Abstract
Background: The present study investigated the functional maturity of oligodendrocyte derived from rat bone marrow stromal cells (BMSC). Methods: The BMSC were isolated from female Sprague-Dawley rats and evaluated for different markers, such as fibronectin, CD106, CD90, Oct-4 and CD45. Transdifferentiation of OLC from BMSC was obtained by exposing the BMSC to DMSO and 1 µM all-trans-retinoic acid during the pre-induction stage and then induced by heregulin (HRG), platelet-derived growth factor AA (PDGFR-α), fibroblast growth factor and T3. The neuroprogenitor cells (NPC) were evaluated for nestin, neurofilament 68, neurofilament 160 and glial fibrillary acidic protein gene expression using immunocytochemistry. The OLC were assessed by immunocytochemistry for O4, oligo2, O1 and MBP marker and gene expression of PDGFR-α was examined by RT-PCR. Results: Our results showed that the fibronectin, CD106, CD90, CD45 and Oct-4 were expressed after the fourth passage. Also, the yield of OLC differentiation was about 71% when using the O1, O4 and oligo2 markers. Likewise, the expression of PDGFR-α in pre-oligodendrocytes was noticed, while MBP expression was detected in oligodendrocyte after 6 days of the induction. Conclusion: The conclusion of the study showed that BMSC can be induced to transdifferentiate into mature OLC.
Key Words: Bone marrow stromal cell, Triiodothyronine, Platelet-derived growth factor α
Introduction
Oligodendrocytes produce the myelin ensheathing axons, which is essential for rapid conduction of nervous impulses [1]. Myelin damage can lead to the loss of nerve function, which is seen in many central nervous system damages such as multiple sclerosis and spinal cord injury. Oligo-dendrocyte-based cell therapy causes remyelination of a demyelinated axon [2]. In order to obtain oligo-dendrocytes with a high purity, primary mechanical enrichment was used, while the positive selection was done based on the presence of expressed markers on the cell surface [3]. Several studies have reported the differentiation of various types of stem cells into oligodendrocytes [4], such as olfactory ensheathing cells, neural stem cells, embryonic stem cells and bone marrow stromal cells (BMSC) [5]. Moreover, in vivo administration of BMSC could improve the oligodendrogenesis [6]. Sher and co-workers' finding [5] suggested the possibility of generating oligodendrocytes in vitro, which could be a source for cell therapy. One of the most interesting methods to perform a safe transplant was the use of an autologous source such as BMSC, since there was minimal immunological rejection [7]. Therefore, BMSC can be considered as a feasible source for neurological disorder therapy [8]. Although trans-differentiation of BMSC into oligodendrocytes was performed [9], the maturity has not been evaluated. The goal of this study was to improve the induction technique for in vitro transdifferentiation of BMSC into oligodendrocyte-like cells (OLC) and to evaluate their maturity.
MATERIALS AND METHODS
Bone marrow stromal cell extraction and culturing. Sprague-Dawley female rats, weighing 200-250 g (Razi Institute for Serums and Vaccine, Karaj, Iran), were kept under a controlled light/dark cycle (lights on at 6 a.m. and lights off at 6 p.m.) at a temperature of 18-25oC. All animal studies were conducted in accordance with the principles and procedures approved by the Ethical Committee of the Faculty of Medical Sciences, Tarbiat Modares University (Iran). The bone marrow was extracted from rats’ long (200-250 g) bones and cultured in DMEM/F12 (Stem Cell Technology Company, Tehran, Iran), supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin and 2 mM/ml L-glutamine and incubated in a humidified incubator with 5% CO2 at 37°C. The cells were then immunostained for fibronectin, CD45, CD90 and CD106. The neuron D and the stemness gene (Oct-4) were evaluated using RT-PCR.
Pre-induction. BMSC pre-induction and induction was done according to Kaka et al. [9]. Briefly, The BMSC were pre-induced (4th passage) using DMSO (2%) in DMEM/F12 medium without fetal bovine serum for 1 day. Then, the medium was replaced with DMEM/F12 containing 15% FBS and 1 µM all-trans-retinoic acid (Sigma-Aldrich, St. Luis, MO, USA) for the following 3 days. The BMSC were plated on gelatin-coated flasks (BD-Biosciences, India) or on 6-well plates containing gelatin-coated glass coverslips. The pre-induced cells were evaluated with nestin, neurofilament 68 (NF68), neurofilament 160 (NF160) and glial fibrilliary acidic protein (GFAP).
