). The statistical analysis showed that there was a significant difference between HO-1 protein level at 6 and 9 h with control (P<0.001 and P = 0.004, respectively). The statistical analysis also showed that there was a significant difference between HO-1 protein level in different treatment time (P<0.001).
Fig. 3.
Quantitative real-time RT-PCR analysis of samples from HepG2. Values represent the means ± S.E. of four separate experiments. The bar graph shows the quantization of HO-1 gene expression
Fig. 4.
Expression of HO-1 protein in HepG2 cells exposed to homocysteine. (a) Western blot of samples from HepG2. Values represent the means ± S.E. of three separate experiments. The bar graph shows the quantization of HO-1 protein. (b) Western blot analysis of HO-1 in HepG2 cells. The cells were incubated with 50 μM homocysteine for 3, 6 and 9 h, and the cell extracts were electrophoresed, protein transferred, and blotted with the polyclonal antibody to HO-1. HO-1 is a major band appearing at approximately 32 kDa. β -actin was used as loading control
DISCUSSION
Elevated levels of homocysteine play an important role in the pathogenesis of various diseases including hepatic dysfunction. The underlying mechanisms of how hyperhomocysteinemia contributes to patho-genesis of liver injury are still poorly understood and may be multifactorial.
We used HepG2 cells as a cellular model to consider the role of the self-protecting systems in the cells which arises upon exposure to homocysteine. In the present study we used 50 µM D,L-homocysteine for the treatment of the cells. This concentration of homocysteine that can be found in hyper-homocysteinemic individual [17] did not affect overall cell number or viability as we showed by Trypan blue assay. Studies on the other cultured cells also indicate that exogenous homocysteine in concentration up to 5 mM do not impair cell viability and only increase intracellular levels of homocysteine [18, 19].
Using quantitative real time PCR analysis and Western-blotting, an increase in mRNA and protein levels for HO-1 was demonstrated. We identified that exposure to homocysteine at micro molar level is inducer of HO-1 in HepG2 cells. We found that HO-1 induction occurs via an ARE within the HO-1 promoter. Furthermore, we found that this induction is dependent on Nrf2-ARE pathway. The promoter region of the HO-1 gene contains multiple copies of ARE [13]. To elucidate the role of Nrf2 in transcriptional activation of ARE in response to homocysteine, electromobility shift assay (EMSA) was performed. Using EMSA, we were able to demonstrate that homocysteine induces increased binding of Nrf2 nuclear proteins in HepG2 cells to ARE containing oligonucleotide specific for the HO-1 promoter. It is well established that Nrf2 nuclear accumulation is indicator of Nrf2 activation [20, 21]. Consistent with the results of EMSA, Western-blot analysis and increasing Nrf2 nuclear proteins after stimulation with homocysteine revealed that transcriptional factor Nrf2 plays a critical role in cellular response against homocysteine-induced oxidative stress. Our data also indicated that Nrf2 is rapidly stabilized and mobilized to the nucleus in response to homocysteine treatment. The translocation of Nrf2 protein into nucleus following homocysteine treatment was associated with a marked increase in expression of HO-1.
Increased expression of HO-1 as an anti-oxidant has been reported in many studies [10, 11]. Induction of HO-1 expression under regulation of Nrf2-ARE is consistent with observation of Huang et al. [22], who reported that up-regulation of HO-1 expression inhibited oxidative stress in association with increased Nrf2-ARE-binding activity in hepatocytes. This study showed that some protective compounds inhibit oxidative stress in hepatocytes by up-regulation of HO-1. On the other hand, up-regulation of HO-1 in chronic liver diseases has been suggested in different studies [23-25]. In this context, the results obtained by Wei et al. [23] in patients with liver cirrhosis showed that HO-1 mRNA and protein expression were increased in hepatocytes of cirrhotic rat liver. Bessa et al. [24] showed that HO-1 mRNA expression in patients with chronic liver disease was significantly increased. There was a significant relationship between the increased HO-1 expression and oxidative stress biomarkers in patients with chronic liver diseases. Regarding clinical cases with non-alcoholic steatohepatitis, Malaguarnora et al. [25] showed that HO-1 expression was significantly increased in these patients, and the increase reflected the severity of the disease. Moreover non-alcoholic steatohepatitis patients with lower glutathione level exhibited higher expression of HO-1.