Induction. At the induction stage, the cells were initially incubated with DMEM/F12 medium containing 5 ng/ml platelet-derived growth factor AA (PDGF-AA) (Sigma-Aldrich, St. Luis, MO, USA), 10 ng/ml basic fibroblast growth factor (bFGF) (Sigma-Aldrich, St. Luis, MO, USA) and 200 ng/ml heregulin (HRG) (Sigma-Aldrich, St. Luis, MO, USA) for 2 days, followed by induction with different concen-trations of triiodothyronine (T3): 0, 5, 12.5, 25, 50, 100, 200 ng/ml (Sigma-Aldrich, St. Luis, MO, USA) for 2 days. The cells were immunostained for O4, oligo2, O1 and myelin basic protein (MBP), while platelet-derived growth factor α (PDGFR-α) was evaluated by RT-PCR.
Viability assay. The dye exclusion test (trypan blue exclusion test) was used to determine the number of viable cells that present in cell suspension. Live cells possess intact cell membranes that exclude certain dyes such as trypan blue, whereas dead cells do not. Cell suspension was simply mixed with dye and then visually examined to determine whether cells take up or exclude the dye. In the protocol presented here, a viable cell will have a clear cytoplasm, whereas a nonviable cell will have a blue cytoplasm.
Immunocytochemical method. The cultured cells were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 20 min. After permeabilization, cells were blocked by 5% bovine serum albumin for 30 min. Immunostaining was done on BMSC, pre-induced and induced cells. We used mouse anti- fibronectin monoclonal antibody (1:100), mouse anti-CD45 monoclonal antibody (1:100), mouse anti-CD106 monoclonal antibody (1:200) and mouse anti-CD90 monoclonal antibody (1:200) specific markers for mesenchymal stem cells. In addition, mouse anti-NF68 monoclonal antibody (1:50) and rat anti-NF160 monoclonal antibody (1:100), markers for neuroprogenitor cells NPC and neurons, respectively; rat anti-GFAP monoclonal antibody (1:100), a specific marker for astrocyte cells; mouse anti-O4 monoclonal antibody (1:100), mouse anti-O1 monoclonal antibody (1:100) and mouse anti-oligo2 monoclonal antibody (1:100), specific markers for immature OLC (all antibodies from EMD Millipore Corporation, Billerica, MA, USA) and mouse anti-MBP monoclonal antibody (1:1000, Covance, Berkeley and CA), a specific marker for mature oligodendrocytes were applied. The cells were incubated with FITC conjugated rabbit anti-mouse secondary antibody (1:100, EMD Millipore Corporation, Billerica, MA and USA) for 2 hours at room temperature. The cells were counterstained with 1:10,000 ethidium bromide (Sigma-Aldrich, St. Luis, MO, USA) for 1 min.
RT-PCR. The BMSC at the end of the fourth passage, rat neonate brain cells (controls), pre-induced cells and induced cells were evaluated for the expression of Oct-4, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), NeuroD and PDGFR-α genes. Using the RNX-Plus Kit (Fermentas Inc., Maryland, USA), 2 µg of total RNA from each sample was treated with DNase I (Fermentas Inc., Maryland, USA). The purity and integrity of the extracted RNA were evaluated by optical density measurements and electrophoresis on 1% agarose gel. Extracted RNA (1 µg) was converted to cDNA using the First Strand cDNA Synthesis Kit (Ferments Inc. Maryland, USA). An amount of 50 ng of cDNA was added to the PCR reaction for 35 cycles with denaturation at 95°C for 45 seconds, annealing at 58°C for 45 seconds, and elongation at 72°C for 30 seconds. After amplification, the products were separated on 2% agarose gel and visualized using ethidium bromide under UV light. Each experiment was repeated at least 3 times in order to ensure reproducibility. Primer sequences (forward and reverse), the size of the product and PCR conditions were as follows: expression of rat Oct-4 gene (a marker for BMSC stemness) was done using the 5´ AAGCTGCTGAAACAGAAGAGG 3´ Oct-4 forward primer and the 5´ACACGGTTCTCAATG CTAGTC3´ forward and backward primers, (210 bp, accession number: N001009178, annealing at 62ºC). GAPDH has served as an internal control gene: 5´ CCACAACTCTTCCATTCTC 3´ and 5´ CCAAGAT TCACGGTAGATAC 3´, forward and backward primers, respectively (400 bp, accession number: NP_002037.2, annealing at 58ºC). The expression of rat PDGFR-α gene, (a marker for immature oligodendrocytes) was assessed using the 5´CTAATTCACATTCGGAAGGTTG 3´ and 5´GGAC GATGGGCGACTAGAC 3´ (forward and backward primers, respectively, 190 bp, accession number: M63837.1, annealing at 57ºC). The expression of rat NeuroD (a neuroprogenitor marker) was assessed using the 5´CAGATGATGGCACAAAGGGTAG3´ and 5´G ACCGAGAGCATCGCATATTG 3´, (forward and backward primers, respectively, 220 bp, accession number NM-001105729.3, annealing at 59ºC).
Statistical analysis. All data were compared by one way analysis of variance (ANOVA) with Turkey’s test and Student’s t-test method.