Regarding alcoholic steatohepatitis, Yao et al. [26] have shown that the induction of HO-1 by Ginkgo biloba is associated with a decrease in liver damage caused by ethanol feeding in rats. This is probably due to the enhanced anti-oxidative capacity against ethanol- induced oxidative stress and maintenance of cellular redox balance [27, 28]. Similar results were also obtained by Nepal et al. [29] in ethanol-induced apoptosis in HepG2 cells. They showed that HO-1 induction protects HepG2 cells from apoptosis. This protective effect is mediated via Nrf2 pathway.
Our results showed that Nrf2-dependent HO-1 induction is an adoptive response against oxidative stress induced by homocysteine. This was supported by a recent study by Abiko et al. [30] who reported that exposure of HepG2 cells to arsenic caused persistent induction of HO-1 accompanied by prolonged Nrf2 activation. Taken together, the results obtained from our study and the others [22, 27-30] showed that HO-1 induction not only is a general response in hepatocytes against oxidative stress but also is evoked by different protective compounds which have the potential to protect hepatic cells against oxidative stress-mediated chronic diseases.
Our study has suggested that the pathophysiological effects of homocysteine on hepatic cells are mediated via oxidative stress. The evidence from the present study suggests that the effects of homocysteine on hepatic cells are mediated via oxidative stress. First there was a significant decrease in glutathione (GSH) content in HepG2 cells treated with homocysteine. GSH is the most prevalent non-protein thiol and plays a fundamental role in defending against cellular injuries induced by oxidative stress in liver [31]. In this study, GSH levels significantly decreased after 15 min of treatment. This initial decline shows that to combat against oxidative stress induced by homocysteine, glutathione might be the first line of anti-oxidant defense. Perhaps this lowered GSH level which was caused the stimulation of Nrf2/ARE system and induced HO-1 expression. Second, homocysteine induces Nrf2/ARE pathway. This pathway is important in the cellular anti-oxidant defense system and is evoked when oxidative stress happens [20].Third, HO-1 mRNA and protein expression are up-regulated following oxidative stress and cellular injury [32].
Exposure to homocysteine causes GSH depletion, and changes in the redox status of the cell, which triggers nuclear translocation of Nrf2 and activation of the Nrf2/ARE pathway, in turn leading to up-regulation of HO-1. This suggests that an auto-regulation anti-oxidant mechanism exists against homocysteine and it is largely dependent on the activation of Nrf2 pathway. From this study, we conclude that HO-1 mRNA and protein expression are significantly increased in HepG2 cells exposed to homocysteine. Our results also show that this increase is under regulation of Nrf2/ARE pathway. As Nrf2/ARE pathway has been shown to provide protection against several oxidative impacts in different organs [33-35], induction of HO-1 under regulation of Nrf2/ARE might be a therapeutic option for liver dysfunctions in cases with severe hyperhomocys-teinemia. However, clinical studies are needed to clarify the exact role of HO-1 system in defending against homocysteine-induced oxidative stress and the potential clinical application of inducing the HO-1 system.
ACKNOWLEDGEMENTS
This work was financially supported by a grant No. 10501 from the Tehran University of Medical Science, Iran.