RESULTS
After the fourth passage of the isolated BMSC from the rat bone marrow, the viability of the cells was 98.18 ± 0.94% (mean ± SEM). The cellular phenotype was characterized by immunocytochemistry for fibronectin, CD90 and CD106 (Fig. 1). The percentages of immunoreactive cells were 94.32 ± 0.45%, 95.48 ± 0.24% and 97.16 ± 0.82%, respectively. Also, none or very few of the cells expressed CD45, nestin, NF68, NF160, O4, O1, oligo2 and GFAP (data not shown).
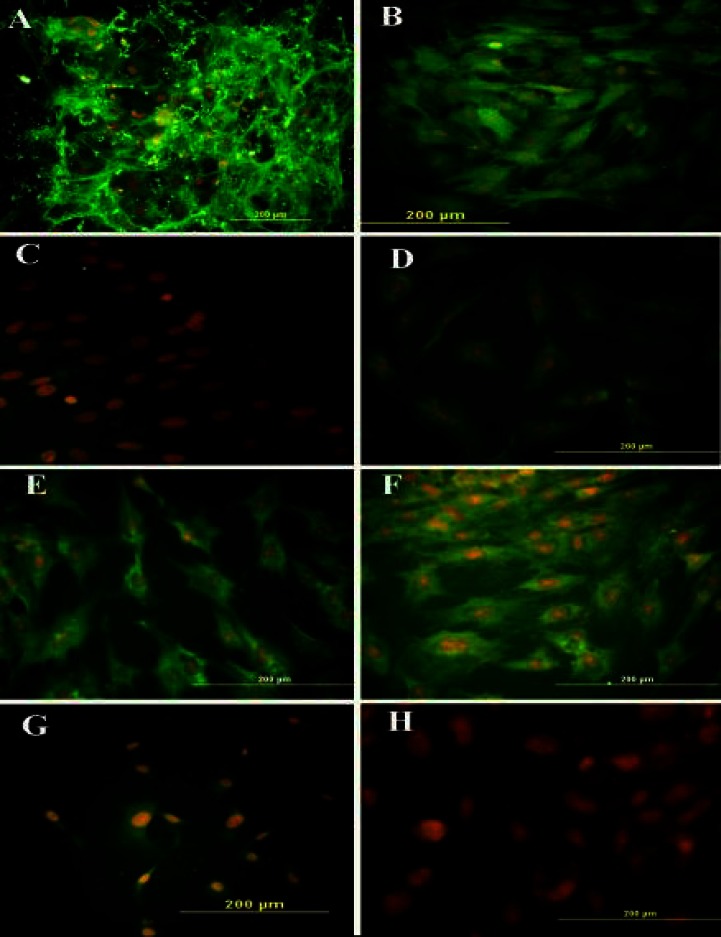
Fig. 1.
Immunocytochemistry representation of different cell markers. Following the treatment of bone marrow stromal cells with DMSO-retinoic acid at pre-induction stage, the cells were labeled with primary antibodies, followed by incubation of FITC-conjugated secondary antibody and counterstaining with ethidium bromide. Immunostained cells with (A) anti-fibronectin antibody, (B) anti-CD90 antibody, (C) anti-CD45 antibody, (D) anti-CD106 antibody, (E) anti-nestin, (F) anti-neurofilament 160 (NF-160) antibody, (G) anti-NF68 antibody and (H) anti-glial fibrilliary acidic protein antibody
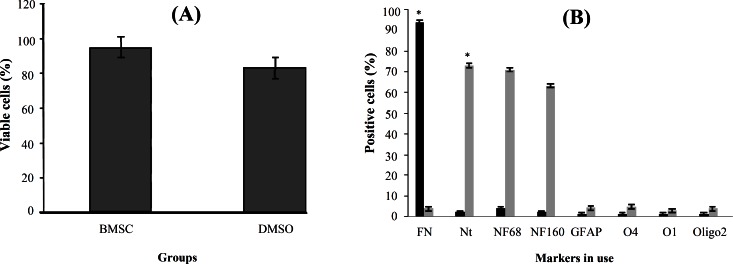
Pre-induction . The viability of BMSC treated with pre-inducers (79.36 ± 4.82%) (DMSO-retinoic acid) was significantly lower viability than untreated BMSC (94.26 ± 1.44%) (Fig. 2A). The immunostaining for Nestin, NF68 and NF160 was used to study the pre-induction of BMSC (Fig. 1). Also, the pre-induced cells expressed the NeuroD protein (Fig. 3, upper panel). The expression of fibronectin was decreased to 3.10 ± 0.49% during the pre-induction stage (Fig. 2B). The pre-induced BMSC were evaluated for nestin and NF68 antibodies (markers for NPC). The mean percentages of immunoreactive cells to nestin and NF68 were 73.2 ± 2.64% and 71.34 ± 2.65%, respectively (Fig. 2B).
Fig. 2.