References
- 1.Aparna P, Betigeri AM, Pasupathi P. Homocysteine and oxidative stress markers and inflammation in patients with coronary artery disease. Int J Biol Med Res. 2010;1(4):125–9. [Google Scholar]
- 2.Kang SS, Wong PW, Malinow MR. Hyperhomocystein-emia as a risk factor for occlusive vascular disease. Annu Rev Nutr. 1992;12:279–98. doi: 10.1146/annurev.nu.12.070192.001431. [DOI] [PubMed] [Google Scholar]
- 3.Mudd SH, Levy HL, Kraus JP. Disorders of transsulfuration. In: Beaudet AL, Sly WS, Valle D, Child B, Kinzler KW, Vogelstein B, editors. The metabolic and molecular bases of inherited disease. Scriver CR. 2001. pp. 2007–56. [Google Scholar]
- 4.Hamelet J, Demuth K, Paul JL, Delabar JM, Janel N. Hyperhomocysteinemia due to cystathionine beta synthase deficiency induces dysregulation of genes involved in hepatic lipid homeostasis in mice. J Hepatol. 2007 Jan;46(1):151–9. doi: 10.1016/j.jhep.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Robert K, Nehme J, Bourdon E, Pivert G, Friquet B, Delcayre C, et al. Cystathionine beta synthase deficiency promotes oxidative stress, fibrosis, and steatosis in mice liver. Gastroenterology. 2005 May;128(5):1405–15. doi: 10.1053/j.gastro.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 6.Medici M, Peerson JM, Stabler SP, French SW, Gregory JF, Virata MC, et al. Impaired homocysteine trans-sulfuration is an indicator of alcoholic liver disease. J hepatol. 2010 Sep;53(3):551–7. doi: 10.1016/j.jhep.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu SC, Tsukamoto H, Mato JM. Role of abnormal methionine metabolism in alcoholic liver injury. Alcohol. 2002 Jul;27(3):155–62. doi: 10.1016/s0741-8329(02)00226-4. [DOI] [PubMed] [Google Scholar]
- 8.Choi J, Ou JH. Mechanisms of Liver Injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. Am J Physiol Gastrointest Liver Physiol. May;290(5):G847–51. doi: 10.1152/ajpgi.00522.2005. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury A, Santra A, Bhattacharjee K, Ghatak S, Saha DR, Dhali GK. Mitochondrial oxidative stress and permeability transition in Isoniazid and rifampicin induced liver injury in mice. J Hepatol. 2006 Jul;45(1):117–26. doi: 10.1016/j.jhep.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T, Morita K, Akaghi R, Sassa S. Heme oxygenase-1: A novel therapeutic target in oxidative tissue injury. Curr Med Chem. 2004 Jun;11(12):1545–61. doi: 10.2174/0929867043365080. [DOI] [PubMed] [Google Scholar]
- 11.Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006 Sep;39(50):479–91. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–60. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 13.Motterlini R, Green CJ, Foresti R. Regulation of heme oxygenase-1 by redox signals involving nitric oxide. Antioxid Redox Signal. 2002 Aug;4(4):615–24. doi: 10.1089/15230860260220111. [DOI] [PubMed] [Google Scholar]
- 14.Dinkova-kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005 Dec;18(12):1779–91. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 15.Lee OH, Jain AK, Papusha V, Jaiswal AK. An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance. J Biol Chem. 2007 Dec;282(50):36412–20. doi: 10.1074/jbc.M706517200. [DOI] [PubMed] [Google Scholar]
- 16.Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005 Nov-Dec;7(11-12):1664–73. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 17.Perta-Kajan J, Twardowski T, Jakubowski H. Mechanisms of homocysteine toxicity in humans. Amino Acids. 2007;32(4):561–72. doi: 10.1007/s00726-006-0432-9. [DOI] [PubMed] [Google Scholar]
- 18.Outinen PA, Sood SK, Pfeifer SI, Pamidi S, Podor TJ, Li J, et al. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood. 1999 Aug;94(3):959–67. [PubMed] [Google Scholar]