Histograms of quantitative analysis of viability and different markers by immunocytochemistry at pre-induction and induction stages. (A) The percentage of the viable cells in untreated and treated bone marrow stromal cells (BMSC) at pre-induction stage. The histogram shows the viable cells in untreated BMSC (control) and the DMSO-retinoic acid treated cells. The viability was higher in the control group compared to the other group. (B) Quantitative analysis of different markers by immunocytochemistry at pre-induction stage. Black columns shows untreated BMSC and light gray columns shows BMSC treated with dimethyl sulfoxide-retinoic acid. The fibronectin (FN) shows a significant difference in each group. *indicates statistical significance between BMSC and the other experimental groups (P<0.001). Nt, nestin; NF, neurofilament; GFAP, glial fibrilliary acidic protein.
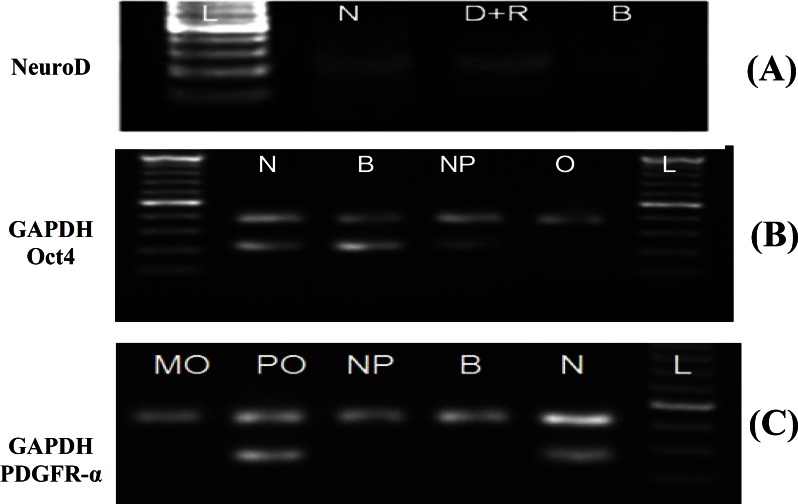
Fig. 3.
Electrophorograms of glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 400 bp), NeuroD (220 bp), Oct-4 (210 bp) and platelet-derived growth factor α (PDGFR-α, 190 bp) using RT-PCR. (A) The electrophorogram of NeuroD gene expression profile at positive control (rat neonate brain, N), pre-induction stage (dimethyl sulfoxide-retinoic acid, D+R) and a negative control (untreated bone marrow stromal cells [BMSC], B). (B) The electrophorogram of Oct-4 gene expression profile in rat neonate brain (N), BMSC (B), neuroprogenitor (NP) cells and oligodendrocyte-like cells (O). (C) The electrophorogram of PDGFR-α gene expression profile in mature oligodendrocyte (MO), pre-oligodendrocyte (PO), neuroprogenitor cells (NP), BMSC (B) and rat neonate brain (N). L shows DNA ladder
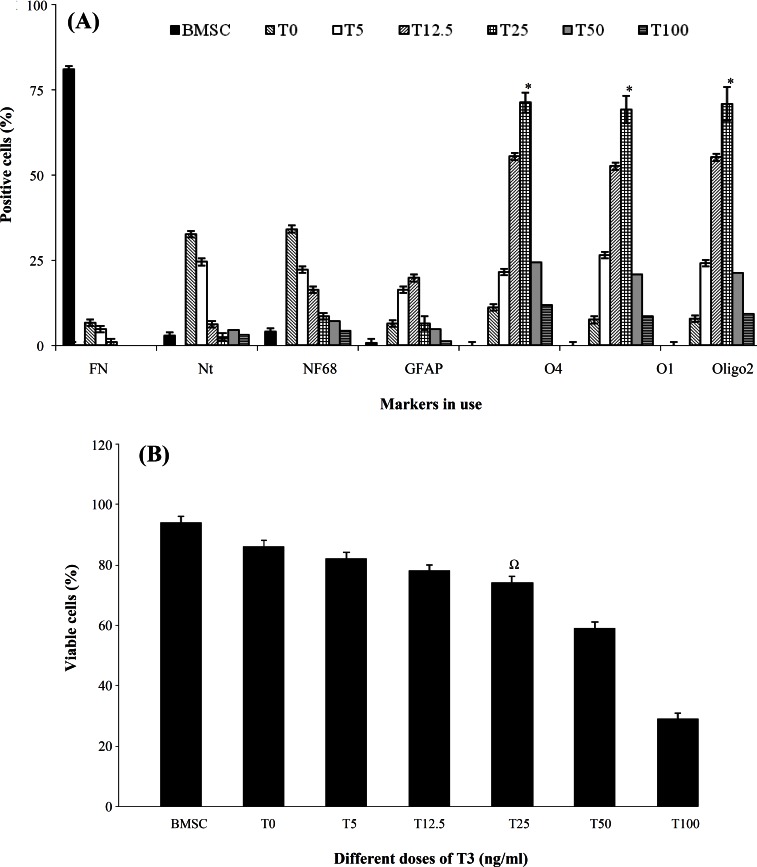
Induction. The expression of Oct-4 was not detected at induction stage, while untreated BMSC expressed this gene (Fig. 3). The percentages of undifferentiated and pre-induced cells were estimated by assessing immunopositivity for fibronectin, nestin, NF68, NF160, O1, oligo2, O4 and GFAP (Fig. 2B). Figure 3C depicts the marker expression in the induced BMSC (bFGF, PDGF and HRG), followed by treatment with T3 at 0, 5, 12.5, 25, 50, 100 and 200 ng/mL. The viability in the T3-treated group at the induction stage was the highest with no significant differences at concentrations of 25, 5 and 12.5 ng/mL. However, the viability was significantly lower at concentrations of 50, 100 and 200 ng/mL (Fig. 4D). Figure 5 demonstrates the immunostaining of BMSC induced by O4, oligo2 O1 and MBP and the transdifferentiated cells were immunoreactive to these markers.
Fig. 4.
Histograms of quantitative analysis of viability and different markers by immunocytochemistry at induction stages. (A) The percentage of the immunoreactive cells. Triiodothyronine (T3) was added to the induction stage in concentrations of 0, 5, 12.5, 25, 50, 100 and 200 ng/ml. Untreated bone marrow stromal cells (BMSC) were used as a control group. In addition to T3, the cells were pre-induced with dimethyl sulfoxide-retinoic acid and induced with platelet-derived growth factor (PDGF), fibroblast growth factor and heregulin. The assessed markers were: fibronectin (FN), nestin (Nt), neurofilament 68 (NF68), glial fibrilliary acidic protein (GFAP), O4, O1 and Oligo2. There were statistically significant differences among the groups in the same stage. A significant increase was noted for percentages of O4, O1 and oligo2 (P<0.05), while NF68 was significantly increased in the pre-induction stage. ∗indicates a significant difference among cells treated with 0, 5 and 12.5 ng/ml of T3. (B) The percentage of viable cells at the induction stage in a dose response of T3 (0, 5, 12.5, 25, 50, 100 and 200 ng/mL) as an inducer following pre-induction with PDGF, basic fibroblast growth factor and heregulin. Increased T3 concentrations (50, 100 and 200 ng/ml) in the medium caused lower viability. Ω indicates a significant difference among cells treated with 0, 5 and 12.5 ng/ml of T3
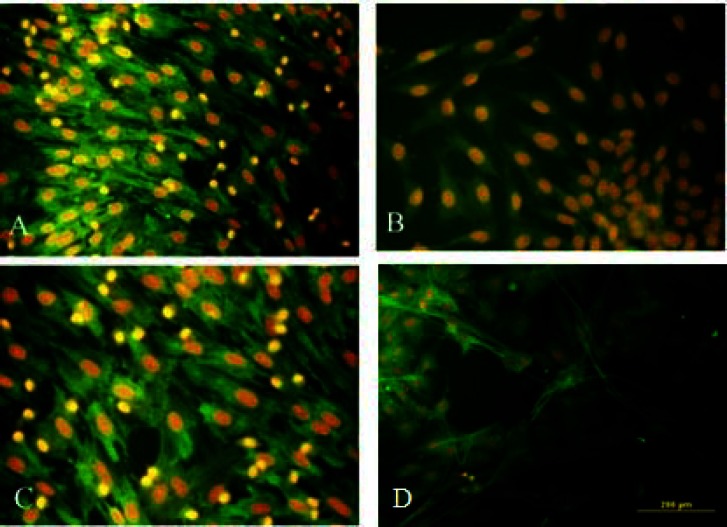
Fig. 5.
Immunocytochemistry analysis of the transdifferentiated bone marrow stromal cells into oligodendrocyte-like cells. Immunostained cells with (A) anti-O1, (B) anti-oligo2, (C) anti-O4 and (D) anti-myelin basic protein antibodies
RT-PCR. The results of RT-PCR of NeuroD, Oct-4 and PDGFR-α showed that NeuroD was expressed in pre-induced cells, Oct-4 expressed in BMSC and NPC and PDGFR-α expressed in pre-oligodendrocytes, while NPC showed no band (Fig. 4).
Discussion
The generation of OLC from BMSC necessitates the generation of NPC at the pre-induction stage and our results correlate with findings of Neri et al. [10], who reported the generation of oligodendrocyte from the neural stem cells.
The results of this research showed that when the fourth passage of the BMSC was grown in the existence of DMSO and followed by retinoic acid, it produced mainly NPC, which were expression to NeuroD. NeuroD expression was not detected in the untreated BMSC, which is consistent with the results of Yeu et al. [11], who found no detectable levels of NeuroD in undifferentiated BMSC. However, there were reports confirming the NeuroD gene expression in BMSC and in marrow stromal cells at very low levels [12]. This issue may be due to the spontaneous differentiation of BMSC into neuron-like cells [13]. The expression of NeuroD in rat neonate brain (used as a positive control in this study) is consistent with other investigations that have reported the expression of NeuroD in rat neonate brain [14]. Also, we identified its expression at pre-induction stage, which is in agreement with the finding of Naghdi et al. [15].