- 19.Outinen PA, Sood SK, Liaw PC, Sarge KD, Maeda N, Hirsh J, et al. Characterization of the stress-inducing effects of homocysteine. Biochem J. 1998 May;332(Pt 1):213–21. doi: 10.1042/bj3320213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med. 2004 Aug;37(4):433–41. doi: 10.1016/j.freeradbiomed.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Niture SK, Kaspar JW, Shen J, Jaiswal AK. Nrf2 signaling and cell survival. Toxicol Appl Pharmacol. 2010 Apr;244(1):37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang CS, Lii CK, Lin AH, Yeh YW, Yao HT, LI Cc, Et al. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch Toxicol. 2012 Jan;87(1):167–78. doi: 10.1007/s00204-012-0913-4. [DOI] [PubMed] [Google Scholar]
- 23.Wei CL, Lee KH, Khoo HE, Hon WM. Expression of haem oxygenase in cirrhotic rat liver. J Pathol. 2003 Mar;199(3):324–34. doi: 10.1002/path.1284. [DOI] [PubMed] [Google Scholar]
- 24.Bessa SS, Mohamed Ali EM, Abd El-Wahab AE, Nor El-Din SA. Heme oxygenase-1 mRNA expression in Egyptian patients with chronic liver disease. Hepat Mon. 2012 Apr;12(4):278–85. doi: 10.5812/hepatmon.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malaguarnera L, Madeddu R, Palio E, Arena N, Malaguarnera M. Heme oxygenase-1 levels and oxidative stress-related parameters in non-alcoholic fatty liver disease patients. J Hepatol. 2005 Apr;42(4):585–91. doi: 10.1016/j.jhep.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 26.Yao P, Li K, Song F, Zhou S, Sun X, Zhang X, et al. Heme oxygenase-1 upregulated by Ginkgo biloba extract: potential protection against ethanol-induced oxidative liver damage. Food Chem Toxicol. 2007 Aug;45(8):1333–42. doi: 10.1016/j.fct.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Yao P, Hao L, Nussler N, Lehmann A, Song F, Zhao J, et al. The protective role of HO-1 and its generated products (CO, bilirubin, and Fe) in ethanol-induced human hepatocyte damage. Am J Physiol Gastrointest Liver Physiol. 2009 Jun;296(6):G1318–23. doi: 10.1152/ajpgi.00555.2007. [DOI] [PubMed] [Google Scholar]
- 28.Nussler AK, Hao L, Knobeloch D, Yao P, Nussler NC, Wang Z, et al. Protective role of HO-1 for alcohol-dependent liver damage. Dig Dis. 2010;28(6):792–8. doi: 10.1159/000324287. [DOI] [PubMed] [Google Scholar]
- 29.Nepal S, Kim MJ, Subedi A, Lee ES, Young CS, Kim JA, et al. Globular adiponectin inhibits ethanol-induced apoptosis in HepG2 cells through heme oxygenase-1 induction. Biochem Pharmacol. 2012 Oct;84(7):974–83. doi: 10.1016/j.bcp.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Abiko Y, Shinkai Y, Sumi D, Kumagai Y. Reduction of arsenic-induced cytotoxicity through Nrf2/HO-1 signaling in HepG2 cells. J Toxicol Sci. 2010 Jun;35(3):419–23. doi: 10.2131/jts.35.419. [DOI] [PubMed] [Google Scholar]
- 31.DeLeve LD, Kaplowitz N. Importance and regulation of hepatic glutathione. Semin Liver Dis. 1990 Nov;10(4):251–66. doi: 10.1055/s-2008-1040481. [DOI] [PubMed] [Google Scholar]
- 32.Guo X, Shin VY, Cho Ch. Modulation of heme oxygenase in tissue injury and its implication in protection against gastrointestinal diseases. Life Sci. 2001 Nov;69(25-26):3113–9. doi: 10.1016/s0024-3205(01)01417-5. [DOI] [PubMed] [Google Scholar]
- 33.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid RedoxSignal. 2006 Jan-Feb;8(1-2):76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- 34.Chen XL, Kusch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004;10(8):879–91. doi: 10.2174/1381612043452901. [DOI] [PubMed] [Google Scholar]
- 35.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003 Apr;278(14):12029–38. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]