After performing pre-induction with DMSO-retinoic acid, the cells were induced by PDGF, bFGF and HRG, followed by different concentrations of T3 (dose response). The highest percentage of OLC was observed at T3 concentration of 25 ng/mL. However, at higher concentrations of T3, a viability decline occurred, probably due to toxicity. This finding was also reported by Kaka et al. [9]. On the other hand, the optimum expression of O1, O4 and Oligo2 was achieved at dose of 25 ng/mL. The differentiation of BMSC into OLC at this dose was 71%, while Kaka et al. [9] reported a 58% differentiation at T3 concentration of 10 ng/mL.
T3 increases morphological and functional maturation of postmitotic oligodendrocytes, as indicated by a well-developed network of branched processes and by the expression of myelin oligodendrocyte glycoprotein and glutamine synthetase [16]. The T3 deficiency changes the distribution of oligodendrocyte/myelin markers during oligodendroglial differentiation in vitro [9]. However, our results show that T3 at concentration of 25 ng/mL is more effective than 10-ng/mL concentration. Kang et al. [17] have generated NPC by exposing human embryonic stem cells to several inducing agents in DMEM/F12 supplement and then by subjecting them to growth factors EGF, PDGF, bFGF and T3 (30 ng/mL). By this method, they achieved 81% differentiation into OLC [17]. However, this result may be due to the source of cells used for differentiation, which resulted in a higher percentage of oligodendrocytes. The current study supports the results of previous research on the effect of T3 on the differentiation stem cells into OLC [18].
Our RT-PCR results showed that OLC expressed PDGFR-α, while untreated BMSC and NPC did not. It was first reported that PDGF could be the cause of proliferation and differentiation of oligodendrocyte progenitor cells. The PDGF and its receptors are widely expressed in both embryonic and adult central nervous system [19]. In previous studies, the expression of PDGF in OLC derived from different regions of the central nervous system such as brain has been studied, but the expression on bone marrow-derived OLC has not been studied [20]. Kaka et al. [9] could differentiate OLC from BMSC, but PDGFR gene expression was not evaluated. Our data showed that OLC-derived BMSC can express. Several studies show that PDGF has a neurotrophic effect, neuroprotection, and neuronal differentiation effect [21] on glial and neural cells. In vitro, PDGF is a survival factor and also an effective mitogen for oligodendrocyte progenitor cells, but it prompts only a limited number of cell division [22]. In our study, PDGF along with other inducers was added to culture medium that caused the differentiation and proliferation of NPC into OLC. We observed that apoptosis of OLC was increased in lack of PDGF.
Recently, it has been demonstrated that PDGF is a survival factor for oligodendrocyte progenitors in impaired oligodendrocyte development in the PDGF-A deficient mice [23]. These mice are characterized by a reduction in the numbers of PDGFR-α progenitors and oligodendrocytes in the spinal cord and cerebellum and the medulla. Injection of PDGF into mice greatly reduced apoptosis [24]. It seems that PDGF influences on development of oligodendrocyte progenitor cells by blocking the intracellular signaling pathways from the PDGF receptor to the gene expression [25]. We recognized olig2 expression at induction stage, which is in agreement with the findings of other investigations [26]. OPC and mature oligodendrocyte express Olig2 transcription factors, the importance of Olig2 for the differentiation of neural progenitor cells into the oligodendroglial lineage have discovered in other studies [27], over expression of Olig2 stimulates differentiation of neural stem cells into mature oligodendrocytes in vitro [28]. Disruption of Olig2 causes an interrupted development of oligodendrocytes in the spinal cord and lack of oligodendrocyte progenitor cells called NG2 cells [29]. Mature oligodendrocytes expressed low levels of Olig2 transcription factors [30]. The differentiated oligo-dendrocytes also expressed MBP, which is an important marker for maturity [31].
Moreover, MBP was considered as a functional marker for oligodendrocytes [32] and confirmed in time-lapse imaging [33]. The prior expression of PDGFR-α (at pre-oligodendrocytes) with immune-positivity for MBP (in differentiated oligodendrocytes) may indicate the necessity for the presence of PDGF for maturation of these cells [34]. Amur-Umarjee et al. [35] reported that MBP mRNA is transported into the oligodendrocytes. Double immunostaining showed the simultaneous expression of MBP and PDGFR-α, suggesting the importance of these receptors for the maturation of oligodendrocytes.
Our results show that transdifferentiation of BMSC into the OLC can be achieved by pre-induction with DMSO + retinoic acid and using bFGF, PDGF, HRG and T3 (25 ng/ml) as inducers. The OLC obtained through the described procedure can be used as a potential cell source for transplantation and treatment of central nervous system disorders.
ACKNOWLEDGMENTS
The project (Grant # 86-N-105) was funded by the Shefa Neurosciences Research Center at Khatam Al-Anbia Hospital, Tehran. We are also grateful for the support of the Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
References
- 1.Stoffel W, Bosio A. Myelin glycolipids and their functions. Curr Opin Neurobiol. 1997 Oct;7(5):654–61. doi: 10.1016/s0959-4388(97)80085-2. [DOI] [PubMed] [Google Scholar]
- 2.Keirstead HS. Stem cells for the treatment of myelin loss. Trends Neurosci. 2005 Dec;28(12):677–83. doi: 10.1016/j.tins.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Cizkova D, Cizek M, Nagyova M, Slovinska L, Novotna I, Jergova S, et al. Enrichment of rat oligodendrocyte progenitor cells by magnetic cell sorting. J Neurosci Methods. 2009 Oct;184(1):88–94. doi: 10.1016/j.jneumeth.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Sher F, Balasubramaniyan V, Boddeke E, Copray S. Oligodendrocyte differentiation and implantation: new insights for remyelinating cell therapy. Curr Opin Neurobiol. 2008 Oct;21(5):607–14. doi: 10.1097/WCO.0b013e32830f1e50. [DOI] [PubMed] [Google Scholar]
- 5.Bartolomei JC, Greer CA. Olfactory ensheathing cells: bridging the gap in spinal cord injury. Neurosurgery. 2000 Nov;47(5):1057–69. doi: 10.1097/00006123-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Li Y, Zhang ZG, Lu M. Bone marrow stromal cells increase oligodendrogenesis after stroke. J Cereb Blood Flow Metab. 2009 Jun;29(6):1166–74. doi: 10.1038/jcbfm.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang YH, Wang HF, Liu W, Wei B, Bing LJ, Gao YM. Insulin-producing cells derived from rat bone marrow and their autologous transplantation in the duodenal wall for treating diabetes. Anat Rec (Hoboken) 2009 May;292(5):728–35. doi: 10.1002/ar.20892. [DOI] [PubMed] [Google Scholar]
- 8.Chopp M, Li Y. Transplantation of bone marrow stromal cells for treatment of Central nervous system diseases. Adv Exp Med Biol. 2006;585:49–64. doi: 10.1007/978-0-387-34133-0_4. [DOI] [PubMed] [Google Scholar]
- 9.Kaka GR, Tiraihi T, Delshad A, Arabkheradmand J, Kazemi H. In vitro differentiation of bone marrow stromal cells into oligodendrocyte-like cells using triiodothyronine as inducer. Int J Neurosci. 2012 May;122(5):237–47. doi: 10.3109/00207454.2011.642037. [DOI] [PubMed] [Google Scholar]
- 10.Neri M, Maderna C, Ferrari D, Cavazzin C, Vescovi AL, Gritti A. Robust generation of oligodendrocyte progenitors from human neural stem cells and engraftment in experimental demyelination models in mice. PLoS ONE. 2010 Apr;5(4):e10145. doi: 10.1371/journal.pone.0010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeu IS, Lee HJ, Yi JS, Yang JH, Lee IW, Lee HK. The survival and migration pattern of the bone marrow stromal cells after intra cerebral transplantation in rats. Korean Neurosurg Soc. 2004 Jul;36(5):400–4. [Google Scholar]
- 12.Woodbury D, Reynolds K, Black IB. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J Neurosci Res. 2002 Sep;69(6):908–17. doi: 10.1002/jnr.10365. [DOI] [PubMed] [Google Scholar]
- 13.Ni WF, Yin LH, Lu J, Xu HZ, Chi YL, Wu JB, et al. In vitro neural differentiation of bone marrow stromal cells induced by Co cultured olfactory ensheathing cells. Neurosci Lett. 2010 May;475(2):99–103. doi: 10.1016/j.neulet.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 14.Cowan WM, Jessell TM, Zipursky SL. The Determination of the Neuronal Phenotype. London: Oxford University Press; 1997. Molecular and Cellular Approaches to Neural Development; pp. 26–43. [Google Scholar]
- 15.Naghdi M, Tiraihi T, Mesbah-Namin SA, Arabkheradmand J. Induction of Bone Marrow Stromal Cells into Cholinergic-Like Cells by Nerve Growth Factor. Iran Biomed J. 2009 Apr;13(2):117–23. [PubMed] [Google Scholar]
- 16.Jones SA, Jolson DM, Cuta KK, Mariash CN, Anderson GW. Triiodothyronine is a survival factor for developing oligodendrocytes. Mol Cell Endocrinol. 2003 Jan;199(1-2):49–60. doi: 10.1016/s0303-7207(02)00296-4. [DOI] [PubMed] [Google Scholar]
- 17.Kang SM, Cho MS, Seo H, Yoon CJ, Oh SK, Choi YM, et al. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007 Feb;25(2):419–24. doi: 10.1634/stemcells.2005-0482. [DOI] [PubMed] [Google Scholar]
- 18.Johe KK, Hazel TG, Muller T, Dugich-Diordievic MM, McKay RD. Single factors directs the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996 Dec;10(24):3129–40. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 19.Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD. Platelet-derived growth factor from astrocytes drives the clock that times oligo-dendrocyte development in culture. Nature. 1988 Jun;333(6173):562–5. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- 20.Smits A, Kato M, Westermark B, Nister M, Heldin CH, Funa K. Neurotrophic activity of platelet-derived growth factor (PDGF): Rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci USA. 1991 Sep;88(18):8159–63. doi: 10.1073/pnas.88.18.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietz K, Odin P, Funa K, Lindvall O. Protective effect of platelet derived growth factor against 6-hydroxydopamine-induced lesion of rat dopamine-ergic neurons in culture. Neurosci Lett. 1999 Feb;204(1-2):101–4. doi: 10.1016/0304-3940(96)12326-0. [DOI] [PubMed] [Google Scholar]
- 22.Gao FB, Apperly J, Raff M. Cell-intrinsic timers and thyroid hormone regulate the probability of cell-cycle withdrawal and differentiation of oligodendrocyte precursor cells. Dev Biol. 1998 May;197(1):54–66. doi: 10.1006/dbio.1998.8877. [DOI] [PubMed] [Google Scholar]
- 23.Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver Ar, Bostrom H, et al. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999 Feb;126(3):457–67. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- 24.Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993 May;118:283–95. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- 25.Hart IK, Richardson WD, Bolsover SR, Raff MC. PDGF and intracellular signaling in the timing of oligodendrocyte differentiation. J Cell Biol. 1989 Dec;109(6 Pt 2):3411–7. doi: 10.1083/jcb.109.6.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fumagalli M, Daniele S, Lecca D, Lee PR, Parravicini C, Fields RD, et al. Phenotypic changes, signaling pathway, and functional correlates of GPR17-expressing neural precursor cells during oligodendrocyte differentiation. J Biol Chem. 2011 Mar;286(12):10593–604. doi: 10.1074/jbc.M110.162867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, et al. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci. 2006;103(20):7853–8. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copray S, Balasubramaniyan V, Levenga J, de Bruijn J, Liem R, Boddeke E. Olig2 overe-xpression induces the in vitro differentiation of neural stem cells into mature oligodendrocytes. Stem Cells. 2006 Apr;24(4):1001–10. doi: 10.1634/stemcells.2005-0239. [DOI] [PubMed] [Google Scholar]
- 29.Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, et al. Common developmental requirement for Olig2 function indicates a motor neuron/oligo-dendrocyte connection. Cell. 2002 Apr;109(1):75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 30.Kitada M, Rowitch DH. Transcription factor co-expression patterns indicate heterogeneity of oligodendroglial subpopulations in adult spinal cord. Glia. 2006 Jul;54(1):35–46. doi: 10.1002/glia.20354. [DOI] [PubMed] [Google Scholar]
- 31.Lin T, Xiang Z, Cui L, Stallcup W, Reeves SA. New mouse oligodendrocyte precursor (mOP) cells for studies on oligodendrocyte maturation and function. J Neurosci Methods. 2006 Oct;157(2):187–94. doi: 10.1016/j.jneumeth.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Tracy ET, Zhang CY, Gentry T, Shoulars KW, Kurtzberg J. Isolation and expansion of oligo-dendrocyte progenitor cells from cryopreserved human umbilical cord blood. Cytotherapy. 2011 Jul;13(6):722–9. doi: 10.3109/14653249.2011.553592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ioannidou K, Anderson KI, Strachan D, Edgar JM, Barnett SC. Time-lapse imaging of the dynamics of CNS glial-axonal interactions in vitro and ex vivo. PLoS One. 2012 Jan;7(1):307–75. doi: 10.1371/journal.pone.0030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butt AM, Hornby MF, Ibrahim M, Kirvell S, Graham A, Berry M. PDGF-Alpha receptor and myelin basic protein mRNAs are not coexpressed by oligodendrocytes in vivo: a double in situ hybridization study in the anterior medullary velum of the neonatal rat. Mol Cell Neurosci. 1997;8(5):311–22. doi: 10.1006/mcne.1996.0590. [DOI] [PubMed] [Google Scholar]
- 35.Amur-Umarjee S, Schonmann V, Campagnoni AT. Neuronal regulation of myelin basic protein mRNA translocation in oligodendrocytes is mediated by platelet derived growth factor. Dev Neurosci. 1997;19(2):143–51. doi: 10.1159/000111200. [DOI] [PubMed] [Google Scholar]